Abstract
Background
Available data on Mismatch Repair system (MMR) deficiency are conflicting and derived from small studies. Our study aimed to evaluate the therapeutic implications of MMR status in patients with locally advanced rectal cancer (LARC).
Methods
We retrospectively collected data from 318 patients affected by LARC treated in Italy at the Medical Oncology Units of the University Hospital of Cagliari, Istituto Nazionale dei Tumori Milan, and AOU Ospedali Riuniti Ancona. All patients underwent neoadjuvant chemoradiotherapy. The primary objective was major TRG while secondary objectives were pathological complete response, disease-free survival (DFS) and overall survival (OS).
Results
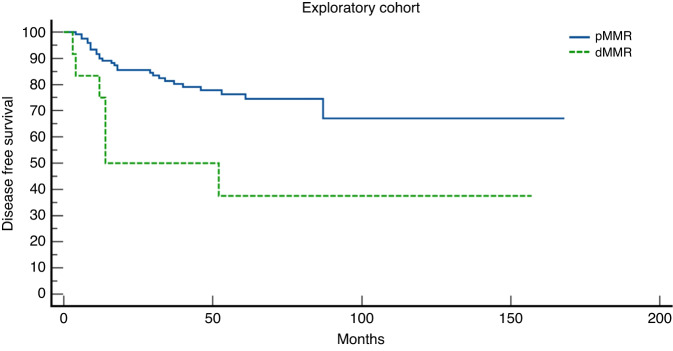
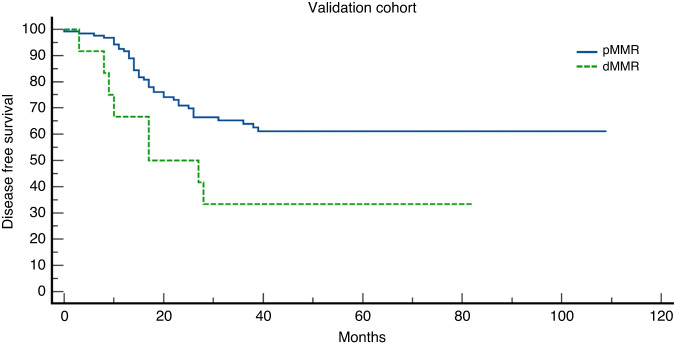
One hundred sixty patients (148 pMMR and 12 dMMR) were included in the exploratory cohort and 158 (146 pMMR and 12 dMMR) were included in the validation cohort. A major TRG has been shown in 42.6% and 43.1% patients with pMMR in exploratory and validation cohort, respectively; while no major TRG have been shown in dMMR patients in both cohorts. Exploratory and validation cohorts showed a statistically significant higher mDFS in pMMR patients compared to dMMR: NR vs. 14 months and NR vs. 17 months, respectively.
Conclusion
Our results indicated an association between dMMR and poor response to preoperative chemoradiotherapy and they represent a hypothesis-generating data for new neoadjuvant strategies.
Subject terms: Rectal cancer, Rectal cancer
Introduction
Colorectal cancer (CRC) is the third leading cancer and the second cause of cancer-related deaths worldwide [1]. A better prognosis in rectal cancer patients than in those with colon cancer has been reported [1]. Multimodal treatment, consisting of neoadjuvant chemoradiotherapy (CRT), surgical total mesorectal excision (TME) followed by adjuvant chemotherapy, has become the standard of care in patients with locally advanced rectal cancer (LARC) [2]. However, the response to neoadjuvant CRT in patients with LARC is variable [3]. An alternative strategy, the total neoadjuvant therapy (TNT), was taking root in recent years. TNT consists in administering CRT plus neoadjuvant chemotherapy before surgery to provide uninterrupted systemic therapy to eradicate micrometastases [4–8].
Furthermore, identifying biomarkers to predict response is desirable to guide treatment decisions and improve CRT outcomes. Recently, several studies have investigated the molecular cancer profiles in different CRC settings to better define prognosis and to find a valid guide in the therapeutic choice, to improve responses [9–16].
In this context, growing interest in the role played by microsatellite instability (MSI) as a predictor of response to CRT is emerged. MSI, the hallmark of deficient mismatch repair (dMMR) tumors, may be caused by a germline mutation in one of the (MMR) genes (MLH1, MSH2, MSH6, PMS2, and EPCAM deletion), consistent with the Lynch syndrome, or by epigenetic silencing of the MMR genes promoter regions [17]. There are two distinct MSI phenotypes: high-MSI (MSI-H) and low-MSI (MSI-L), which may be identified on the type and number of microsatellites analyzed. However, there is no evidence that MSI-L CRCs differ in their clinicopathological or molecular features from stable microsatellite (MSS) tumors [18]. Conversely, it is well known that dMMR/MSI-H CRCs differ from proficient MMR (pMMRs)/MSS tumors in several aspects, including prognosis, response to treatment, and metastatic spread pattern [19]. dMMR occurs in 15–20% of cases, and studies of resected early-stage CRC evidenced a better prognosis and no improvement from adjuvant 5-Fluoruracil therapy in MSI-H patients [20–22]. The prognostic impact of dMMR appears to decrease as the stage of the disease progresses. It is considerably less common in the metastatic setting and occurs in 3–5% of cases. Although data are currently lacking and inconsistent, dMMR tumors appear less responsive to fluoropyrimidines and oxaliplatin chemotherapy, in metastatic CRC (mCRC) [23, 24]. Furthermore, in this setting MSI/dMMR mCRC achieves long-lasting responses with immune checkpoint inhibitors [25–28]. It has been hypothesized that the beneficial effect of immunotherapy in these patients might depend on the increased somatic mutational load, the abundant infiltration of immune cells in the tumor microenvironment (TME) and on increasing tumor neoantigens [29–31]. For this reason, screening for dMMR expression is now recommended for all CRC patients [32].
Compared to colon cancer, the prevalence of dMMR in rectal cancer is less frequent, around 10%. Considering that colon cancer differs from rectal cancer, there are many questions about the role of MMR in LARC, particularly regarding its prognostic and predictive role in response to fluoropyrimidine-based CRT. Currently, we have only few conflicting data in the literature. De Rosa et al. showed that dMMR rectal cancer had an excellent prognosis and pathologic response with fluoropyrimidine-based CRT [33]. Conversely, Cercek et al. demonstrated that (total neoadjuvant) TNT regimen, including mFOLFOX or fluoropyrimidine-based CRT, is far less efficacious in dMMR than in pMMR rectal cancer [34].
In recent years, there has also been a growing interest regarding the role of MSI in the neoadjuvant/adjuvant setting in other cancers. For example, some studies suggested a potential lack of benefit of perioperative or adjuvant chemotherapy in patients with MSI gastric cancer undergoing surgery [35–37].
Based on these considerations, we have conducted a retrospective analysis to evaluate the frequency and therapeutic implications of dMMR status in patients with LARC treated in our hospital.
Methods
Patients and methods
We retrospectively collected data from 318 patients affected by LARC adenocarcinoma (cT3-4 ± N1-2) treated at the Medical Oncology Unit of the University Hospital of Cagliari, Italy, the Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy and at the Medical Oncology Unit, AOU Ospedali Riuniti, Ancona. Italy.
All patients included in the study underwent neoadjuvant concurrent capecitabine (825 mg/m2/bid) and long-course radiotherapy (RT) (total dose of Gy 50.4). CT and MRI were performed at baseline and before surgery to verify the radiological response, according to RECIST v1.1 criteria. Subsequently, all patients underwent total mesorectal excision (TME) at the local Colo-rectal Surgery Unit. All patients included in the evaluation obtained an R0 resection.
MMR expression was evaluated through immunohistochemistry. Immuno-histochemical investigations were performed on the surgical samples to evaluate Mismatch Repair Proteins expression (MLH1, PMS2, MSH6, MSH2, and EPCAM). Patients with BRAF mutant disease were not included in the study, in order to avoid bias in the outcomes evaluation.
The primary objective was major TRG (0–1 Ryan’s score) while secondary objectives were pathological complete response, disease-free survival (DFS) and overall survival (OS). TRG evaluation was made on the surgical sample according to Ryan’s score [38–40] and Dworak’s score [41] to describe the tissue response to chemo-radiotherapy. Secondary objectives were disease-free survival (DFS), overall response rate (ORR) and overall survival (OS).
Statistical analysis
Statistical analysis was performed with the MedCalc Statistical Software Version 20.2016 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2022). The association between categorical variables was estimated by the Fisher exact test for categorical binomial variables or by the chi-square test in all other instances. Survival probability over time was estimated by the Kaplan–Meier method. Significant differences in the probability of survival between the strata were evaluated by the log-rank test. The independent role of variables that were statistically significant at univariate analysis was assessed with a logistic regression analysis.
Major Tumor Regression Grade rate was defined as the percentage of patients who achieved a complete (TRG-0) or near complete response (TRG-1) on tissue samples, according to Ryan’s score.
Disease-free survival was defined as the time from treatment start until the first cancer-related event, second cancer, or death from any cause. Overall survival was defined as the time interval between the date of the treatment start to death or the last follow-up visit for patients who were lost at follow-up. Overall response rate (ORR) is defined as the proportion of patients who have a partial or complete response to therapy. Disease control rate was defined as the percentage of patients with stable disease or partial/complete response to treatment.
Based on the results from the 160 patients of the exploratory cohort, we tried to validate the findings in a validation cohort. Then, we identified the validation group sample size according to major TRG rate and survival analysis, from the exploratory cohort. To validate the difference in terms of major TRG in pMMR patients (around 40%) and dMMR patients (around 5%), assuming a probability alpha of 0.05 and a beta of 0.20), with a two group ratio of 12, the required sample size would have been 155 patients (143 + 12), using a “comparison of proportion test”.
Results
Patients characteristics
Globally, 318 patients affected by LARC adenocarcinoma (cT3-4 ± N1-2) were included in the study, 160 patients (148 pMMR and 12 dMMR) were included in the exploratory cohort and 158 (146 pMMR and 12 dMMR) were included in the validation cohort. Median age was 68 ± 11 both in exploratory and validation cohort. Stage III patient rate was 64% and 63% in the exploratory and the validation cohort, respectively. Mismatch Repair system deficiency rate was 7.5% and 7.6% in the exploratory and the validation cohort, respectively. MRF involvement was evenly distributed in the two groups of patients. Patient baseline characteristics are detailed in Tables 1 and 2.
Table 1.
Exploratory cohort: patients baseline characteristics.
| pMMR | dMMR | Tot. | p value | |
|---|---|---|---|---|
| N. | 148 | 12 | 160 | |
| M | 92 | 10 | p = 0.213 | |
| F | 56 | 2 | ||
| ECOG-PS 0 | 105 | 10 | 115 | p = 0.094 |
| ECOG-PS 1 | 41 | 1 | 42 | |
| ECOG-PS 2 | 2 | 1 | 3 | |
| Stage II | 53 | 4 | 57 | p = 1 |
| Stage III | 95 | 8 | 103 | |
| Upper rectum | 15 | 1 | 16 | p = 0.980 |
| Medium rectum | 85 | 7 | 92 | |
| Lower rectum | 48 | 4 | 52 | |
| MRF+ | 51 | 4 | 55 | p = 0.9371 |
| MRF− | 97 | 8 | 105 | |
| Adjuvant CHT+ | 56 | 7 | 63 | p = 0.1636 |
| Adjuvant CHT− | 92 | 5 | 97 |
Table 2.
Validation cohort: patient baseline characteristics.
| pMMR | dMMR | Tot. | p value | |
|---|---|---|---|---|
| N. | 146 | 12 | 158 | |
| M | 95 | 7 | 102 | p = 0.755 |
| F | 51 | 5 | 56 | |
| ECOG-PS 0 | 105 | 11 | 116 | p = 0.296 |
| ECOG-PS 1 | 38 | 1 | 39 | |
| ECOG-PS 2 | 5 | – | 5 | |
| Stage II | 54 | 3 | 47 | p = 0.538 |
| Stage III | 92 | 9 | 111 | |
| Upper rectum | 16 | 1 | 17 | p = 0.149 |
| Medium rectum | 81 | 10 | 91 | |
| Lower rectum | 49 | 1 | 50 | |
| MRF+ | 3 | 45 | 55 | p = 0.6743 |
| MRF− | 9 | 101 | 105 | |
| Adjuvant CHT+ | ||||
| Adjuvant CHT− |
Tumor regression grade and clinical outcomes
In the exploratory cohort a major TRG has been shown in 64/148 (42.6%) pMMR patients: 14.2% achieved a TRG-0 and 28.4% achieved a TRG-1; while no major TRG have been shown in dMMR (0%): 2/12 patients achieved a TRG-2 and 10/12 patients achieved a TRG-3. Afterwards, we evaluated the differences in median disease-free survival between pMMR and dMMR patients. pMMR showed a statistically significant higher median DFS: NR versus 14 months (p = 0.003) (Table 3).
Table 3.
Tumor regression grade according to Ryan’s scores.
| Ryan’s scorea | Tot (n.) | p value | |||||
|---|---|---|---|---|---|---|---|
| TRG-0 | TRG-1 | TRG-2 | TRG-3 | ||||
| Exploratory cohort | pMMR | 21 | 43 | 75 | 9 | 148 | p < 0.0001 |
| dMMR | – | – | 2 | 10 | 12 | ||
| Validation cohort | pMMR | 19 | 44 | 72 | 11 | 146 | p < 0.0001 |
| dMMR | – | – | 3 | 9 | 12 | ||
aRyan tumor regression (TRG) score: TRG-0 no visible cancer cells (complete response); TRG-1 single cells or small group of cancer cells (near-complete response); TRG-2 residual cancer with evident tumor regression, but more than single cells or rare small group of cancer cells (partial response); TRG-3 extensive residual cancer with no evident tumor regression (poor or no response).
The percentage of patients with positive postoperative lymph nodes in exploratory cohort was as follows: 66.7% of dMMR patients and 20.9% of pMMR patients. While in validation cohort 58.3% dMMR patients and 24.7% pMMR patients were N+.
Results were confirmed in the validation cohort in which a major TRG has been found in 63/146 (43.1%) pMMR patients: 13% achieved a TRG-0 and 30.1% achieved a TRG-1. No major TRG have been shown in dMMR (0%): 3/12 patients achieved a TRG-2 and 9/12 patients achieved a TRG-3. Then, evaluating the differences in median DFS, pMMR showed a statistically significant higher median DFS: NR versus 17 months (p = 0.02) (Figs. 1 and 2).
Fig. 1. Kaplan–Meier curves of disease-free survival (DFS) in the exploratory cohort.
Proficient Mismatch Repair (pMMR) patients represented by the blue line and deficient Mismatch Repair (dMMR) patients represented by the green line.
Fig. 2. Kaplan–Meier curves of disease-free survival (DFS) in the validation cohort.
Proficient Mismatch Repair (pMMR) patients represented by the blue line and deficient Mismatch Repair (dMMR) patients represented by the green line.
Regarding ORR, the exploratory cohort obtained the following results: among dMMR patients 25% achieved a partial response (PR) while 66.7% a stable disease (SD) and 8.3% a progressive disease (PD); amid pMMR patients 17.6% reached a CR while 69.6% a PR and 12.8% a SD. Validation Cohort achieved the following results: amid dMMR patients 33.4% obtained a PR while 50% a SD and 16.6% a PD; amidst pMMR patients 16.4% reached a complete response (CR) while 69.2% a PR and 14.4% a SD (Table 4).
Table 4.
Overall response rate.
| Exploratory cohort | Validation cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| pMMR | dMMR | pMMR | dMMR | |||||
| No. of patients | % | No. of patients | % | No. of patients | % | No. of patients | % | |
| Complete response | 26 | 17.6 | 0 | – | 24 | 16.4 | 0 | – |
| Partial response | 103 | 69.6 | 3 | 25 | 101 | 69.2 | 4 | 33.4 |
| Stable disease | 19 | 12.8 | 8 | 66.7 | 21 | 14.4 | 6 | 50 |
| Progressive disease | 0 | – | 1 | 8.3 | 0 | – | 2 | 16.6 |
| ORR | 129 | 87.2 | 3 | 25 | 125 | 85.6 | 4 | 33.4 |
| p = 0.0114 | p = 0.035 | |||||||
At present, our findings on median overall survival in both exploratory and validation cohorts are still immature to be able to obtain conclusive results.
Discussion
Our findings show biological resistance to capecitabine-based chemoradiotherapy in patients with LARC adenocarcinoma and dMMR in a real life population. To the best of our knowledge, in the literature only few retrospective studies evaluated small groups of dMMR patients, with conflicting results in terms of pCR. De Rosa et al. described the response to multimodality treatment (chemoradiotherapy plus TME) in 62 dMMR rectal adenocarcinomas patients who achieved a high pCR rate (27.6%), however these results might be related to patients’ selection (stage I patients were also included in this study) [33]. On the other hand, Cercek et al. evaluated the outcome of 50 dMMR patients after chemoradiotherapy, compared with a corresponding group of pMMR patients, showing a poor treatment response in the dMMR group [34]. These observations laid the basis for the initiation of a prospective phase 2 study in which single-agent dostarlimab, an anti-PD-1 monoclonal antibody, was administered every 3 weeks for 6 months in patients with dMMR stage II or III rectal adenocarcinoma. Notably, among the 12 patients who completed treatment with dostarlimab, the authors reported 100% of clinical complete response [42].
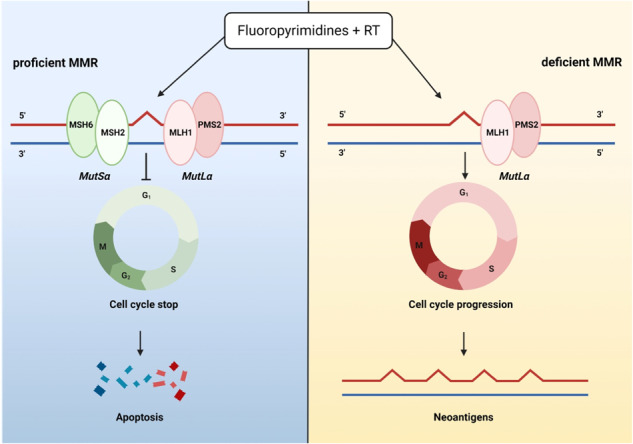
A possible biological explanation for these results lies in the MMR protein’s biological function. Ten proteins have a role in this process, and all of these combine to obtain two types of functional heterodimer: MutS and MutL [43]. MutL proteins are ATPases of the GHKL family, which have ATPase in the N-terminal domain and the dimerization domain at the C-terminal [44–48]. Human cells express 4 MutL homologs: MLH1, MLH3, PMS1 and PMS2, which combine into three different heterodimer subtypes: MutLα (MLH1/PMS2), MutLβ (MLH1/PMS1) and MutLγ (MLH1/MLH3). MutLα plays the most important role in MMR deficient cells that exhibit MSI phenotypes, as in the MSH2 mutated cells [49–53].
Several preclinical studies investigated the association between MMR alterations and drug and chemical activity, showing resistance to chemotherapy (fluoropyrimidines and/or oxaliplatin) in dMMR cells [54]. Cancer cells deficient in MMR are significantly more resistant to treatment with methylating agents than cancer cells with proficient MMR. The cytotoxic damage induced by these drugs begins with the methylation of specific nucleotide residues that may lead to cell cycle arrest, if intercepted correctly. Instead, in cells with deficient proteins in the repair system, these alterations persist, and tumor cells survive with a large load of mutations [55, 56]. These data are consistent with the responses described in our study, where pMMR tumors showed a lower tumor regression grade (evaluated according to both Ryan’s and Dworak’s scores). However, few patients with pMMR showed a poor response, whereas few patients with dMMR showed an appropriate response to treatment. The reason for these conflicting results could be related to the immunohistochemical (IHC) technique utilized and/or to tumor biology. IHC detection for MMR proteins has a similar performance to PCR-based analysis for MSI, with a concordance ranging from 90.4% up to 99.6%, depending on the case series [57, 58]. However, other studies evaluating the sensitivity and specificity of detection of MLH1 and MSH2 mutations suggested that these may reach high values (74% and 91% sensitivity and 81 and 90% specificity, respectively) [59]. Therefore, despite the excellent performance of IHC, in some cases the results may not reflect the truthful state of MMR. For example, missense mutations of MMR genes may produce non-functioning proteins that can bind antibodies. In these cases, a percentage of MSI-H ranging from 5% to 11% could still be positive for IHC stains [60]. Therefore, despite showing positive staining for MMR proteins, patients may have MSI-H status and may not respond adequately to neoadjuvant treatment. In addition, many other factors can influence the response to treatment, including the extracellular matrix, the immune cell infiltrates, and inter-individual variability.
Our study also showed a high frequency of MSH2/MSH6 and MLH1/PMS2 deficiencies, which is consistent with the data available in the literature, which predict a higher frequency of these mutations in rectal adenocarcinoma rather than in colon adenocarcinoma [33, 34]. Based on available genetic evaluation data, in the exploratory cohort, 7 out of 12 patients (58%) confirmed the diagnosis of s. of Lynch, while in the validation cohort 5 out of 12 patients (42%). Survival data showed a better median DFS in pMMR patients, while OS data are immature for an evaluation.
Our study has some limitations, mainly for its retrospective nature that by definition is hypothesis generating and could represent the first step toward a future validation of these findings in a prospective study. Moreover, MMR proteins were only evaluated in the resected specimen and a comparison with diagnostic biopsy tissue was not performed, implicating a potential bias for a full interpretation of the results. Although exploratory we believe that our findings, along with those deriving from ongoing trials, could represent an important step forward in the definition of the optimal treatment strategy for dMMR/MSI-H locally advanced rectal cancer patients.
Mismatch repair deficient/High Microsatellite Instability locally advanced rectal cancers showed a lower response to standard chemoradiotherapy. Moreover, we now know that they might have an excellent response to treatment with immune checkpoint inhibitors. The emerging picture is showing dMMR/MSI-H rectal cancers as a disease completely different from its pMMR/MSS counterpart, and furthermore, they might deserve a completely different diagnostic and therapeutic strategy.
Author contributions
AP: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing, visualization. PZ and RG: resources, data curation, formal analysis, writing—original draft, writing—review and editing. G Pinna and CD: resources, data curation, writing—original draft. GR, FL, GD, EP, FM and FS: resources, data curation. AR, SM, MAD, VP, MP, EL, AR, LZ, R Barbara, and R Berardi: resources. G Pretta and CS: writing—review and editing. GF: resources, data curation, writing—original draft, writing—review and editing. FP: resources, data curation, writing—original draft, writing—review and editing. MS: methodology, validation, formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing.
Data availability
Datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Ethics Committee approval was obtained for the study (Protocol number 2020/10912—code: EMIBIOCCOR) from Cagliari Independent Ethics Committee and written informed consent was obtained from all participants for their tissues to be utilized for this work. This study was performed in accordance with the study protocol, the ethical principles stated in the Declaration of Helsinki as well as those indicated in the International Conference on Harmonization (ICH) Note for Guidance on Good Clinical Practice (GCP; ICH E6, 1995), and all applicable regulatory requirements. All patients signed a written informed consent before study entry. Adequate information was given to eligible patients by the principal investigator or co-investigators in accordance with local regulations. The declaration of informed consent was personally signed and dated by the subject, and by the investigator/person designated by the investigator to conduct the informed consent discussion.
Consent for publication
Patients signed an informed consent regarding the publication of their data.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. 10.3322/caac.21492. [DOI] [PubMed]
- 2.Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment, and follow-up. Ann Oncol. 2017;28:iv22–40. 10.1093/annonc/mdx224. [DOI] [PubMed]
- 3.Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadounet RJ, EORTC Radiation Oncology Group, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomized study. Lancet Oncol. 2014;15:184–90. doi: 10.1016/S1470-2045(13)70599-0. [DOI] [PubMed] [Google Scholar]
- 4.Weiser MR. Total neoadjuvant therapy for locally advanced rectal cancer: PRODIGE 23 trial. Ann Surg Oncol. 2022;29:1493–5. 10.1245/s10434-021-11104-9. [DOI] [PubMed]
- 5.Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Meershoek-Klein Kranenbarg E, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomized, open-label, phase 3 trial. Lancet Oncol. 2021;22:29–42. 10.1016/S1470-2045(20)30555-6. [DOI] [PubMed]
- 6.Garcia-Aguilar J, Patil S, Kim JK, Yuval JJB, Thompson H, Verheijet F, et al. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. J Clin Oncol. 2020;38:4008a. doi: 10.1200/JCO.2020.38.15_suppl.4008. [DOI] [Google Scholar]
- 7.Giunta EF, Bregni G, Pretta A, Deleporte A, Liberale G, Bali AM, et al. Total neoadjuvant therapy for rectal cancer: making sense of the results from the RAPIDO and PRODIGE 23 trials. Cancer Treat Rev. 2021;96:102177. 10.1016/j.ctrv.2021.102177. [DOI] [PubMed]
- 8.Bregni G, Vandeputte C, Pretta A, Senti C, Trevisi E, Acedo Reina E, et al. Rationale and design of REGINA, a phase II trial of neoadjuvant regorafenib, nivolumab, and short-course radiotherapy in stage II and III rectal cancer. Acta Oncol. 2021;60:549–53. 10.1080/0284186X.2020.1871067. [DOI] [PubMed]
- 9.Gutierrez ME, Price KS, Lanman RB, Nagy RJ, Shah I, Mathura S, et al. Genomic profiling for KRAS, NRAS, BRAF, microsatellite instability, and mismatch repair deficiency among patients with metastatic colon cancer. JCO Precis Oncol. 2019;3:PO.19.00274. 10.1200/PO.19.00274. [DOI] [PMC free article] [PubMed]
- 10.Ziranu P, Lai E, Schirripa M, Puzzoni M, Persano M, Pretta A, et al. The role of p53 expression in patients with RAS/BRAF wild-type metastatic colorectal cancer receiving irinotecan and cetuximab as later line treatment. Target Oncol. 2021;16:517–27. 10.1007/s11523-021-00816-3. [DOI] [PMC free article] [PubMed]
- 11.Vega-Benedetti AF, Loi E, Moi L, Restivo A, Cabras F, Deidda S, et al. Colorectal cancer promoter methylation alteration affects the expression of glutamate ionotropic receptor AMPA type subunit 4 alternative isoforms potentially relevant in colon tissue. Hum Cell. 2022;35:310–9. doi: 10.1007/s13577-021-00640-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giampieri R, Ziranu P, Daniele B, Zizzi A, Ferrari D, Lonardi S, et al. From CENTRAL to SENTRAL (SErum aNgiogenesis cenTRAL): circulating predictive biomarkers to anti-VEGFR therapy. Cancers. 2020;12:1330. doi: 10.3390/cancers12051330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giampieri R, Lupi A, Ziranu P, Bittoni A, Pretta A, Pecci F, et al. Retrospective comparative analysis of KRAS G12C vs. other KRAS mutations in mCRC patients treated with first-line chemotherapy doublet + bevacizumab. Front Oncol. 2021;11:736104. doi: 10.3389/fonc.2021.736104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai E, Liscia N, Donisi C, Mariani S, Tolu S, Pretta A, et al. Molecular-biology-driven treatment for metastatic colorectal cancer. Cancers. 2020;12:1214. doi: 10.3390/cancers12051214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasqualetti G, Schirripa M, Dochy E, Fassan M, Ziranu P, Puzzoni M, et al. Thyroid hormones ratio is a major prognostic marker in advanced metastatic colorectal cancer: results from the phase III randomised CORRECT trial. Eur J Cancer. 2020;133:66–73. doi: 10.1016/j.ejca.2020.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Puzzoni M, Ziranu P, Demurtas L, Lai E, Mariani S, Liscia N, et al. Why precision medicine should be applied across the continuum of care for metastatic colorectal cancer patients. Future Oncol. 2020;16:4337–9. doi: 10.2217/fon-2019-0624. [DOI] [PubMed] [Google Scholar]
- 17.Herman JG, Umar A, Polyak K, Graff JR, Ahuja N, Issa JP, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci USA. 1998;95:6870–5. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timothy MP, Chandrajit PR, Rodriguez-Bigas MA. Colorectal carcinogenesis: MSI-H versus MSI-L. Dis Markers. 2004;20:199–206. doi: 10.1155/2004/368680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623–32. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merok MA, Ahlquist T, Royrvik EC, Tufteland KF, Hektoen M, Sjo OH, et al. Microsatellite instability has a positive prognostic impact on stage II colorectal cancer after complete resection: results from a large, consecutive Norwegian series. Ann Oncol. 2013;24:1274–82. doi: 10.1093/annonc/mds614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–26. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sargent DJ, Marsoni S, Thibodeau SN, Labianca R, Hamilton SR, Torri V, et al. Confirmation of deficient mismatch repair (dMMR) as a predictive marker for lack of benefit from 5-FU based chemotherapy in stage II and III colon cancer (CC): a pooled molecular reanalysis of randomized chemotherapy trials. J Clin Oncol. 2008;26:15s. 10.1200/jco.2008.26.15_suppl.4008.
- 23.Brueckl WM, Moesch C, Brabletz T, Koebnick C, Riedel C, Jung A, et al. Relationship between microsatellite instability, response and survival in palliative patients with colorectal cancer undergoing first-line chemotherapy. Anticancer Res. 2003;23:1773–7. [PubMed]
- 24.Alex AK, Siqueira S, Coudry R, Santos J, Alves M, Hoff PM, et al. Response to chemotherapy and prognosis in metastatic colorectal cancer with DNA deficient mismatch repair. Clin Colorectal Cancer. 2017;16:228–39. doi: 10.1016/j.clcc.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair—deficient/microsatellite instability—high metastatic colorectal cancer. JCO. 2018;36:773–9. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 27.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Nivolumab (NIVO) + low-dose ipilimumab (IPI) in previously treated patients (pts) with microsatellite instability-high/mismatch repair-deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC): long-term follow-up. JCO. 2019;37:635. doi: 10.1200/JCO.2019.37.4_suppl.635. [DOI] [Google Scholar]
- 28.Andre T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt CJA, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer: the phase 3 KEYNOTE-177 study. In: Proceedings of the ASCO Annual Meeting 2020, Virtual Scientific Program, Chicago, IL, USA, 29–31 May 2020. 10.1200/JCO.2020.38.18_suppl.LBA4.
- 29.Becht E, de Reyniès A, Giraldo NA, Pilati C, Buttard B, Lacroix L, et al. Immune and stromal classification of colorectal cancer is associated with molecular subtypes and relevant for precision immunotherapy. Clin Cancer Res. 2016;22:4057–66. doi: 10.1158/1078-0432.CCR-15-2879. [DOI] [PubMed] [Google Scholar]
- 30.Giannakis M, Mu XJ, Shukla SA, Qian ZR, Cohen O, Nishihara R, et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. 2016;15:857–65. doi: 10.1016/j.celrep.2016.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salem ME, Bodor JN, Puccini A, Xiu J, Goldberg RM, Grothey A, et al. Relationship between MLH1, PMS2, MSH2, and MSH6 gene-specific alterations and tumor mutational burden in 1057 microsatellite instability-high solid tumors. Int J Cancer. 2020. 10.1002/ijc.33115. [DOI] [PMC free article] [PubMed]
- 32.Benson AB, III, Venook AP, Cederquist L, Chan E, Chen YJ, Cooper HS, et al. Colon cancer, version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2017;15:370–98. doi: 10.6004/jnccn.2017.0036. [DOI] [PubMed] [Google Scholar]
- 33.de Rosa N, Rodriguez-Bigas MA, Chang GJ, Veerapong J, Borras E, Krishnan S, et al. DNA mismatch repair deficiency in rectal cancer: benchmarking its impact on prognosis, neoadjuvant response prediction, and clinical cancer genetics. J Clin Oncol. 2016;34:3039–46. doi: 10.1200/JCO.2016.66.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cercek A, Dos Santos Fernandes G, Roxburgh CS, Ganesh K, Ng S, Sanchez-Vega F, et al. Mismatch repair-deficient rectal cancer and resistance to neoadjuvant chemotherapy. Clin Cancer Res. 2020;26:3271–9. doi: 10.1158/1078-0432.CCR-19-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi YY, Kim H, Shin SJ, Kim HY, Lee J, Yang HK, et al. Microsatellite instability and programmed cell death-ligand 1 expression in stage II/III gastric cancer: post hoc analysis of the CLASSIC randomized controlled study. Ann Surg. 2019;270:309–16. doi: 10.1097/SLA.0000000000002803. [DOI] [PubMed] [Google Scholar]
- 36.Smyth EC, Wotherspoon A, Peckitt C, Gonzalez D, Hulkki-Wilson S, Eltahir Z, et al. Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the medical research council adjuvant gastric infusional chemotherapy (MAGIC) trial. JAMA Oncol. 2017;3:1197–203. doi: 10.1001/jamaoncol.2016.6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pietrantonio F, Miceli R, Raimondi A, Kim YW, Kang WK, Langley RE, et al. Individual patient data meta-analysis of the value of microsatellite instability as a biomarker in gastric cancer. J Clin Oncol. 2019;37:3392–3400. doi: 10.1200/JCO.19.01124. [DOI] [PubMed] [Google Scholar]
- 38.Ryan R, Gibbons D, Hyland JM, Treanor D, White A, Mulcahy HE, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47:141–6. doi: 10.1111/j.1365-2559.2005.02176.x. [DOI] [PubMed] [Google Scholar]
- 39.Huh JW, Kim HC, Kim SH, Park YA, Cho YN, Yun SH, et al. Tumor regression grade as a clinically useful outcome predictor in patients with rectal cancer after preoperative chemoradiotherapy. Surgery. 2019;165:579–85. 10.1016/j.surg.2018.08.026. [DOI] [PubMed]
- 40.Jäger T, Neureiter D, Urbas R, Klieser E, Hitzl W, Emmanuelet K. Applicability of American Joint Committee on cancer and College of American Pathologists Regression Grading System in rectal cancer. Dis Colon Rectum. 2017;60:815–26. 10.1097/DCR.0000000000000806. [DOI] [PubMed]
- 41.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23. 10.1007/s003840050072. [DOI] [PubMed]
- 42.Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med. 2022;386:2363–76. doi: 10.1056/NEJMoa2201445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 2006;7:335–46. doi: 10.1038/nrm1907. [DOI] [PubMed] [Google Scholar]
- 44.Palombo F, Gallinari P, Iaccarino I, Lettieri T, Hughes M, D’Arrigo A, et al. GTBP, a 160-kilodalton protein essential for mismatch-binding activity in human cells. Science. 1995;268:1912–4. doi: 10.1126/science.7604265. [DOI] [PubMed] [Google Scholar]
- 45.Drummond JT, Li GM, Longley MJ, Modrich P. Isolation of an hMSH2–p160 heterodimer that restores DNA mismatch repair to tumor cells. Science. 1995;268:1909–12. doi: 10.1126/science.7604264. [DOI] [PubMed] [Google Scholar]
- 46.Acharya S, Wilson T, Gradia S, Kane MF, Guerrette S, Marsischky GT, et al. hMSH2 forms specific mispairbinding complexes with hMSH3 and hMSH6. Proc Natl Acad Sci USA. 1996;93:13629–34. doi: 10.1073/pnas.93.24.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palombo F, Iaccarino I, Nakajima E, Ikejima M, Shimada T, Jiricnyet J. hMutSβ, a heterodimer of hMSH2 and hMSH3, binds to insertion/deletion loops in DNA. Curr Biol. 1996;6:1181–4. doi: 10.1016/s0960-9822(02)70685-4. [DOI] [PubMed] [Google Scholar]
- 48.Gradia S, Acharya S, Fishel R. The human mismatch recognition complex hMSH2–hMSH6 functions as a novel molecular switch. Cell. 1997;91:995–1005. doi: 10.1016/s0092-8674(00)80490-0. [DOI] [PubMed] [Google Scholar]
- 49.Prolla TA, Baker SM, Harris AC, Tsao JL, Yao X, Bronneret CE, et al. Tumour susceptibility and spontaneous mutation in mice deficient in Mlh1, Pms1 and Pms2 DNA mismatch repair. Nature Genet. 1998;18:276–9. doi: 10.1038/ng0398-276. [DOI] [PubMed] [Google Scholar]
- 50.Raschle M, Marra G, Nystrom-Lahti M, Schar P, Jiricny J. Identification of hMutLβ, a heterodimer of hMLH1 and hPMS1. J Biol Chem. 1999;274:32368–75. doi: 10.1074/jbc.274.45.32368. [DOI] [PubMed] [Google Scholar]
- 51.Chen PC, Dudley S, Hagen W, Dizon D, Paxton L, Reichow D, et al. Contributions by MutL homologue Mlh3 and Pms2 to DNA mismatch repair and tumor suppression in the mouse. Cancer Res. 2005;65:8662–70. doi: 10.1158/0008-5472.CAN-05-0742. [DOI] [PubMed] [Google Scholar]
- 52.Cannavo E, Marra G, Sabates-Bellver J, Menigatti M, Lipkin SM, Fischeret F, et al. Expression of the MutL homologue hMLH3 in human cells and its role in DNA mismatch repair. Cancer Res. 2005;65:10759–66. doi: 10.1158/0008-5472.CAN-05-2528. [DOI] [PubMed] [Google Scholar]
- 53.Flores-Rozas H, Kolodner RD. The Saccharomyces cerevisiae MLH3 gene functions in MSH3-dependent suppression of frameshift mutations. Proc Natl Acad Sci USA. 1998;95:12404–9. doi: 10.1073/pnas.95.21.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stojic L, Brun R, Jiricny J. Mismatch repair, and DNA damage signaling. DNA Repair. 2004;3:1091–101. doi: 10.1016/j.dnarep.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 55.Duckett DR, Drummond JT, Murchie AI, Reardon JT, Sancar A, Lilley DM, et al. Human MutSα recognizes damaged DNA base pairs containing O6-methylguanine, O4-methylthymine, or the cisplatin(GpG) adduct. Proc Natl Acad Sci USA. 1996;93:6443–7. doi: 10.1073/pnas.93.13.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karran P. Mechanisms of tolerance to DNA damaging therapeutic drugs. Carcinogenesis. 2001;22:1931–7. doi: 10.1093/carcin/22.12.1931. [DOI] [PubMed] [Google Scholar]
- 57.Cheah PL, Li J, Looi LM, Koh CC, Lau TP, Chang SW, et al. Screening for microsatellite instability in colorectal carcinoma: practical utility of immunohistochemistry and PCR with fragment analysis in a diagnostic histopathology setting. Malays J Pathol. 2019;41:91–100. [PubMed] [Google Scholar]
- 58.Hissong E, Crowe EP, Yantiss RK, Chen YT. Assessing colorectal cancer mismatch repair status in the modern era: a survey of current practices and re-evaluation of the role of microsatellite instability testing. Mod Pathol. 2018;31:1756–66. 10.1038/s41379-018-0094-7. [DOI] [PubMed]
- 59.Shia J, Klimstra DS, Nafa K, Offit K, Guillem JG, Markowitz AJ, et al. Value of immunohistochemical detection of DNA mismatch repair proteins in predicting germline mutation in hereditary colorectal neoplasms. Am J Surg Pathol. 2005;29:96–104. doi: 10.1097/01.pas.0000146009.85309.3b. [DOI] [PubMed] [Google Scholar]
- 60.Baretti M, Le DT. DNA mismatch repair in cancer. Pharmacol Ther. 2018;189:45–62. doi: 10.1016/j.pharmthera.2018.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.