Abstract
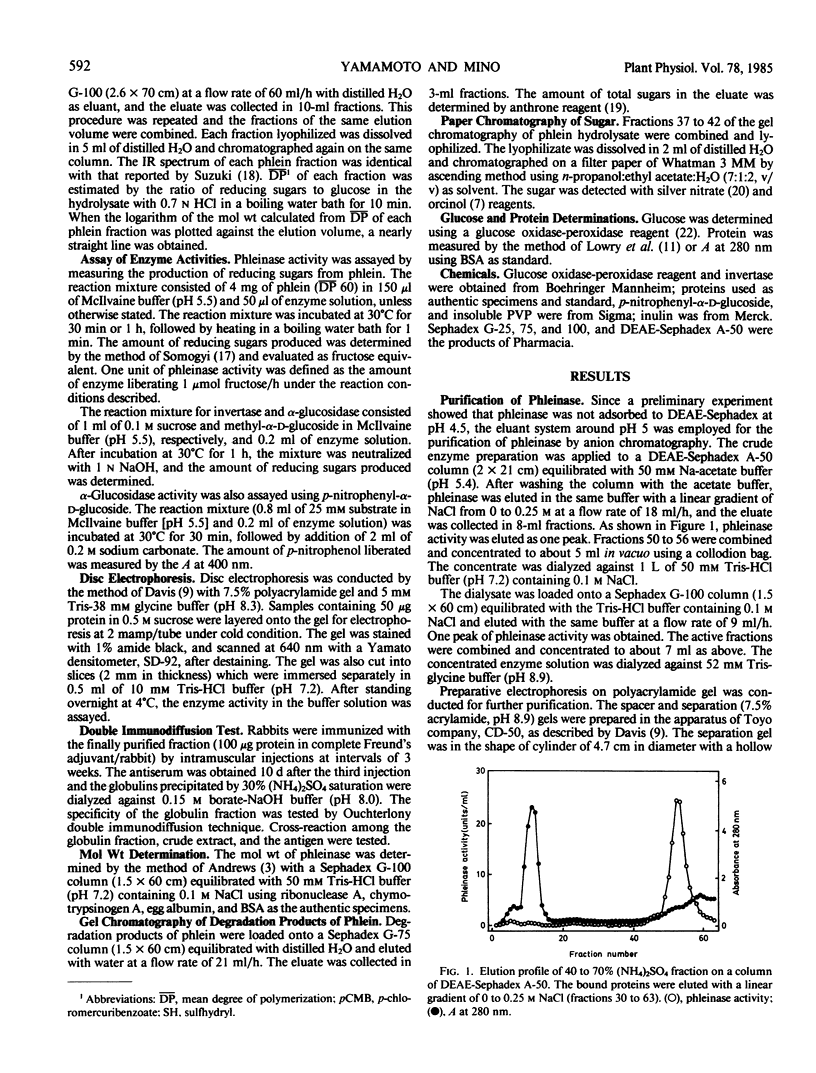
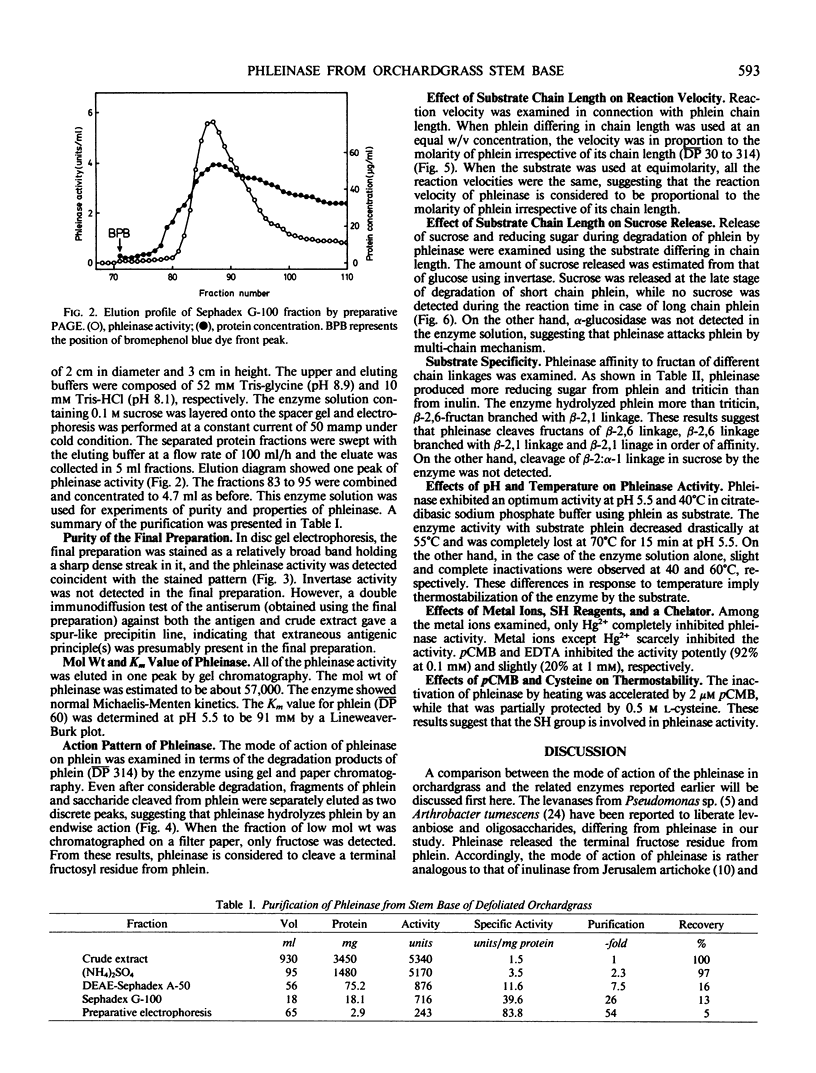
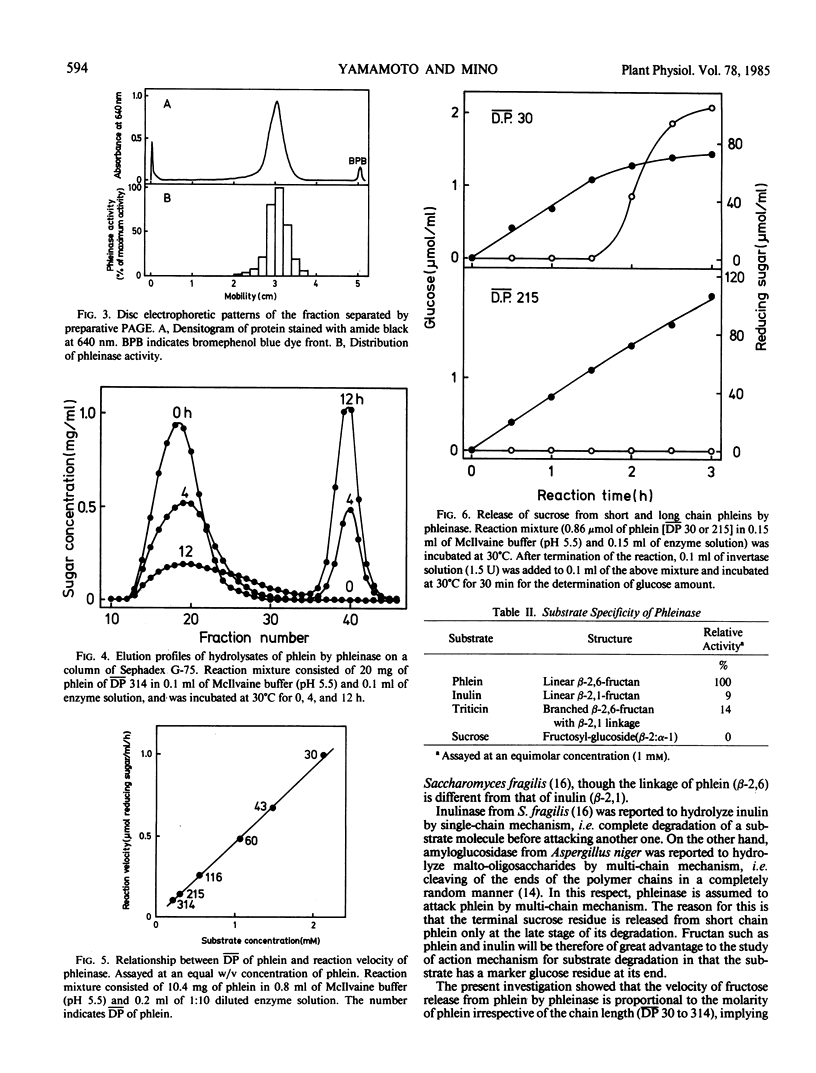
Phleinase induced in stem base of orchardgrass (Dactylis glomerata L.) after defoliation was partially purified with ammonium sulfate precipitation, DEAE-Sephadex chromatography, gel filtration, and preparative polyacrylamide gel electrophoresis. The molecular weight of phleinase was 57,000 as determined by gel chromatography. The enzyme showed normal Michaelis-Menten kinetics and its Km value was 91 millimolar for phlein of mean degree of polymerization 60 as substrate. Reaction velocity of the enzyme was proportional to molarity of phlein irrespective of its chain length (mean degree of polymerization, 30 to 314). Phleinase attacked terminal fructosyl linkage of phlein by multi-chain mechanism. Phleinase cleaved β-2,6 linkage, β-2,6 linkage branched with β-2,1 linkage, and β-2,1 linkage of fructan in order of affinity, but not sucrose. Phleinase exhibited an optimum activity at pH 5.5 at 40°C. Its complete inactivation occurred at 60 and 70°C without and with phlein, respectively. Heat inactivation of the enzyme was enhanced by p-chloromercuribenzoate and protected partially by l-cysteine. The enzyme was inhibited by sulfhydryl reagents such as p-chloromercuribenzoate and Hg2+. The modes of action of phleinase were compared with those of the related enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEVENUE A., WILLIAMS K. T. Further evidence indicating the specificity of the orcinol spray reagent for ketoheptoses on paper chromatograms. Arch Biochem Biophys. 1951 Nov;34(1):225–227. doi: 10.1016/s0003-9861(51)80032-8. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Edelman J., Jefford T. G. The metabolism of fructose polymers in plants. 4. Beta-fructofuranosidases of tubers of Helianthus tuberosus L. Biochem J. 1964 Oct;93(1):148–161. doi: 10.1042/bj0930148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PAZUR J. H., ANDO T. The action of an amyloglucosidase of Aspergillus niger on starch and malto-oligosaccharides. J Biol Chem. 1959 Aug;234(8):1966–1970. [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- SNYDER H. E., PHAFF H. J. The pattern of action of inulinase from Saccharomyces fragilis on inulin. J Biol Chem. 1962 Aug;237:2438–2441. [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELIKSON R., HESTRIN S. Limited hydrolysis of levan by a levanpolyase system. Biochem J. 1961 Apr;79:71–79. doi: 10.1042/bj0790071. [DOI] [PMC free article] [PubMed] [Google Scholar]


