Abstract
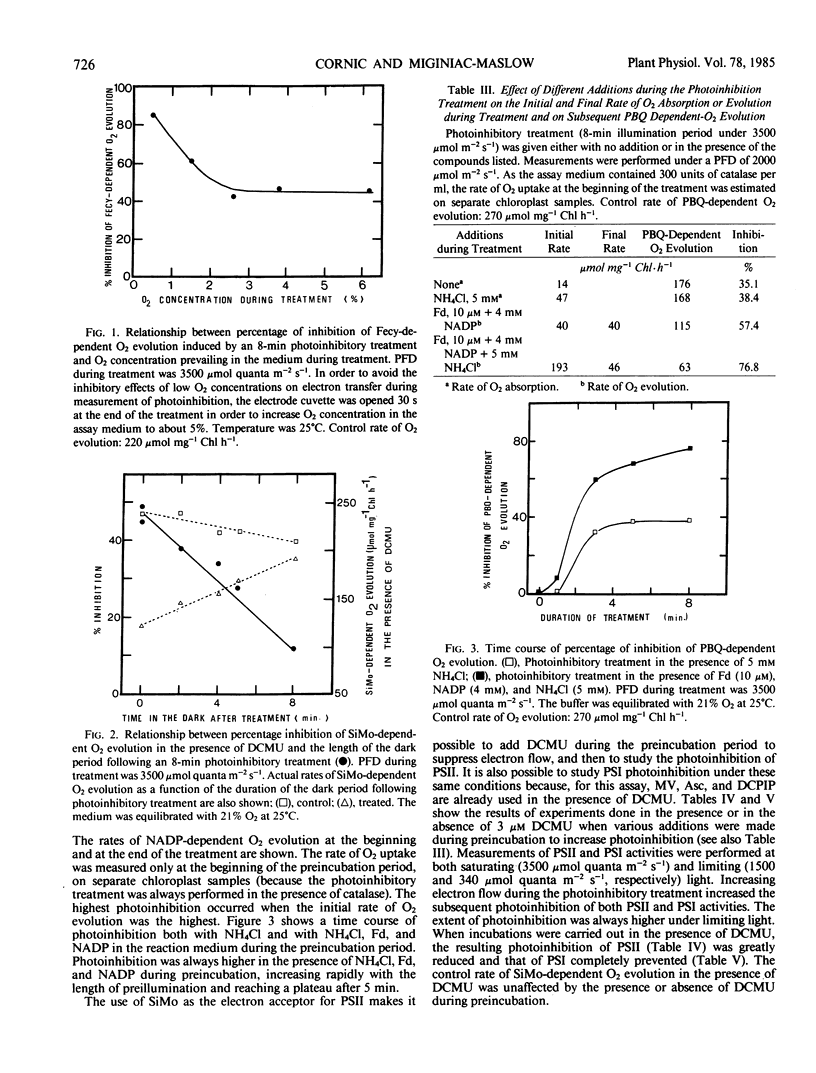
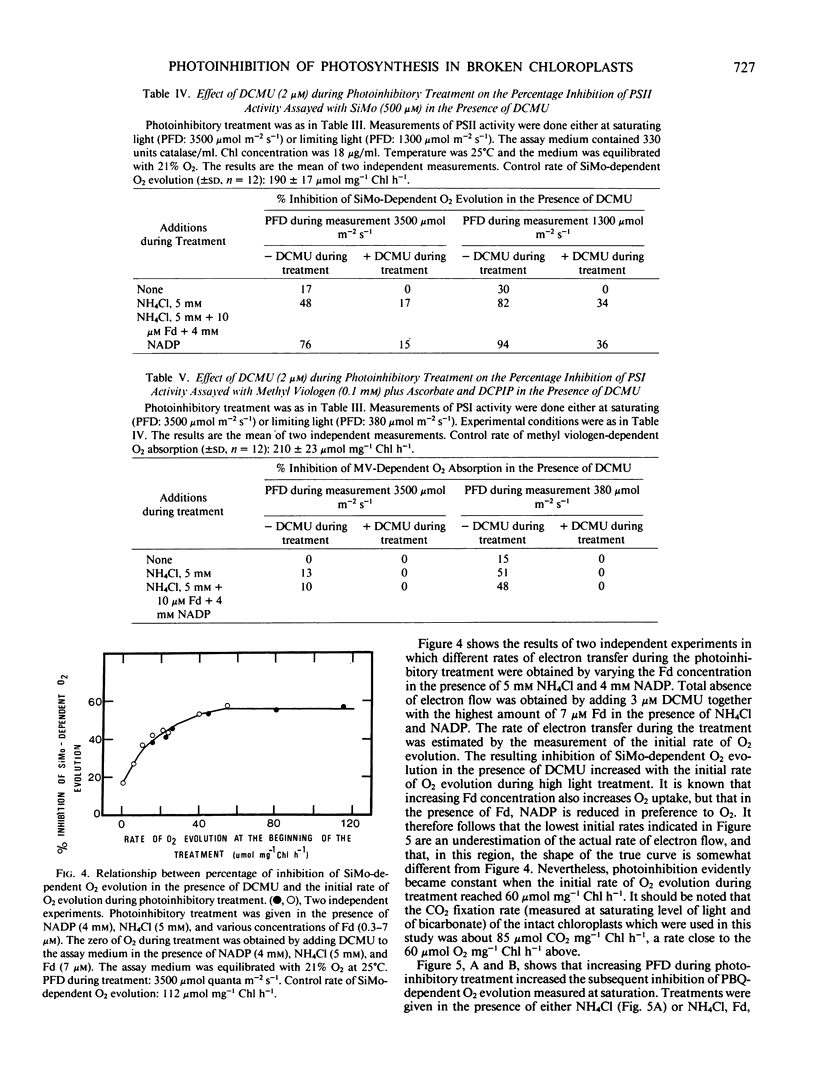
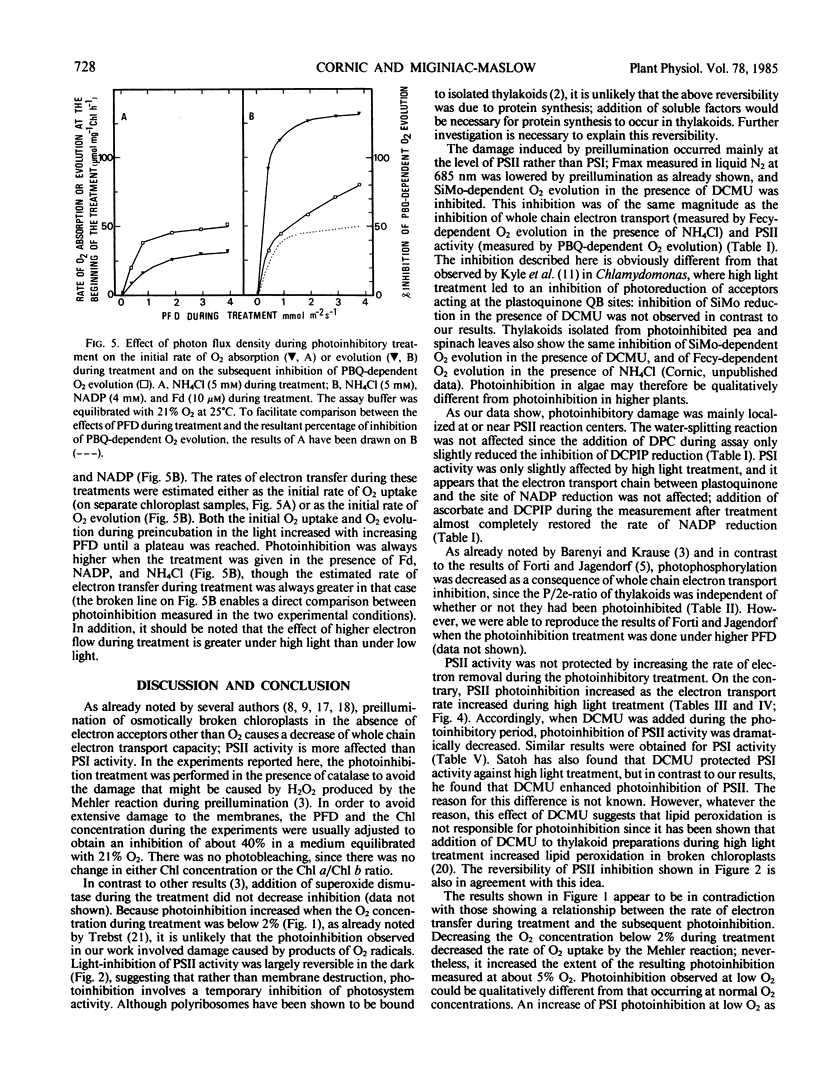
Photoinhibition was studied in osmotically broken chloroplasts isolated from spinach leaves (Spinacia oleracea L.). Both whole chain electron transport (measured as ferricyanide-dependent O2 evolution in the presence of NH4Cl) and photosystem II activity (measured as O2 evolution in the presence of either silicomolybdate plus 3-(3,4-diphenyl)-1,1 dimethylurea or parabenzoquinone) showed similar decreases in activity in response to a photoinhibitory treatment (8 minutes of high light given in the absence of an electron acceptor other than O2). Photosystem I activity was less affected. Photoinhibition of silicomolybdate reduction was largely reversible by an 8 minute dark incubation following the light treatment. Decreasing the O2 concentration during photoinhibition below 2% increased photoinhibition of whole chain electron transport. Addition of superoxide dismutase to the reaction medium did not affect photoinhibition. Photoinhibition of both photosystem I and photosystem II activity increased as the rate of electron transfer during the treatment increased, and was largely prevented when 3-(3,4-diphenyl)-1,1-dimethylurea was present during the photoinhibition period. Noncyclic photophosphorylation was decreased as a consequence of whole chain electron transfer photoinhibition. Since diphenyl carbazide added after light treatment did not relieve photoinhibition of dichlorophenol indophenol reduction, we conclude that the site of inhibition is located within or near the photosystem II reaction center.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Alscher-Herman R., Jagendorf A. T., Grumet R. Ribosome-thylakoid association in peas: influence of anoxia. Plant Physiol. 1979 Aug;64(2):232–235. doi: 10.1104/pp.64.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORTI G., JAGENDORF A. T. Inactivation by light of the phosphorylative activity of chloroplasts. Biochim Biophys Acta. 1960 Oct 21;44:34–40. doi: 10.1016/0006-3002(60)91519-5. [DOI] [PubMed] [Google Scholar]
- Heber U. Stoichiometry of reduction and phosphorylation during illumination of intact chloroplasts. Biochim Biophys Acta. 1973 Apr 27;305(1):140–152. doi: 10.1016/0005-2728(73)90239-9. [DOI] [PubMed] [Google Scholar]
- Jones L. W., Kok B. Photoinhibition of Chloroplast Reactions. II. Multiple Effects. Plant Physiol. 1966 Jun;41(6):1044–1049. doi: 10.1104/pp.41.6.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. W., Kok B. Photoinhibition of chloroplast reactions. I. Kinetics and action spectra. Plant Physiol. 1966 Jun;41(6):1037–1043. doi: 10.1104/pp.41.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle D. J., Ohad I., Arntzen C. J. Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew S. G. Nondenaturing procedure for rapid preparation of ferredoxin from Clostridium pasteurianum. Anal Biochem. 1971 Jul;42(1):191–194. doi: 10.1016/0003-2697(71)90025-x. [DOI] [PubMed] [Google Scholar]
- Powles S. B., Critchley C. Effect of Light Intensity during Growth on Photoinhibition of Intact Attached Bean Leaflets. Plant Physiol. 1980 Jun;65(6):1181–1187. doi: 10.1104/pp.65.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saillard C., Vignault J. C., Gadeau A., Carle P., Garnier M., Fos A., Bove J. M., Tully J. G., Whitcomb R. F. Discovery of a new plant-pathogenic spiroplasma. Isr J Med Sci. 1984 Oct;20(10):1013–1015. [PubMed] [Google Scholar]
- Shneyour A., Avron M. Disproportionation of I,5 -diphenylcarbazone. A new reaction catalysed by photosystem I. Biochim Biophys Acta. 1971 Dec 7;253(2):412–420. doi: 10.1016/0005-2728(71)90044-2. [DOI] [PubMed] [Google Scholar]


