Abstract
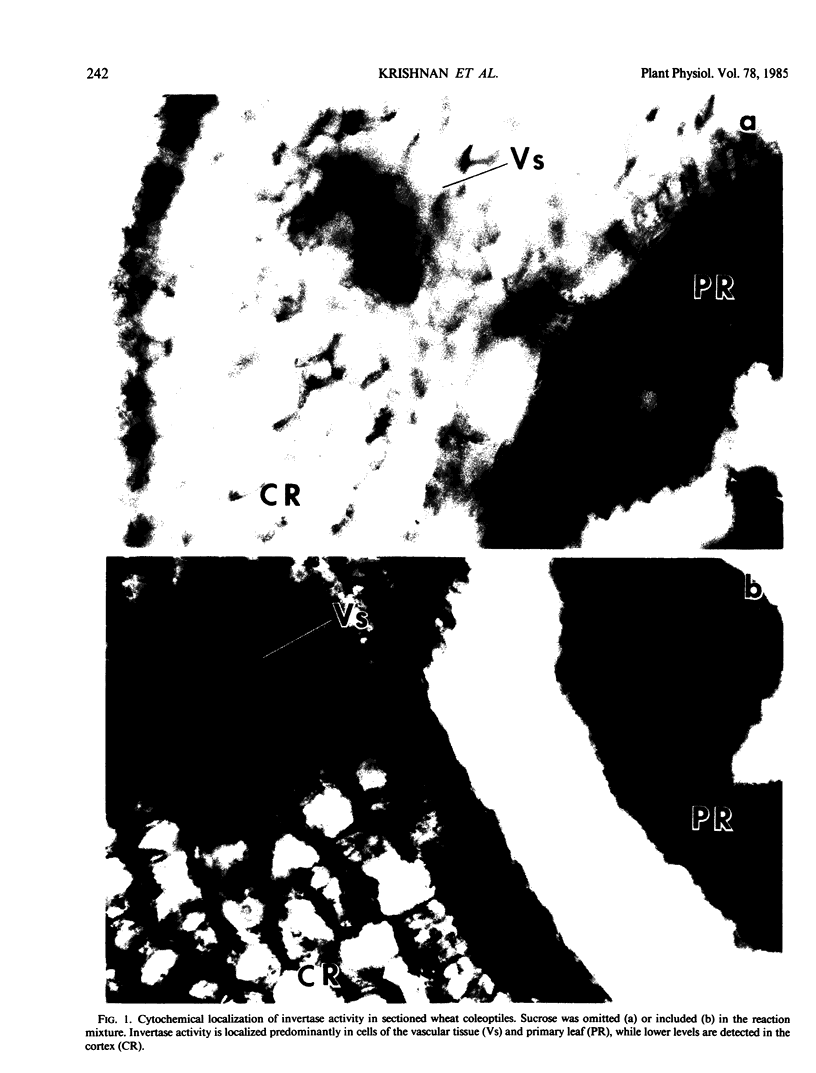
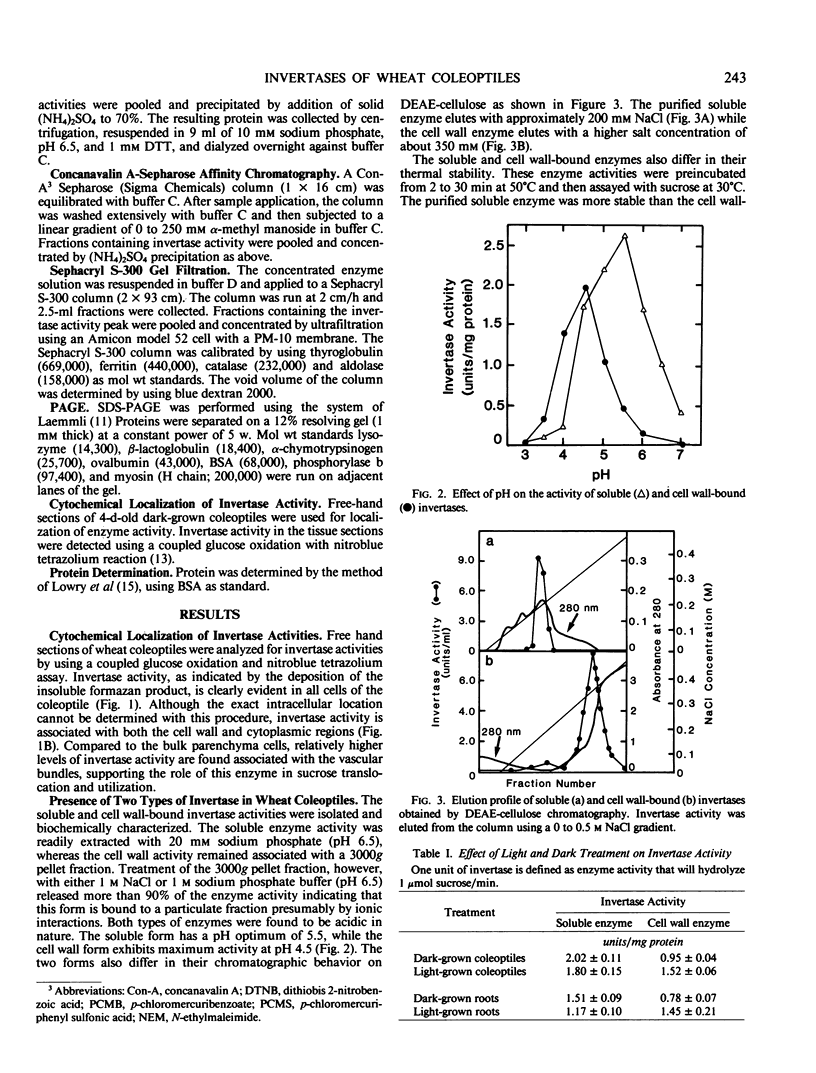
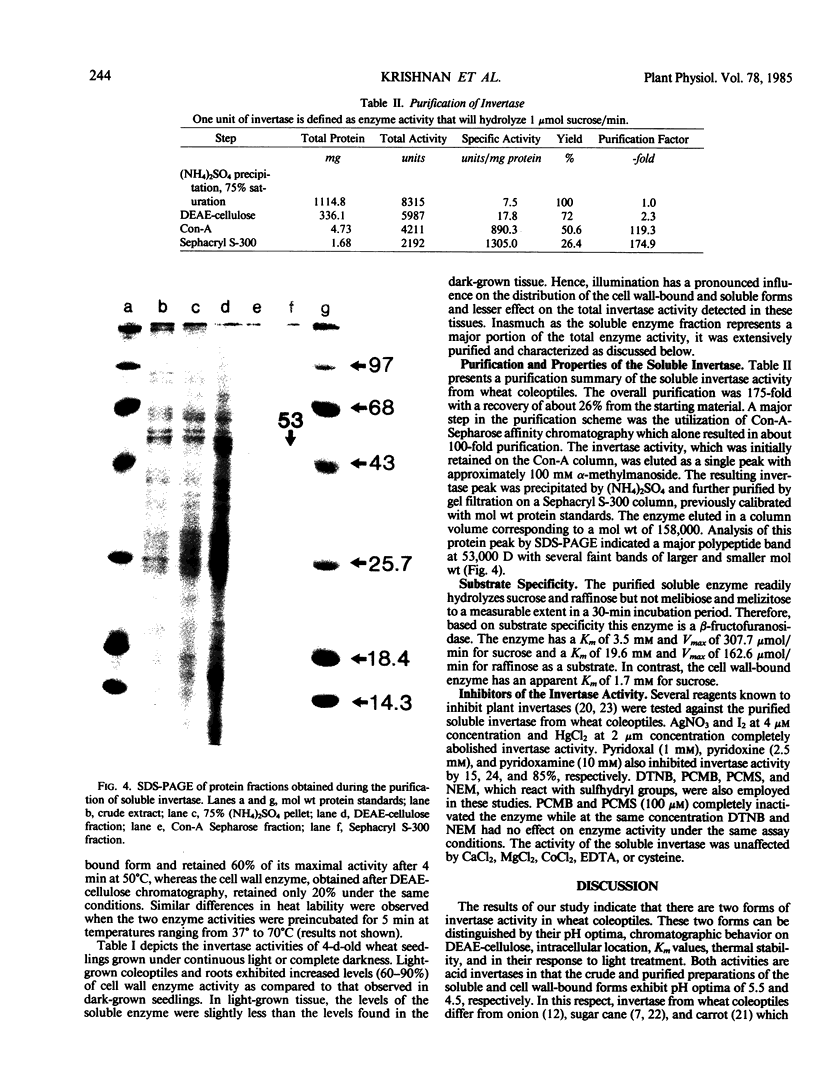
Wheat coleoptiles have two distinct invertases, a soluble and a cell wall-bound form as indicated by results from cytochemical and biochemical studies. These enzyme activities differ in their pH optima, chromatographic behavior on diethylaminoethyl cellulose, kinetic properties, thermal stability, and response to light treatment. The soluble invertase was purified to near homogeneity by diethylaminoethyl-cellulose, concanavalin-A Sepharose, and Sephacryl S-300 chromatography. The overall purification was 175-fold with a recovery of about 26%. The holoenzyme has an apparent molecular weight of 158,000 and subunit molecular weight of 53,000 as estimated by polyacrylamide gel electrophoresis under denaturing conditions. Illumination of wheat seedlings caused an increase in the cell wall, but not the soluble, invertase activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hatch M. D., Sacher J. A., Glasziou K. T. Sugar Accumulation Cycle in Sugar Cane. I. Studies on Enzymes of the Cycle. Plant Physiol. 1963 May;38(3):338–343. doi: 10.1104/pp.38.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard H. F., Witham F. H. Invertase activity and the kinetin-stimulated enlargement of detached radish cotyledons. Plant Physiol. 1983 Oct;73(2):304–308. doi: 10.1104/pp.73.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman P. B., Ghosheh N. S., Lacroix J. D., Soni S. L., Ikuma H. Regulation of invertase levels in Avena stem segments by gibberellic Acid, sucrose, glucose, and fructose. Plant Physiol. 1973 Sep;52(3):221–228. doi: 10.1104/pp.52.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman P. B., Ghosheh N., Ikuma H. Promotion of growth and invertase activity by gibberellic Acid in developing Avena internodes. Plant Physiol. 1968 Jan;43(1):29–34. doi: 10.1104/pp.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Uritani I. Change in invertase activity of sweet potato in response to wounding and purification and properties of its invertases. Plant Physiol. 1974 Jul;54(1):60–66. doi: 10.1104/pp.54.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann N. P., Lampen J. O. Purification and properties of yeast invertase. Biochemistry. 1967 Feb;6(2):468–475. doi: 10.1021/bi00854a015. [DOI] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O., Cannon L. E. Presecretory and cytoplasmic invertase polypeptides encoded by distinct mRNAs derived from the same structural gene differ by a signal sequence. Proc Natl Acad Sci U S A. 1982 Feb;79(3):781–785. doi: 10.1073/pnas.79.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R., Avants J. K. Invertases in Oat Seedlings: SEPARATION, PROPERTIES, AND CHANGES IN ACTIVITIES IN SEEDLING SEGMENTS. Plant Physiol. 1980 Jan;65(1):136–140. doi: 10.1104/pp.65.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. B., Knox R. B. Invertases of Lilium Pollen : Characterization and Activity during In Vitro Germination. Plant Physiol. 1984 Mar;74(3):510–515. doi: 10.1104/pp.74.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]