Abstract
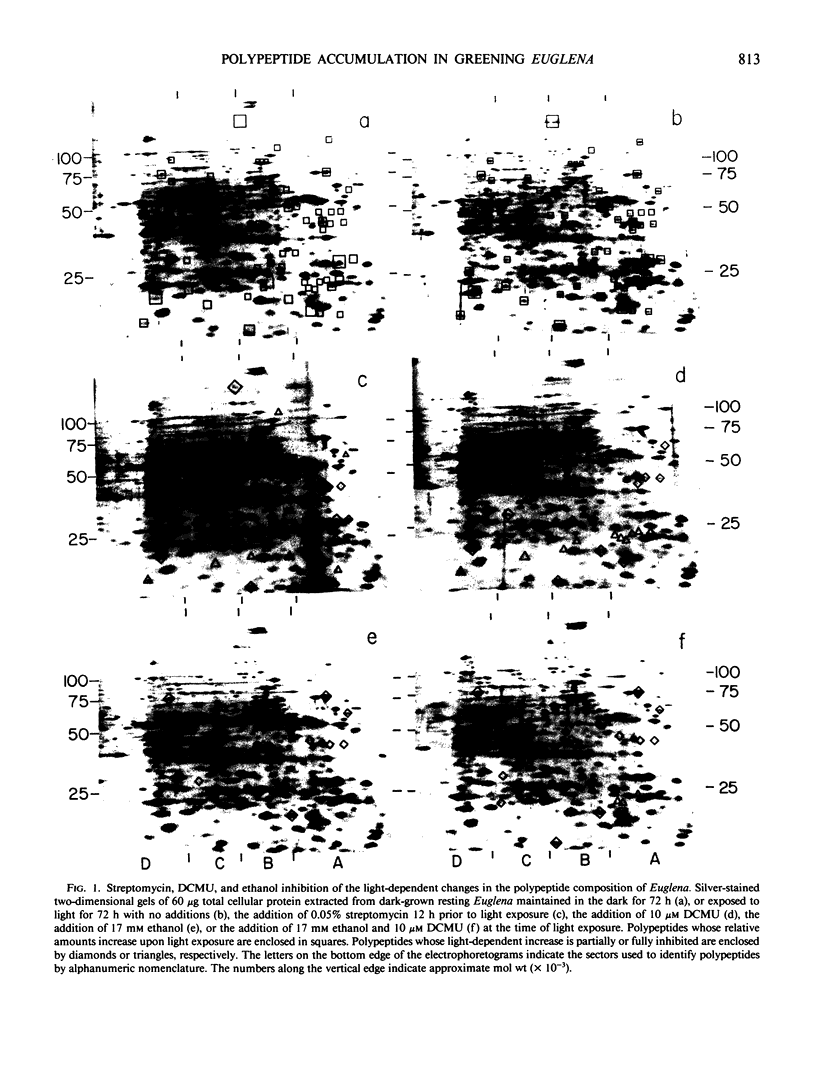
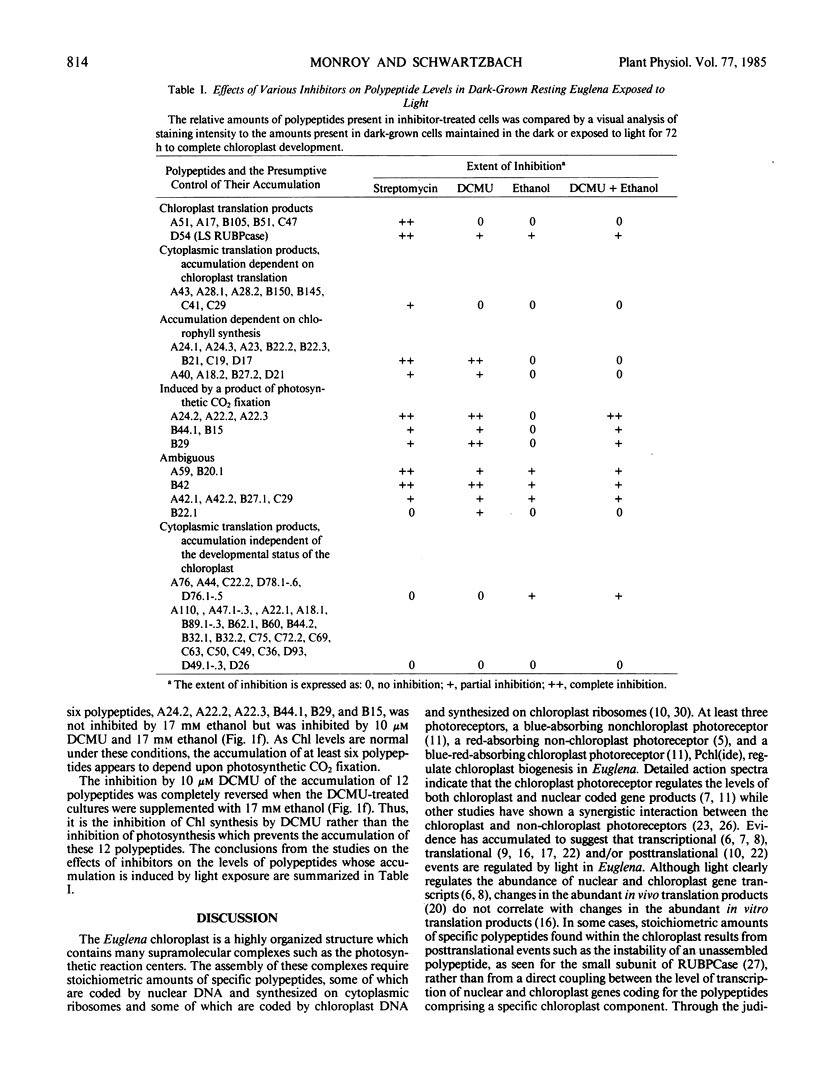
Two-dimensional gel electrophoresis resolves total cellular protein from Euglena gracilis klebs var bacillaris Cori into 640 polypeptides, 79 of which are induced by light exposure. The inhibition of chloroplast translation by streptomycin, the direct inhibition of photosynthesis as well as the indirect inhibition of chlorophyll synthesis by 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) and the specific inhibition of photosynthesis but not chlorophyll synthesis by DCMU in the presence of 17 millimolar ethanol failed to inhibit the accumulation of 40 polypeptides. These polypeptides appear to be synthesized on cytoplasmic ribosomes and their accumulation is independent of the developmental status of the chloroplast. Streptomycin but not DCMU completely inhibited the accumulation of six polypeptides which are undetectable in mutants lacking chloroplast DNA suggesting that these polypeptides are translated on chloroplast ribosomes. The accumulation of seven polypeptides which are detectable in mutants lacking chloroplast DNA was also inhibited by streptomycin but not by DCMU suggesting that the accumulation of these polypeptides is dependent upon stabilization by a chloroplast translation product. The accumulation of 12 polypeptides was inhibited by streptomycin and by DCMU under conditions in which chlorophyll synthesis was inhibited, but not under conditions in which chlorophyll synthesis was unaffected by DCMU. The inhibition by DCMU of the accumulation of these polypeptides appears to be due to the inhibition of chlorophyll synthesis suggesting that they are components of pigment protein complexes. The accumulation of six polypeptides was inhibited under all conditions in which photosynthesis was inhibited suggesting that the accumulation of these polypeptides is dependent upon a product of photosynthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bovarnick J. G., Chang S. W., Schiff J. A., Schwartzbach S. D. Events surrounding the early development of Euglena chloroplasts: experiments with streptomycin in non-dividing cells. J Gen Microbiol. 1974 Jul;83(0):51–62. doi: 10.1099/00221287-83-1-51. [DOI] [PubMed] [Google Scholar]
- Bovarnick J. G., Schiff J. A., Freedman Z., Egan J. M. Events surrounding the early development of Euglena chloroplasts: cellular origins of chloroplast enzymes in euglena. J Gen Microbiol. 1974 Jul;83(0):63–71. doi: 10.1099/00221287-83-1-63. [DOI] [PubMed] [Google Scholar]
- Chelm B. K., Hallick R. B., Gray P. W. Transcription program of the chloroplast genome of Euglena gracilis during chloroplast development. Proc Natl Acad Sci U S A. 1979 May;76(5):2258–2262. doi: 10.1073/pnas.76.5.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D., Schiff J. A. Events surrounding the early development of Euglena chloroplasts. Photoregulation of the transcription of chloroplastic and cytoplasmic ribosomal RNAs. Arch Biochem Biophys. 1976 Nov;177(1):201–216. doi: 10.1016/0003-9861(76)90430-6. [DOI] [PubMed] [Google Scholar]
- Curtis S. E., Rawson J. R. Measurement of the transcription of nuclear single-copy deoxyribonucleic acid during chloroplast development in Euglena gracilis. Biochemistry. 1979 Nov 27;18(24):5299–5304. doi: 10.1021/bi00591a006. [DOI] [PubMed] [Google Scholar]
- Egan J. M., Dorsky D., Schiff J. A. Events Surrounding the Early Development of Euglena Chloroplasts: VI. Action Spectra for the Formation of Chlorophyll, Lag Elimination in Chlorophyll Synthesis, and Appearance of TPN-dependent Triose Phosphate Dehydrogenase and Alkaline DNase Activities. Plant Physiol. 1975 Aug;56(2):318–323. doi: 10.1104/pp.56.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrum M. A., Schwartzbach S. D. Nutritional Regulation of Organelle Biogenesis in Euglena: INDUCTION OF MICROBODIES. Plant Physiol. 1981 Aug;68(2):430–434. doi: 10.1104/pp.68.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrum M. A., Schwartzbach S. D. Nutritional Regulation of Organelle Biogenesis in Euglena: REPRESSION OF CHLOROPHYLL AND NADP-GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE SYNTHESIS. Plant Physiol. 1980 Feb;65(2):382–386. doi: 10.1104/pp.65.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B., Gingrich J. C., Stiegler G. L., Farley M. A., Delius H., Hallick R. B. Nine introns with conserved boundary sequences in the Euglena gracilis chloroplast ribulose-1,5-bisphosphate carboxylase gene. Cell. 1984 Feb;36(2):545–553. doi: 10.1016/0092-8674(84)90247-2. [DOI] [PubMed] [Google Scholar]
- Miller M. E., Jurgenson J. E., Reardon E. M., Price C. A. Plastid translation in organello and in vitro during light-induced development in Euglena. J Biol Chem. 1983 Dec 10;258(23):14478–14484. [PubMed] [Google Scholar]
- Monroy A. F., Schwartzbach S. D. Catabolite repression of chloroplast development in Euglena. Proc Natl Acad Sci U S A. 1984 May;81(9):2786–2790. doi: 10.1073/pnas.81.9.2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon E. M., Price C. A. Cytoplasmic regulation of chloroplast translation in Euglena gracilis. Arch Biochem Biophys. 1983 Oct 15;226(2):433–440. doi: 10.1016/0003-9861(83)90312-0. [DOI] [PubMed] [Google Scholar]
- Russell G. K., Draffan A. G. Light-induced Enzyme Formation in a Chlorophyll-less Mutant of Euglena gracilis. Plant Physiol. 1978 Nov;62(5):678–682. doi: 10.1104/pp.62.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff J. A., Zeldin M. H., Rubman J. Chlorophyll Formation and Photosynthetic Competence in Euglena During Light-Induced Chloroplast Development in the Presence of 3, (3,4-dichlorophenyl) 1,1-Dimethyl Urea (DCMU). Plant Physiol. 1967 Dec;42(12):1716–1725. doi: 10.1104/pp.42.12.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. Rapid degradation of unassembled ribulose 1,5-bisphosphate carboxylase small subunits in chloroplasts. Proc Natl Acad Sci U S A. 1983 May;80(9):2632–2636. doi: 10.1073/pnas.80.9.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzbach S. D., Schiff J. A. Chloroplast and cytoplasmic ribosomes of Euglena: selective binding of dihydrostreptomycin to chloroplast ribosomes. J Bacteriol. 1974 Oct;120(1):334–341. doi: 10.1128/jb.120.1.334-341.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzbach S. D., Schiff J. A., Goldstein N. H. Events surrounding the early development of euglena chloroplasts: v. Control of paramylum degradation. Plant Physiol. 1975 Aug;56(2):313–317. doi: 10.1104/pp.56.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]



