Abstract
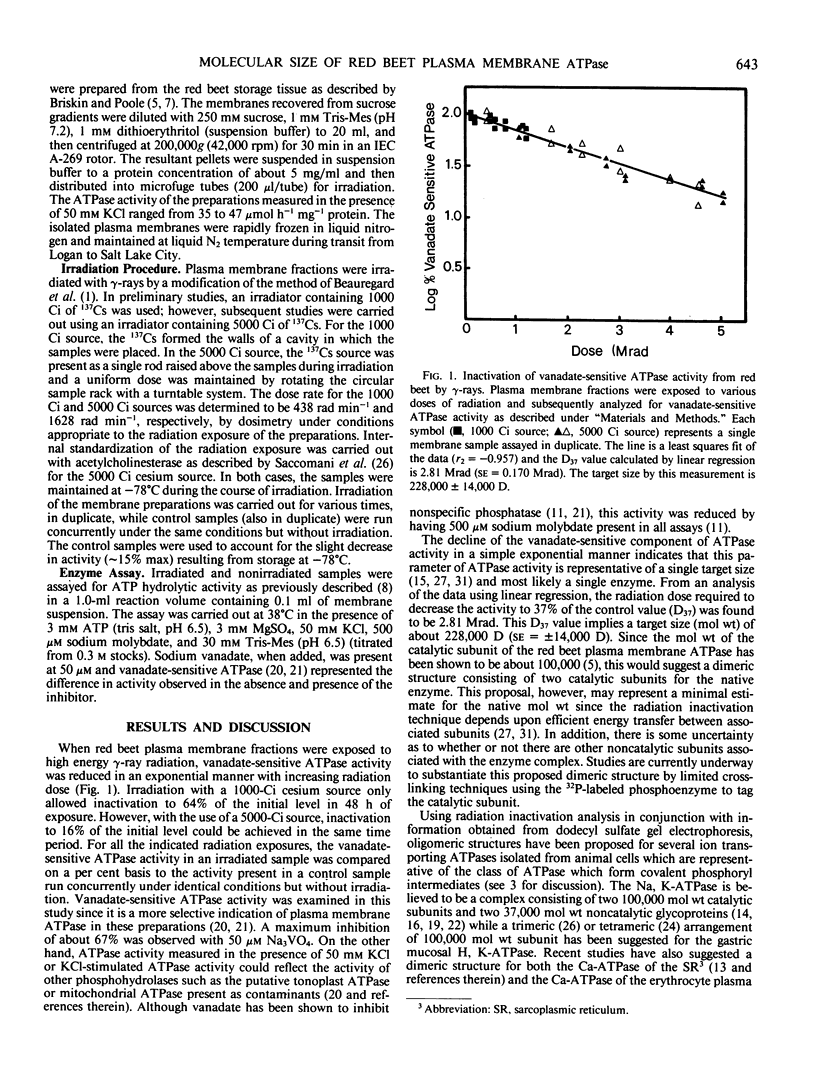
Radiation inactivation of the red beet (Beta vulgaris L.) plasma membrane ATPase was carried out using γ-ray radiation from a 137Cs source. Inactivation of vanadate-sensitive ATPase activity by γ-ray radiation followed an exponential decline with increasing total dose, indicating a single target size calculated to have a molecular weight of about 228,000. Since the catalytic subunit of the red beet plasma membrane ATPase has been demonstrated to have a molecular weight of about 100,000 by dodecyl-sulfate gel electrophoresis following 32P-phosphorylation, it is suggested that the native enzyme may exist, at least, as a dimer of catalytic subunits.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauregard G., Giroux S., Potier M. Target size analysis by radiation inactivation: a large capacity tube rack for irradiation in a Gammacell 220. Anal Biochem. 1983 Jul 15;132(2):362–364. doi: 10.1016/0003-2697(83)90021-0. [DOI] [PubMed] [Google Scholar]
- Boyer P. D., Kohlbrenner W. E., McIntosh D. B., Smith L. T., O'Neal C. C. ATP and ADP modulations of catalysis by F1 and Ca2+, Mg2+-ATPases. Ann N Y Acad Sci. 1982;402:65–83. doi: 10.1111/j.1749-6632.1982.tb25732.x. [DOI] [PubMed] [Google Scholar]
- Briskin D. P., Leonard R. T. Partial characterization of a phosphorylated intermediate associated with the plasma membrane ATPase of corn roots. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6922–6926. doi: 10.1073/pnas.79.22.6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Poole R. J. Characterization of a k-stimulated adenosine triphosphatase associated with the plasma membrane of red beet. Plant Physiol. 1983 Feb;71(2):350–355. doi: 10.1104/pp.71.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Poole R. J. Characterization of the solubilized plasma membrane ATPase of red beet. Plant Physiol. 1984 Sep;76(1):26–30. doi: 10.1104/pp.76.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Poole R. J. Evidence for a beta-Aspartyl Phosphate Residue in the Phosphorylated Intermediate of the Red Beet Plasma Membrane ATPase. Plant Physiol. 1983 Aug;72(4):1133–1135. doi: 10.1104/pp.72.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Poole R. J. Plasma membrane ATPase of red beet forms a phosphorylated intermediate. Plant Physiol. 1983 Mar;71(3):507–512. doi: 10.1104/pp.71.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller L., Jackson R., Malinowska D., Mukidjam E., Rabon E., Saccomani G., Sachs G., Smolka A. Mechanistic aspects of gastric (H+ + K+)-ATPase. Ann N Y Acad Sci. 1982;402:146–163. doi: 10.1111/j.1749-6632.1982.tb25738.x. [DOI] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffeau A., Amory A., Villalobo A., Dufour J. P. The H+-ATPase of the yeast plasma membrane. Ann N Y Acad Sci. 1982;402:91–98. doi: 10.1111/j.1749-6632.1982.tb25734.x. [DOI] [PubMed] [Google Scholar]
- Hymel L., Maurer A., Berenski C., Jung C. Y., Fleischer S. Target size of calcium pump protein from skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1984 Apr 25;259(8):4890–4895. [PubMed] [Google Scholar]
- Jørgensen P. L. Mechanism of the Na+, K+ pump. Protein structure and conformations of the pure (Na+ +K+)-ATPase. Biochim Biophys Acta. 1982 Aug 11;694(1):27–68. doi: 10.1016/0304-4157(82)90013-2. [DOI] [PubMed] [Google Scholar]
- Kempner E. S., Schlegel W. Size determination of enzymes by radiation inactivation. Anal Biochem. 1979 Jan 1;92(1):2–10. doi: 10.1016/0003-2697(79)90617-1. [DOI] [PubMed] [Google Scholar]
- Kepner G. R., Macey R. I. Membrane enzyme systems. Molecular size determinations by radiation inactivation. Biochim Biophys Acta. 1968 Sep 17;163(2):188–203. doi: 10.1016/0005-2736(68)90097-7. [DOI] [PubMed] [Google Scholar]
- Minocherhomjee A. M., Beauregard G., Potier M., Roufogalis B. D. The molecular weight of the calcium-transport-ATPase of the human red blood cell determined by radiation inactivation. Biochem Biophys Res Commun. 1983 Nov 15;116(3):895–900. doi: 10.1016/s0006-291x(83)80226-5. [DOI] [PubMed] [Google Scholar]
- Nakao M., Nagano K., Nakao T., Mizuno N., Tashima Y., Fujita M., Maeda H., Matsudaira H. Molecular weight of Na, K-ATPase approximated by the radiation inactivation method. Biochem Biophys Res Commun. 1967 Nov 30;29(4):588–592. doi: 10.1016/0006-291x(67)90526-8. [DOI] [PubMed] [Google Scholar]
- O'neill S. D., Bennett A. B., Spanswick R. M. Characterization of a NO(3)-Sensitive H-ATPase from Corn Roots. Plant Physiol. 1983 Jul;72(3):837–846. doi: 10.1104/pp.72.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'neill S. D., Spanswick R. M. Effects of vanadate on the plasma membrane ATPase of red beet and corn. Plant Physiol. 1984 Jul;75(3):586–591. doi: 10.1104/pp.75.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolenghi P., Ellory J. C. Radiation inactivation of (Na,K)-ATPase, an enzyme showing multiple radiation-sensitive domains. J Biol Chem. 1983 Dec 25;258(24):14895–14907. [PubMed] [Google Scholar]
- Periyasamy S. M., Huang W. H., Askari A. Subunit associations of (Na+ + K+)-dependent adenosine triphosphatase. Chemical cross-linking studies. J Biol Chem. 1983 Aug 25;258(16):9878–9885. [PubMed] [Google Scholar]
- Peters W. H., Fleuren-Jakobs A. M., Schrijen J. J., De Pont J. J., Bonting S. L. Studies on (K+ + H+)-ATPase V. Chemical composition and molecular weight of the catalytic subunit. Biochim Biophys Acta. 1982 Sep 9;690(2):251–260. doi: 10.1016/0005-2736(82)90329-7. [DOI] [PubMed] [Google Scholar]
- Peterson G. L., Hokin L. E. Molecular weight and stoichiometry of the sodium- and potassium-activated adenosine triphosphatase subunits. J Biol Chem. 1981 Apr 25;256(8):3751–3761. [PubMed] [Google Scholar]
- Saccomani G., Sachs G., Cuppoletti J., Jung C. Y. Target molecular weight of the gastric (H+ + K+)-ATPase functional and structural molecular size. J Biol Chem. 1981 Aug 10;256(15):7727–7729. [PubMed] [Google Scholar]
- Simon P., Swillens S., Dumont J. E. Size determination of an equilibrium enzymic system by radiation inactivation: theoretical considerations. Biochem J. 1982 Sep 1;205(3):477–483. doi: 10.1042/bj2050477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C. Mechanism of free energy coupling in active transport. Annu Rev Biochem. 1983;52:379–409. doi: 10.1146/annurev.bi.52.070183.002115. [DOI] [PubMed] [Google Scholar]
- Vara F., Serrano R. Phosphorylated intermediate of the ATPase of plant plasma membranes. J Biol Chem. 1983 May 10;258(9):5334–5336. [PubMed] [Google Scholar]
- Verkman A. S., Skorecki K., Ausiello D. A. Radiation inactivation of oligomeric enzyme systems: theoretical considerations. Proc Natl Acad Sci U S A. 1984 Jan;81(1):150–154. doi: 10.1073/pnas.81.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]


