Abstract
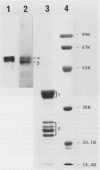
In vitro studies to explore the biosynthesis of 11S globulin developing cotyledons of pumpkin (Cucurbita sp.) demonstrated that 11S globulin is synthesized on membrane-bound polysomes. Mr of the translation products (preproglobulin) synthesized by the poly(A)+-RNA isolated from developing cotyledons were determined to be 64,000 and 59,000, which are larger than those of the mature globulin subunit (62,000 and 57,000). Preproglobulin is then cotranslationally processed by cleavage of the signal peptide to produce proglobulin. In vivo pulse-chase experiments showed the sequential transformation of the single-chain proglobulin to mature globulin subunit (disulfide-linked doublet polypeptides) indicating posttranslational modification of the proglobulin.
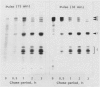
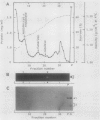
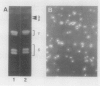
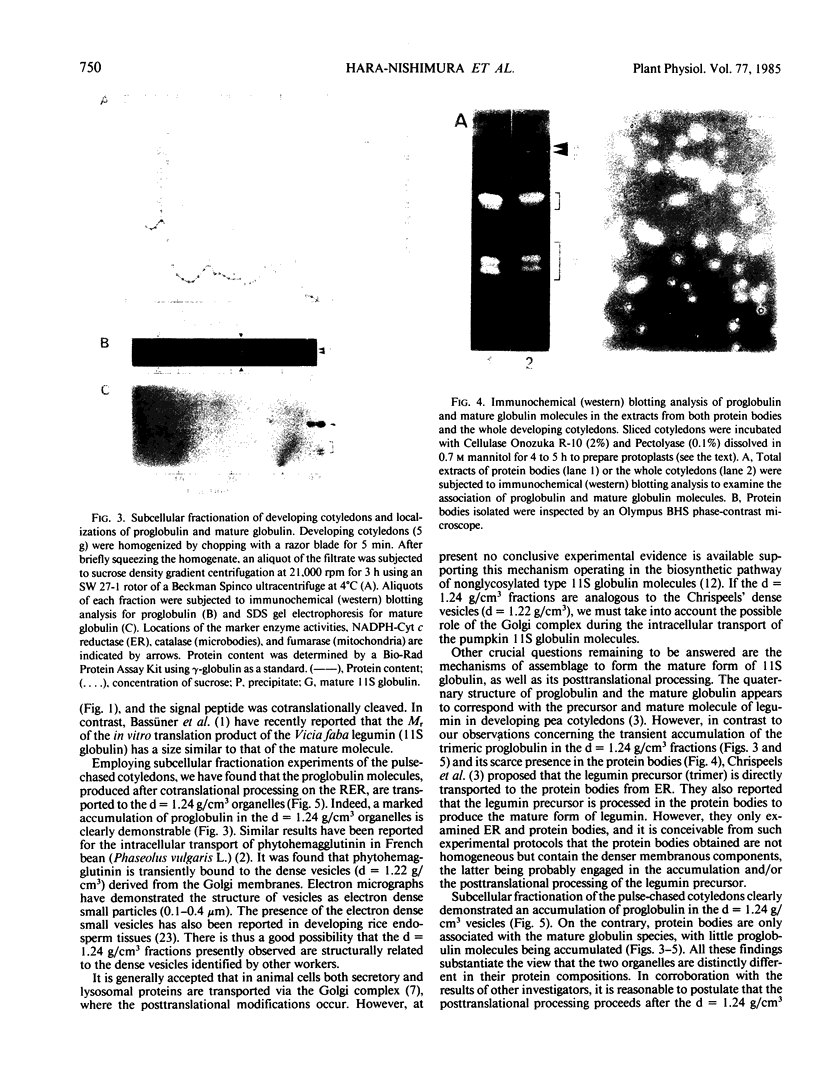
Subcellular fractionation of the pulse-chased intact cotyledons showed that the [35S]methionine label is detectable in proglobulin in rough endoplasmic reticulum shortly after the pulse label. With time, the labeled proteins move into other cellular fractions: proglobulin in the density = 1.24 grams per cubic centimeter fractions after 30 minutes and mature globulin subunit associated with protein bodies after 1 to 2 hours. The distribution of proglobulin in sucrose density gradients did not correspond with those of catalase (microbody marker) or fumarase (mitochondria marker). An accumulation of proglobulin occurred in the density = 1.24 grams per cubic centimeter fractions, whereas the mature globulin was scarcely detectable in this fraction. In contrast, proglobulin was not detected by immunochemical blotting analysis in the protein bodies prepared under the mild conditions from cotyledon protoplasts. The results suggest that the d = 1.24 grams per cubic centimeter fractions are engaged in the translocation of proglobulin into the protein bodies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrispeels M. J., Higgins T. J., Spencer D. Assembly of storage protein oligomers in the endoplasmic reticulum and processing of the polypeptides in the protein bodies of developing pea cotyledons. J Cell Biol. 1982 May;93(2):306–313. doi: 10.1083/jcb.93.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Carroll R. J., Steiner D. F. Conversion of proinsulin to insulin: involvement of a 31,500 molecular weight thiol protease. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4613–4617. doi: 10.1073/pnas.79.15.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura I., Nishimura M., Matsubara H., Akazawa T. Suborganellar localization of proteinase catalyzing the limited hydrolysis of pumpkin globulin. Plant Physiol. 1982 Sep;70(3):699–703. doi: 10.1104/pp.70.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins T. J., Chrispeels M. J., Chandler P. M., Spencer D. Intracellular sites of synthesis and processing of lectin in developing pea cotyledons. J Biol Chem. 1983 Aug 10;258(15):9550–9552. [PubMed] [Google Scholar]
- Larkins B. A., Bracker C. E., Tsai C. Y. Storage Protein Synthesis in Maize: Isolation of Zein-synthesizing Polyribosomes. Plant Physiol. 1976 May;57(5):740–745. doi: 10.1104/pp.57.5.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Miyata S., Okamoto K., Watanabe A., Akazawa T. Enzymic Mechanism of Starch Breakdown in Germinating Rice Seeds: 10. IN VIVO AND IN VITRO SYNTHESIS OF alpha-AMYLASE IN RICE SEED SCUTELLUM. Plant Physiol. 1981 Dec;68(6):1314–1318. doi: 10.1104/pp.68.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira M. A., Hermodson M. A., Larkins B. A., Nielsen N. C. Partial characterization of the acidic and basic polypeptides of glycinin. J Biol Chem. 1979 Oct 10;254(19):9921–9926. [PubMed] [Google Scholar]
- Nishimura M., Beevers H. Hydrolases in vacuoles from castor bean endosperm. Plant Physiol. 1978 Jul;62(1):44–48. doi: 10.1104/pp.62.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plietz P., Damaschun G., Müller J. J., Schwenke K. D. The structure of 11-S globulins from sunflower and rape seed. A small-angle X-ray scattering study. Eur J Biochem. 1983 Feb 1;130(2):315–320. doi: 10.1111/j.1432-1033.1983.tb07154.x. [DOI] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaessen R. T., Kreike J., Groot G. S. Protein transfer to nitrocellulose filters. A simple method for quantitation of single proteins in complex mixtures. FEBS Lett. 1981 Feb 23;124(2):193–196. doi: 10.1016/0014-5793(81)80134-2. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J., Nishimura M., Akazawa T. Maturation of catalase precursor proceeds to a different extent in glyoxysomes and leaf peroxisomes of pumpkin cotyledons. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4809–4813. doi: 10.1073/pnas.81.15.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]