Abstract
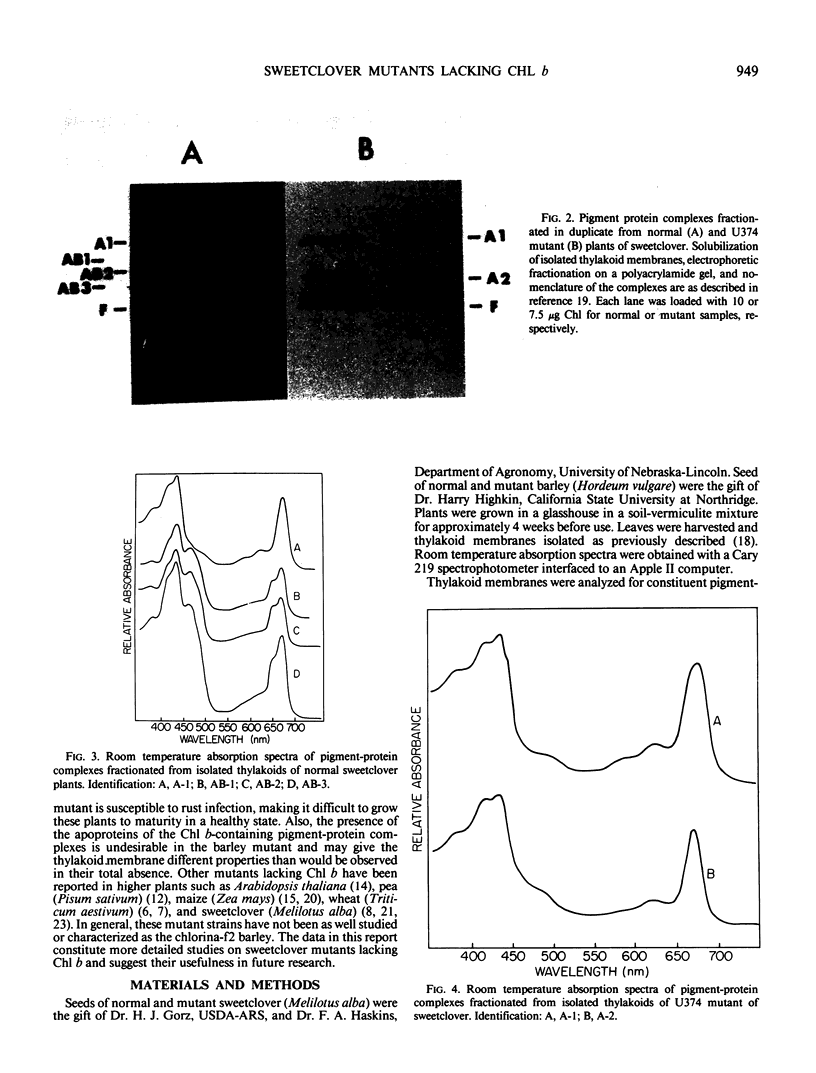
Mutants of sweetclover (Melilotus alba) with defects in the nuclear ch5 locus were examined. Using thin-layer chromatography and absorption spectroscopy, three of these mutants were found to lack chlorophyll (Chl) b. One of these three mutants, U374, possessed thylakoid membranes lacking the three Chl b-containing pigment-protein complexes (AB-1, AB-2, and AB-3) while still containing A-1 and A-2, Chl a complexes derived from photosystems I and II, respectively. Complete solubilization and denaturation of the thylakoid proteins from this mutant revealed very little apoprotein from the Chl b-containing light-harvesting complexes, the major thylakoid proteins in normal plants. The normal and mutant sweetclover plants had active thylakoid protein kinase activities and numerous polypeptides were labeled following incubation with [γ-32P]ATP. With the U374 mutant, however, there was very little detectable label co-migrating with the light-harvesting complex apoproteins on polyacrylamide gels. The Chl b-deficient chlorina-f2 mutant of barley (Hordeum vulgare) also had an active protein kinase activity capable of phosphorylating numerous polypeptides, including ones migrating with the same mobility as the light-harvesting complex apoproteins. These results indicate that the sweetclover mutants may be useful systems for studies on the function and organization of Chl b in thylakoid membranes of higher plants.
Full text
PDF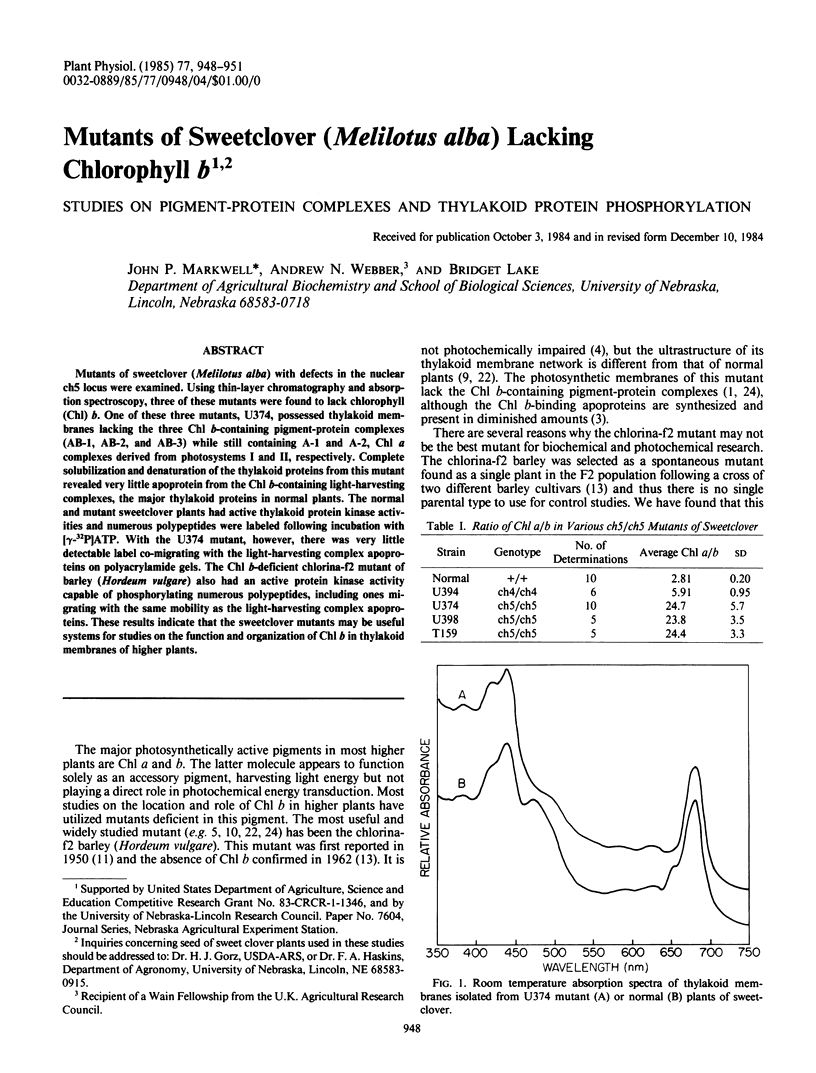


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemare G., Bartlett S. G., Chua N. H. Biosynthesis of chlorophyll a/b-binding polypeptides in wild type and the chlorina f2 mutant of barley. J Biol Chem. 1982 Jul 10;257(13):7762–7767. [PubMed] [Google Scholar]
- Boardman N. K., Highkin H. R. Studies on a barley mutant lacking chlorophyll b. I. Photochemical activity of isolated chloroplasts. Biochim Biophys Acta. 1966 Oct 10;126(2):189–199. doi: 10.1016/0926-6585(66)90054-9. [DOI] [PubMed] [Google Scholar]
- Burke J. J., Steinback K. E., Arntzen C. J. Analysis of the Light-harvesting Pigment-Protein Complex of Wild Type and a Chlorophyll-b-less Mutant of Barley. Plant Physiol. 1979 Feb;63(2):237–243. doi: 10.1104/pp.63.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysen M. E., Freeman T. P., Williams N. D., Olson L. L. Regulation of excitation energy in a wheat mutant deficient in light-harvesting pigment protein complex. Plant Physiol. 1984 Nov;76(3):561–566. doi: 10.1104/pp.76.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild D. J., Highkin H. R., Boardman N. K. The fine structure of chloroplasts in a barley mutant lacking chlorophyll B. Exp Cell Res. 1966 Oct;43(3):684–688. doi: 10.1016/0014-4827(66)90045-0. [DOI] [PubMed] [Google Scholar]
- Haworth P., Kyle D. J., Arntzen C. J. Protein phosphorylation and excitation energy distribution in normal intermittent-light-grown, and a chlorophyll b-less mutant of barley. Arch Biochem Biophys. 1982 Oct 1;218(1):199–206. doi: 10.1016/0003-9861(82)90336-8. [DOI] [PubMed] [Google Scholar]
- Highkin H. R., Boardman N. K., Goodchild D. J. Photosynthetic Studies on a Pea-mutant Deficient in Chlorophyll. Plant Physiol. 1969 Sep;44(9):1310–1320. doi: 10.1104/pp.44.9.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highkin H. R. CHLOROPHYLL STUDIES ON BARLEY MUTANTS. Plant Physiol. 1950 Apr;25(2):294–306. doi: 10.1104/pp.25.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highkin H. R., Frenkel A. W. Studies of growth & metabolism of a barley mutant lacking chlorophyll b. Plant Physiol. 1962 Nov;37(6):814–820. doi: 10.1104/pp.37.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell J. P., Baker N. R., Bradbury M., Thornber J. P. Use of zinc ions to study thylakoid protein phosphorylation and the state 1-state 2 transition in vitro. Plant Physiol. 1984 Feb;74(2):348–354. doi: 10.1104/pp.74.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell J. P., Reinman S., Thornber J. P. Chlorophyll-protein complexes from higher plants: a procedure for improved stability and fractionation. Arch Biochem Biophys. 1978 Sep;190(1):136–141. doi: 10.1016/0003-9861(78)90260-6. [DOI] [PubMed] [Google Scholar]
- Miles C. D., Markwell J. P., Thornber J. P. Effect of nuclear mutation in maize on photosynthetic activity and content of chlorophyll-protein complexes. Plant Physiol. 1979 Nov;64(5):690–694. doi: 10.1104/pp.64.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornber J. P., Highkin H. R. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974 Jan 3;41(1):109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]