Key Points
-
•
Liso-cel significantly improved EFS, CR rate, and PFS vs chemotherapy ± ASCT as a second-line treatment for LBCL.
-
•
Liso-cel was well tolerated as a second-line therapy, with low rates of any grade or severe cytokine release syndrome and neurological events.
Visual Abstract
Abstract
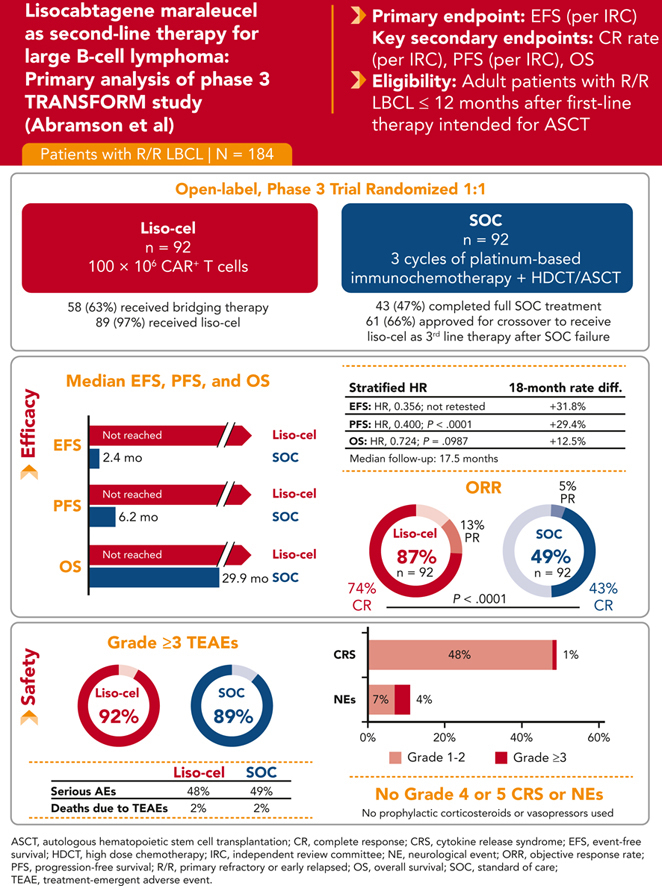
This global phase 3 study compared lisocabtagene maraleucel (liso-cel) with a standard of care (SOC) as second-line therapy for primary refractory or early relapsed (≤12 months) large B-cell lymphoma (LBCL). Adults eligible for autologous stem cell transplantation (ASCT; N = 184) were randomly assigned in a 1:1 ratio to liso-cel (100 × 106 chimeric antigen receptor–positive T cells) or SOC (3 cycles of platinum-based immunochemotherapy followed by high-dose chemotherapy and ASCT in responders). The primary end point was event-free survival (EFS). In this primary analysis with a 17.5-month median follow-up, median EFS was not reached (NR) for liso-cel vs 2.4 months for SOC. Complete response (CR) rate was 74% for liso-cel vs 43% for SOC (P < .0001) and median progression-free survival (PFS) was NR for liso-cel vs 6.2 months for SOC (hazard ratio [HR] = 0.400; P < .0001). Median overall survival (OS) was NR for liso-cel vs 29.9 months for SOC (HR = 0.724; P = .0987). When adjusted for crossover from SOC to liso-cel, 18-month OS rates were 73% for liso-cel and 54% for SOC (HR = 0.415). Grade 3 cytokine release syndrome and neurological events occurred in 1% and 4% of patients in the liso-cel arm, respectively (no grade 4 or 5 events). These data show significant improvements in EFS, CR rate, and PFS for liso-cel compared with SOC and support liso-cel as a preferred second-line treatment compared with SOC in patients with primary refractory or early relapsed LBCL. This trial was registered at www.clinicaltrials.gov as #NCT03575351.
Abramson and colleagues report on the primary analysis of the TRANSFORM study, a randomized trial of lisocabtagene maraleucel (liso-cel) following a cycle of bridging therapy (if needed) vs standard-of-care salvage chemotherapy and autologous transplantation in second-line therapy of patients with primary refractory or early relapse large B-cell lymphoma. Liso-cel significantly improves event-free survival as well as complete response rate and progression-free survival but not overall survival after a median of 18 months follow-up. These data establish liso-cel as a standard of care for these patients with a previous poor prognosis.
Introduction
For patients with relapsed or refractory large B-cell lymphoma (LBCL), platinum-based immunochemotherapy followed by high-dose chemotherapy and autologous stem cell transplantation (ASCT) in patients who are sensitive to chemotherapy has been the standard of care (SOC) for decades.1,2 Up to half of the patients who undergo a second-line immunochemotherapy do not proceed to undergo transplantation because of chemotherapy-insensitive disease, and <30% of patients are cured.3, 4, 5, 6 Recent studies have demonstrated that patients with relapsed or refractory LBCL benefit from chimeric antigen receptor (CAR) T-cell therapies as the second-line therapy.7, 8, 9
Lisocabtagene maraleucel (liso-cel) is an autologous, CD19-directed, 4-1BB costimulated CAR T-cell product administered at equal target doses of CD8+ and CD4+ CAR+ T cells. Results of a prespecified interim analysis of the TRANSFORM study (www.clinicaltrials.gov, #NCT03575351), performed at a median follow-up of 6.2 months, demonstrated superior efficacy of liso-cel than that of SOC as a second-line treatment for patients with LBCL primary refractory to or relapsed within 12 months of first-line therapy; the overall survival (OS) data were immature.9 Results of the event-driven primary analysis of TRANSFORM are reported here, with a median follow-up of 17.5 months.
Methods
Study design
TRANSFORM is a global, randomized, open-label, phase 3 study of liso-cel vs SOC second-line therapy in adults with relapsed or refractory LBCL. Details of the study design have been previously described.9 Briefly, the study enrolled adults (18-75 years of age) having positron emission tomography (PET)–positive LBCL, per Lugano 2014 criteria,10 refractory (stable disease, progressive disease, partial response [PR], or complete response [CR] with relapse ≤3 months) or relapsed (CR with relapse ≤12 months) disease after CD20 antibody and anthracycline-containing first-line therapy, with an Eastern Cooperative Oncology Group performance status ≤1, adequate organ function, and eligible for high-dose chemotherapy and ASCT.
The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guidelines, and applicable regulatory requirements. Institutional review boards at participating institutions approved the study protocol and amendments. All patients provided written informed consent before any study-related procedures.
All patients underwent leukapheresis before being randomly assigned, in a ratio of 1:1, to either the liso-cel or SOC arm. Randomization was stratified based on response to the first-line therapy and secondary age-adjusted International Prognostic Index. Patients randomly assigned to the liso-cel arm received lymphodepleting chemotherapy (fludarabine, 30 mg/m2 and cyclophosphamide, 300 mg/m2 daily for 3 days), followed by a liso-cel infusion at a dose of 100 × 106 CAR+ T cells. Bridging therapy with a single cycle of one of the protocol-defined SOC platinum-based chemotherapy regimens was allowed as per investigator discretion during liso-cel manufacturing; patients were reassessed using PET scan before receiving lymphodepleting therapy to confirm the presence of PET-positive disease, but no formal response assessment was conducted at that time. Patients randomly assigned to the SOC arm received 3 cycles of immunochemotherapy (investigator choice of R-DHAP [rituximab, dexamethasone, cytarabine, and cisplatin]; R-ICE [rituximab, ifosfamide, carboplatin, and etoposide]; or R-GDP [rituximab, gemcitabine, dexamethasone, and cisplatin]), with response evaluated using PET/computed tomography scan after 3 cycles. Responding patients (CR or PR) were to proceed to high-dose chemotherapy (carmustine, etoposide, cytarabine, and melphalan) and ASCT. Patients in the SOC arm were allowed to cross over and receive liso-cel upon independent review committee (IRC) confirmation of a failed response after 3 cycles of SOC, disease progression at any time, or absence of CR 18 weeks after randomization.
End points and assessments
The primary efficacy end point was event-free survival (EFS) based on the IRC evaluation per Lugano 2014 criteria,10 defined as the time from randomization to death from any cause, progressive disease, failure to achieve CR or PR by 9 weeks after randomization (evaluated after 3 cycles of SOC and 5 weeks after liso-cel infusion), or the start of new antineoplastic therapy because of efficacy concerns, whichever occurred first. Key secondary efficacy end points were CR rate, progression-free survival (PFS), and OS. Additional secondary efficacy end points included overall response rate (ORR) and duration of response (DOR). Exploratory efficacy end points included EFS, CR rate, PFS, OS, ORR, and DOR for crossover patients.
Safety end points included the type, frequency, and severity of adverse events (AEs), serious AEs, and laboratory abnormalities. Exploratory end points included cellular kinetics. See supplemental Table 1, available on the Blood website, for the complete list of study end points.
Efficacy was assessed per Lugano 2014 criteria10 by an IRC based on PET/computed tomography scans at week 9 (after 3 cycles of SOC and 5 weeks after liso-cel infusion), week 18 (8 weeks after start of high-dose chemotherapy and 14 weeks after liso-cel infusion), and months 6, 9, 12, 18, 24, and 36. AEs were evaluated by investigators. AEs and laboratory abnormalities were graded per the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Cytokine release syndrome (CRS) was graded per Lee 2014 criteria.11 Neurological events (NEs) were defined as investigator-identified neurological AEs related to liso-cel. Cellular kinetics were analyzed in peripheral blood samples via a droplet digital polymerase chain reaction to detect the liso-cel CAR transgene.9
Statistical analysis
Efficacy analyses were conducted on the intention-to-treat set, safety analyses in the safety set, and cellular kinetic analyses in the cellular kinetic set. See supplemental Table 2 for definitions of all analysis sets.
It was calculated that 119 events would provide ≥90% power at a 2.5%, 1-sided significance level to reject the null hypothesis of a hazard ratio (HR) ≥1 for the primary end point during the primary analysis. A sample size of 182 patients was planned to be randomized. A hierarchical testing strategy was used for the primary (EFS) and key secondary end points (CR rate, PFS, and OS) to control the type I error rate.9 As described in the statistical analysis plan, all key secondary end points were to be retested at the primary analysis if the null hypothesis was not rejected for any one of them at the interim analysis. The significance threshold to reject the null hypothesis for the key secondary end points was ≤0.021 at the primary analysis (per the O’Brien-Fleming boundary alpha spending function). For time-to-event end points, the Kaplan-Meier product limit was used to provide summarized information and 95% confidence intervals (CIs); time-to-event rates were computed using the Greenwood formula. HRs were estimated using a stratified Cox proportional hazards model. Prespecified supportive OS analyses, adjusting for patients in the SOC arm crossing over to receive liso-cel as a third-line therapy, were conducted using both the 2-stage accelerated and rank-preserving structural failure time models.12,13 These methodologies aim to estimate survival times that would have been observed in the SOC arm had the crossover not occurred. A stratified Cox proportional hazards regression model was fitted to the observed liso-cel arm survival times and the counterfactual SOC arm survival times to estimate a crossover-adjusted HR (supplemental Appendix, pages 7-8). For the CR rate, the Cochran-Mantel-Haenszel test with stratification factors was used for analysis. Additional statistical analyses are provided in the supplemental Appendix.
Results
Patients
As of the primary analysis data cutoff date of 13 May 2022, the median follow-up was 17.5 months (range, 0.9-37.0). A total of 184 patients were randomly assigned, with 92 patients in each arm. In the SOC arm, 91 were treated with second-line immunochemotherapy (1 withdrew consent), 43 (47%) received high-dose chemotherapy and ASCT, and 61 (66%) were approved for crossover to receive liso-cel, and of them, 58 were infused (57 with liso-cel and 1 with nonconforming product). In the liso-cel arm, 89 received liso-cel infusion (1 withdrew consent, 1 had a manufacturing failure, and 1 received a nonconforming CAR T-cell product). Fifty-eight (63%) patients in the liso-cel arm received bridging therapy (supplemental Figure 1). The most common reasons for receiving bridging therapy, as per investigator assessment, were high tumor burden (28 of 58 [48%]) and rapid progression (23 of 58 [40%]). Nineteen patients (21%) received liso-cel in the outpatient setting.
Baseline characteristics were generally balanced between arms. Overall, 33% of patients were ≥65 years of age, 64% had diffuse LBCL (56% not otherwise specified and 8% transformed from indolent lymphomas), 23% had high-grade B-cell lymphoma (double/triple hit), 74% had refractory disease after first-line therapy, and 26% had relapsed disease within 12 months after the first-line therapy (Table 1).
Table 1.
Demographics and baseline disease characteristics (ITT set)
| Characteristics | Liso-cel (n = 92) | SOC (n = 92) |
|---|---|---|
| Male sex, n (%) | 44 (48) | 61 (66) |
| Age, y | ||
| Median (range) | 60 (20-74) | 58 (26-75) |
| <65, n (%) | 56 (61) | 67 (73) |
| ≥65 to <75, n (%) | 36 (39) | 23 (25) |
| 75, n (%) | 0 | 2 (2) |
| LBCL subtypes,∗n (%) | ||
| DLBCL NOS | 53 (58) | 50 (54) |
| DLBCL transformed from indolent lymphomas | 7 (8) | 8 (9) |
| FL grade 3B | 1 (1) | 0 |
| HGBCL with gene rearrangements in MYC and BCL2, BCL6, or both† | 22 (24) | 21 (23) |
| PMBCL | 8 (9) | 9 (10) |
| THRBCL | 1 (1) | 4 (4) |
| ECOG PS, n (%) | ||
| 0 | 48 (52) | 57 (62) |
| 1 | 44 (48) | 35 (38) |
| sAAIPI, n (%) | ||
| 0 or 1 | 56 (61) | 55 (60) |
| 2 or 3 | 36 (39) | 37 (40) |
| Prior response status, n (%) | ||
| Refractory‡ | 67 (73) | 70 (76) |
| Relapsed§ | 25 (27) | 22 (24) |
| Ann Arbor stage, n (%) | ||
| 1 | 8 (9) | 14 (15) |
| 2 | 16 (17) | 15 (16) |
| 3 | 18 (20) | 13 (14) |
| 4 | 50 (54) | 50 (54) |
| SPD, median (range), cm2 | 11.4 (1-120) | 15.7 (1-224) |
| SPD >50 cm2, n (%) | 10 (11) | 10 (11) |
| Missing | 5 (5) | 6 (7) |
| Secondary CNS lymphoma, n (%) | 1 (1) | 3 (3) |
| Best response to first-line therapy, n (%) | ||
| CR | 30 (33) | 28 (30) |
| PR | 36 (39) | 46 (50) |
| Stable disease | 7 (8) | 5 (5) |
| Progressive disease | 19 (21) | 13 (14) |
| Not evaluable | 0 | 0 |
| Median (range) time from initial diagnosis to randomization, mo | 7.6 (2.0-21.5) | 7.7 (2.5-25.4) |
CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; FL, follicular lymphoma; HGBCL, high-grade B-cell lymphoma; ITT, intention-to-treat; NOS, not otherwise specified; PMBCL, primary mediastinal large B-cell lymphoma; sAAIPI, secondary age-adjusted International Prognostic Index; SPD, sum of the product of perpendicular diameters; THRBCL, T-cell/histiocyte-rich large B-cell lymphoma.
Based on World Health Organization 2016 classification, as reported by the investigator.
Fluorescence in situ hybridization results were assessed locally but subsequently confirmed by a central laboratory.
Defined as stable disease, progressive disease, PR, or CR with relapse <3 months after first-line therapy.
Defined as CR with relapse on or after 3 months within 12 months after first-line therapy.
Efficacy
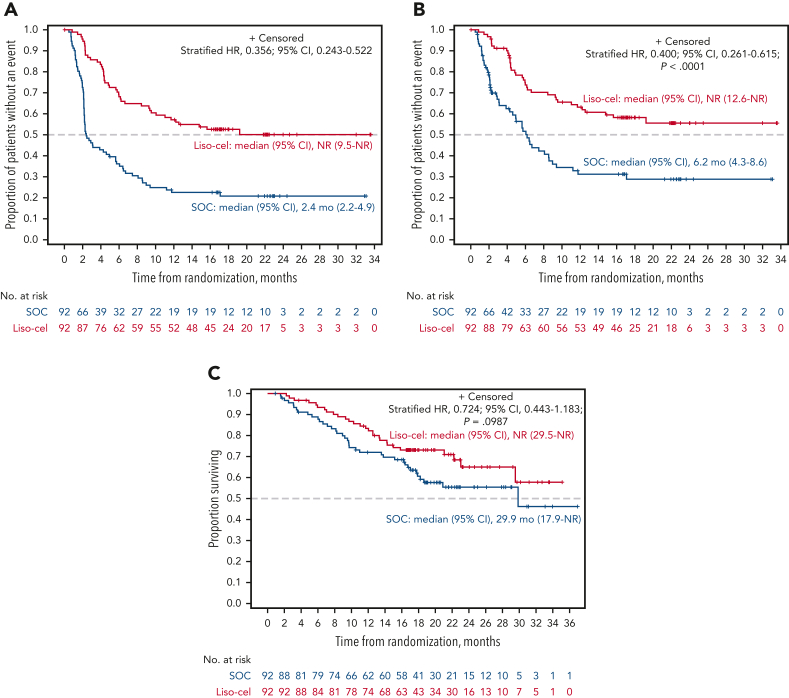
The median EFS was not reached (NR; 95% CI, 9.5 to NR) for liso-cel vs 2.4 months (95% CI, 2.2-4.9) for SOC (HR, 0.356; 95% CI, 0.243-0.522) (Figure 1A). EFS rates at 18 months were 52.6% (95% CI, 42.3-62.9) for liso-cel vs 20.8% (95% CI, 12.2-29.5) for SOC. In subgroup analyses, EFS favored liso-cel across all prespecified subgroups (supplemental Figure 2).
Figure 1.
EFS, PFS, and OS (ITT set). The graphs show Kaplan-Meier estimates of EFS (primary end point) (A), PFS per IRC (B), and OS based on the ITT principle (C).
The key secondary end points of CR rate and PFS were met, demonstrating the superiority of liso-cel over SOC. The CR rate was 74% (95% CI, 63.7-82.5) for liso-cel vs 43% (95% CI, 33.2-54.2) for SOC (P < .0001). Of 26 patients with the best overall response of PR at the interim analysis, the response deepened to CR for 9 patients at the primary analysis (6/18 in the liso-cel arm and 3/8 in the SOC arm). The median PFS was NR (95% CI, 12.6 to NR) for liso-cel vs 6.2 months (95% CI, 4.3-8.6) for SOC (HR, 0.400; 95% CI, 0.261-0.615; P < .0001) (Table 2; Figure 1B). The PFS rates at 18 months were 58.2% (95% CI, 47.7-68.7) for liso-cel vs 28.8% (95% CI, 17.7-40.0) for SOC.
Table 2.
Summary of primary, key secondary, and secondary efficacy outcomes (ITT set)
| Liso-cel (n = 92) | SOC (n = 92) | Liso-cel vs SOC | |
|---|---|---|---|
| Primary efficacy end point per IRC | |||
| EFS | Stratified HR (95% CI) | ||
| Patients with events, n (%) | 44 (48) | 71 (77) | — |
| Median (95% CI) EFS, mo∗ | NR (9.5-NR) | 2.4 (2.2-4.9) | 0.356 (0.243-0.522) |
| 12-mo EFS rate, % (95% CI)† | 57.1 (47.0-67.3) | 22.5 (13.9-31.2) | — |
| 18-mo EFS rate, % (95% CI)† | 52.6 (42.3-62.9) | 20.8 (12.2-29.5) | — |
| Key secondary efficacy end points | |||
| CR rate per IRC | Stratified 1-sided P value | ||
| CR, n (%); 95% CI‡ | 68 (74); 63.7-82.5 | 40 (43); 33.2-54.2 | <.0001 |
| PFS per IRC | Stratified HR (95% CI); P value§ | ||
| Patients with events, n (%) | 37 (40) | 52 (57) | — |
| Median (95% CI) PFS, mo∗ | NR (12.6-NR) | 6.2 (4.3-8.6) | 0.400 (0.261-0.615); <.0001 |
| 12-mo PFS rate, % (95% CI)† | 63.1 (53.0-73.3) | 31.2 (20.2-42.3) | — |
| 18-mo PFS rate, % (95% CI)† | 58.2 (47.7-68.7) | 28.8 (17.7-40.0) | — |
| OS | Stratified HR (95% CI); P value§ | ||
| Patients with events, n (%) | 28 (30) | 38 (41) | — |
| Median (95% CI) OS, mo∗ | NR (29.5-NR) | 29.9 (17.9-NR) | 0.724 (0.443-1.183); .0987 |
| 12-mo OS rate, % (95% CI)† | 83.4 (75.7-91.1) | 72.0 (62.7-81.3) | — |
| 18-mo OS rate, % (95% CI)† | 73.1 (63.9-82.3) | 60.6 (50.2-71.1) | — |
| Secondary efficacy end points per IRC | |||
| ORR | |||
| ORR, n (%); 95% CI‡ | 80 (87); 78.3-93.1 | 45 (49); 38.3-59.6 | — |
| DOR | Stratified HR (95% CI) | ||
| Patients with events, n/N (%) | 31/80 (39) | 25/45 (56) | — |
| Median (95% CI) DOR, mo∗ | NR (13.4-NR) | 9.1 (5.1-NR) | 0.579 (0.340-0.984) |
| Duration of CR | |||
| Patients with events, n/N (%) | 21/68 (31) | 21/40 (52.5) | — |
| Median (95% CI) DOR, mo∗ | NR (NR-NR) | 9.3 (5.1-NR) | 0.483 (0.262-0.890) |
All percentages are rounded to whole numbers except those with “.5%.”
— denotes not available or not reported.
Median estimates of time to event were Kaplan-Meier product-limit estimates.
Based on Greenwood formula; 2-sided CI.
Based on Cochran-Mantel-Haenszel test; 2-sided CI.
Based on a stratified Cox proportional hazards model; 1-sided P value.
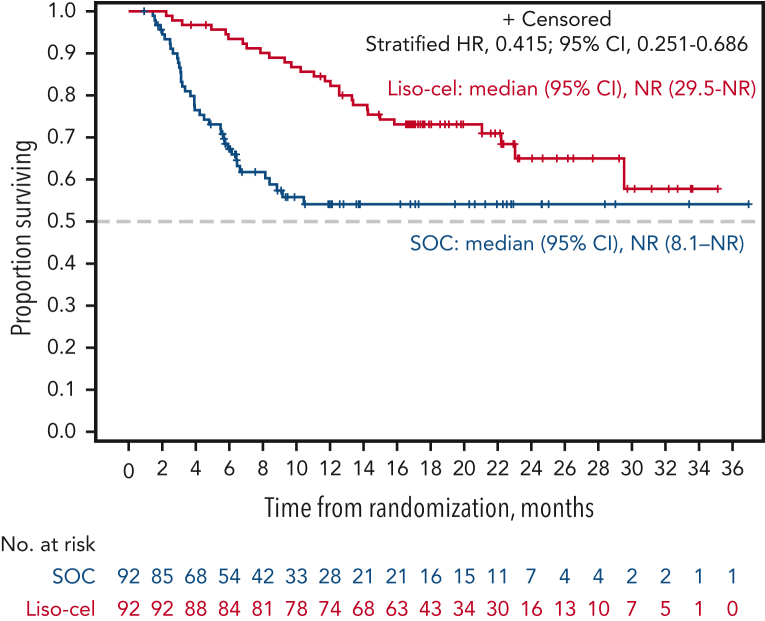
There were 28 deaths in the liso-cel arm and 38 deaths in the SOC arm. The median OS was NR (95% CI, 29.5 to NR) for liso-cel vs 29.9 months (95% CI, 17.9 to NR) for SOC (HR, 0.724; 95% CI, 0.443-1.183; P = .0987) (Figure 1C). OS rates at 18 months were 73.1% (95% CI, 63.9-82.3) for liso-cel vs 60.6% (95% CI, 50.2-71.1) for SOC. In a prespecified supportive OS analysis using the 2-stage accelerated failure time model, conducted to adjust for the treatment effect of patients in the SOC arm crossing over to receive liso-cel, the median OS was NR for both liso-cel and SOC (HR, 0.415; 95% CI, 0.251-0.686) (Figure 2), with 18-month OS rates of 73.1% (95% CI, 63.9-82.3) for liso-cel and 54.1% (95% CI, 43.1-65.2) for SOC (supplemental Table 3). Consistent with results from the 2-stage accelerated failure time model, an additional prespecified OS analysis using the rank-preserving structural failure time model showed an HR of 0.279 (95% CI, 0.145-0.537), also favoring the liso-cel arm (supplemental Table 3).
Figure 2.
OS adjusted for crossover from SOC to liso-cel using a 2-stage accelerated failure time model. OS for SOC was estimated based on the outcome if crossover had not occurred (ie, hypothetical scenario to isolate the relative effect of liso-cel vs SOC alone without subsequent anticancer therapy).
Secondary efficacy end points of ORR, DOR, and duration of CR also favored liso-cel (Table 2). The median duration of CR was NR (95% CI, NR to NR) with liso-cel vs 9.3 months (95% CI, 5.1 to NR) with SOC. Of the 58 patients who received bridging therapy, 47 were tested to be PET-positive and 9 were PET-negative after bridging therapy; 2 patients did not have a prelymphodepleting chemotherapy assessment. When analyzed based on bridging therapy use, the EFS, CR rate, and PFS consistently favored liso-cel over SOC, regardless of whether patients had PET-positive or PET-negative disease after bridging therapy (supplemental Table 4).
In the liso-cel arm, 30 patients received at least 1 subsequent therapy: 30 (33%) received systemic anticancer therapy, 10 (11%) received stem cell transplantation (SCT; 3 ASCT and 7 allogeneic SCT), and 4 (4%) received radiation therapy. In the SOC arm, 65 patients received at least 1 subsequent therapy: 58 (63%) crossed over and received CAR+ T cells (57 received liso-cel and 1 received nonconforming product), 23 (25%) received systemic anticancer therapy, and 2 (2%) received allogeneic SCT. Information about patients who crossed over to receive liso-cel is provided in the supplemental Appendix (supplemental Tables 5-7). The median time from crossover approval to liso-cel infusion was 15 days (range, 8-95), which was shorter than in the liso-cel arm because the product manufacturing started before randomization for all patients. Crossover patients had an ORR of 61%, a CR rate of 53%, and a median EFS of 5.9 months (95% CI, 3.1-15.1) (supplemental Table 7).
Safety
Treatment-emergent AEs (TEAEs) were experienced by almost all patients in both arms (Table 3). The most common TEAEs of any grade were neutropenia, anemia, thrombocytopenia, and nausea, which were reported in more than half of the patients in each arm. The most common grade ≥3 AEs in both arms were neutropenia, thrombocytopenia, and anemia (Table 3; supplemental Table 8). Sixty-six patients died on the study, 28 in the liso-cel arm and 38 in the SOC arm, including 29 after crossover (supplemental Table 9). The most frequent cause of death was disease progression.
Table 3.
TEAEs (safety set)
| TEAEs∗ | Liso-cel (n = 92) |
SOC (n = 91) |
||
|---|---|---|---|---|
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| Patients experiencing any TEAE, n (%) | 92 (100) | 85 (92) | 90 (99) | 81 (89) |
| Patients experiencing any serious TEAE, n (%) | 44 (48) | —† | 45 (49) | —† |
| Deaths due to TEAEs, n (%) | 2 (2) | 2 (2) | ||
| Most common TEAEs (occurring in ≥15% of patients in either arm), n (%) | ||||
| Neutropenia | 76 (83) | 75 (82) | 50 (55) | 47 (52) |
| Anemia | 62 (67) | 48 (52) | 62 (68) | 51 (56) |
| Thrombocytopenia | 55 (60) | 46 (50) | 66 (73) | 62 (68) |
| Nausea | 49 (53) | 3 (3) | 53 (58) | 4 (4) |
| CRS | 45 (49) | 1 (1) | 0 | 0 |
| Headache | 40 (43) | 4 (4) | 21 (23) | 1 (1) |
| Fatigue | 37 (40) | 0 | 37 (41) | 2 (2) |
| Constipation | 30 (33) | 2 (2) | 24 (26) | 0 |
| Pyrexia | 28 (30) | 0 | 23 (25) | 0 |
| Lymphopenia | 25 (27) | 24 (26) | 11 (12) | 9 (10) |
| Diarrhea | 23 (25) | 0 | 39 (43) | 3 (3) |
| Dizziness | 22 (24) | 0 | 13 (14) | 0 |
| Decreased appetite | 21 (23) | 1 (1) | 32 (35) | 4 (4) |
| Hypokalemia | 21 (23) | 4 (4) | 22 (24) | 4 (4) |
| Hypotension | 19 (21) | 3 (3) | 6 (7) | 0 |
| Insomnia | 19 (21) | 0 | 10 (11) | 0 |
| Vomiting | 18 (20) | 1 (1) | 27 (30) | 2 (2) |
| Leukopenia | 17 (18) | 15 (16) | 13 (14) | 11 (12) |
| Febrile neutropenia | 15 (16) | 11 (12) | 24 (26) | 21 (23) |
| Peripheral edema | 15 (16) | 1 (1) | 17 (19) | 0 |
| Hypomagnesemia | 15 (16) | 0 | 21 (23) | 1 (1) |
| Back pain | 14 (15) | 1 (1) | 16 (18) | 2 (2) |
TEAEs were defined as AEs occurring or worsening within 90 days after liso-cel infusion (liso-cel arm or crossover patients), the last dose of chemotherapy (SOC arm), or the start of new antineoplastic therapy, whichever occurred first, and treatment-related AEs occurring at any time thereafter.
Not applicable; serious TEAE can be of any grade.
TEAEs of special interest for CAR T-cell therapies and their management are reported in Table 4. The rates of any-grade CRS and NEs were 49% and 11%, respectively, with grade 3 CRS and NEs in only 1% and 4%, respectively; there were no grade 4 or 5 events. Severe infections were reported in 14 (15%) patients in the liso-cel arm and 19 (21%) in the SOC arm. Prolonged cytopenias were reported in 40 (43%) patients in the liso-cel arm, and most (73%) had recovered to grade ≤2 within 2 months after liso-cel infusion. In the SOC arm, prolonged cytopenias were reported in 3 (3%) patients. Additional details on the lineage and duration of prolonged cytopenias are provided in Table 4. Infections were reported in 2 of 92 patients >90 days after liso-cel infusion (herpes zoster and pneumonia, 1 each); no bleeding events were reported. In the liso-cel arm, 40 patients received at least 1 dose of granulocyte colony-stimulating factor, 12 received immunoglobulins, 32 received at least 1 packed red blood cell transfusion, and 34 received at least 1 platelet transfusion on or after study day 64 after randomization. Second primary malignancies were reported in 3 (3%) and 7 (8%, including 3 after crossover) patients in the liso-cel and SOC arms, respectively.
Table 4.
TEAEs of special interest (safety set)
| AEs of special interest of CRS or NEs | Liso-cel (n = 92) | |
|---|---|---|
| CRS, n (%)∗ | ||
| Any grade | 45 (49) | |
| Grade 1 | 34 (37) | |
| Grade 2 | 10 (11) | |
| Grade 3 | 1 (1) | |
| Grade 4/5 | 0 | |
| Median (range) time to onset, d | 5.0 (1-63) | |
| Median (range) time to resolution, d | 4.0 (1-16) | |
| NEs, n (%)† | ||
| Any grade | 10 (11) | |
| Grade 1 | 4 (4) | |
| Grade 2 | 2 (2) | |
| Grade 3 | 4 (4) | |
| Grade 4/5 | 0 | |
| Median (range) time to onset, d | 11.0 (7-17) | |
| Median (range) time to resolution, d | 4.5 (1-30) | |
| Clinical management of CRS and/or NEs, n (%) | ||
| Tocilizumab, corticosteroids, or both | 24 (26) | |
| Tocilizumab only | 9 (10) | |
| Tocilizumab and corticosteroids | 13 (14) | |
| Corticosteroids only | 2 (2) | |
| Vasopressors | 0 | |
| Other AEs of special interest | Liso-cel (n = 92) | SOC (n = 91) |
|---|---|---|
| Severe infections, n (%) | 14 (15) | 19 (21) |
| Hypogammaglobulinemia, n (%) | 10 (11) | 3 (3) |
| Grade ≥3 cytopenia at study d 64, n (%)‡ | 40 (43) | 3 (3) |
| Grade ≥3 neutropenia at study d 64‡ | 34 (37) | 2 (2) |
| Recovered to grade ≤2 within 35 d§ | 25 (74) | 1 (100) |
| Recovered to grade ≤2 within 62 d§ | 4 (12) | 0 |
| Recovered to grade ≤2 by end of study§ | 4 (12) | 0 |
| Grade ≥3 thrombocytopenia at study d 64‡ | 34 (37) | 0 |
| Recovered to grade ≤2 within 35 d§ | 24 (73) | 0 |
| Recovered to grade ≤2 within 62 d§ | 3 (9) | 0 |
| Recovered to grade ≤2 by end of study§ | 1 (3) | 0 |
| Grade ≥3 anemia at study d 64, n (%) | 11 (12) | 1 (1) |
| Recovered to grade ≤2 within 35 d§ | 8 (73) | 1 (100) |
| Recovered to grade ≤2 within 62 d§ | 2 (18) | 0 |
| Recovered to grade ≤2 by end of study§ | 0 | 0 |
Graded per the Lee 2014 criteria.11
Defined as investigator-identified neurological AEs related to liso-cel and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Defined as grade ≥3 central laboratory results at 35 days after liso-cel infusion or after the start of the last chemotherapy in the SOC arm. Prolonged cytopenia was assessed at the study day 64 visit for patients in the liso-cel arm (35 days after liso-cel infusion) or 35 days after the start of the last cycle of chemotherapy, including high-dose chemotherapy, for patients in the SOC arm. A window of ±6 days around these target dates was considered; within this window, the closest central laboratory assessment to the target date was used, and in case 2 assessments within the same window were equidistant from the target date, the worst result was taken. Laboratory assessments performed after starting a new antineoplastic therapy were not considered for analysis.
In the liso-cel arm, poststudy day 64 laboratory results were available for 34, 33, and 11 patients with grade ≥3 neutropenia, thrombocytopenia, and anemia, respectively. In the SOC arm, poststudy day 64 laboratory results were available for 1, 0, and 1 patient with grade ≥3 neutropenia, thrombocytopenia, and anemia, respectively. Recovery to grade ≤2 cytopenia was assessed on days 35 and 62 days after the initial prolonged cytopenia assessment and at the end of the study.
For patients randomly assigned to the SOC arm who crossed over to receive liso-cel, TEAEs after liso-cel infusion are shown in supplemental Table 10. The rates of CRS and NEs among crossover patients were similar to those in patients randomly assigned to the liso-cel arm (supplemental Table 11).
Cellular kinetics
Among evaluable patients in the liso-cel arm, the median time to maximum expansion was 10 days (range, 6-22), median maximum expansion was 33 285 copies per μg (interquartile range, 13 848-94 913), and median area under the curve from 0 to 28 days after infusion was 268 911 day × copies per μg (interquartile range, 114 626-779 701) (supplemental Table 12). Cellular kinetics for the crossover subgroup were similar to those of the liso-cel arm. Persistence of the liso-cel transgene was observed up to 23 months after infusion (5 of 15 evaluable patients) (supplemental Table 13).
Discussion
With a median follow-up of 17.5 months, the primary analysis of the TRANSFORM study confirmed the superiority of liso-cel over SOC. Treatment with liso-cel resulted in significant improvements in EFS, CR rate, and PFS. Consistent with previous studies of liso-cel,8,9,14 rates of any-grade and severe CRS and NEs were relatively low (CRS: 49% and 1%; NEs: 11% and 4%, respectively; no grade 4 or 5 events), with no prophylactic steroid use.
For patients treated with liso-cel, median EFS and PFS were NR compared with only 2.4 and 6.2 months, respectively, for SOC, and a plateau in both curves was observed at ∼12 months. At 18 months, EFS and PFS rates with liso-cel were more than double of those with SOC. In addition, significantly more patients achieved CR with liso-cel than with SOC (74% vs 43.5%), and among patients with a CR, the duration of CR was longer with liso-cel than with SOC (NR vs 9.3 months), demonstrating more sustained disease control with liso-cel, even compared with SOC that included ASCT. OS numerically favored liso-cel; however, the difference was not statistically significant. This was possibly because of the limited number of events (ie, deaths) and the treatment effect of the crossover, given that 66% of patients in the SOC arm crossed over to receive liso-cel. Both supportive OS analyses, which were adjusted for the treatment effect of the crossover, favored liso-cel over SOC.
TRANSFORM allowed patients in the liso-cel arm to receive a cycle of bridging therapy with one of the immunochemotherapy regimens in the SOC arm, which allowed patients with a high tumor burden or rapidly progressing disease to participate in the study. Regardless of whether patients were PET-negative (n = 9) or PET-positive (n = 47) after bridging therapy or did not receive bridging therapy at all (n = 34), the EFS, CR rate, and PFS consistently favored liso-cel over SOC.
The incidence of CAR T-cell therapy–specific AEs was manageable and consistent with those of previous studies of liso-cel.8,9,14 Prolonged cytopenias were observed in 43% of patients in the liso-cel arm, and most patients recovered to grade ≤2 within 2 months after infusion. The prolonged cytopenias did not result in a higher rate of severe infections compared with that in the SOC arm. Longer follow-up did not show an increased risk of long-term AEs such as secondary malignancies in the liso-cel arm.
These data add to recent phase 3 studies of other CAR T-cell therapies in similar patient populations with second-line LBCL. ZUMA-7, with a median follow-up of 24.9 months, demonstrated that axicabtagene ciloleucel led to a significant improvement in the EFS (median EFS, 8.3 vs 2.0 months) and CR rate (65% vs 32%) compared with SOC.7 The rates of any-grade and severe CRS were 92% and 6%, respectively, and rates of any-grade and severe NEs were 60% and 21%, respectively. In contrast, tisagenlecleucel was not shown to be superior to SOC in the BELINDA study, with a median EFS of 3.0 months and a CR rate of 28% in both arms.15 The median EFS for liso-cel in TRANSFORM had not been reached at a median follow-up of 17.5 months, and the CR rate was 74%. Incidences of any-grade and severe CRS and NEs were also notably lower for liso-cel than reported with axicabtagene ciloleucel in ZUMA-7. In TRANSFORM, 63% of patients in the SOC arm received liso-cel as a third-line therapy, and in ZUMA-7, 56% of patients in the SOC arm received commercial CAR T-cell therapy as the third-line therapy.7 The main differences were that 1 cycle of bridging therapy with 1 of the protocol-defined SOC regimens was allowed in TRANSFORM, whereas bridging therapy was limited to glucocorticoids in ZUMA-7; moreover, TRANSFORM included a built-in crossover, enabling immediate treatment with liso-cel (median, 15 days), which would not be achievable in a real-world setting. These results support the use of CAR T-cell therapy as a second-line treatment for patients with LBCL. Interestingly, patients in the liso-cel arm had longer EFS and higher response rates than those who crossed over to receive liso-cel as third-line treatment. This finding suggests that patients who experienced a failure with the second line of immunochemotherapy before CAR T-cell treatment might have developed more highly resistant or rapidly progressive disease. These outcomes are consistent with those reported for patients in the SOC arm of ZUMA-7, who received CAR T-cell therapy as subsequent third-line therapy.16 Overall, these data support the importance of earlier treatment with CAR T-cell therapy. The data also complement data from the PILOT study, which demonstrated a clinical benefit of liso-cel in patients with relapsed or refractory LBCL who were not intended to undergo transplantation, supporting the efficacy of liso-cel as a second-line therapy across a broad population of patients with LBCL.8
In summary, liso-cel demonstrated superior efficacy as a second-line treatment in patients with primary refractory or early relapsed LBCL compared with the SOC platinum-based immunochemotherapy followed by high-dose chemotherapy and ASCT for chemotherapy-sensitive patients and had a favorable safety profile. These data support the use of liso-cel as a preferred second-line treatment compared with SOC for patients with primary refractory or early relapsed LBCL.
Conflict-of-interest disclosure: J.S.A. reports consulting fees from Bristol Myers Squibb (BMS), AbbVie, Genentech, Epizyme, BeiGene, Kymera, bluebird bio, Incyte, Kite Pharma, Genmab, Ono Pharmaceutical, Mustang Bio, MorphoSys, Regeneron, Century, AstraZeneca, Eli Lilly, and Janssen and received research funding from BMS and Seattle Genetics. J.A. reports honoraria from BMS. B.G. is a consultant for BMS, Roche, Gilead, and Novartis; reports research funding from Roche; is a part of speaker’s bureau at BMS, Roche, and Novartis; is on the advisory boards of BMS and Roche; and is a current employee of Helios Klinikum Berlin-Buch. S.I. is an equity holder in Karyopharm Therapeutics. S. Mielke reports honoraria from Novartis and Celgene, a Bristol-Myers Squibb Company; travel support from Kite/Gilead; and is on the data safety monitoring boards for Miltenyi Biotec and Immunicum. P.M. reports grants or contracts from AstraZeneca and consultancy fees from GlaxoSmithKline. F.H.-I. is on the advisory boards of Kite; Novartis; Celgene, a Bristol-Myers Squibb Company; BMS; Epizyme; Incyte; and AbbVie. K.I. reports consulting fees from BMS, BeiGene, AstraZeneca, Ono Pharmaceutical, AbbVie, Novartis, and Chugai; honoraria from BMS, AstraZeneca, Ono Pharmaceutical, AbbVie, Novartis, Chugai, MSD, Eisai, Janssen, Kyowa Kirin, Daiichi Sankyo, Eli Lilly, SymBio Pharmaceuticals Limited, and Takeda; and research funding from BeiGene, AstraZeneca, Ono Pharmaceutical, AbbVie, Chugai, MSD, Eisai, Incyte, Janssen, Yakult Pharmaceutical Industry Co, Ltd, Daiichi Sankyo, Loxo Oncology, and Genmab. F.M. reports consulting fees from Roche, Gilead, and AbbVie and membership on an entity’s board of directors or advisory committees for Roche, Gilead, Novartis, BMS, AbbVie, Genmab, Miltenyi Biotec, Allogene Therapeutics, AstraZeneca, and Janssen. M.L. reports consulting fees from AbbVie, Acrotech Biopharma, ADC Therapeutics, Astellas, AstraZeneca, BMS, Daiichi Sankyo, EUSA Pharma, Fate Therapeutics, Genentech, Genmab, Janssen/Pharmacyclics, Kite, MorphoSys, Nurix Therapeutics, Pharmacyclics, Seattle Genetics, and TG Therapeutics and research funding from BMS and Curis. A.C. and A.P. are current employees of Celgene, a Bristol-Myers Squibb Company and current equity holders in BMS. S. Montheard is a current employee of Celgene, a Bristol-Myers Squibb Company and a current equity holder in BMS, Novartis Pharma AG, and Alcon. K.O. is a current employee of BMS and current equity holder in BMS. M.K. reports consulting fees from TG Therapeutics, Genentech, AbbVie, AstraZeneca, Adaptive Technologies, ADC Therapeutics, BeiGene, and ImpactBio; reports honoraria from Seagen; and reports research funding and/or data monitoring committee from TG Therapeutics, Genentech, Novartis, and Celgene, a Bristol-Myers Squibb Company. The remaining authors declare no competing financial interests.
Acknowledgments
Writing and editorial assistance was provided by Amy Agbonbhase, of The Lockwood Group (Stamford, CT), funded by Bristol Myers Squibb.
This study was funded by Celgene, a Bristol-Myers Squibb Company.
Authorship
Contribution: J.S.A., S.R.S., J.A., P.B.J., B.G., V.B., S.I., S. Mielke, P.M., F.H.-I., K.I., F.M., M.L., and M.K. contributed to data acquisition; A.C., S. Montheard, A.P., and K.O. contributed to the study conception or design and data analysis; J.S.A., M.L., A.C., S. Montheard, A.P., and K.O. contributed to data interpretation; and all authors contributed to writing of the manuscript.
Footnotes
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
A complete list of the TRANSFORM study sites is provided in the supplemental Appendix.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Supplementary Material
References
- 1.Gandhi S, Kallab AM, Hernandez-Ilizaliturri FJ. Diffuse large B-cell lymphoma (DLBCL) guidelines. https://emedicine.medscape.com/article/202969-guidelines Updated 18 November 2022.
- 2.Tilly H, Gomes da Silva M, Vitolo U, et al. ESMO Guidelines Committee Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v116–125. doi: 10.1093/annonc/mdv304. [DOI] [PubMed] [Google Scholar]
- 3.Van Den Neste E, Schmitz N, Mounier N, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant. 2016;51(1):51–57. doi: 10.1038/bmt.2015.213. [DOI] [PubMed] [Google Scholar]
- 4.Crump M, Kuruvilla J, Couban S, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol. 2014;32(31):3490–3496. doi: 10.1200/JCO.2013.53.9593. [DOI] [PubMed] [Google Scholar]
- 5.van Imhoff GW, McMillan A, Matasar MJ, et al. Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse large B-cell lymphoma: the ORCHARRD study. J Clin Oncol. 2017;35(5):544–551. doi: 10.1200/JCO.2016.69.0198. [DOI] [PubMed] [Google Scholar]
- 6.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Locke FL, Miklos DB, Jacobson CA, et al. ZUMA-7 Investigators and Contributing Kite Members Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–654. doi: 10.1056/NEJMoa2116133. [DOI] [PubMed] [Google Scholar]
- 8.Sehgal A, Hoda D, Riedell PA, et al. Lisocabtagene maraleucel as second-line therapy in adults with relapsed or refractory large B-cell lymphoma who were not intended for haematopoietic stem cell transplantation (PILOT): an open-label, phase 2 study. Lancet Oncol. 2022;23(8):1066–1077. doi: 10.1016/S1470-2045(22)00339-4. [DOI] [PubMed] [Google Scholar]
- 9.Kamdar M, Solomon SR, Arnason J, et al. TRANSFORM Investigators Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–2308. doi: 10.1016/S0140-6736(22)00662-6. [DOI] [PubMed] [Google Scholar]
- 10.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latimer NR, Abrams KR, Lambert PC, et al. Adjusting for treatment switching in randomised controlled trials - a simulation study and a simplified two-stage method. Stat Methods Med Res. 2017;26(2):724–751. doi: 10.1177/0962280214557578. [DOI] [PubMed] [Google Scholar]
- 13.Ishak KJ, Proskorovsky I, Korytowsky B, Sandin R, Faivre S, Valle J. Methods for adjusting for bias due to crossover in oncology trials. Pharmacoeconomics. 2014;32(6):533–546. doi: 10.1007/s40273-014-0145-y. [DOI] [PubMed] [Google Scholar]
- 14.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 15.Bishop MR, Dickinson M, Purtill D, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386(7):629–639. doi: 10.1056/NEJMoa2116596. [DOI] [PubMed] [Google Scholar]
- 16.Ghobadi AJM, Westin J, Locke FL, et al. Outcomes of subsequent anti-lymphoma therapies in patients (pts) with large B-cell lymphoma (LBCL) treated with axicabtagene ciloleucel (axi-cel) or standard of care (SOC) in the second-line (2L) ZUMA-7 study. Blood. 2022;140(suppl 1):1595–1597. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.