Abstract
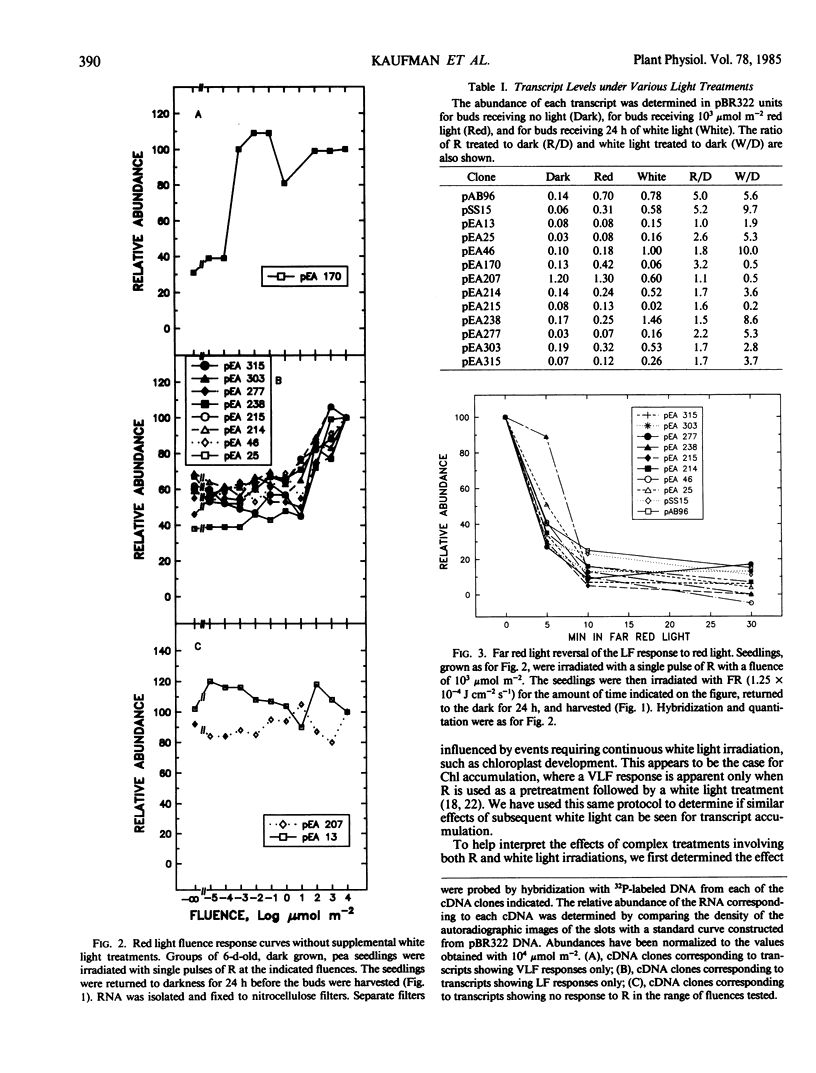
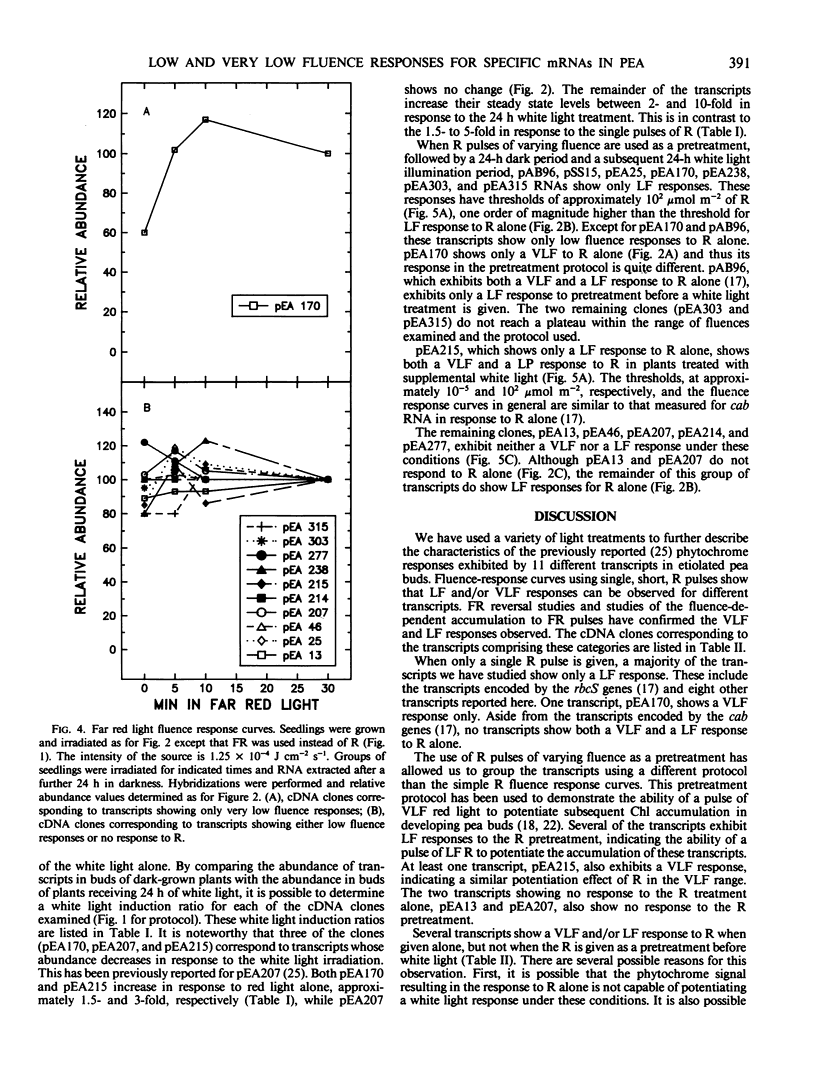
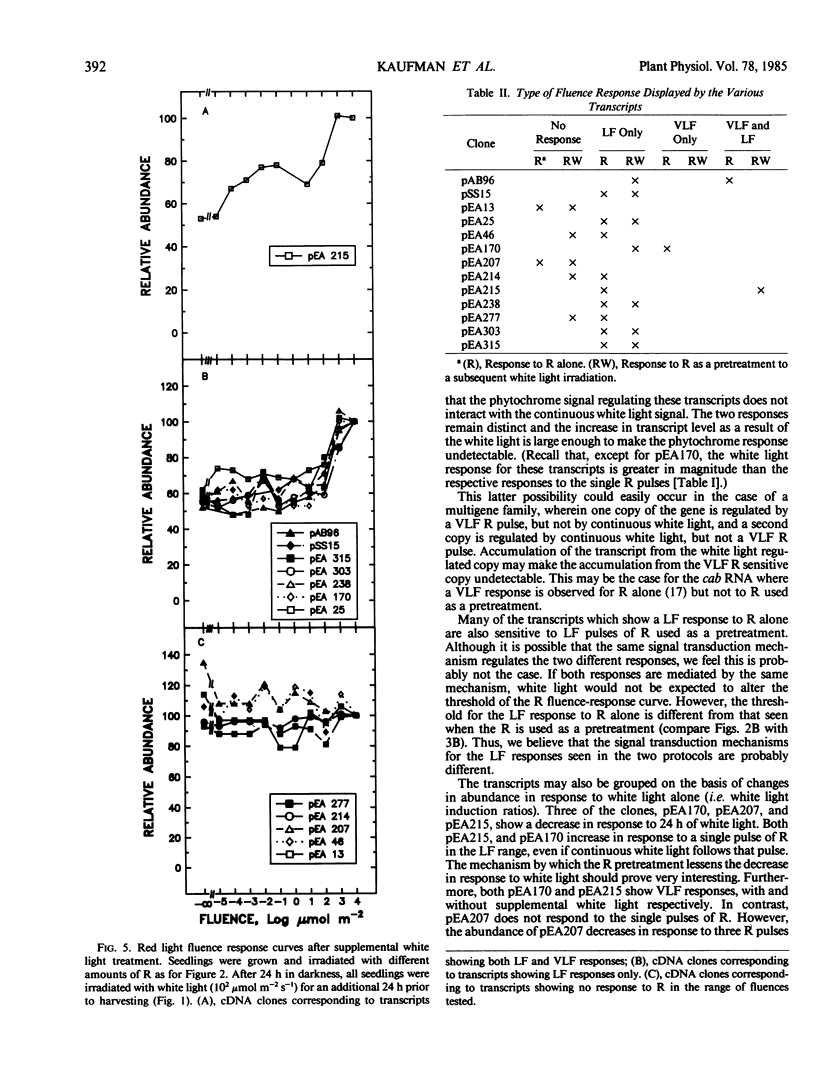
We have examined phytochrome regulated changes in transcript abundance for 11 different light regulated mRNAs in developing pea buds. Fluence-response curves were measured for changes in transcript abundance in response to red light pulses in both the low and very low fluence ranges. Most transcripts show only low fluence responses, with a threshold of approximately 10 micromoles per square meter. All of the low fluence responses are reversible by far red light. One transcript shows a very low fluence response, with a threshold of approximately 10−4 micromoles per square meter. As expected, the very low fluence response is not far red reversible and in fact can be induced by far red light.
Various fluences of red light were also used as pretreatments before transferring seedlings to continuous white light. One transcript responds to pretreatments in the very low fluence range, several respond to pretreatments in the low fluence range (including chlorophyll a/b binding protein RNA and ribulose-1,5-bisphosphate carboxylase RNA), and several show no response to the red light under these conditions. The threshold of these low fluence responses is approximately 102 micromoles per square meter, one order of magnitude greater than the threshold of the low fluence responses to red light alone.
The transcripts may also be grouped by their responses to white light treatment alone. Three of the clones correspond to transcripts whose abundance decreases after a 24 hour white light treatment. The remainder of the mRNAs increase between 2- and 10-fold in response to the 24 hour white light.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K. Phytochrome-induced appearance of mRNA activity for the apoprotein of the light-harvesting chlorophyll a/b protein of barley (Hordeum vulgare). Eur J Biochem. 1979 Jun;97(1):183–188. doi: 10.1111/j.1432-1033.1979.tb13101.x. [DOI] [PubMed] [Google Scholar]
- Apel K. The protochlorophyllide holochrome of barley (Hordeum vulgare L.). Phytochrome-induced decrease of translatable mRNA coding for the NADPH: protochlorophyllide oxidoreductase. Eur J Biochem. 1981 Nov;120(1):89–93. doi: 10.1111/j.1432-1033.1981.tb05673.x. [DOI] [PubMed] [Google Scholar]
- Colbert J. T., Hershey H. P., Quail P. H. Autoregulatory control of translatable phytochrome mRNA levels. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2248–2252. doi: 10.1073/pnas.80.8.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Cashmore A., Chua N. H. Nucleotide sequences of two pea cDNA clones encoding the small subunit of ribulose 1,5-bisphosphate carboxylase and the major chlorophyll a/b-binding thylakoid polypeptide. J Biol Chem. 1983 Feb 10;258(3):1399–1402. [PubMed] [Google Scholar]
- Dunsmuir P., Smith S. M., Bedbrook J. The major chlorophyll a/b binding protein of petunia is composed of several polypeptides encoded by a number of distinct nuclear genes. J Mol Appl Genet. 1983;2(3):285–300. [PubMed] [Google Scholar]
- Dunsmuir P., Smith S., Bedbrook J. A number of different nuclear genes for the small subunit of RuBPCase are transcribed in petunia. Nucleic Acids Res. 1983 Jun 25;11(12):4177–4183. doi: 10.1093/nar/11.12.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T. F., Ellis R. J. Light-stimulated transcription of genes for two chloroplast polypeptides in isolated pea leaf nuclei. EMBO J. 1982;1(12):1493–1498. doi: 10.1002/j.1460-2075.1982.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorton H. L., Briggs W. R. Phytochrome Responses to End-of-Day Irradiations in Light-grown Corn Grown in the Presence and Absence of Sandoz 9789. Plant Physiol. 1980 Dec;66(6):1024–1026. doi: 10.1104/pp.66.6.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L. S., Thompson W. F., Briggs W. R. Different Red Light Requirements for Phytochrome-Induced Accumulation of cab RNA and rbcS RNA. Science. 1984 Dec 21;226(4681):1447–1449. doi: 10.1126/science.226.4681.1447. [DOI] [PubMed] [Google Scholar]
- Mandoli D. F., Briggs W. R. Phytochrome control of two low-irradiance responses in etiolated oat seedlings. Plant Physiol. 1981 Apr;67(4):733–739. doi: 10.1104/pp.67.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G., Kloppstech K. A rapidly light-induced chloroplast protein with a high turnover coded for by pea nuclear DNA. Eur J Biochem. 1984 Jan 2;138(1):201–207. doi: 10.1111/j.1432-1033.1984.tb07900.x. [DOI] [PubMed] [Google Scholar]
- Silverthorne J., Tobin E. M. Demonstration of transcriptional regulation of specific genes by phytochrome action. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1112–1116. doi: 10.1073/pnas.81.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]


