Abstract
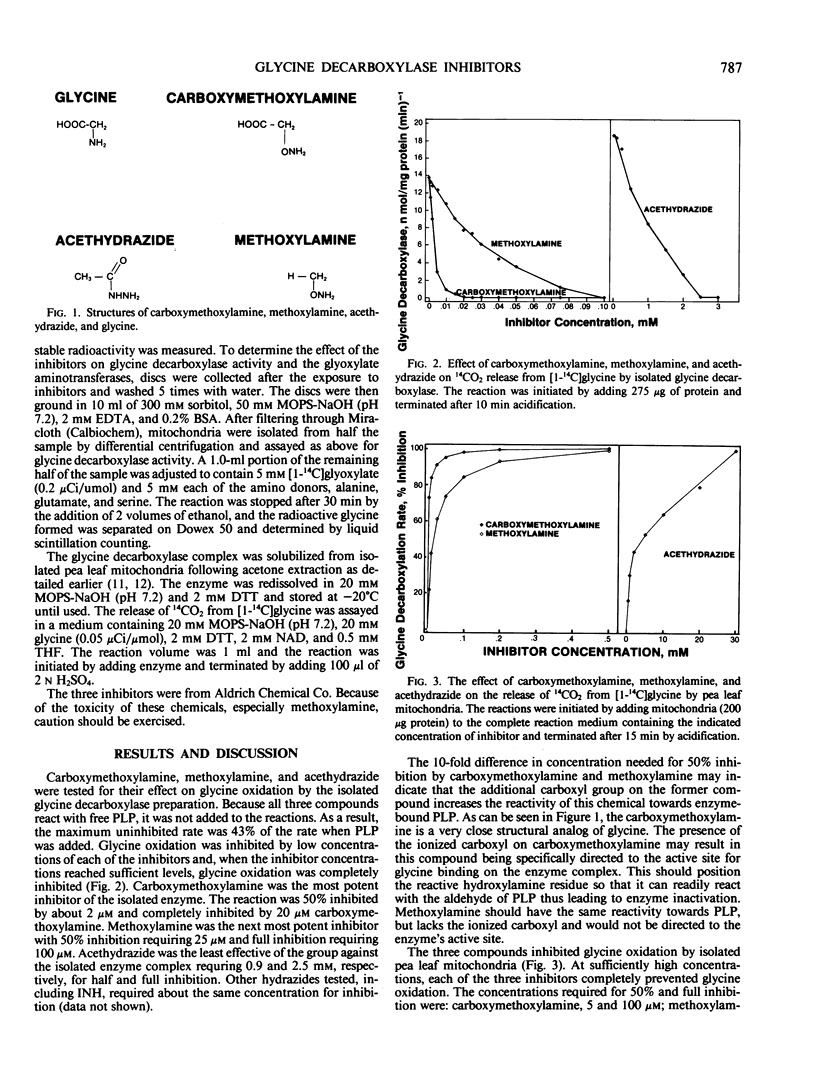
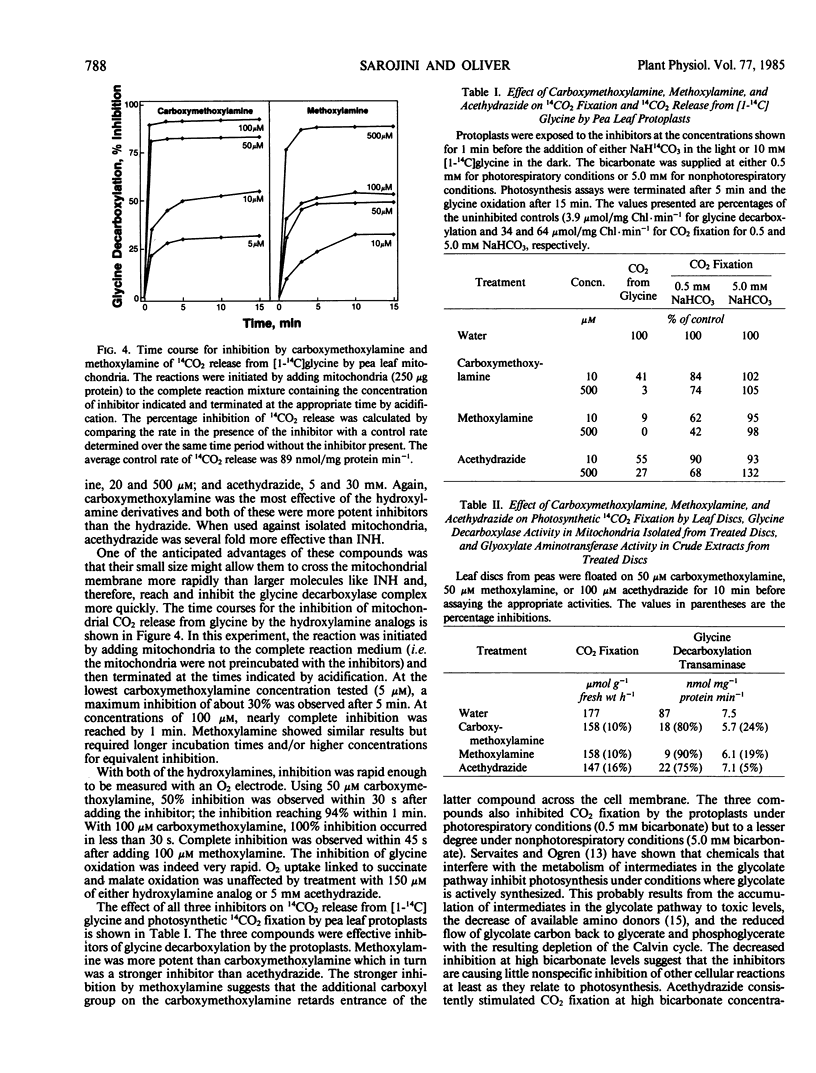
Carboxymethoxylamine (amino-oxyacetate), methoxylamine, and acethydrazide are shown to be effective, although not completely specific, inhibitors of glycine oxidation by the isolated glycine decarboxylase multienzyme complex, mitochondria, protoplasts, and leaf discs from peas. The inhibition probably results from a reaction between these compounds and the pyridoxal 5-phosphate cofactor of the enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., Done J. Polarographic study of ammonia assimilation by isolated chloroplasts. Plant Physiol. 1977 Oct;60(4):504–508. doi: 10.1104/pp.60.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi G. The glycine cleavage system: composition, reaction mechanism, and physiological significance. Mol Cell Biochem. 1973 Jun 27;1(2):169–187. doi: 10.1007/BF01659328. [DOI] [PubMed] [Google Scholar]
- Lawyer A. L., Zelitch I. Inhibition of glycine decarboxylation and serine formation in tobacco by glycine hydroxamate and its effect on photorespiratory carbon flow. Plant Physiol. 1979 Nov;64(5):706–711. doi: 10.1104/pp.64.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea P. J., Miflin B. J. The occurrence of glutamate synthase in algae. Biochem Biophys Res Commun. 1975 Jan 2;64(3):856–862. doi: 10.1016/0006-291x(75)90126-6. [DOI] [PubMed] [Google Scholar]
- Moore A. L., Jackson C., Halliwell B., Dench J. E., Hall D. O. Intramitochondrial localisation of glycine decarboxylase in spinach leaves. Biochem Biophys Res Commun. 1977 Sep 23;78(2):483–491. doi: 10.1016/0006-291x(77)90204-2. [DOI] [PubMed] [Google Scholar]
- Sarojini G., Oliver D. J. Extraction and partial characterization of the glycine decarboxylase multienzyme complex from pea leaf mitochondria. Plant Physiol. 1983 May;72(1):194–199. doi: 10.1104/pp.72.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C. Chemical inhibition of the glycolate pathway in soybean leaf cells. Plant Physiol. 1977 Oct;60(4):461–466. doi: 10.1104/pp.60.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. B., Briggs S., Triebwasser K. C., Freedland R. A. Re-evaluation of amino-oxyacetate as an inhibitor. Biochem J. 1977 Feb 15;162(2):453–455. doi: 10.1042/bj1620453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C. R., Ogren W. L. Photorespiration-deficient Mutants of Arabidopsis thaliana Lacking Mitochondrial Serine Transhydroxymethylase Activity. Plant Physiol. 1981 Apr;67(4):666–671. doi: 10.1104/pp.67.4.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. H., Oliver D. J., Sarojini G. Simultaneous oxidation of glycine and malate by pea leaf mitochondria. Plant Physiol. 1982 Nov;70(5):1465–1469. doi: 10.1104/pp.70.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. H., Sarojini G., Oliver D. J. Identification of a glycine transporter from pea leaf mitochondria. Biochem Biophys Res Commun. 1982 Aug;107(3):856–861. doi: 10.1016/0006-291x(82)90601-5. [DOI] [PubMed] [Google Scholar]


