Abstract
Stomatal conductance is coupled to leaf photosynthetic rate over a broad range of environmental conditions. We have investigated the extent to which chloroplasts in guard cells may contribute to this coupling through their photosynthetic activity. Guard cells were isolated by sonication of abaxial epidermal peels of Vicia faba. The electrochromic band shift of isolated guard cells was probed in vivo as a means of studying the electric field that is generated across the thylakoid membranes by photosynthetic electron transport and dissipated by photophosphorylation. Both guard cells and mesophyll cells exhibited fast and slow components in the formation of the flash-induced electrochromic change. The spectrum of electrochromic absorbance changes in guard cells was the same as in the leaf mesophyll and was typical of that observed in isolated chloroplasts. This observation indicates that electron transport and photophosphorylation occur in guard cell chloroplasts. Neither the fast nor the slow component of the absorbance change was observed in the presence of the uncoupler carbonylcyanide p-trifluoromethoxy-phenylhydrazone which confirms that the absorbance change was caused by the electric field across the thylakoid membranes. The magnitude of the fast rise was reduced by half in the presence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea. Therefore, photosystem II is functional and roughly equal in concentration to photosystem I in guard cell chloroplasts. The slow rise was abolished by 2,5-dibromo-3-methyl-6-isopropyl-1,4-benzoquinone indicating the involvement of the cytochrome b6/f complex in electron transport between the two photosystems. Relaxation of the absorbance change was irreversibly retarded in cells treated with the energy transfer inhibitor, N,N′-dicyclohexylcarbodiimide. The slowing of the rapid decay kinetics by N,N′-dicyclohexylcarbodiimide confirms that the electrical potential across the thyalkoid membrane is dissipated by photophosphorylation. These results show that guard cell chloroplasts conduct photosynthetic electron transport in a manner similar to that in mesophyll cells and provide the first evidence that photophosphorylation occurs in guard cells in vivo.
Full text
PDF

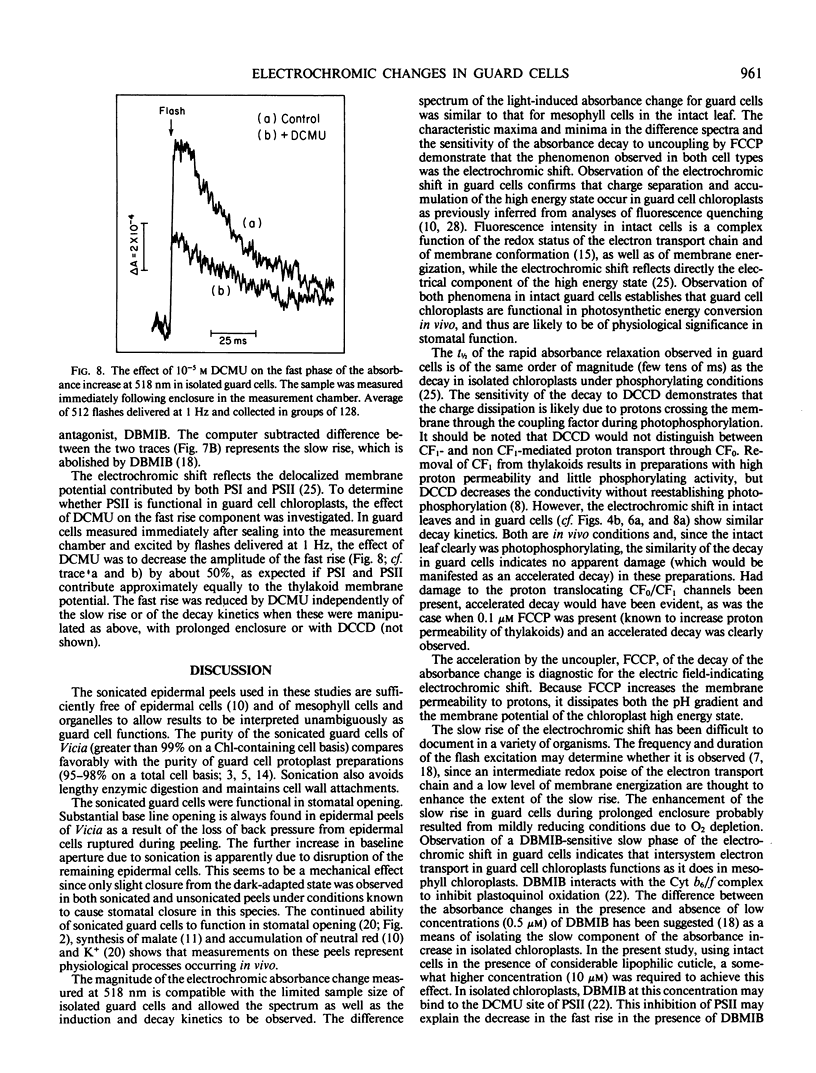

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hsiao T. C., Allaway W. G. Action Spectra for Guard Cell Rb Uptake and Stomatal Opening in Vivia faba. Plant Physiol. 1973 Jan;51(1):82–88. doi: 10.1104/pp.51.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot P., Delosme R. Flash-induced 519 nm absorption change in green algae. Biochim Biophys Acta. 1974 Aug 23;357(2):267–284. doi: 10.1016/0005-2728(74)90066-8. [DOI] [PubMed] [Google Scholar]
- Melis A., Zeiger E. Chlorophyll a Fluorescence Transients in Mesophyll and Guard Cells : MODULATION OF GUARD CELL PHOTOPHOSPHORYLATION BY CO(2). Plant Physiol. 1982 Mar;69(3):642–647. doi: 10.1104/pp.69.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Grantz D., Boyer J., Govindjee Effects of Cations and Abscisic Acid on Chlorophyll a Fluorescence in Guard Cells of Vicia faba. Plant Physiol. 1982 May;69(5):1140–1144. doi: 10.1104/pp.69.5.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Manchester J., Dicamelli C. A., Randall D. D., Rapp B., Veith G. M. Photosynthetic carbon reduction pathway is absent in chloroplasts of Vicia faba guard cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6371–6375. doi: 10.1073/pnas.76.12.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Mayne B. C., Zenger V. E., Manchester J. Presence of Both Photosystems in Guard Cells of Vicia faba L: IMPLICATIONS FOR ENVIRONMENTAL SIGNAL PROCESSING. Plant Physiol. 1981 Jan;67(1):12–16. doi: 10.1104/pp.67.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao I. M., Anderson L. E. Light and stomatal metabolism : I. Possible involvement of light modulation of enzymes in stomatal movement. Plant Physiol. 1983 Mar;71(3):451–455. doi: 10.1104/pp.71.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T. D., Raschke K. Separation and measurement of direct and indirect effects of light on stomata. Plant Physiol. 1981 Jul;68(1):33–40. doi: 10.1104/pp.68.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn K. C., Outlaw W. H. Cytochemical and cytofluorometric evidence for guard cell photosystems. Plant Physiol. 1983 Feb;71(2):420–424. doi: 10.1104/pp.71.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velthuys B. R. A third site of porton translocation in green plant photosynthetic electron transport. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6031–6034. doi: 10.1073/pnas.75.12.6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt H. T. Coupling of quanta, electrons, fields, ions and phosphrylation in the functional membrane of photosynthesis. Results by pulse spectroscopic methods. Q Rev Biophys. 1971 Nov;4(4):365–477. doi: 10.1017/s0033583500000834. [DOI] [PubMed] [Google Scholar]
- Zeiger E., Armond P., Melis A. Fluorescence Properties of Guard Cell Chloroplasts: EVIDENCE FOR LINEAR ELECTRON TRANSPORT AND LIGHT-HARVESTING PIGMENTS OF PHOTOSYSTEMS I AND II. Plant Physiol. 1981 Jan;67(1):17–20. doi: 10.1104/pp.67.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger E., Hepler P. K. Light and stomatal function: blue light stimulates swelling of guard cell protoplasts. Science. 1977 May 20;196(4292):887–889. doi: 10.1126/science.196.4292.887. [DOI] [PubMed] [Google Scholar]