Abstract
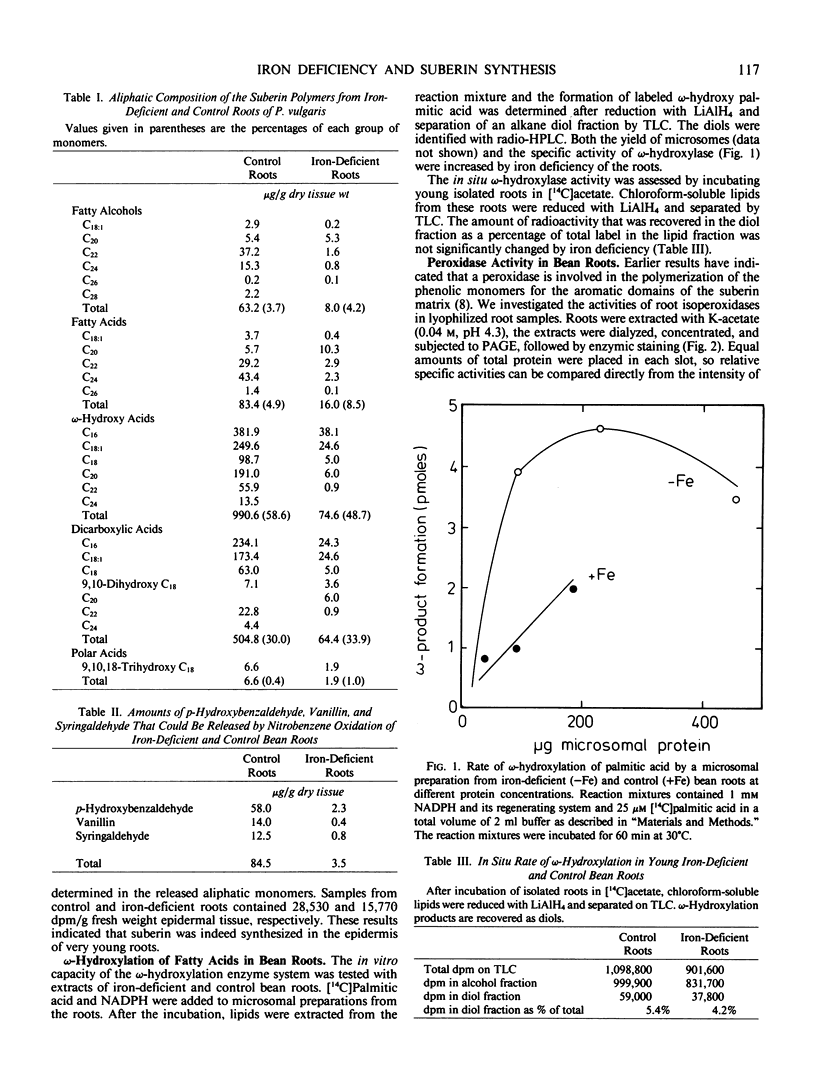
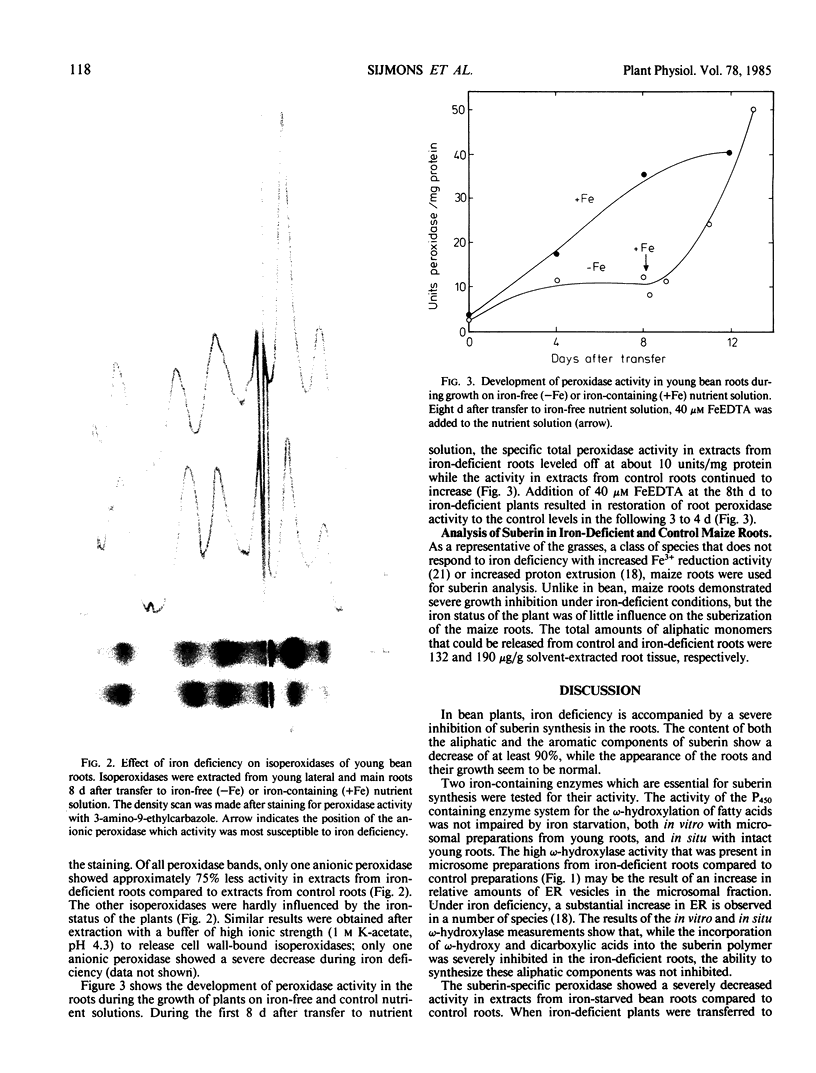
The suberin content of young root parts of iron-deficient and iron-sufficient Phaseolus vulgaris L. cv Prélude was determined. The aliphatic components that could be released from suberin-enriched fractions by LiAID4 depolymerization were identified by gas chromatography-mass spectrometry. In the normal roots, the major aliphatic components were ω-hydroxy acids and dicarboxylic acids in which saturated C16 and monounsaturated C18 were the dominant homologues. Iron-deficient bean roots contained only 11% of the aliphatic components of suberin found in control roots although the relative composition of the constituents was not significantly affected by iron deficiency. Analysis of the aromatic components of the suberin polymer that could be released by alkaline nitrobenzene oxidation of bean root samples showed a 95% decrease in p-hydroxybenzaldehyde, vanillin, and syringaldehyde under iron-deficient conditions. The inhibition of suberin synthesis in bean roots was not due to a decrease in Fe-dependent ω-hydroxylase activity since normal ω-hydroxylation could be demonstrated, both in vitro with microsomal preparations and in situ by labeling of ω-hydroxy and dicarboxylic acids with [14C]acetate. The level of the isozyme of peroxidase that is specifically associated with suberization was suppressed by iron deficiency to 25% of that found in control roots. None of the other extracted isozymes of peroxidase was affected by the iron nutritional status. The activity of the suberin-associated peroxidase was restored within 3 to 4 days after application of iron to the growth medium. The results suggest that, in bean roots, iron deficiency causes inhibition of suberization by causing a decrease in the level of isoperoxidase activity which is required for polymerization of the aromatic domains of suberin, while the ability to synthesize the aliphatic components of the suberin polymer is not impaired.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borchert R. Simultaneous separation of acidic and basic isoperoxidases in wounded potato tissue by acrylamide gel electrophoresis. Plant Physiol. 1978 Nov;62(5):794–797. doi: 10.1104/pp.62.5.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchert R. Time course and spatial distribution of phenylalanine ammonia-lyase and peroxidase activity in wounded potato tuber tissue. Plant Physiol. 1978 Nov;62(5):789–793. doi: 10.1104/pp.62.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chaney R. L., Brown J. C., Tiffin L. O. Obligatory reduction of ferric chelates in iron uptake by soybeans. Plant Physiol. 1972 Aug;50(2):208–213. doi: 10.1104/pp.50.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottle W., Kolattukudy P. E. Abscisic Acid stimulation of suberization : induction of enzymes and deposition of polymeric components and associated waxes in tissue cultures of potato tuber. Plant Physiol. 1982 Sep;70(3):775–780. doi: 10.1104/pp.70.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottle W., Kolattukudy P. E. Biosynthesis, deposition, and partial characterization of potato suberin phenolics. Plant Physiol. 1982 Feb;69(2):393–399. doi: 10.1104/pp.69.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy P. E. Biopolyester membranes of plants: cutin and suberin. Science. 1980 May 30;208(4447):990–1000. doi: 10.1126/science.208.4447.990. [DOI] [PubMed] [Google Scholar]
- Pozuelo J. M., Espelie K. E., Kolattukudy P. E. Magnesium deficiency results in increased suberization in endodermis and hypodermis of corn roots. Plant Physiol. 1984 Feb;74(2):256–260. doi: 10.1104/pp.74.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römheld V., Müller C., Marschner H. Localization and capacity of proton pumps in roots of intact sunflower plants. Plant Physiol. 1984 Nov;76(3):603–606. doi: 10.1104/pp.76.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijmons P. C., Lanfermeijer F. C., de Boer A. H., Prins H. B., Bienfait H. F. Depolarization of Cell Membrane Potential during Trans-Plasma Membrane Electron Transfer to Extracellular Electron Acceptors in Iron-Deficient Roots of Phaseolus vulgaris L. Plant Physiol. 1984 Dec;76(4):943–946. doi: 10.1104/pp.76.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijmons P. C., van den Briel W., Bienfait H. F. Cytosolic NADPH is the electron donor for extracellular fe reduction in iron-deficient bean roots. Plant Physiol. 1984 May;75(1):219–221. doi: 10.1104/pp.75.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliday C. L., Kolattukudy P. E. Biosynthesis of Cutin omega-Hydroxylation of Fatty Acids by a Microsomal Preparation from Germinating Vicia faba. Plant Physiol. 1977 Jun;59(6):1116–1121. doi: 10.1104/pp.59.6.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]