Abstract
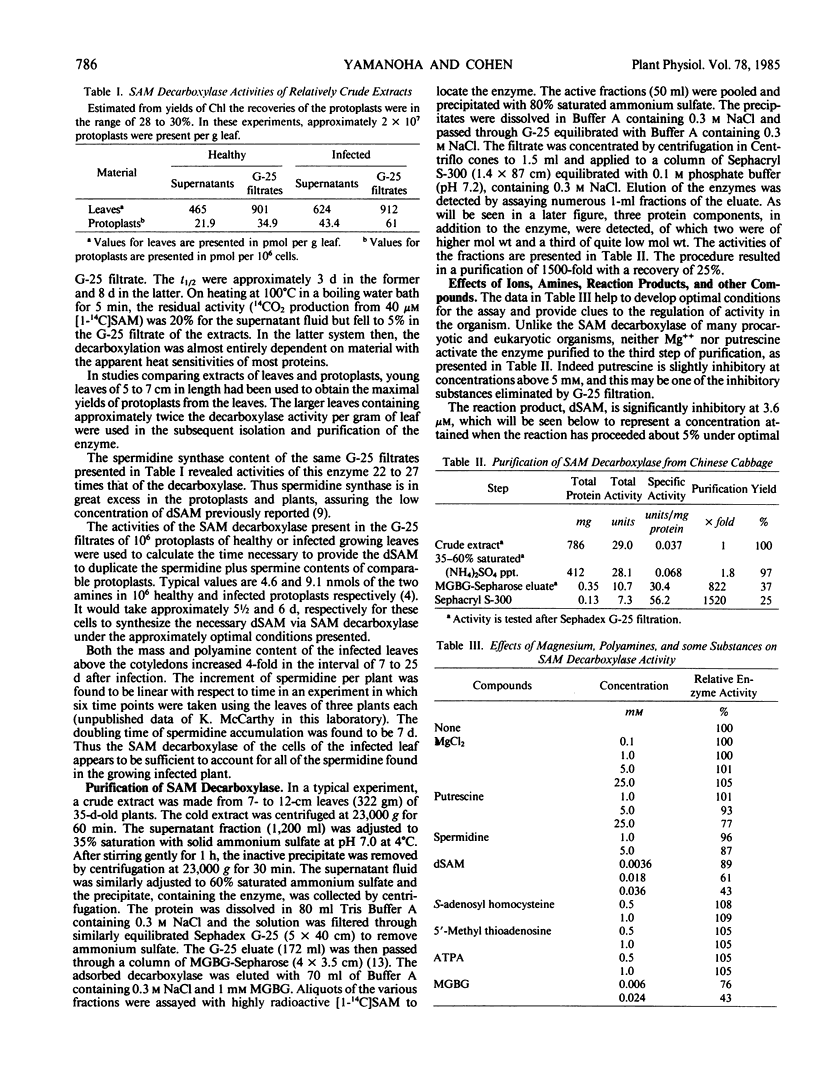
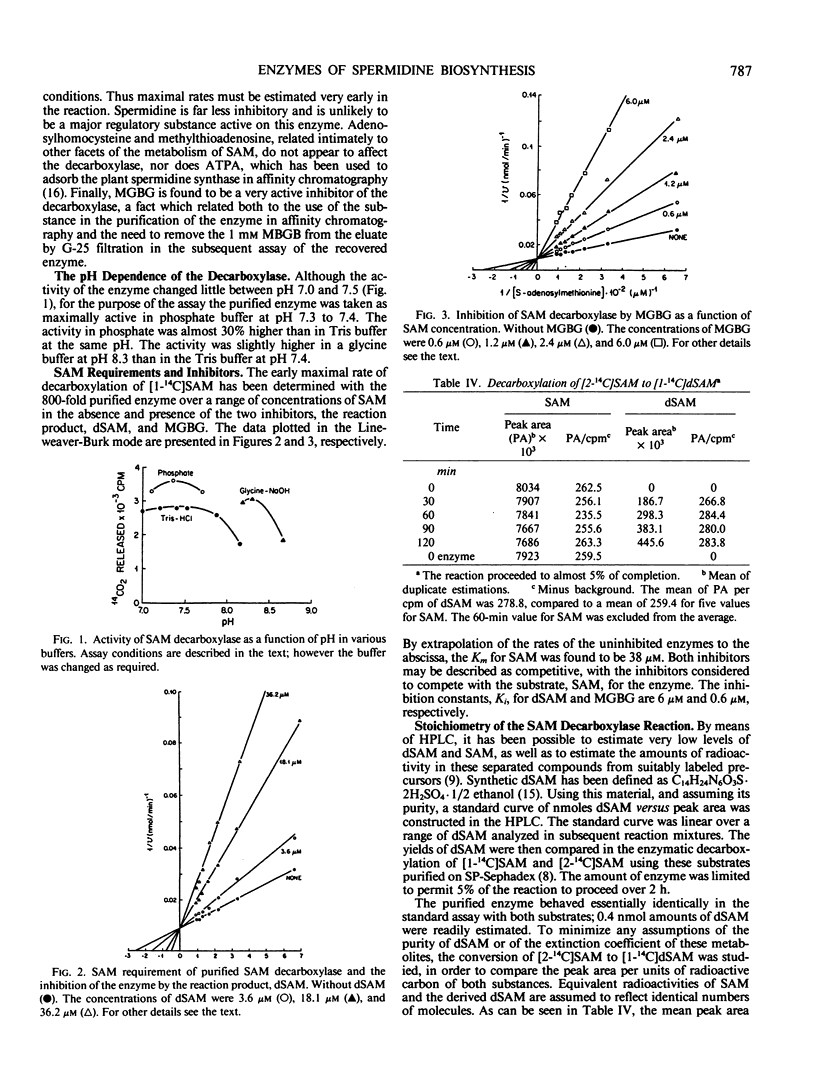
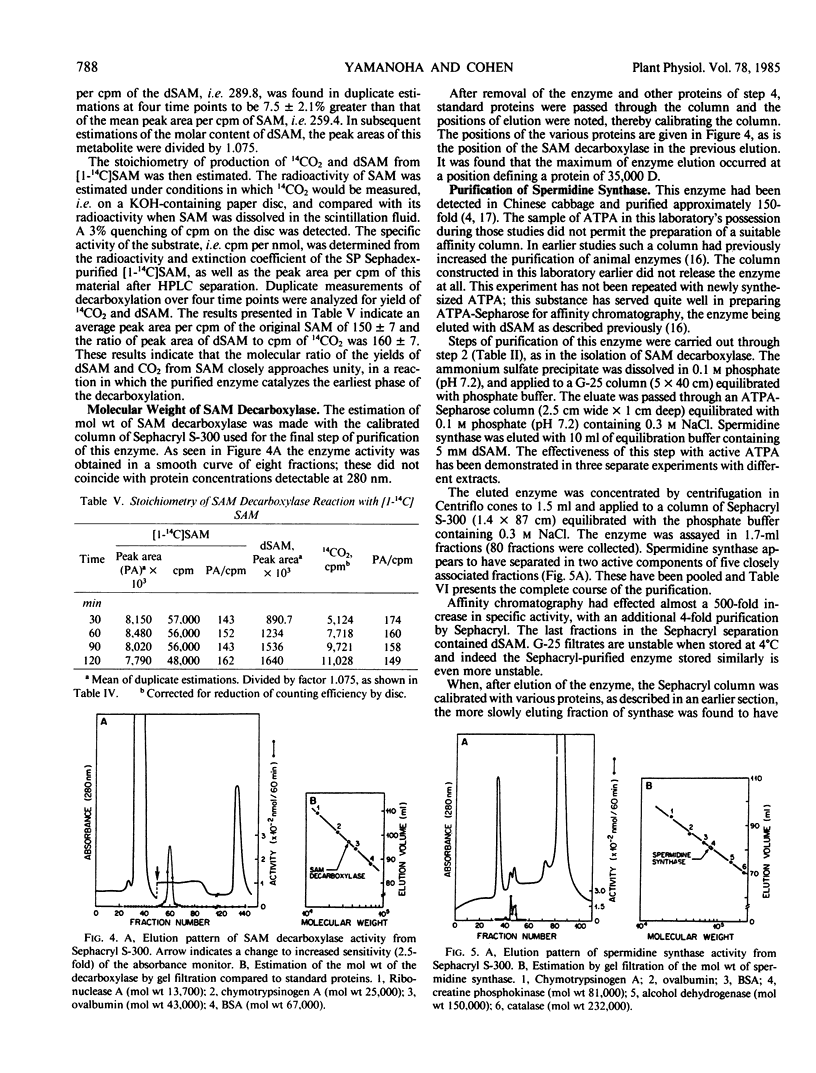
The enzyme, S-adenosylmethionine (SAM) decarboxylase (EC 4.1.1.50), has been demonstrated in leaves of Chinese cabbage, (Brassica pekinensis var Pak Choy). All of the enzyme can be found in extracts of the protoplasts obtained from the leaves of growing healthy or virus-infected cabbage. The protein has been purified approximately 1500-fold in several steps involving ammonium sulfate precipitation, affinity chromatography, and Sephacryl S-300 filtration. The reaction catalyzed by the purified enzyme has been shown to lead to the equimolar production of CO2 and of decarboxylated S-adenosylmethionine (dSAM). The Km for SAM is 38 micromolar. The reaction is not stimulated by Mg++ or putrescine, and is inhibited by dSAM competitively with SAM. It is also inhibited strongly by methylglyoxal bis(guanylhydrazone). The enzyme, spermidine synthase (EC 2.5.1.16), present in leaf or protoplast extracts in many fold excess over SAM decarboxylase, has been purified approximately 1900-fold in steps involving ammonium sulfate precipitation, affinity chromatography, and gel filtration on Sephacryl S-300. Standardization of the Sephacryl column by proteins of known molecular weight yielded values of 35,000 and 81,000 for the decarboxylase and synthase, respectively.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S., Balint R., Sindhu R. K. The synthesis of polyamines from methionine in intact and disrupted leaf protoplasts of virus-infected chinese cabbage. Plant Physiol. 1981 Nov;68(5):1150–1155. doi: 10.1104/pp.68.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Identification of a pyruvoyl residue in S-adenosylmethionine decarboxylase from Saccharomyces cerevisiae. J Biol Chem. 1977 Nov 25;252(22):8212–8216. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Glazer R. I., Peale A. L. Measurement of S-adenosyl-L-methionine levels by SP Sephadex chromatography. Anal Biochem. 1978 Dec;91(2):516–520. doi: 10.1016/0003-2697(78)90538-9. [DOI] [PubMed] [Google Scholar]
- Kaur-Sawhney R., Flores H. E., Galston A. W. Polyamine oxidase in oat leaves: a cell wall-localized enzyme. Plant Physiol. 1981 Aug;68(2):494–498. doi: 10.1104/pp.68.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manen C. A., Russell D. H. Comparative properties of rat liver and sea urchin eggs S-adenosyl-L-methionine decarboxylase. Biochemistry. 1974 Nov 5;13(23):4729–4735. doi: 10.1021/bi00720a008. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Purification of rat liver S-adenosyl-L-methionine decarboxylase. Biochem J. 1974 Aug;141(2):581–583. doi: 10.1042/bj1410581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. Stimulation of the decarboxylation of S-adenosylmethionine by putrescine in mammalian tissues. Biochem Biophys Res Commun. 1968 Jan 11;30(1):76–82. doi: 10.1016/0006-291x(68)90715-8. [DOI] [PubMed] [Google Scholar]
- Samejima K., Yamanoha B. Purification of spermidine synthase from rat ventral prostate by affinity chromatography on immobilized S-adenosyl(5')-3-thiopropylamine. Arch Biochem Biophys. 1982 Jun;216(1):213–222. doi: 10.1016/0003-9861(82)90206-5. [DOI] [PubMed] [Google Scholar]
- Sindhu R. K., Cohen S. S. Propylamine transferases in chinese cabbage leaves. Plant Physiol. 1984 Mar;74(3):645–649. doi: 10.1104/pp.74.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhu R. K., Cohen S. S. Subcellular localization of spermidine synthase in the protoplasts of chinese cabbage leaves. Plant Physiol. 1984 Sep;76(1):219–223. doi: 10.1104/pp.76.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh M. R., Adiga P. R. Putrescine-sensitive (artifactual) and insensitive (biosynthetic) S-adenosyl-L-methionine decarboxylase activities of Lathyrus sativus seedlings. Eur J Biochem. 1977 Oct 3;79(2):511–518. doi: 10.1111/j.1432-1033.1977.tb11835.x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Hirasawa E. S-adenosylmethionine decarboxylase of corn seedlings. Plant Physiol. 1980 Dec;66(6):1091–1094. doi: 10.1104/pp.66.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABOR H., ROSENTHAL S. M., TABOR C. W. The biosynthesis of spermidine and spermine from putrescine and methionine. J Biol Chem. 1958 Oct;233(4):907–914. [PubMed] [Google Scholar]
- Wilson J., Corti A., Hawkins M., Williams-Ashman H. G., Pegg A. E. The decarboxylation of S-adenosylmethionine by detergent-treated extracts of rat liver. Biochem J. 1979 Jun 15;180(3):515–522. doi: 10.1042/bj1800515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanoha B., Samejima K., Nakajima T., Yasuhara T. Differences between homogeneous spermidine synthases isolated from rat and pig liver. J Biochem. 1984 Oct;96(4):1273–1281. doi: 10.1093/oxfordjournals.jbchem.a134946. [DOI] [PubMed] [Google Scholar]
- Zappia V., Zydek-Cwick R., Schlenk F. The specificity of S-adenosylmethionine derivatives in methyl transfer reactions. J Biol Chem. 1969 Aug 25;244(16):4499–4509. [PubMed] [Google Scholar]


