Abstract
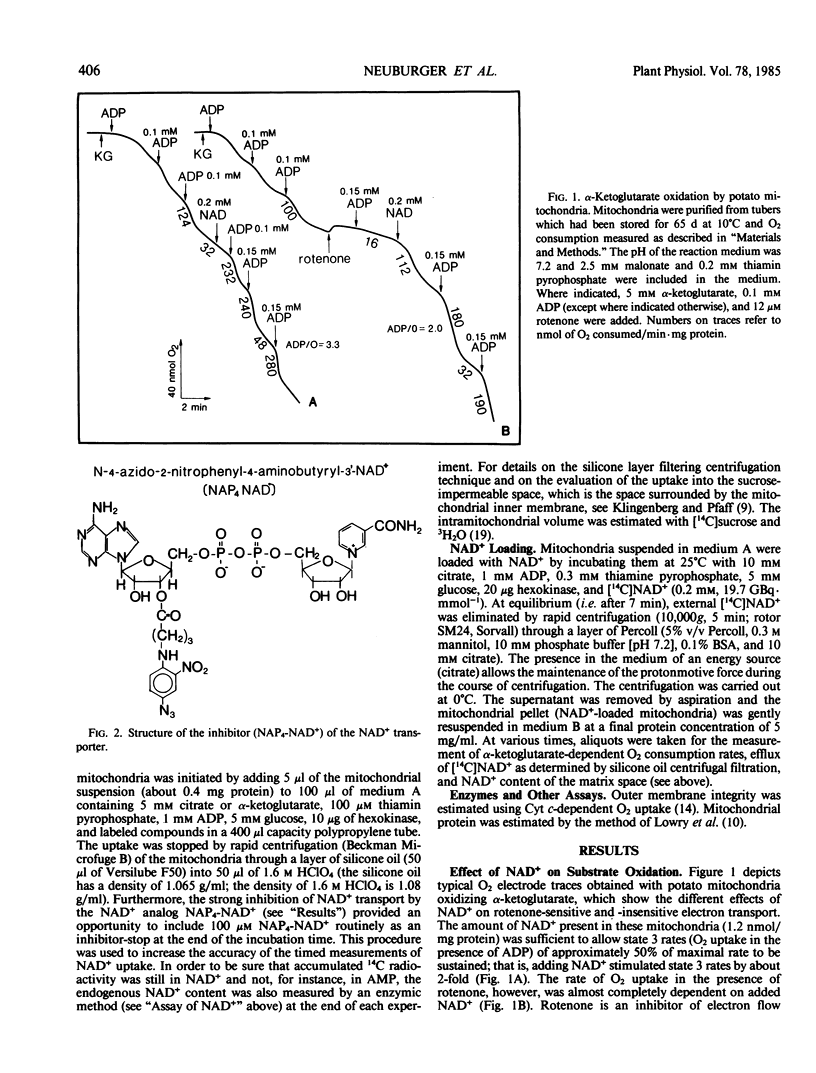
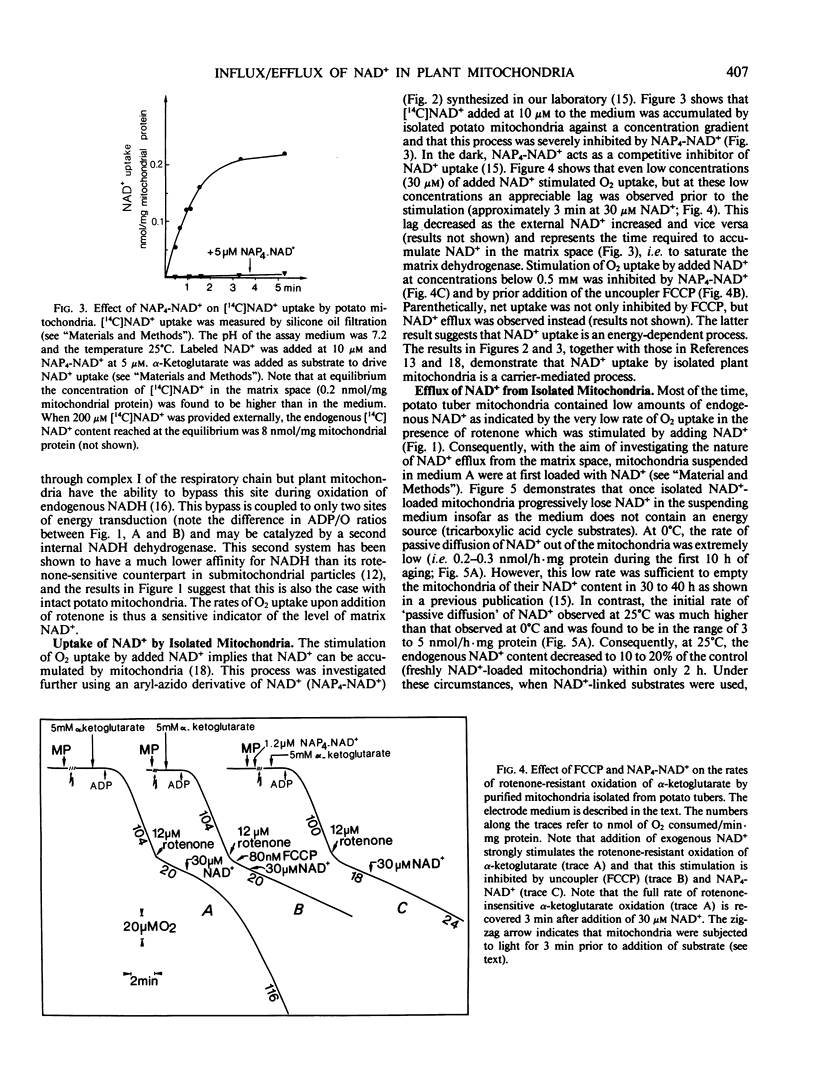
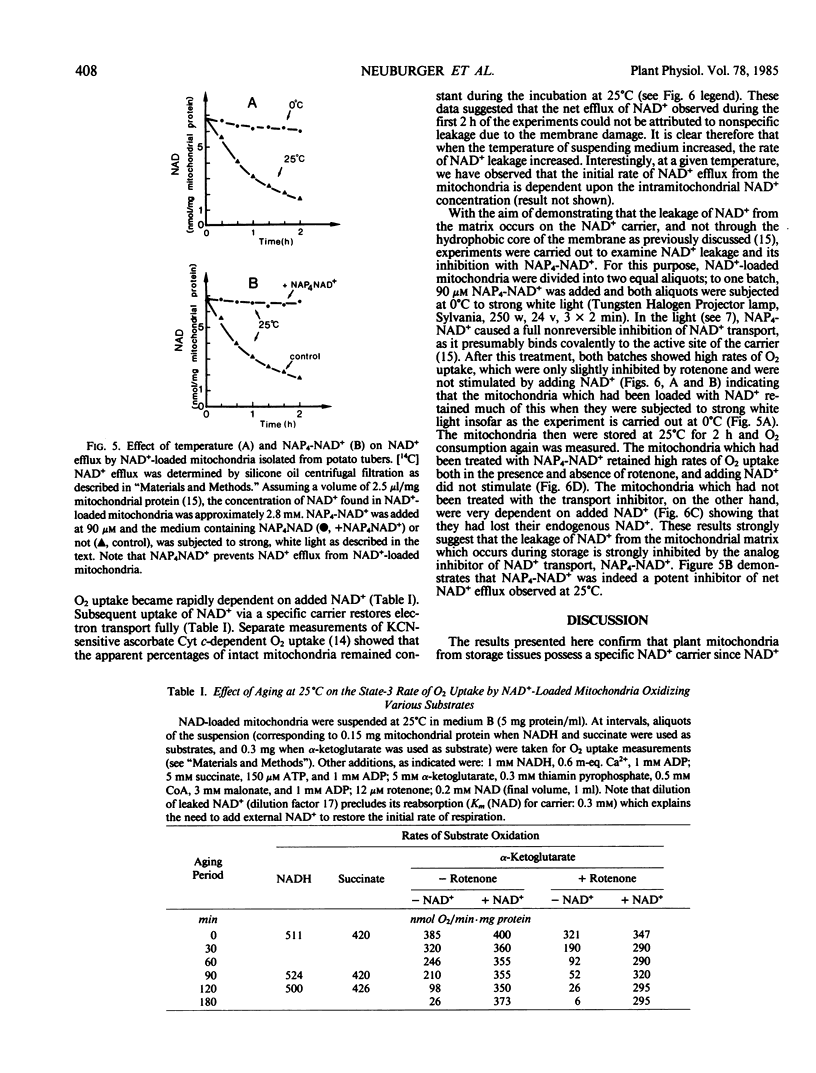
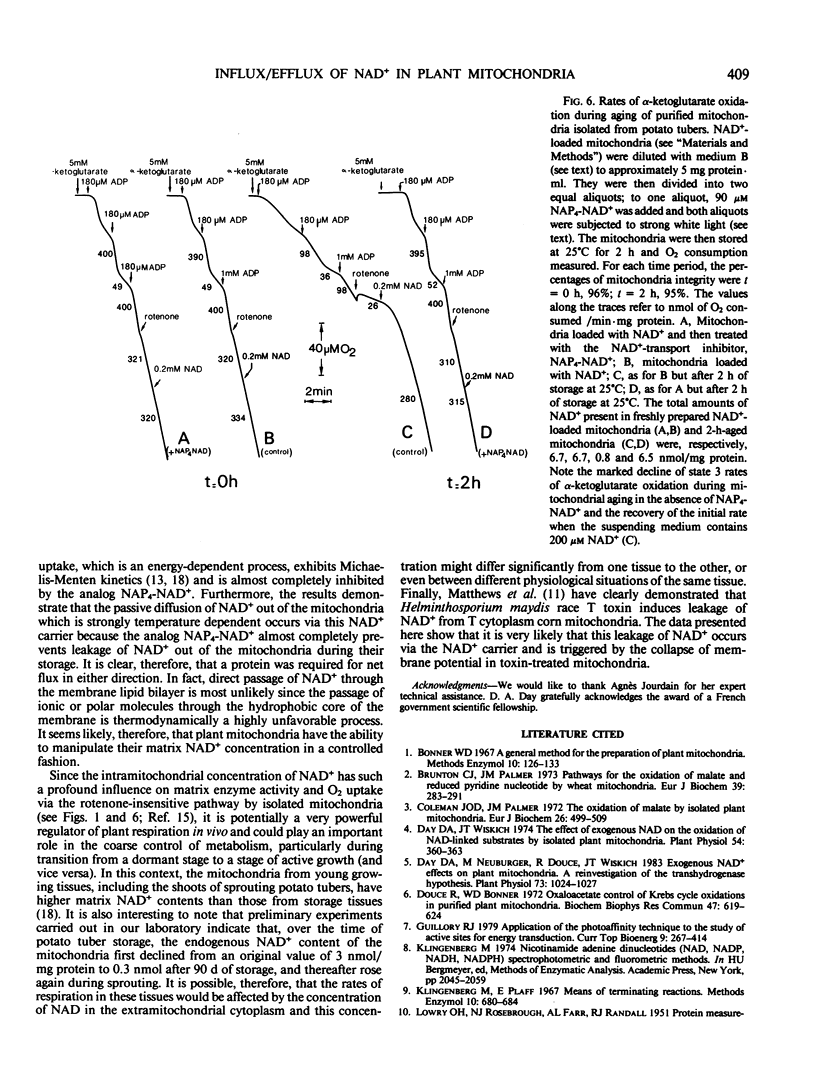
A mechanism by which intact potato (Solanum tuberosum) mitochondria may regulate the matrix NAD content was studied in vitro. If mitochondria were incubated with NAD+ at 25°C in 0.3 molar mannitol, 10 millimolar phosphate buffer (pH 7.4), 5 millimolar MgCl2, and 5 millimolar α-ketoglutarate, the NAD pool size increased with time. In the presence of uncouplers, net uptake was not only inhibited, but NAD+ efflux was observed instead. Furthermore, the rate of NAD+ accumulation in the matrix space was strongly inhibited by the analog N-4-azido-2-nitrophenyl-4-aminobutyryl-3′-NAD+. When suspended in a medium that avoided rupture of the outer membrane, intact purified mitochondria progressively lost their NAD+ content. This led to a slow decrease of NAD+-linked substrates oxidation by isolated mitochondria The rate of NAD+ efflux from the matrix space was strongly temperature dependent and was inhibited by the analog inhibitor of NAD+ transport indicating that a carrier was required for net flux in either direction. It is proposed that uptake and efflux operate to regulate the total matrix NAD pool size.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunton C. J., Palmer J. M. Pathways for the oxidation of malate and reduced pyridine nucleotide by wheat mitochondria. Eur J Biochem. 1973 Nov 1;39(1):283–291. doi: 10.1111/j.1432-1033.1973.tb03125.x. [DOI] [PubMed] [Google Scholar]
- Coleman J. O., Palmer J. M. The oxidation of malate by isolated plant mitochondria. Eur J Biochem. 1972 Apr 24;26(4):499–509. doi: 10.1111/j.1432-1033.1972.tb01792.x. [DOI] [PubMed] [Google Scholar]
- Day D. A., Neuburger M., Douce R., Wiskich J. T. Exogenous NAD Effects on Plant Mitochondria: A Reinvestigation of the Transhydrogenase Hypothesis. Plant Physiol. 1983 Dec;73(4):1024–1027. doi: 10.1104/pp.73.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. The Effect of Exogenous Nicotinamide Adenine Dinucleotide on the Oxidation of Nicotinamide Adenine Dinucleotide-linked Substrates by Isolated Plant Mitochondria. Plant Physiol. 1974 Sep;54(3):360–363. doi: 10.1104/pp.54.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Bonner W. D., Jr Oxalacetate control of Krebs cycle oxidations in purified plant mitochondria. Biochem Biophys Res Commun. 1972 May 12;47(3):619–624. doi: 10.1016/0006-291x(72)90923-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matthews D. E., Gregory P., Gracen V. E. Helminthosporium maydis Race T Toxin Induces Leakage of NAD from T Cytoplasm Corn Mitochondria. Plant Physiol. 1979 Jun;63(6):1149–1153. doi: 10.1104/pp.63.6.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuburger M., Douce R. Slow passive diffusion of NAD+ between intact isolated plant mitochondria and suspending medium. Biochem J. 1983 Nov 15;216(2):443–450. doi: 10.1042/bj2160443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuburger M., Journet E. P., Bligny R., Carde J. P., Douce R. Purification of plant mitochondria by isopycnic centrifugation in density gradients of Percoll. Arch Biochem Biophys. 1982 Aug;217(1):312–323. doi: 10.1016/0003-9861(82)90507-0. [DOI] [PubMed] [Google Scholar]
- Palmer J. M., Schwitzguébel J. P., Møller I. M. Regulation of malate oxidation in plant mitochondria. Response to rotenone and exogenous NAD+. Biochem J. 1982 Dec 15;208(3):703–711. doi: 10.1042/bj2080703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin A., Djerdjour B., Journet E., Neuburger M., Douce R. Effect of NAD on Malate Oxidation in Intact Plant Mitochondria. Plant Physiol. 1980 Aug;66(2):225–229. doi: 10.1104/pp.66.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Jagow G., Klingenberg M. Pathways of hydrogen in mitochondria of Saccharomyces carlsbergensis. Eur J Biochem. 1970 Feb;12(3):583–592. doi: 10.1111/j.1432-1033.1970.tb00890.x. [DOI] [PubMed] [Google Scholar]


