Abstract
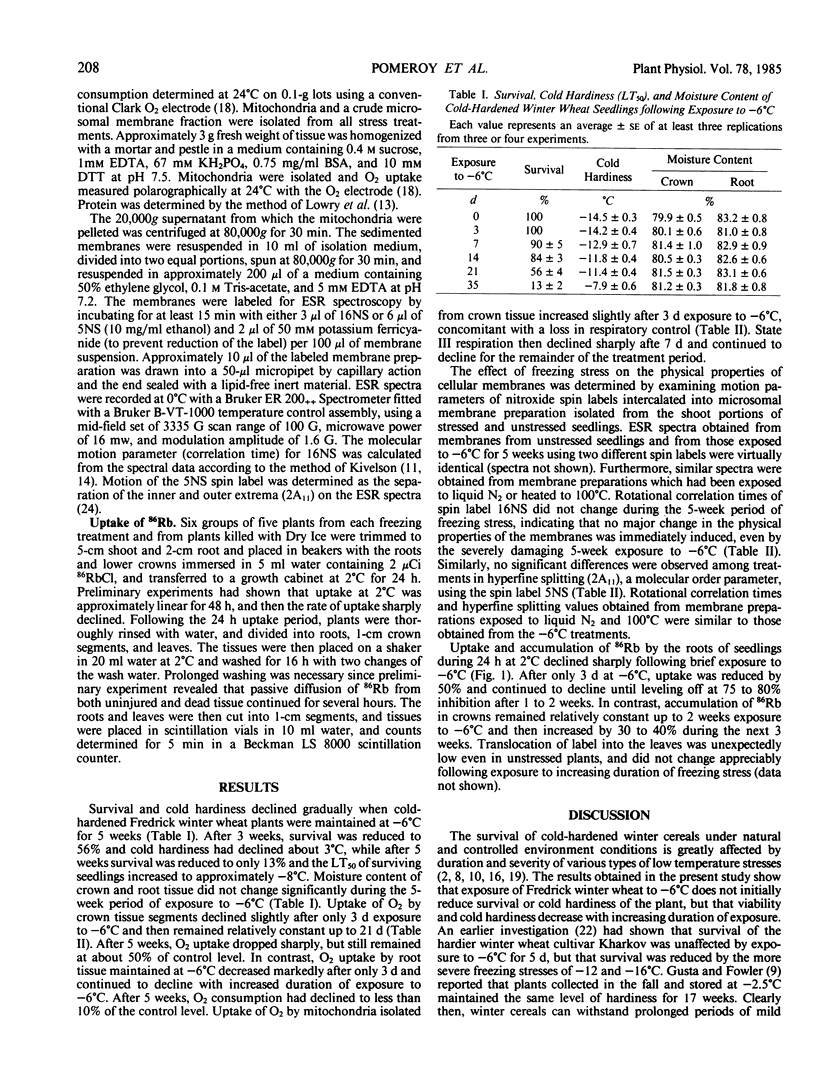
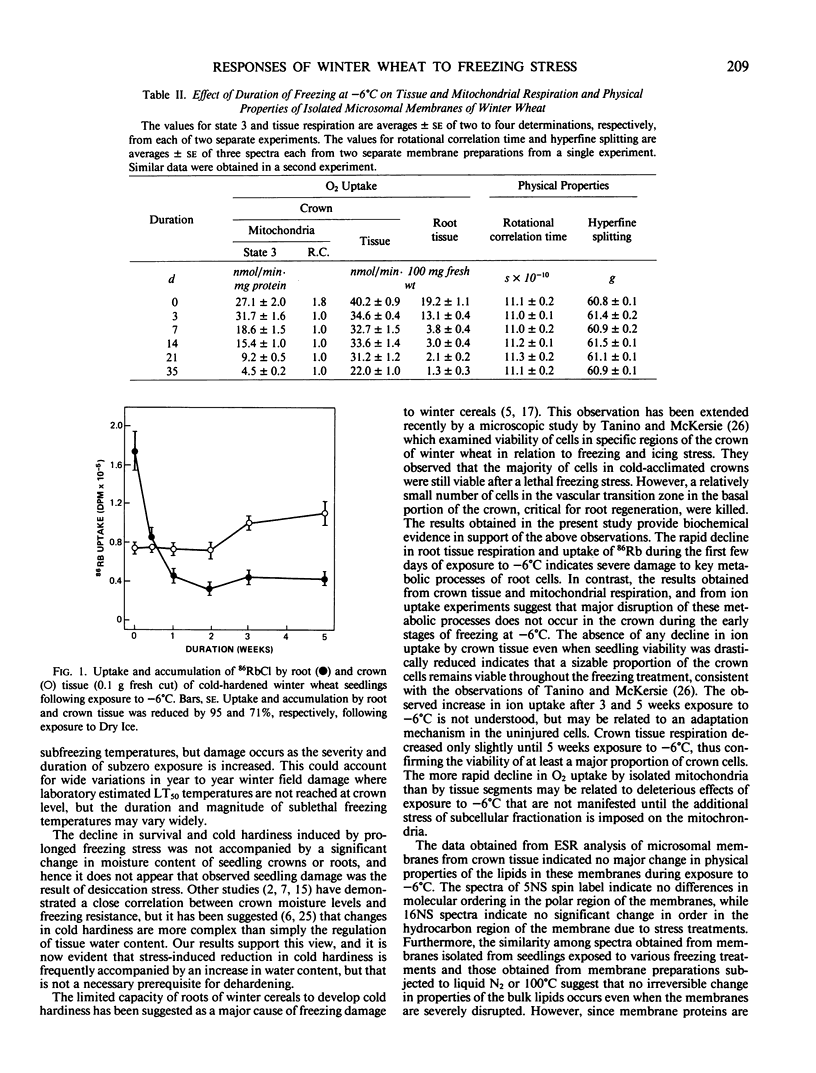
Survival and cold hardiness declined gradually when cold-hardened Fredrick winter wheat (Triticum aestivum L.) was maintained at −6°C for several weeks. Moisture content of crown and root tissue did not change significantly during this period. Uptake of O2 and accumulation of 86Rb by root tissue declined abruptly upon exposure to −6°C, whereas a concomitant negative effect of freezing on these metabolic processes was not observed in crown tissue. Electron spin resonance spectroscopic analysis of microsomal membrane preparations from crown tissue revealed no evidence of gross changes in the physical properties of the bulk lipids even when seedlings were killed. The results provide biochemical evidence that seedling damage due to prolonged exposure to a mild freezing stress is due to disruption of key metabolic process in the root while cells within the crown remain viable.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen T. H., Gusta L. V., Fowler D. B. Freezing injury and root development in winter cereals. Plant Physiol. 1983 Nov;73(3):773–777. doi: 10.1104/pp.73.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J. Y., Andrews C. J., Pomeroy M. K. Interactions among Flooding, Freezing, and Ice Encasement in Winter Wheat. Plant Physiol. 1983 Jun;72(2):303–307. doi: 10.1104/pp.72.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pomeroy M. K., Andrews C. J. Metabolic and Ultrastructural Changes in Winter Wheat during Ice Encasement Under Field Conditions. Plant Physiol. 1978 May;61(5):806–811. doi: 10.1104/pp.61.5.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy M. K., Pihakaski S. J., Andrews C. J. Membrane properties of isolated winter wheat cells in relation to icing stress. Plant Physiol. 1983 Jun;72(2):535–539. doi: 10.1104/pp.72.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy M. K., Raison J. K. Maintenance of Membrane Fluidity during Development of Freezing Tolerance of Winter Wheat Seedlings. Plant Physiol. 1981 Aug;68(2):382–385. doi: 10.1104/pp.68.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy M. K. Studies on the respiratory properties of mitochondria isolated from developing winter wheat seedlings. Plant Physiol. 1974 Apr;53(4):653–657. doi: 10.1104/pp.53.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J., Miller R. W. Spin-label Studies of Membranes in Rye Protoplasts during Extracellular Freezing. Plant Physiol. 1980 Aug;66(2):349–352. doi: 10.1104/pp.66.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. Studies on Freezing Injury of Plant Cells: I. Relation between Thermotropic Properties of Isolated Plasma Membrane Vesicles and Freezing Injury. Plant Physiol. 1984 May;75(1):38–42. doi: 10.1104/pp.75.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]


