Abstract
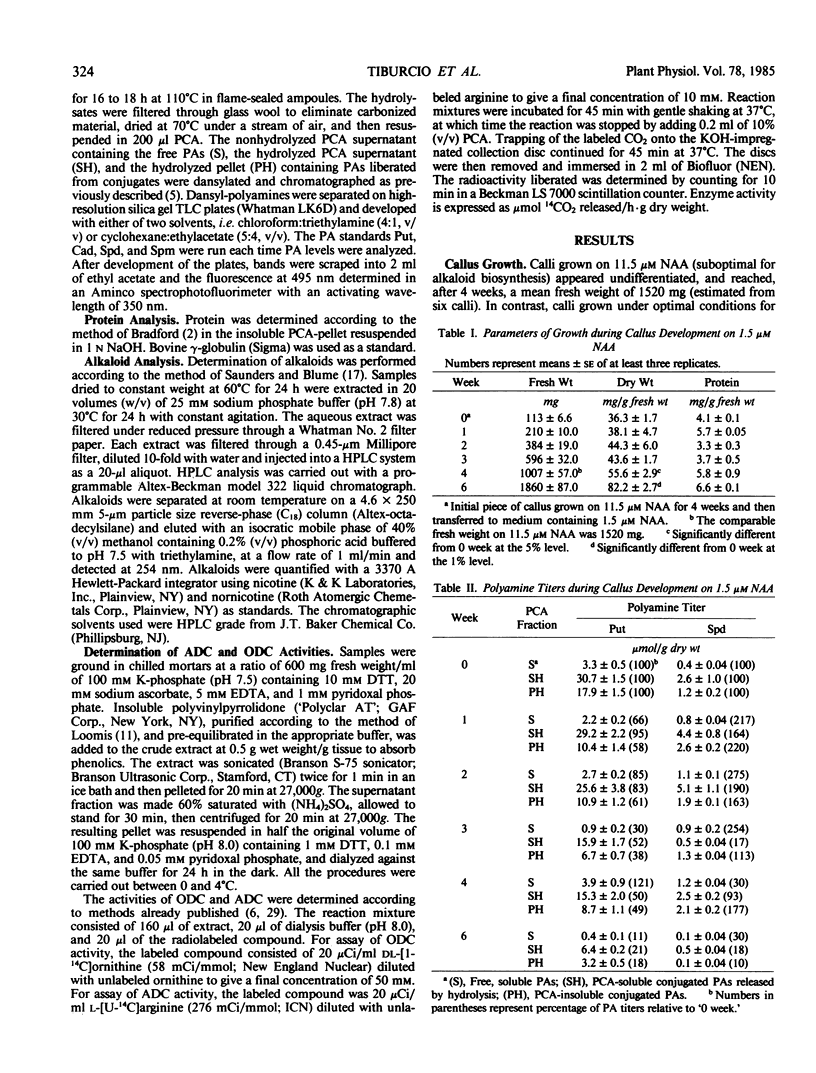
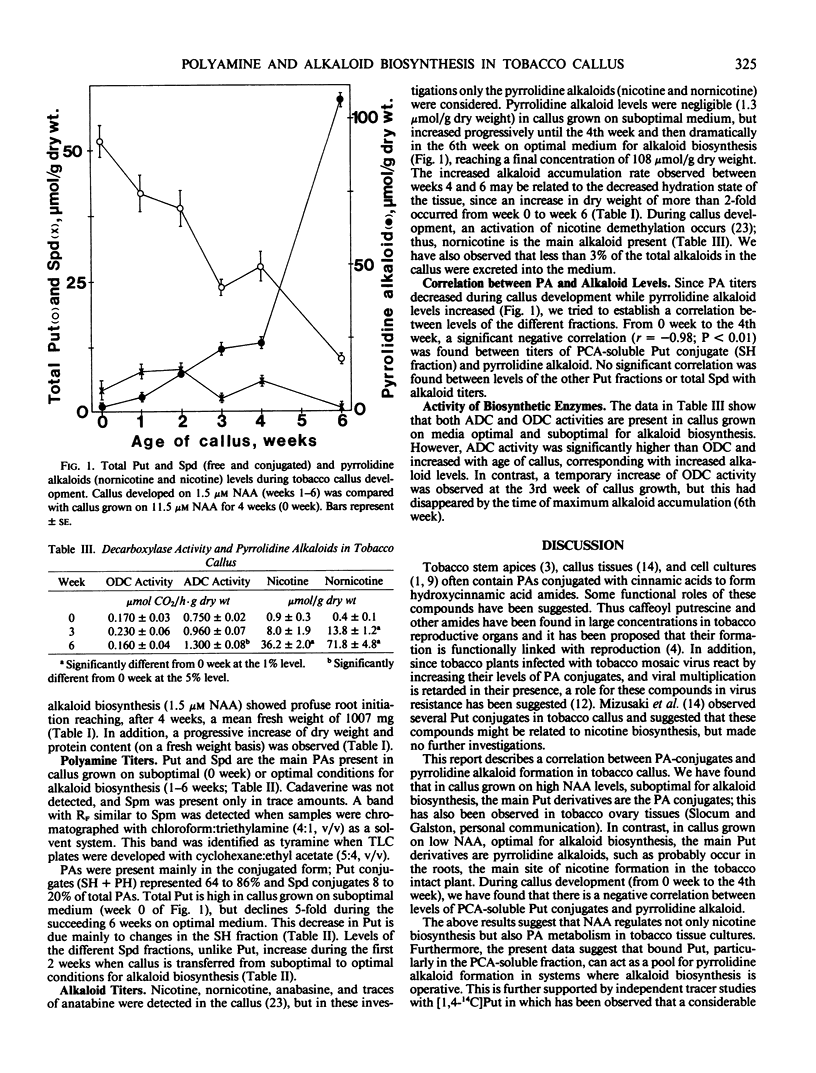
Since the diamine putrescine can be metabolized into the pyrrolidine ring of tobacco alkaloids as well as into the higher polyamines, we have investigated the quantitative relationship between putrescine and these metabolites in tobacco callus cultured in vitro. We measured levels of free and conjugated putrescine and spermidine, and pyrrolidine alkaloids, as well as activities of the putrescine-biosynthetic enzymes arginine and ornithine decarboxylase. In callus grown on high (11.5 micromolar) α-naphthalene acetic acid, suboptimal for alkaloid biosynthesis, putrescine and spermidine conjugates were the main putrescine derivatives, while in callus grown on low (1.5 micromolar) α-naphthalene acetic acid, optimal for alkaloid formation, nornicotine and nicotine were the main putrescine derivatives. During callus development, a significant negative correlation was found between levels of perchloric acid-soluble putrescine conjugates and pyrrolidine alkaloids. The results suggest that bound putrescine can act as a pool for pyrrolidine alkaloid formation in systems where alkaloid biosynthesis is active. In addition, changes in arginine decarboxylase activity corresponding to increased alkaloid levels suggest a role for this enzyme in the overall biosynthesis of pyrrolidine alkaloids.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Flores H. E., Galston A. W. Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol. 1982 Mar;69(3):701–706. doi: 10.1104/pp.69.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores H. E., Galston A. W. Osmotic stress-induced polyamine accumulation in cereal leaves : I. Physiological parameters of the response. Plant Physiol. 1984 May;75(1):102–109. doi: 10.1104/pp.75.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis W. D. Overcoming problems of phenolics and quinones in the isolation of plant enzymes and organelles. Methods Enzymol. 1974;31:528–544. doi: 10.1016/0076-6879(74)31057-9. [DOI] [PubMed] [Google Scholar]
- Martin-Tanguy J., Martin C., Gallet M., Vernoy R. Sur de puissants inhibiteurs naturels de multiplication du virus de la mosaique du tabac. C R Acad Sci Hebd Seances Acad Sci D. 1976 Jun 28;282(24):2231–2234. [PubMed] [Google Scholar]
- Mizusaki S., Kisaki T., Tamaki E. Phytochemical Studies on the Tobacco Alkaloids. XII. Identification of gamma-Methylaminobutyraldehyde and its Precursor Role in Nicotine Biosynthesis. Plant Physiol. 1968 Jan;43(1):93–98. doi: 10.1104/pp.43.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solt M. L. Nicotine Production and Growth of Excised Tobacco Root Cultures. Plant Physiol. 1957 Sep;32(5):480–484. doi: 10.1104/pp.32.5.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N. D., Galston A. W. Putrescine and Acid Stress : Induction of Arginine Decarboxylase Activity and Putrescine Accumulation by Low pH. Plant Physiol. 1983 Apr;71(4):767–771. doi: 10.1104/pp.71.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]


