Abstract
The CO2/O2 specificity factor of sucrose gradient purified ribulose 1,5-bisphosphate carboxylase/oxygenase from the C3-C4 intermediate plants Moricandia arvensis (79 ± 1) and Panicum milioides (89 ± 2) was similar to the respective values of the enzyme from the closely related C3 species, Moricandia foetida (80 ± 5) and Panicum laxum (86 ± 2). Thus, the kinetic properties of this bifunctional enzyme do not explain the reduced rates of photorespiration exhibited by either of these intermediate species.
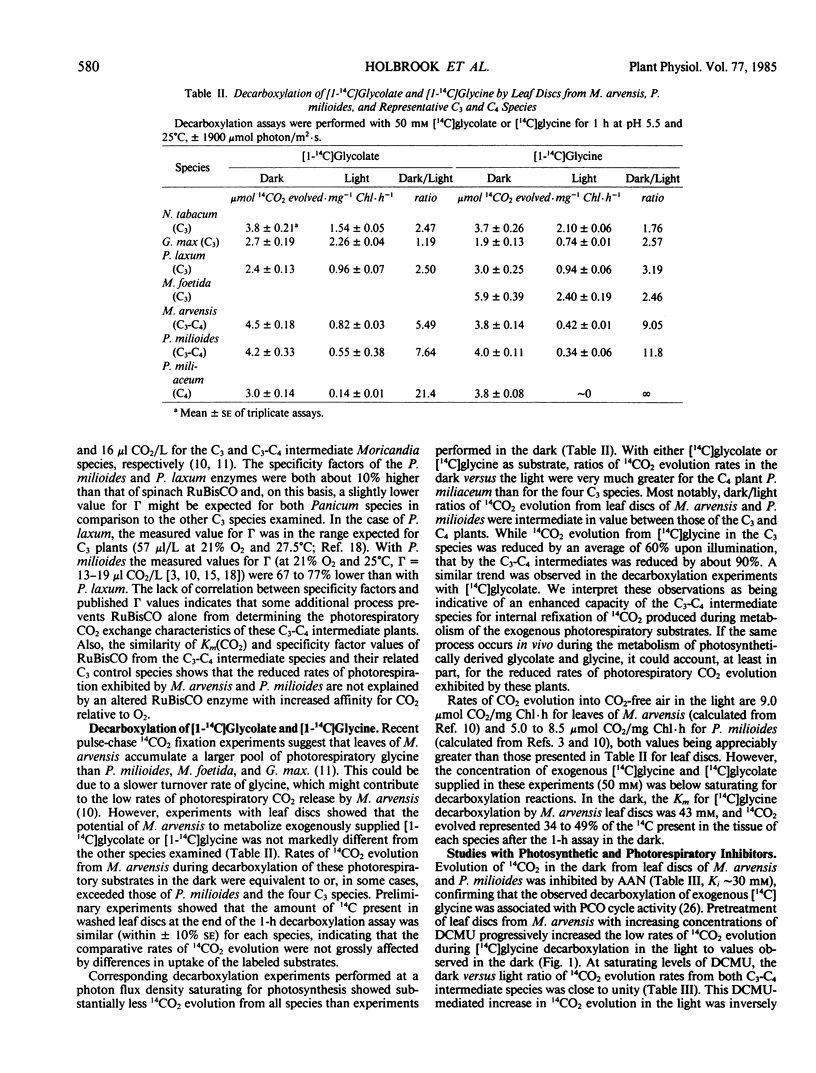
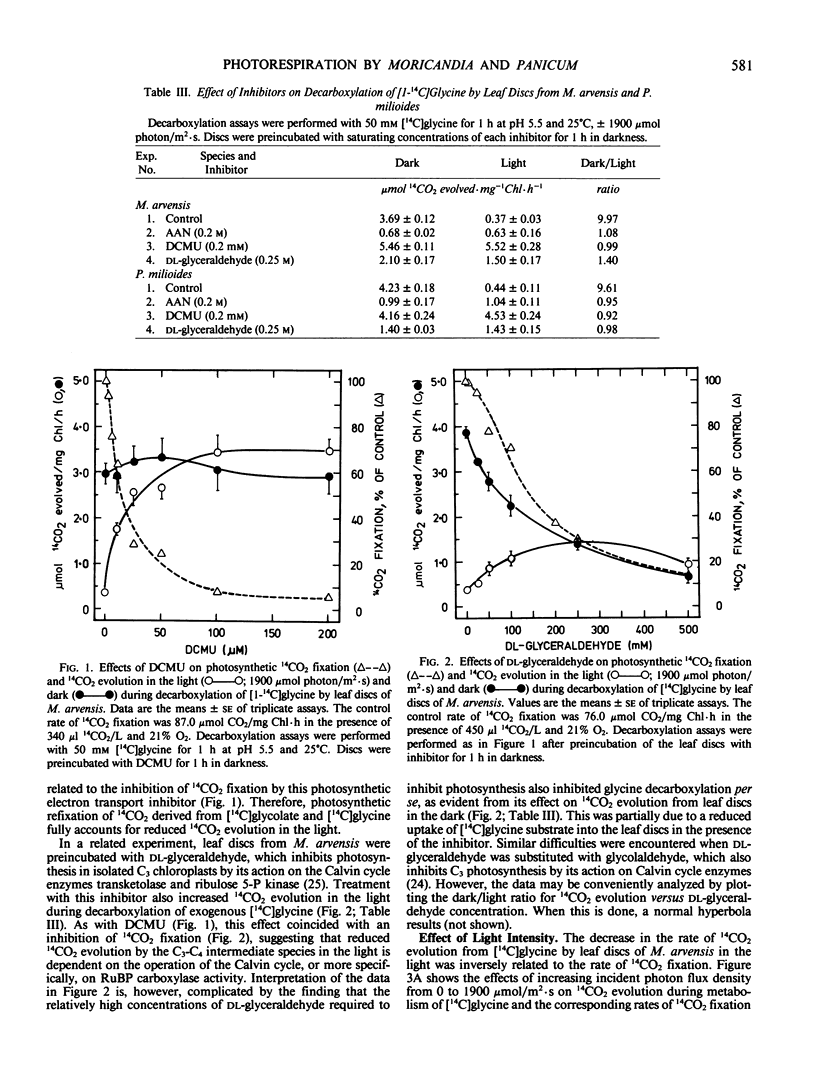
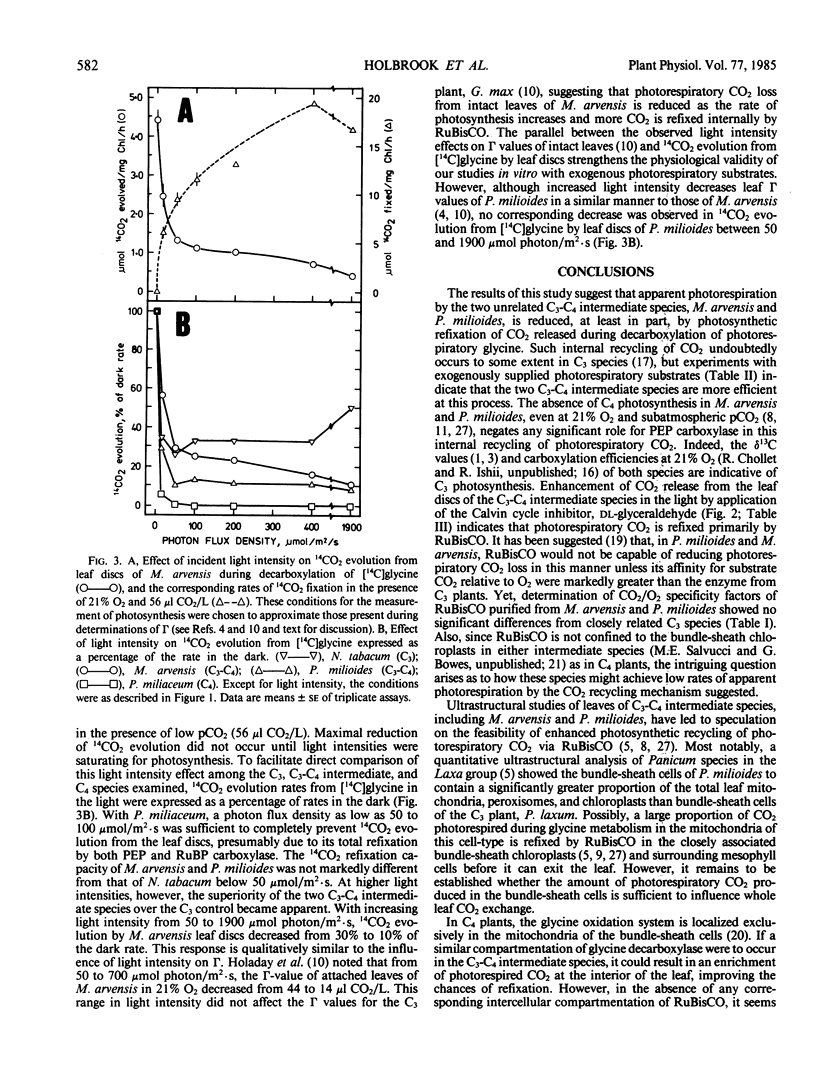
Dark/light ratios for aminoacetonitrile-sensitive 14CO2 evolution during decarboxylation of exogenous [1-14C]glycine by leaf discs had values of 9.0 with M. arvensis and 11.8 with P. milioides. Equivalent ratios with M. foetida and P. laxum were 2.5 and 3.2, respectively. Similar results were obtained using [1-14C]glycolate as the exogenous photorespiratory substrate, with dark/light 14CO2 evolution ratios for the C3-C4 and C3 leaf discs averaging 6.6 and 2.0, respectively. Stimulating photosynthetic CO2 fixation by progressively increasing photon flux density from 0 to 1900 micromoles per square meter per second caused a concomitant reduction in 14CO2 evolution from leaf discs of M. arvensis and P. milioides supplied with [1-14C]glycine. Conversely, inhibition of photosynthesis by DCMU or the Calvin cycle inhibitor dl-glyceraldehyde increased 14CO2 evolution in the light to rates comparable to those in the dark. The data suggest that P. milioides and M. arvensis are capable of a more efficient internal recycling of photorespiratory CO2 via ribulose bisphosphate carboxylase/oxygenase than closely related C3 plants, and that this may partially account for the reduced rates of apparent photorespiration by these intermediate species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown R. H., Bouton J. H., Rigsby L., Rigler M. Photosynthesis of Grass Species Differing in Carbon Dioxide Fixation Pathways : VIII. Ultrastructural Characteristics of Panicum Species in the Laxa Group. Plant Physiol. 1983 Feb;71(2):425–431. doi: 10.1104/pp.71.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. H., Morgan J. A. Photosynthesis of Grass Species Differing in Carbon Dioxide Fixation Pathways : VI. DIFFERENTIAL EFFECTS OF TEMPERATURE AND LIGHT INTENSITY ON PHOTORESPIRATION IN C(3), C(4), AND INTERMEDIATE SPECIES. Plant Physiol. 1980 Oct;66(4):541–544. doi: 10.1104/pp.66.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canvin D. T., Fock H. Measurement of photorespiration. Methods Enzymol. 1972;24:246–260. doi: 10.1016/0076-6879(72)24072-1. [DOI] [PubMed] [Google Scholar]
- Chollet R. Evaluation of the light/dark C assay of photorespiration: tobacco leaf disk studies with glycidate and glyoxylate. Plant Physiol. 1978 Jun;61(6):929–932. doi: 10.1104/pp.61.6.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaday A. S., Chollet R. Photosynthetic/Photorespiratory Carbon Metabolism in the C(3)-C(4) Intermediate Species, Moricandia arvensis and Panicum milioides. Plant Physiol. 1983 Nov;73(3):740–745. doi: 10.1104/pp.73.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D. B., Ogren W. L. A Sensitive Assay Procedure for Simultaneous Determination of Ribulose-1,5-bisphosphate Carboxylase and Oxygenase Activities. Plant Physiol. 1981 Feb;67(2):237–245. doi: 10.1104/pp.67.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D. B., Ogren W. L. Species variation in kinetic properties of ribulose 1,5-bisphosphate carboxylase/oxygenase. Arch Biochem Biophys. 1983 Dec;227(2):425–433. doi: 10.1016/0003-9861(83)90472-1. [DOI] [PubMed] [Google Scholar]
- Keck R. W. Differential Oxygen Response of Photosynthesis in Soybean and Panicum milioides. Plant Physiol. 1976 Oct;58(4):552–555. doi: 10.1104/pp.58.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. A., Brown R. H. Photosynthesis in Grass Species Differing in Carbon Dioxide Fixation Pathways: II. A Search for Species with Intermediate Gas Exchange and Anatomical Characteristics. Plant Physiol. 1979 Aug;64(2):257–262. doi: 10.1104/pp.64.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes D. M., Walker D. A. Photosynthesis by isolated chloroplasts. Inhibition by DL-glyceraldehyde of carbon dioxide assimilation. Biochem J. 1972 Aug;128(5):1147–1157. doi: 10.1042/bj1281147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K., Usuda H., Tsuzuki M., Schmitt M., Edwards G. E., Thomas R. J., Evert R. F. Influence of Nitrate and Ammonia on Photosynthetic Characteristics and Leaf Anatomy of Moricandia arvensis. Plant Physiol. 1982 Aug;70(2):616–625. doi: 10.1104/pp.70.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- Yeoh H. H., Badger M. R., Watson L. Variations in Kinetic Properties of Ribulose-1,5-bisphosphate Carboxylases among Plants. Plant Physiol. 1981 Jun;67(6):1151–1155. doi: 10.1104/pp.67.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]


