Abstract
Three treatments which altered translocation rate were applied to cucumber plants: Girdling of source leaf petiole; removal of all aerial sinks; removal of all source leaves except one. Two different effects were observed, one short-term (during the initial 6 hours), and one long-term (detected after several days).
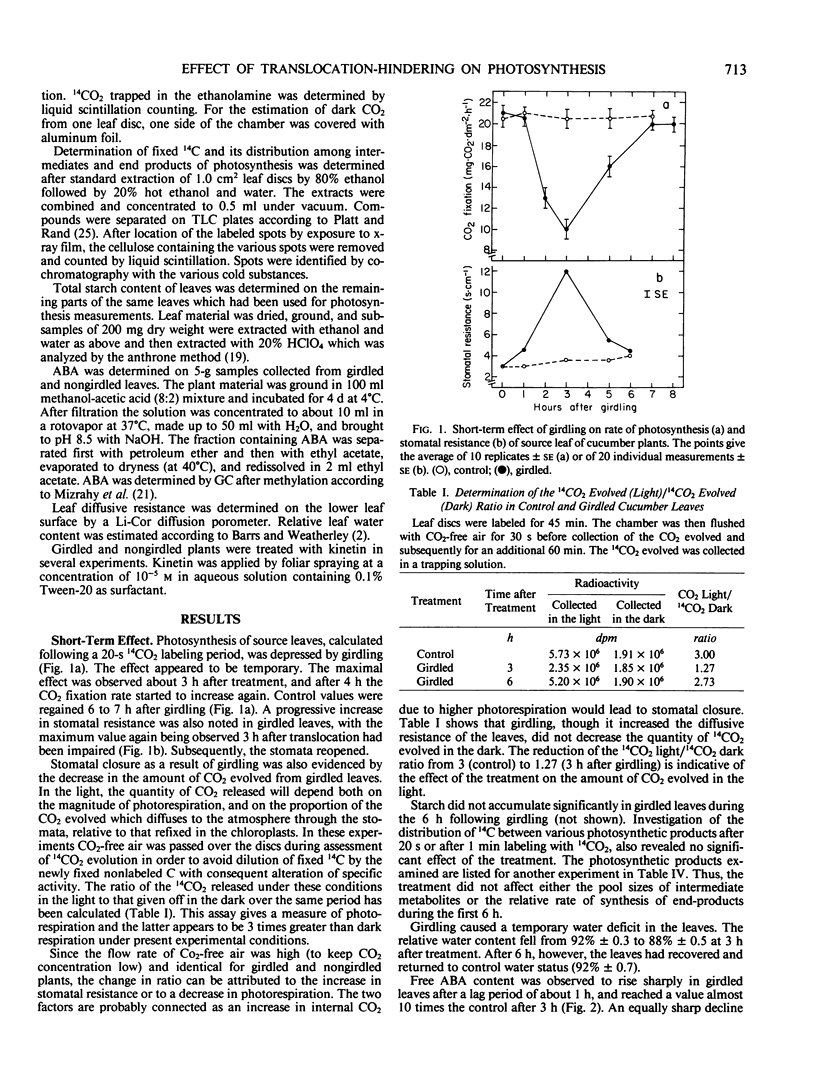
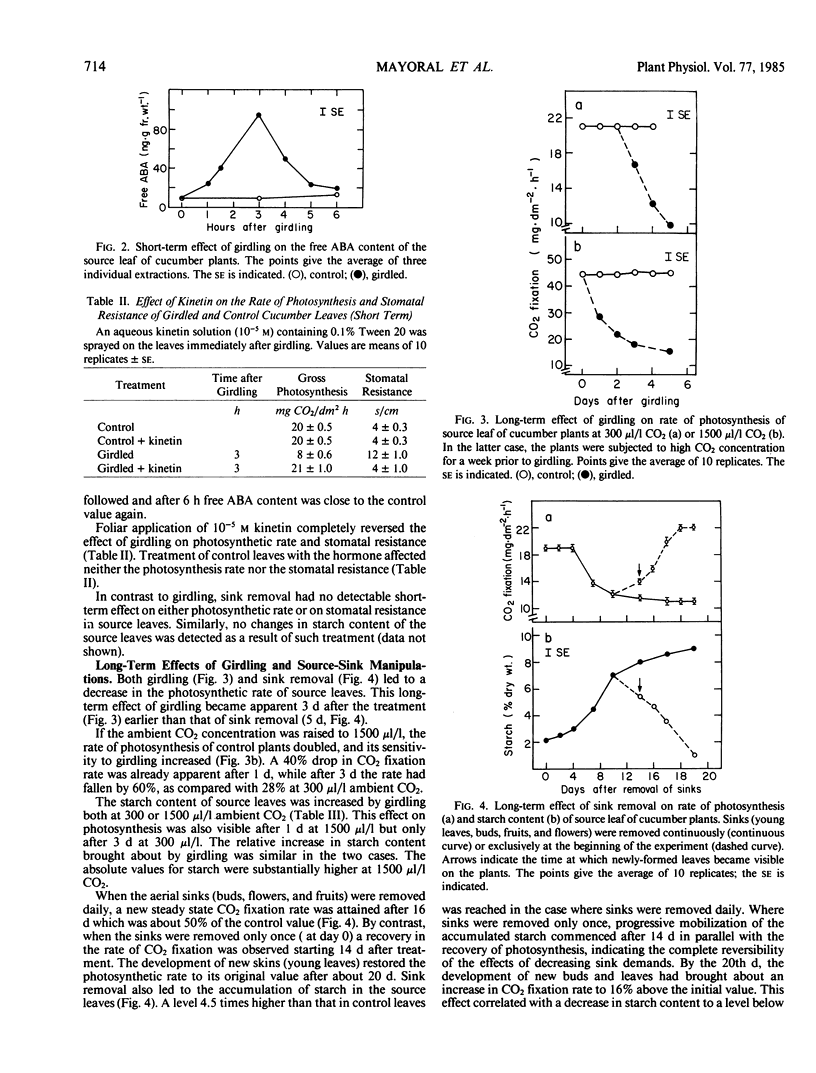
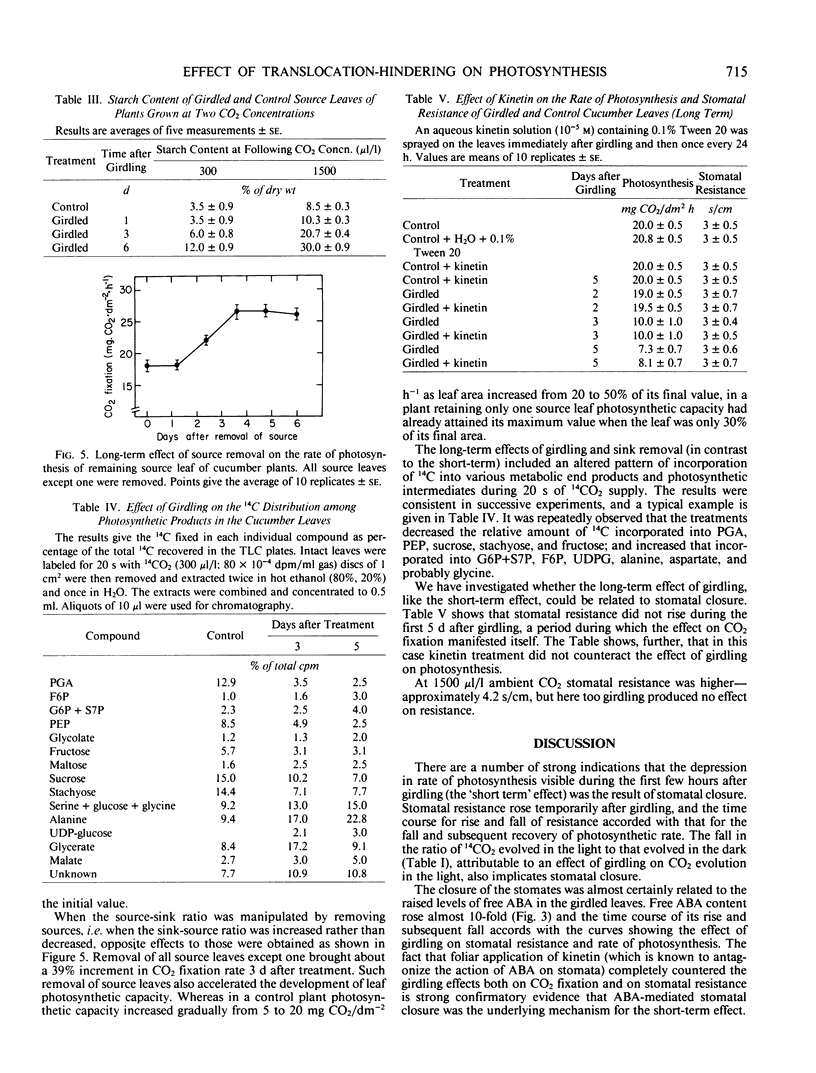
The short-term effect was observed exclusively in girdled leaves and involved a reduction in 14CO2 fixation rate paralleled by an increase in stomatal resistance. The effects were maximal after 3 hours with subsequent recovery. Stomatal closure apparently resulted from the 5 to 10% water deficit temporarily detected in girdled leaves which probably induced the observed temporary increases in abscisic acid content. Kinetin counteracted the effects of girdling.
The long-term effect was detected 3 days after girdling and 3 to 5 days after sink manipulation. An increase or decrease in 14CO2 fixation rate was observed when the sink-source ratio was increased or decreased respectively, accompanied by a respective decrease or increase in starch content. Changes in the relative amount of 14CO2 incorporated into various photosynthetic products were also observed. Stomatal closure was not involved, and the decrease in CO2 fixation was not counteracted by kinetin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beitler G. A., Hendrix J. E. Stachyose: an early product of photosynthesis in squash leaves. Plant Physiol. 1974 May;53(5):674–676. doi: 10.1104/pp.53.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers-Zampini C., Glamm A. B., Hoddinott J., Swanson C. A. Alterations in source-sink patterns by modifications of source strength. Plant Physiol. 1980 Jun;65(6):1116–1120. doi: 10.1104/pp.65.6.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Nafziger E. D., Koller H. R. Influence of Leaf Starch Concentration on CO(2) Assimilation in Soybean. Plant Physiol. 1976 Apr;57(4):560–563. doi: 10.1104/pp.57.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter T. L., Brun W. A. Stomatal closure and photosynthetic inhibition in soybean leaves induced by petiole girdling and pod removal. Plant Physiol. 1980 May;65(5):884–887. doi: 10.1104/pp.65.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb K. L., Burley J. W. Stachyose Translocation in Plants. Plant Physiol. 1964 Nov;39(6):973–977. doi: 10.1104/pp.39.6.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe H. H., Prosser R. J. Influence of temperature gradients on leaf water potential. Plant Physiol. 1977 Feb;59(2):256–258. doi: 10.1104/pp.59.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]


