Abstract
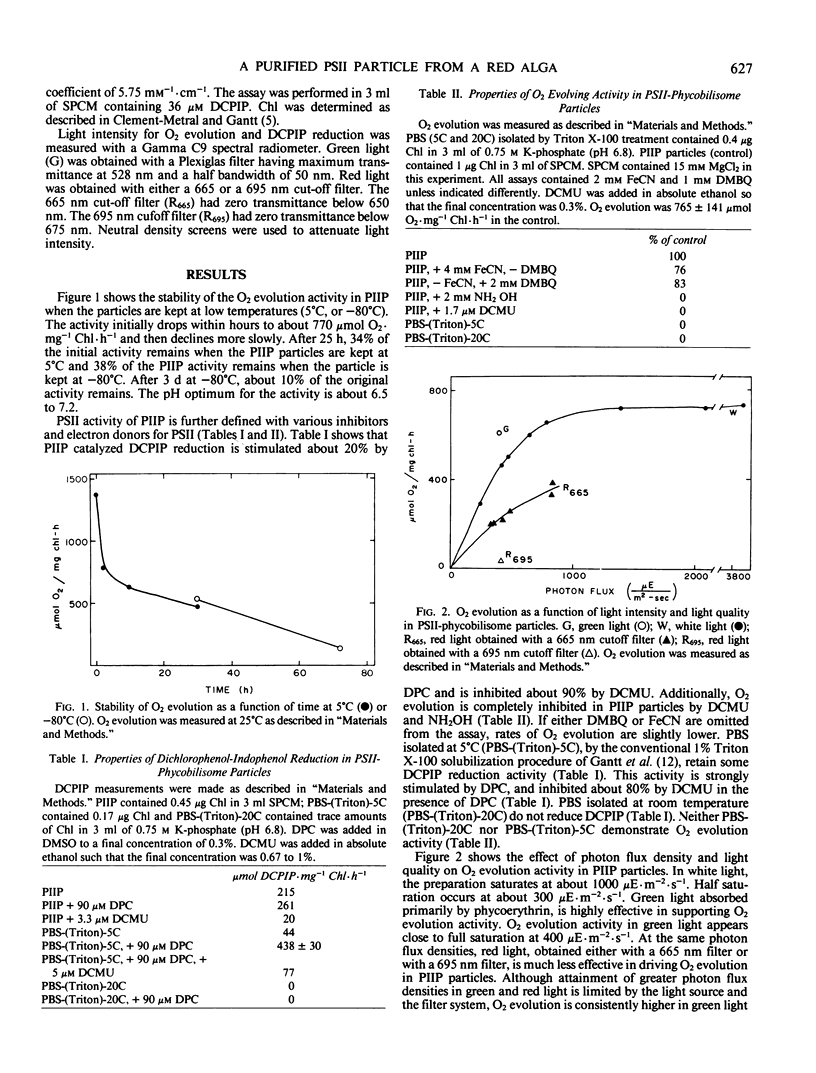
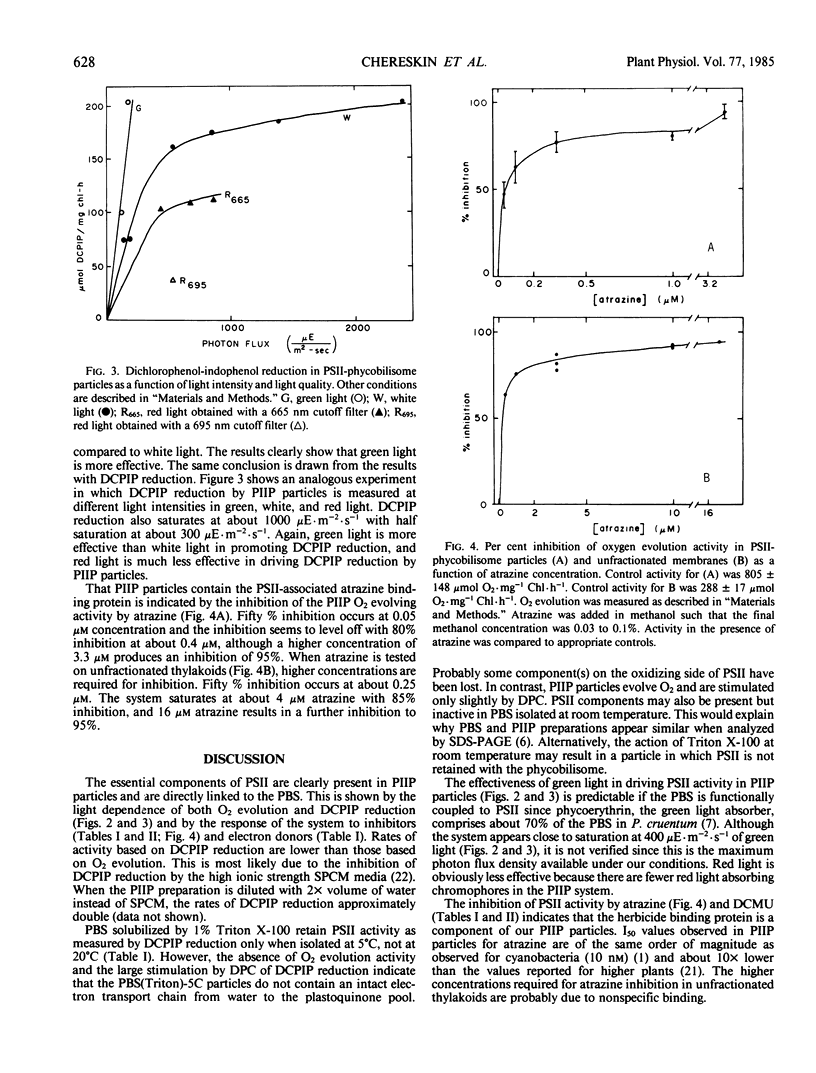
Detergent preparations isolated from thylakoids of the red alga Porphyridium cruentum, in a sucrose, phosphate, citrate, magnesium chloride medium consist of phycobilisomes and possess high rates of photosystem II activity. Characterization of these particles shows that the O2-evolving activity is stable for several hours and the pH optimum is about 6.5 to 7.2. Response of the system to light, electron donors and acceptors, and inhibitors verify that the observed activity, measured both as O2 evolution and 2,6-dichlorophenol-indophenol reduction, is due to photosystem II. Furthermore, photosystem II is functionally coupled to the phycobilisome in this preparation since green light, absorbed by phycobilisomes of P. cruentum, is effective in promoting both O2 evolution and 2,6-dichlorophenol-indophenol reduction. Photosystem II activity declines when light with wavelengths shorter than 665 nm is removed. Both 3-(3,4-dichlorophenyl)-1,1-dimethylurea and atrazine inhibit photosystem II activity in this preparation, indicating that the herbicide binding site is a component of the photosystem II-phycobilisome particle.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. M., Turnburke A. C., Lagace E. A., Steinback K. E. Effects of Photosystem II Herbicides on the Photosynthetic Membranes of the Cyanobacterium Aphanocapsa 6308. Plant Physiol. 1983 Feb;71(2):388–392. doi: 10.1104/pp.71.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth M. F., Gantt E. Phycobilisome-thylakoid Topography on Photosynthetically Active Vesicles of Porphyridium cruentum. Plant Physiol. 1981 Apr;67(4):608–612. doi: 10.1104/pp.67.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diner B. A., Wollman F. A. Functional Comparison of the Photosystem II Center-Antenna Complex of a Phycocyanin-less Mutant of Cyanidium caldarium with That of Chlorella pyrenoidosa. Plant Physiol. 1979 Jan;63(1):20–25. doi: 10.1104/pp.63.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diner B. A., Wollman F. A. Isolation of highly active photosystem II particles from a mutant of Chlamydomonas reinhardtii. Eur J Biochem. 1980 Sep;110(2):521–526. doi: 10.1111/j.1432-1033.1980.tb04894.x. [DOI] [PubMed] [Google Scholar]
- Diner B., Mauzerall D. Photosynthetic oxygen production and the size of the photosynthetic unit in a cellfree preparation from cyanidium caldarium. Biochim Biophys Acta. 1973 Jan 18;292(1):285–290. doi: 10.1016/0005-2728(73)90273-9. [DOI] [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A., Grabowski J., Zimmerman B. K. Phycobilisomes from blue-green and red algae: isolation criteria and dissociation characteristics. Plant Physiol. 1979 Apr;63(4):615–620. doi: 10.1104/pp.63.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley A. C. Effective Absorption Cross-Sections in Porphyridium cruentum: Implications for Energy Transfer between Phycobilisomes and Photosystem II Reaction Centers. Plant Physiol. 1984 Feb;74(2):451–454. doi: 10.1104/pp.74.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimuro M., Fujita Y. Estimation of chlorophyll a distribution in the photosynthetic pigment systems I and II of the blue-green alga Anabaena variabilis. Biochim Biophys Acta. 1977 Mar 11;459(3):376–389. doi: 10.1016/0005-2728(77)90039-1. [DOI] [PubMed] [Google Scholar]
- Mimuro M., Fujita Y. Excitation energy transfer between pigment system II units in blue-green algae. Biochim Biophys Acta. 1978 Dec 7;504(3):406–406. doi: 10.1016/0005-2728(78)90063-4. [DOI] [PubMed] [Google Scholar]
- Pakrasi H. B., Sherman L. A. A Highly Active Oxygen-Evolving Photosystem II Preparation from the Cyanobacterium Anacystis nidulans. Plant Physiol. 1984 Mar;74(3):742–745. doi: 10.1104/pp.74.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J., Cooper J. M. Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem. 1970 Feb;33(2):390–399. doi: 10.1016/0003-2697(70)90310-6. [DOI] [PubMed] [Google Scholar]
- Stewart A. C., Bendall D. S. Preparation of an active, oxygen-evolving photosystem 2 particle from a blue-green alga. FEBS Lett. 1979 Nov 15;107(2):308–312. doi: 10.1016/0014-5793(79)80396-8. [DOI] [PubMed] [Google Scholar]
- Vermaas W. F., Steinback K. E., Arntzen C. J. Characterization of chloroplast thylakoid polypeptides in the 32-kDa region: polypeptide extraction and protein phosphorylation affect binding of photosystem II-directed herbicides. Arch Biochem Biophys. 1984 May 15;231(1):226–232. doi: 10.1016/0003-9861(84)90382-5. [DOI] [PubMed] [Google Scholar]
- Vernon L. P., Shaw E. R. Photoreduction of 2,6-dichlorophenolindophenol by diphenylcarbazide: a photosystem 2 reaction catalyzed by tris-washed chloroplasts and subchloroplast fragments. Plant Physiol. 1969 Nov;44(11):1645–1649. doi: 10.1104/pp.44.11.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi A., Katoh S. Two chlorophyll-binding subunits of the photosystem 2 reaction center complex isolated from the thermophilic cyanobacterium Synechococcus sp. Arch Biochem Biophys. 1983 Sep;225(2):836–846. doi: 10.1016/0003-9861(83)90096-6. [DOI] [PubMed] [Google Scholar]


