Abstract
Illicium verum Hook. F., also known as star anise, is one of the most important plants of the genus Anise in the family Magnoliaceae. I. verum not only has the functions of warming Yang, dispersing cold, regulating Qi and relieving pain but can also be used as a condiment to increase flavor as well as reconcile and remove fish smells. Currently, 201 chemical constituents have been identified from star anise; among these, star anise oil and shikimic acid are the two most widely used and studied chemical components in star anise, with the oil accounting for a large proportion of the total. This review integrates, classifies and updates studies related to the botany, pharmacology, phytochemistry, traditional and modern uses and quality control of star anise, with a special reference to its phytochemical composition and pharmacological activity. It will provide a reference for further research on this important medicinal plant. In addition, the broad applications and research profiles of star anise essential oil and shikimic acid are highlighted. Our review indicates that the research prospects regarding star anise are very broad and worthy of further investigation.
Keywords: Illicium verum, star anise, traditional uses, phytochemistry, pharmacology, toxicology, quality control
1. Introduction
Plant-based Chinese medicine is a generic term for Chinese medicines derived from plant resources, which are processed from natural plants or parts of naturally occurring flora under the guidance of the Traditional Chinese Medicine (TCM) doctrine. The medicinal parts of plants for Chinese medicines include roots, rhizomes, stems, leaves, flowers, fruits, seeds, skins and whole herbs [1]. Before the rapid development of medicinal chemistry in the 20th century, human beings fought diseases with plants and other natural medicines. Even in the rapidly developing 21st century, the use of herbal remedies for various diseases is a very common practice around the world [2]. In addition, many plant-based Chinese medicines have been found to be used both as medicines to treat illnesses and as food in culinary dishes, and star anise is one of them.
Star anise, a plant in the genus Anise in the Magnolia family, is mainly produced in China and Vietnam. The genus name Illicium is derived from the Latin word “illicere” meaning “allure”, indicating that the branches and leaves of star anise have an attractive fragrance [3]. The fruit of star anise is star shaped and it has an aromatic smell. It is an important medicinal plant and is also a commonly used as a spice. In China, the use of star anise as a spice can be traced back to the Song Dynasty (AD960–AD1279) and as a medicine to the Ming Dynasty (AD1368–AD1644). Qi in TCM refers to a very delicate substance in the human body, one of the basic components that make up the human body and maintain life activities. Yang (or Yang Qi) is a special and significant concept in TCM. It can warm the body and produce a feeling of excitement, thus allowing Qi and blood to flow smoothly. The Chinese Pharmacopoeia (2005 edition) documented the main effects star anise as warming Yang and dispersing cold, regulating Qi and relieving pain. As a well-known spice, star anise was first introduced to Europe in the seventeenth century, and it gets its distinctive licorice taste from the presence of star anise essential oil (SAO). It is one of the first traditional Chinese medicines announced by the Ministry of Health of the People’s Republic of China in 2002 for dual use in medicines and foodstuffs. In additions, it is also one of the Guangxi Zhuang Autonomous Region’s top ten characteristic Traditional Chinese Medicines, Daodi Chinese herbal materials and regional characteristic medicinal materials announced by the Guangxi Zhuang Autonomous Region Administration of Traditional Chinese Medicine (Gvangjsih Bouxcuengh Swcigih Cunghyihyoz Gvanjlijgiz) in 2020. Star anise has been used in China for more than a thousand years, and China is the only country in the world that can produce anise on a large scale.
Star anise is mainly found in East and Southeast Asia and southern North America. In East Asia, China is the main production area of star anise, followed by Vietnam, Cambodia, Myanmar, Sumatra in Indonesia, Kalimantan in the Philippines and other regions and countries. In North America, star anise is found mainly in Mexico, Haiti and Florida. In China, star anise oil, also known as “HuiYou” and “BaJiaoYou” is chemically diverse and has a wide range of uses. It is produced in China but is very popular in Malaysia, Vietnam, Indonesia and other regions (customs.gov.cn, accessed on 20 November 2022). Shikimic acid is the key synthetic precursor of Tamiflu (Roche Group, Basel, Switzerland) and is the only specific drug recommended by the International Health Organization to use against the H5N1 subtype. Star anise has a wide variety of pharmacological effects, including antioxidant, antibacterial, anti-inflammatory, insecticidal and antiviral properties. It is very similar to many plants, and confusing it with others has led to poisoning incidents.
The chemical composition of star anise is diverse, and the most important and widely studied components are SAO and shikimic acid. SAO is volatile oil that is extracted from star anise fruit. It has a unique anise flavor and is widely used in the food and pharmaceutical industries because of its antimicrobial and antioxidant properties. In addition to SAO, the other important compound in star anise, shikimic acid, is the main component of Tamiflu, which was developed by the Swiss Roche Group. Tamiflu is the only specific drug recommended by the International Health Organization to be used to treat the highly pathogenic avian influenza of the H5N1 subtype. Currently, the main industrial use of shikimic acid is for the synthesis of Tamiflu, and this has attracted widespread attention towards organic acids. Before 2000, shikimic acid was generally used as a chemical raw material to be converted into other chemicals and chemical reagents.
So far, a total of 201 chemical components have been isolated from star anise, including hydrocarbons, alcohols and esters, as well as natural products such as star anise oil, flavonoids, phenylpropanoids, organic acids phenols, terpenoids and others. These ingredients have been verified to have a variety of biological activities, and various extraction methods including steam distillation have been established for their extraction. This article reviews the general characteristics, chemical properties and biological activities of the constituents obtained from star anise, focusing particularly on SAO and shikimic acid. The outlook is that the research prospects of star anise are very broad and worthy of further research.
2. Botanical Description, Taxonomy and Geographic Distribution
2.1. Botanical Description and Taxonomy
Illicium verum Hook. F. is the English name of star anise, alias Illicium san-ki. It is a 10–15 m tall arbor plant. The leaves are either alternate or in 3–6 clusters of branches in a whirl at the top and are leathery or thick leathery, obovate-elliptical, oblique lance-shaped or oval, measuring approximately 50–150 mm long and 1–1.5 mm wide. The apex is short and acuminated or slightly obtuse-rounded, the upper midrib is slightly depressed or flat when fresh and the base is cuneate, with 4–6 pairs of lateral veins and 8–20 mm petioles. The flowers are pink to crimson, solitary in leaf axils or subterminal with a 15–40 mm long pedicel. The tepals number 7–12 and often exhibit inconspicuous translucent glandular dots. The largest tepal is broadly elliptical to broadly ovoid, 9–12 mm long and 8–12 mm wide. Its aggregated fruits tend to spread and are 35–40 mm in diameter, whereas the fruit stalks are 20–56 mm long. The seed pods number 7–8 and are 14–20 mm long with an apical rostrum and are obtusely rounded without apices. The seeds are brown and are 7–10 mm long. The star anise tree blooms twice a year, once in March–May with high yields and ripe fruits and again in August–October. According to the Flora of China, the type specimen of star anise was originally obtained from Kew Gardens, England, grown and propagated in Beihai, Guangxi Province, China [4,5]. The medicinal part of star anise is its fruit, which is picked in autumn and winter when the fruit turns from green to yellow [6]. Star anise is classified in the kingdom Plantae, phylum Angiospermae, class Magnoliopsida, order Austrobaileyales, family Magnoliaceae and genus Illicium. The fruit of star anise is cogwheel shaped and consists of an average of eight pods, and therefore it is generally called star anise. The “Huixiang” herbs include fennel, red fennel, star anise and cumin. As mentioned earlier, the genus name Illicium comes from the Latin word “illicere”, which can be translated as “to seduce and attract”, indicating that the fruits and branches of the plant have a seductive fragrance [3,4,5].
2.2. Geographic Distribution
Star anise is suitable for planting in deep, well-drained, fertile, moist and acidic sandy loam or loamy soil. It grows poorly in dry and barren or low-lying, waterlogged areas. It is mainly distributed in Southeast Asia and North America, of which Asia accounts for 80% of its source. In Southeast Asia, the main production area is China, followed by Vietnam, Cambodia and Myanmar (http://www.gxbajiao.org/, accessed on 13 June 2022). In China, star anise is mainly produced in Guangxi, including Baise, Nanning, Qinzhou, Wuzhou and Yulin, where it is mostly cultivated at an altitude of 200–700 m. In Yunnan province, including Funing, Guangnan, Xichou, Pingbian and Lvchun, it is mostly cultivated at its natural distribution levels, which are up to 1600 m in elevation [7]. The deputy director of the Anise Cinnamon Engineering Technology Research Center of the State Forestry Administration of China said that China’s production of star anise accounts for about 80% of the world’s production. Guangxi has the longest cultivation history and the most production, and in 2018, the Guangxi production of this plant accounted for more than 90% of the total national output. Guangxi has the reputation of being “the hometown of star anise” [8].
3. Pharmacology
3.1. Antimicrobial
The antimicrobial effect of star anise is one of the important focuses of modern pharmacological research. SAO has a wide inhibitory spectrum of activities against plant pathogenic fungi [5]. Additionally, some studies have shown that extracts from different parts such as the roots, branches, peels and leaves of star anise have certain antibacterial and antifungal activities. For four tested fungi (Helminthosporiummaydis, Rhizoctonia cerealis, Helminthosporiurn carposaprum, Verticillium dahlia), the antifungal rates of the seedpod and leaf extracts of I. verum were greater than 50%, and those for the root and branch extracts were lower than 50%. The antimicrobial activity of SAO (minimum inhibitory concentration, MIC = 0.5 µL/mL) for Bacillus subtilis was stronger than that of common preservatives, such as paraben [6].
For some fungi such as Aspergillus flavus, Fusarium tricinctum and Candida albicans, star anise also exhibits fungicidal characteristics, with MIC and MFC (minimum fungicidal concentration) values of 2.5–25 μL/mL [7]. Huang et al. determined the IC50 values for 11 plant pathogens (including Alternaria solani, Bipolaris maydis and Botryodiplodia theobromae) using a direct contact assay, and the IC50 values of SAO against mycelar growth ranged from 0.06 to 0.25 mg/mL. Pythium aphanidermatum and Botryodiplodia theobromae were selected to evaluate the antifungal activity of the vapor components the SAO from I. verum as well as that of trans-anethole by using the vapor contact assay. There was also a strong inhibition of Magnaporthe oryzae spore germination when using an inhibition assay, and the IC50 value of the oil was determined to be 0.32 mg/mL. At all concentrations in medium, trans-anethole displayed a very similar inhibitory rate to that of SAO against the test fungi, which suggested again that this was the main active component among the volatiles in the oil [8]. In accordance with these studies, Singh et al. used an inverted petri-plate technique, and they found that the volatile oil exhibited 100% zone inhibition for Fusarium moniliforme. It was also found to be highly effective in controlling the growth of Penicillium citrium, Aspergillus flavus and Penicillium viridicatum by exhibiting more than 75% mycelial zone inhibition, as well as 50% inhibition for Aspergillus niger.
In a 2020 report, Li et al. evaluated the apparent inhibitory effect of SAO on A. flavus by using a contact assay [9]. Mycelial growth was observed after 8 days of SAO treatments at 2.8 and 3.2 μL/mL. They tested the effects of SAO on Aspergillus flavus spore production using various concentrations and found that it was able to effectively inhibit spore production. Furthermore, the antifungal activities of SAO against A. flavus strains CGMCC 3.4408, CGMCC 3.4409 and CGMCC 3.4410 and their MIC and MFC values were determined. The results suggested that SAO exhibited a strong antifungal activity on the growth of the three A. flavus strains [10]. Recent findings from 2019 showed that SAO has antibacterial activity against Penicillium. Not only SAO but also some other anise extracts have similarly good antibacterial activities. A 2010 report stated that the supercritical CO2 and ethanol extracts of I. verum showed substantial antibacterial activities against 67 clinical drug-resistant isolates, including 27 Acinetobacter baumannii, 20 Pseudomonas aeruginosa and 20 methicillin-resistant Staphylococcus aureus strains [7,11,12].
Ibrahim et al. [12] used agar disc diffusion methods, agar plate dilution techniques and MIC and MBC (minimum bactericidal concentration) determinations to evaluate the antibacterial activity of star anise waste residue extract (SAWRE) against the most potent multi-drug-resistant strains of Streptococcus pneumoniae, S. aureus, Klebsiella pneumoniae, A. baumannii, Escherichia coli and P. aeruginosa. The results showed that SAWRE had significant antibacterial activities against all of the tested bacteria, with MIC values between 16 to 128 μL/mL. After binding to SAWRE, the MIC of cephradine, amoxicillin tetracycline and chloramphenicol was reduced by 512-, 64-, 8- and 2-fold, respectively, against A. baumannii. A combination of SAWRE and some antibiotics can represent a novel choice for the treatment of infectious diseases, as the waste extracts may act as activity-modifying agents for the antibiotics. The bacteriostatic mechanism of star anise is mainly through a variety of bacteriostatic components within the extracts. These act synergistically to degrade the bacterial cell walls and cause damage to the cytoplasmic membranes. They may also denature the membrane proteins, resulting in the loss of glucose, proteins and DNA from the cell, causing anabolic disorders and resulting in the death of bacteria and fungi [12].
The isolated compounds from I. verum were tested for anti-HIV activity by using an inhibition assay to assess their cytopathic effects on the virus. The compounds (-)-illicinone-A and 3,4-seco-(24Z)-cycloart-4(28),24-diene-3,26-doic acid, 26-methyl ester, which were isolated from star anise, were found to have anti-HIV activities, with EC50 values of 16.1 and 5.3 µM and with SI values of 18.2 and 15.6, respectively. In addition, it was also demonstrated that star anise extracts had antiviral activities against herpes simplex virus types 1 (HSV-1) and 2 (HSV-2) [13,14,15]. I. verum extracts exhibit excellent antiviral activity against infection with the viral strain SGIV-Gx. The antiviral effects of each type of I. verum extract were assessed using grouper spleen (GS) cells by Q2-AFMP and RT-qPCR. With both techniques, the results showed that the aqueous and ethanolic I. verum extracts displayed dose-dependent antiviral activities against grouper iridovirus. The activities of both extracts achieved >90% inhibition of viral growth. Determining the detailed antiviral mechanisms of I. verum extracts against SGIV-Gx infection is an important research direction for future studies [16]. In addition, (−)-bornyl p-coumarate isolated from star anise in 2022 was also found to have strong antiviral activity, with an IC50 of 1.74 μmol/L against influenza A H1N1 virus. This is better than that of Tamiflu (IC50 = 10.01 μmol/L) and ribavirin (IC50 = 10.76 μmol/L), and it might be considered a potential candidate in drug development for the treatment of influenza A virus (PR8) [16].
3.2. Antioxidant
The fruits of star anise are commonly used as spices, and these are an alternate source of antioxidants that has been used for a long time. The fine powder and extracts of star anise prepared using water and ethanol under normal or under supercritical CO2 conditions as well as SAO have all be found to have antioxidant properties. The antioxidant activity of star anise and its extracts were verified by adopting linoleic acid peroxidation, the β-carotene-linoleic acid system and DPPH (1,1-diphenyl-2-picryhydrazyl) radical-scavenging methods in different studies [17,18]. The addition of star anise fine powder to lemongrass oil enhances its oxidative stability, and together they form a natural antioxidant. The scavenging ability of the star anise antioxidant was evaluated by using the DPPH assay at various concentrations (500, 1000 and 1500 ppm) and was found to be 80.67, 81.38 and 81.73%, respectively [19]. The antioxidant activity of star anise against H2O2-induced DNA damage and human peripheral lymphocyte death was evaluated by assessing lipid peroxide inhibitory, hydroxyl-radical-scavenging, DPPH and superoxide free-radical-scavenging activities. The results showed that the water extracts of star anise had the most effective antioxidant activity. It was also shown that the aqueous extract of star anise acted as an antioxidant at a dose of 25 µg, and this amount provided protection to DNA against peroxides [20].
The DPPH-radical-scavenging method was also used by Yang et al. [21], who showed that the ethyl acetate extracts of star anise possess superior free-radical-scavenging abilities and reducing power compared to many other preparations. The strong correlation between its DPPH and TEAC values and those obtained from the reducing power assay implied that the antioxidants in the extracts were capable of scavenging free radicals and reducing different oxidants. Qualitative DPPH assays were performed on 25 essential oil products on TLC for 1–9 h at a temperature range of 30–70 °C. Two different areas of antioxidant activity with different polarities appeared on all the TLC plates. In addition, the highest antioxidant activity was observed for SAO when the sample was extracted at 60 °C for 1 day (EC50 value = 0.089 ± 0.05 mg/mL). According to numerous reports in the literature, the antioxidant activities of star anise and its extracts could be mainly due to the high polyphenol, carbohydrate, flavonoid and trans-anethole concentrations, along with a combined effect of all the phytochemicals [22]. At present, there is a search for new plant compounds that possess antioxidant activities, and this is a vital area of research in plant medicines. In general plant products are safe and are obtainable at relatively low cost [23,24].
3.3. Anti-Inflammatory
It is well documented that I. verum has anti-inflammatory properties. The anti-inflammatory effects of star anise aqueous extracts were investigated on xylene-induced auricle edemas in mice. The inhibition rate indicated that star anise significantly improved auricle edemas caused by xylene [24]. In another study, subjects in group A used a star aniseed-based mouthwash, and subjects in group B used a placebo (color-tinted water). This confirmed the effective anti-inflammatory properties of I. verum by recording gingival conditions before and after the intervention [25]. The anti-inflammatory activity of I. verum extract was also studied for its ability to inhibit protein denaturation, and the results were read spectrophotometrically. It was found to be effective at inhibiting heat-induced albumin denaturation at different concentrations of 100–500 µg/mL [26]. Five groups of six mice each were treated with distilled water (10 mL/kg), indomethacin (10 mg/kg) or methanolic, ethanolic or aqueous extracts (150, 250 and 350 mg/kg) of I. verum. Edema was induced in the lower metatarsals of the right hind paws of each animal, and the edema-associated paw thickness was measured after 1, 2, 3, 4 and 5 h by using a plethysmometer. The inflammation of paw volume in rats was significantly reduced in the methanol and ethanol I. verum extract test groups when compared to that in the control group. At present, star anise has a good therapeutic effect on acute inflammation such as that associated with the ear canal, oral cavity and airway surfaces, but whether it has an effect on other types of inflammation needs to be further researched [27].
3.4. Insecticidal and Anthelmintic
Phytochemical studies have shown that star anise tree wood is not subject to insect infestation. Using SAO as a grain storage protectant, it was found that the treatment of wheat or whole wheat flour with a 0.1% (w/w) dose of essential oil resulted in 100% inhibition of Tribolium castaneum (Herbest), Rhizopertha dominica Fabricius and Tenebrio molitor Linne. That meant a complete suppression of their reproduction. In addition, the egg dipping method also verified the toxic effects of SAO on the eggs of Tenebrio molitor Linne [28]. The results of the Kim et al. (2016) showed that the fumigation mortality of Drosophila suzukii by SAO could reach 80% within 24 h [29]. Sogatella furcifera was fed rice treated with I. verum extracted with n-hexane and methylene chloride. The results showed the treatment produced satisfactory insecticidal activity against S. furcifera, and this was concentration-dependent [30]. The repellent, antibacterial and insecticidal activities of anise were used to develop a coating material for food packaging, and the physical properties associated with the coating were improved by the addition of a reinforcing SAO filler in order to activate the coating solution. When packaging sliced wheat bread, the film exhibited a strong and long-lasting insect repellent activity, along with the ability to lower the microbial counts. In addition, it provided a better appearance when compared to the samples packaged with a control film [31]. The film prevented insects from approaching the bread as well as inhibiting the growth of microorganisms. Among four solvent-partitioned fractions [(1) n-hexane; (2) ether; (3) ethyl acetate; (4) water], the strongest repellency was found for the n-hexane fraction of star anise extract against Plodia interpunctella larvae [32]. This result is generally consistent with previous studies.
3.5. Other Activities
I. verum extracts affected the spontaneous activity as well as the sound and touch pain responses in mice at a dose of 200 mg/kg and produced moderate or slight depression [33,34]. This effect was produced by inhibiting the nociceptors, and this inhibition did not interfere with motor coordination [34]. Star anise processed products have the effects of warming Yang, dispersing cold and relieving pain [35]. I. verum extracts could increase exhaustive swimming and pole-climbing time periods as well as post exercise hepatic glycogen content in mice. They also raised lactate dehydrogenase activity and decreased lactate and serum urea nitrogen levels. These findings demonstrated that I. verum extracts have noticeable anti-fatigue effects on mice [36]. Good inhibition of aluminum corrosion in concentrated hydrochloric acid solution can be caused by I. verum extracts [37]. A mixture of chamomile and star anise has anti-motility effects and can decrease induced diarrhea in mice [38]. Furthermore, the results of a new study indicate that synthesized magnetite Fe3O4, spinel (2:1) and (4:1) NiFe2O4 using an extract of star anise as a green reducing agent showed high biomedical activities against liver carcinoma cells and non-small lung adenocarcinoma cells [39].
3.6. Toxicology
Star anise, one of the traditional Chinese herbs, has a long history. It was originally used as an ingredient in cooking, and later people gradually explored its medicinal value. According to previous literature, most cases of anise poisoning are due to accidental ingestion of anise analogs, except for cases of dietary overdose which were reported in the 19th century, but the exact mechanism of poisoning is not yet clear. In modern times, the first case of poisoning caused by over-consumption of anise was reported in 1992, in which the patient used star anise plants which were crushed to make cakes and suffered from paroxysmal vomiting, weakness and chills in the extremities after consumption [40]. In 1996, three new compounds extracted from the ethyl acetate extract of star anise, veranisatin A, B, C, were found to have convulsive effects and lethal toxicity at an oral dose of 3 mg/kg in mice, while anisatin caused similar effects at a dose of 1 mg/kg. In addition, several cases of poisoning have been reported in infants who took or were injected with anise, manifesting as neurological and gastrointestinal toxicity, but again the cause of the poisoning is not yet clear [41,42,43]. In 2004, it was speculated that, based on laboratory findings, the cause could be an excess of anise, contaminated anise, or a combination of both [44]. In 2016, a scholar using an aqueous extract of star anise seeds administered by gavage to mice showed that serial types of infusions caused transient behavioral changes consistent with neurological effects [45].
4. Phytochemistry
The chemical composition of star anise has been studied since 1983. Modern research has shown that different parts of this plant (including the roots, leaves and fruits) contain various chemical components, including a volatile oil, phenylpropanoids, sesquiterpene lactones and flavonoids. To date, 201 compounds have been identified, including organic acids, flavonoids, phenylpropanoids, lignans, sesquiterpenes, alcohols and some simple hydrocarbons. This includes 58 new compounds found more recently. Most of the new compounds found in the star anise are phenylpropanoid and lignan compounds. Chemical investigations of the genus Illicium have resulted in the isolation of prenylated C6–C3 compounds, neolignans and seco-prezizaane-type sesquiterpenes, which are characteristic chemical markers of this species. Phenylpropanoids and flavonoids are the most frequently reported components in the chemical composition studies of star anise and have a wide range of pharmacological effects. The chemical constituents that have been identified are listed in tables, and their corresponding structures are represented diagrammatically in the accompanying figures.
4.1. Star Anise Oil
The raw materials for the preparation of SAO are star anise fruits, branches and leaves, which are the main sources of aroma from these plants. The 2020 Chinese Pharmacopoeia classified SAO as a colorless or pale-yellow clear liquid, and its smell is similar to that of star anise. When cold, it often becomes turbid or will precipitate as crystals, and it turns clear after warming. It is readily soluble in 90% ethanol. This highly flavored volatile oil has long been the subject of research, as it is the main chemical component of star anise. SAO can also be used in food flavors such as alcoholic drinks, beverages, candy, baked goods and chewing gum and as a cigarette flavoring agent. It is also a good masking agent that can cover up unpleasant odors and is therefore, used in soap fragrances, mouth gargles and toothpaste. In the fragrance industry, one of its ingredients, anisole, is used to synthesize anisaldehyde, anisol, anisic acid and its esters. These monomer fragrances are widely used in toothpaste, foodstuffs, soap and cosmetics.
SAO has a long history, and it was originally produced as a commodity in Debao County, BaiSe City, Guangxi Province. Debao County was formerly known as “Tianbao”, so SAO has the alias of “Tianbao anise oil”. According to historical records, in the Ming Dynasty (1368–1644), the inhabitants of Debao began to plant anise trees. During the Xianfeng years (1851–1861), SAO, which has a high freezing point and excellent texture, was used as a pure fragrance. They began to steam and boil the leaves of star anise to make the oil. From 1912 to 1949, SAO was exported to Hong Kong, and it was later exported to more than 50 countries around the globe, including France, the United States, Japan and Canada. It is said that the world-famous “Paris perfume” has SAO as one of its ingredients, so there is a saying that “without SAO, the Paris perfume is not fragrant”.
Since 1995, China has maintained a foreign trade of star anise products. The main export areas are the United States, Africa and Europe. The average export volume of SAO is more than 600,000 kg each year. In 2003, the national output of SAO was 1,631,000 kg per year. China accounted for 80% of the total output, and the export volume accounted for 30% of the country’s total output. On 1 January 2010, the China and ASEAN Free Trade Area (CAFTA) was completed, forming a free trade area consisting of 11 countries and covering 1.9 billion consumers. Since then, China’s export of SAO has steadily increased, mainly to 10 countries and regions in Europe, Asia and North America.
The methods for extracting SAO from the fruits of star anise include traditional steam distillation (TSD), organic solvent extraction (OSE), headspace solid-phase microextraction (HSMS), microwave-assisted extraction (MAE), subcritical CO2 (SCOD) and supercritical CO2 fluid extraction (SCFE). The TSD method, which is easy to operate, low in cost and relatively stable in oil yield, has been a commonly used method for extracting SAO. SCFE is a new technology for chemical separation, and it has been widely used in recent years. Compared with the extraction of SAO by TSD, this technique operates at a lower temperature, which is close to ambient temperature but with a higher separation efficiency. In this method, the extraction and separation process are combined into one, and CO2 is used as the solvent, so no solvent residue is produced. Some experiments have shown that the chemical composition of TSD-obtained extracts is significantly reduced compared to that of SCFE and OSE. Extracts obtained using SCFE have a richer chemical composition, including various saturated and unsaturated fatty acids.
Due to the numerous and complex chemical constituents of SAO, most scholars have chosen GC-MS in order to explore its chemical constituents. The 158 chemical constituents identified in SAO are listed in Table 1. The most abundant component was found to be trans-anethole (>80%). SAO has been shown to possess broad-spectrum antibacterial activity. Trans-anethole has also been found in the essential oils of other plants, and it has been shown to possess insecticidal, larvicidal and antimicrobial activities. When the antifungal activity of trans-anethole was compared with that of SAO, the former was found to have similar inhibitory activity towards the test fungi, with IC50 values closed to those of the oil, with an average difference of 0.018. This suggested that trans-anethole is a major contributor to the antifungal properties of SAO [8].
Table 1.
Chemical components of star anise essential oil.
| No. | Chemical Constituents | MF | References |
|---|---|---|---|
| 1 | 2-acetonylcylohexanone | C9H14O2 | [46] |
| 2 | limonene | C10Hl6 | [46] |
| 3 | γ-terpinen | C10Hl6 | [46] |
| 4 | β-linalool | C10H18O | [46] |
| 5 | spiro [4.5] dec-1-ene | C10Hl6 | [46] |
| 6 | 1-terpinen-4-ol | C10H18O | [46] |
| 7 | 3-undecyne | C11H20 | [46] |
| 8 | p-allylanisole | C10H12O | [46] |
| 9 | p-cumic aldehyde | C10H12O | [46] |
| 10 | propanal, 2-methyl-3-phenyl | C10H12O | [46] |
| 11 | trans-anethole | C10H12O | [46] |
| 12 | benzaldehyde, 3-methoxy | C8H8O2 | [46] |
| 13 | p-anisaldehyde | C8H8O2 | [46] |
| 14 | anethole | C10H12O | [46] |
| 15 | anisold methyl ester | C9H10O3 | [46] |
| 16 | anisyl aceton | C10H12O2 | [46] |
| 17 | acetic acid, geraniol ester | C12H20O2 | [46] |
| 18 | copaene | C15H24 | [46] |
| 19 | iso-caryophyllene | C15H24 | [46] |
| 20 | caryophyllene | C15H24 | [46] |
| 21 | 2-norpinene,2,6-d | C15H24 | [46] |
| 22 | β-famesene | C15H24 | [46] |
| 23 | benzene, 1,2-dimethoxy-4-(1-propenyl) | C11H14O2 | [46] |
| 24 | benzenemethanol, 2-(2-aminopropoxy)-3-methyl | C11H17NO2 | [46] |
| 25 | bicyclo [3,1,1] hept-2-ene, 2-ethanol, 6,6-dimethyl | C11H18O2 | [46] |
| 26 | bicyclo [3,1,1] hept-2-ene, 2,6-dimethyl-6-(4-methyl-3-pentenyl) | C15H24 | [46] |
| 27 | γ-elermene | C15H24 | [46] |
| 28 | α-famesene | C15H24 | [46] |
| 29 | cyclohexene, 1-methyl-4-(5-methyl-l-methylene-4-hexenyl) | C15H24 | [46] |
| 30 | germacrene D | C15H24 | [46] |
| 31 | phenylethanolamine | C8H11NO | [46] |
| 32 | acethydrazide | C2H6N2O | [46] |
| 33 | surfynol 102 | C12H22O2 | [46] |
| 34 | p-anisoin | C16H16O4 | [46] |
| 35 | trans-nerolidol | C15H26O | [46] |
| 36 | 1-(3-methyl-2-butenoxy)-4-(1-propenyl) benzene | C14H18O | [46] |
| 37 | bicyclo [2,2,1] heptane-2,3-dione, 6-(acetyloxy)-1,5,5-trimethyl, endo | C12H16O4 | [46] |
| 38 | hydrazinecarboxylic acid, ethyl ester | C3H8N2O2 | [46] |
| 39 | propanoic acid, 2-methyl, 3, 7-dimethyl-2, 6-octadienyl ester(E) | C14H24O2 | [46] |
| 40 | p-allylphen | C9H10O | [46] |
| 41 | hexyl oleate | C24H46O2 | [46] |
| 42 | α-pinene | C10H16 | [47] |
| 43 | cyclotetrasiloxane, octamethyl- | C8H24O4Si4 | [47] |
| 44 | myrcene | C10H16 | [47] |
| 45 | α-phellandrene | C10H16 | [47] |
| 46 | δ-3-carene | C10H16 | [47] |
| 47 | sabinene | C10 H16 | [47] |
| 48 | 1,8-cineole | C10H18O | [47] |
| 49 | linalool | C10H18O | [47] |
| 50 | L-menthone | C10H18O | [47] |
| 51 | 4-terpineol | C10H18O | [47] |
| 52 | estragole | C10H12O | [47] |
| 53 | benzene, 1-methoxy-4-(1-propenyl)- | C10H12O | [47] |
| 54 | trans-ciminnamaldehyde | C9H8O | [47] |
| 55 | β-caryophyllene | C15H24 | [47] |
| 56 | trans-α-bergamotene | C15H24 | [47] |
| 57 | iso-caryophillene | C15H24 | [48] |
| 58 | β-pinene | C10Hl6 | [48] |
| 59 | β-myrcene | C10Hl6 | [48] |
| 60 | Δ3-carene | C10Hl6 | [48] |
| 61 | iso-terpinene | C10Hl6 | [48] |
| 62 | p-cymene | C10Hl4 | [48] |
| 63 | ocimene | C10Hl6 | [48] |
| 64 | α-terpineol | C10H12O | [48] |
| 65 | cis-anethole | C10H12O | [48] |
| 66 | anisaldehyde | C8H8O2 | [48] |
| 67 | α-copaene | C15H24 | [48] |
| 68 | anisketone | C10H12O2 | [48] |
| 69 | elemene | C15H24 | [48] |
| 70 | α-bergamotene | C15H24 | [48] |
| 71 | (Z, E)-α-famesene | C15H24 | [48] |
| 72 | (E, E)-α-famesene | C15H24 | [48] |
| 73 | humulene | C15H24 | [48] |
| 74 | methyl isoeugenol | C11H14O2 | [48] |
| 75 | geranyl valerate | C15H26O2 | [48] |
| 76 | β-bisabolene | C15H24 | [48] |
| 77 | β-cadinene | C15H24 | [48] |
| 78 | feniculine | C14H18O | [48] |
| 79 | cayopyllene oxide | C15H26O | [48] |
| 80 | α-cadinol | C15H26O | [48] |
| 81 | 2,6,10-trimethyltetradecane | C17H36 | [48] |
| 82 | 1-methoxy-4-(1-methyl-2-propenyl) benzene | C11H14O | [48] |
| 83 | 1-(3-methyl-2-butenoxy)-4-(1-propenyl) benzene | C14H18O | [48] |
| 84 | hexadecanoic acid | C16H32O2 | [48] |
| 85 | octadeca-9C,12C,15C trienoic acid | C18H30O2 | [48] |
| 86 | octadeca-9C,12C dienoic acid | C18H30O2 | [48] |
| 87 | octadeca-9C-enoic acid | C18H34O2 | [48] |
| 88 | octadecanoic acid | C18H36O | [48] |
| 89 | camphene | C10H16 | [49] |
| 90 | α-fenchene | C10H16 | [49] |
| 91 | eucalyptol | C10H8O | [49] |
| 92 | 1R-α-pinene | C10H16 | [49] |
| 93 | terpinolene | C10H16 | [49] |
| 94 | cis-linalool oxide | C10H18O2 | [49] |
| 95 | 2,4-dimethylanisole | C9H12O | [49] |
| 96 | fenchyl acetate | C12H20O2 | [49] |
| 97 | 2,5-dimethyl-3-viny-1,4-hexadiene | C10H6 | [49] |
| 98 | α-bisabolene | C15H24 | [49] |
| 99 | α-cedrene | C15H24 | [49] |
| 100 | terpinen-4-ol | C10H18 | [49] |
| 101 | dihydro anethol | C10H14O | [49] |
| 102 | iso-boenyl fomate | C11H8O2 | [49] |
| 103 | γ-muurolene | C15H24 | [49] |
| 104 | α-himachalene | C15H24 | [49] |
| 105 | geimacrene D | C15H24 | [49] |
| 106 | 2,3,4,5,6-pentmethylbenzaldehyde | C12H16O | [49] |
| 107 | benzalacetone | C10H10O | [49] |
| 108 | cubenol | C15H26O | [49] |
| 109 | 1,2,8,9-diepoxy-p-menthane | C10H16O2 | [49] |
| 110 | caryophyllene oxide | C15H24O | [49] |
| 111 | cis-α-santalol | C15H24O | [49] |
| 112 | anisic aldehyde | C8H8O2 | [49] |
| 113 | germacrene D-4-ol | C15H26O | [49] |
| 114 | α-guaiene | C15H24 | [49] |
| 115 | methyl p-anisate | C9H10O3 | [49] |
| 116 | iso-anethole | C10H12O | [49] |
| 117 | Z-α-trans-bergamotol | C15H24O | [49] |
| 118 | spathulenol | C15H24O | [49] |
| 119 | acetylanisole | C9H10O2 | [49] |
| 120 | cinnamyl acetate | C11H12O2 | [49] |
| 121 | p-acetonyl anisole | C10H12O2 | [49] |
| 122 | tau-cadinol | C15H26O2 | [49] |
| 123 | p-methoxypropiophencne | C10H12O2 | [49] |
| 124 | muurolol | C15H26O | [49] |
| 125 | 2-methoxy-α-methyl-benzeneenthanol | C10H14O2 | [49] |
| 126 | foeniculine | C14H8O | [49] |
| 127 | cinnamylalcohol | C9H10O | [49] |
| 128 | 4-methoxy-benzenepropanol | C10H14O2 | [49] |
| 129 | 7-isopropyl-1,4,4a-trimethyl-1,2,3,4,4a,9,10,10a-octaphydrophenanthrene | C20H30 | [49] |
| 130 | acetocumene | C12H8 | [49] |
| 131 | 3-methoxycinnamaldehyde | C10H10O2 | [49] |
| 132 | methylp-methoxycinnamate | C11H13O3 | [49] |
| 133 | ethylp-methoxycinnamate | C12H5O3 | [49] |
| 134 | 1,5,5-trimethyl-6-acetymethyl-cyclohexene | C12H20O | [49] |
| 135 | anisic alcohol | C8H10O2 | [49] |
| 136 | iso-eugenol | C10H12O2 | [49] |
| 137 | oleic acid | C18H34O2 | [49] |
| 138 | β-phellandrene | C10H16 | [50] |
| 139 | 1,4-cineole | C10H18O | [51] |
| 140 | terpinen-1-ol | C10H18O | [10] |
| 141 | γ-terpinene | C10H16 | [50] |
| 142 | α-terpinene | C10H16 | [10] |
| 143 | γ-terpineol | C10H18O | [50] |
| 144 | geraniol | C10H18O | [52] |
| 145 | cis-β-ocimene | C10H16 | [50] |
| 146 | trans-β-ocimene | C10H16 | [50] |
| 147 | borneol | C10H18O | [50] |
| 148 | δ-elemene | C15H24 | [50] |
| 149 | β-elemene | C15H24 | [50] |
| 150 | geranyl acetate | C12H20O2 | [50] |
| 151 | p-anisic acid methyl ester | C9H10O3 | [50] |
| 152 | δ-cadinene | C15H24 | [50] |
| 153 | α-cubebene | C15H24 | [53] |
| 154 | anisylacetone | C11H14O2 | [7] |
| 155 | α-muurolene | C15H24 | [7] |
| 156 | carryophyllene oxide | C15H24O | [53] |
| 157 | trans-chalcone | C15H12O | [54,55] |
| 158 | 4-methoxy-benzaldehyde | C8H8O2 | [54,55] |
4.2. Flavonoids
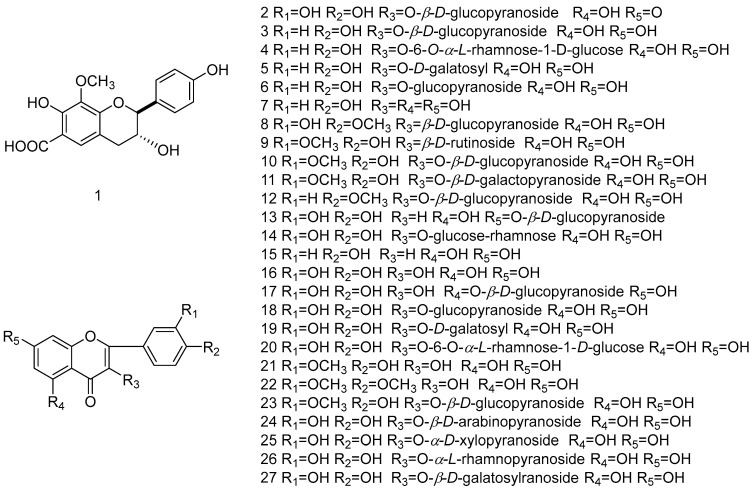
Flavonoids are widely distributed in plants, and most of them combine with sugars to form glycosides; this is also the case for those of I. verum. In addition to the common flavonoids—apigenin accidents (15) [56], star anise also contains high amounts of flavonoid glycosides [57,58,59] (2–14, 17–20 and 23–27) and flavanols [60,61] (16, 21 and 22). The basic parent nucleus of the flavonoids in star anise is a 2-phenyl-chromone. Quercetin, kaempferol and isorhamnetin are the common types of flavonoid aglycones in star anise, and together with D-glucose, D-xylose, L-rhamnose and other monosaccharides or rutinoses, they constitute the flavonoid glycosides found in the plants and its fruits. In 2015 [60], illiciumflavane acid (1), a new flavonoid acid, was discovered in the star anise fruits, and it was also found to have a significant inhibitory effect on A549 cancer cells with an IC50 value of 4.63 µM. The flavonoids isolated from Illicium verum are shown in Table 2, and chemical structure is shown in Figure 1.
Table 2.
The flavonoids isolated from Illicium verum.
| No. | Chemical Constituents | Parts | References |
|---|---|---|---|
| 1 | illiciumflavane acid | Fruit | [60,62] |
| 2 | isoquercitrin | Fruit | [57] |
| 3 | kaempferol-3-O-β-D-glucopyranoside | Fruit | [58] |
| 4 | kaempferol-3-O-rutinoside | Fruit | [58] |
| 5 | kaempferol-3-O-galactosyl | Fruit | [58] |
| 6 | kaempferol-3-O-glucopyranoside | Fruit | [58] |
| 7 | kaempferol | Fruit | [58] |
| 8 | tamarixetin 3-O-neohesperidoside | Fruit | [57] |
| 9 | isorhamnetin-3-O-ruti noside | Fruit | [57] |
| 10 | isorhamnetin-3-O-β-D-glucopyranoside | Fruit | [59] |
| 11 | isorhamnetin-3-O-β-D-galactopyranoside | Fruit | [59] |
| 12 | acacetin-3-O-β-D-glucopyranoside | Fruit | [58] |
| 13 | luteolin-7-O-β-D-glucopyranoside | Fruit | [59] |
| 14 | rutin | Fruit | [59] |
| 15 | apigenin | Fruit | [56] |
| 16 | quercetin | Fruit | [52] |
| 17 | quercetin-5-O-β-D-glucopyranoside | Fruit | [58] |
| 18 | quercetin-3-O-glucopyranoside | Fruit | [58] |
| 19 | quercetin-3-O-galactosyl | Fruit | [58] |
| 20 | quercetin-3-O-rutinoside | Fruit | [58] |
| 21 | 3′-methoxy quercetin | Fruit | [60] |
| 22 | 3′,4′-dimethoxy quercetin | Fruit | [60] |
| 23 | quercetin 3′-O-methyl-3-O-β-D-glucopyranoside | Fruit | [57] |
| 24 | quercetin-3-O-α-L-arabinopyranoside | Fruit | [57] |
| 25 | quercetin-3-O-D-xylopyranoside | Fruit | [57] |
| 26 | quercetin-3-O-α-L-rhamnopyranoside | Fruit | [59] |
| 27 | quercetin-3-O-β-D-galactopyranoside | Fruit | [59] |
Figure 1.
Chemical structures of compounds 1–27 from Illicium verum.
4.3. Phenylpropanoids
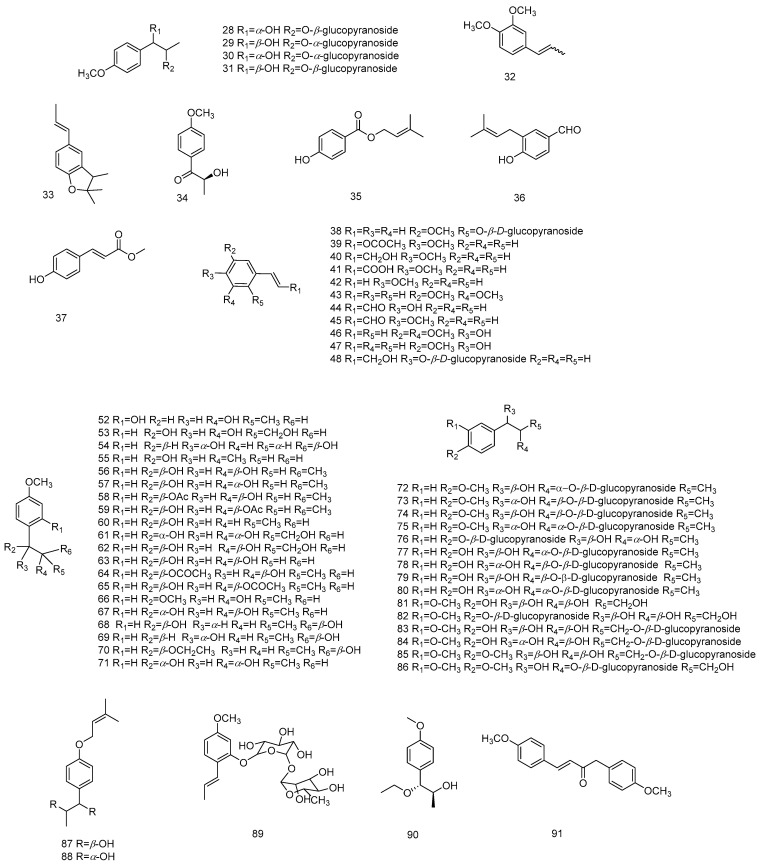
Phenylpropanoids are a group of natural components containing one or more C3–C6 units, i.e., compounds consisting of benzene rings which are linked to three straight-chain carbons. The simple phenylpropanoids include phenylpropylene, phenylpropionic acid and phenylpropionaldehyde. There are a large number of C3–C6-type structural compounds in the genus Anise, and anisole (33) [63,64] was the first to be obtained from SAO produced from anise seeds in 1937. Later, many prenylated C3–C6 compounds were isolated from the fruits, leaves and roots of star anise. Most of the new compounds found in star anise are phenylpropanoids (30, 31, 39, 48, 52, 74–86, 89, 90, 93, 94, 99–104, 107–109, 115–120 and 124–131) [61,65,66]. The phenylpropanoid compounds isolated from star anise have diverse structures. In addition to some simpler phenylpropanoids (32–37, 40–47, 52–67, 70 and 91–95) [54,56,58,60,63,64,65,67,68,69,70], they also include some new phenylpropanoid glycosides formed by combining with glucopyranoside structures (50, 51, 28–31, 38, 48, 49, 72–78, 79, 80 and 82–86) [60,61,71]. Most of these phenylpropanoid compounds come from the fruits of star anise. (E)-1,4-bis(4-methoxyphenyl) but-3-en-2-one (91) [72] is obtained from the bottom of the distillation kettle of star anise and obtained by liquid separation and purification. 1-(4′-methoxyphenyl)-(1R,2S and 1S,2R)-propanediol (50) [61] exhibited the highest stability in a dose-dependent manner in an in vivo experimental model of tumor-necrosis-factor-induced septic shock, and this effect was accompanied by a reduction in plasma alanine aminotransferase levels.
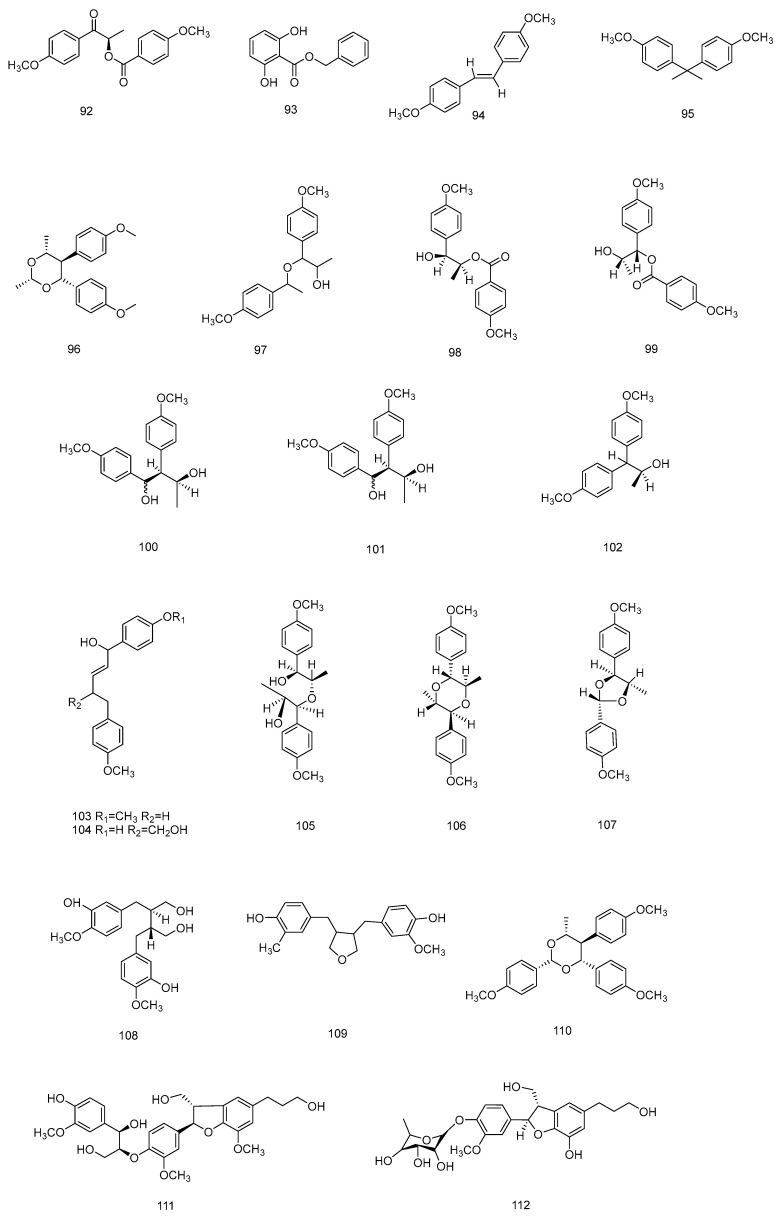
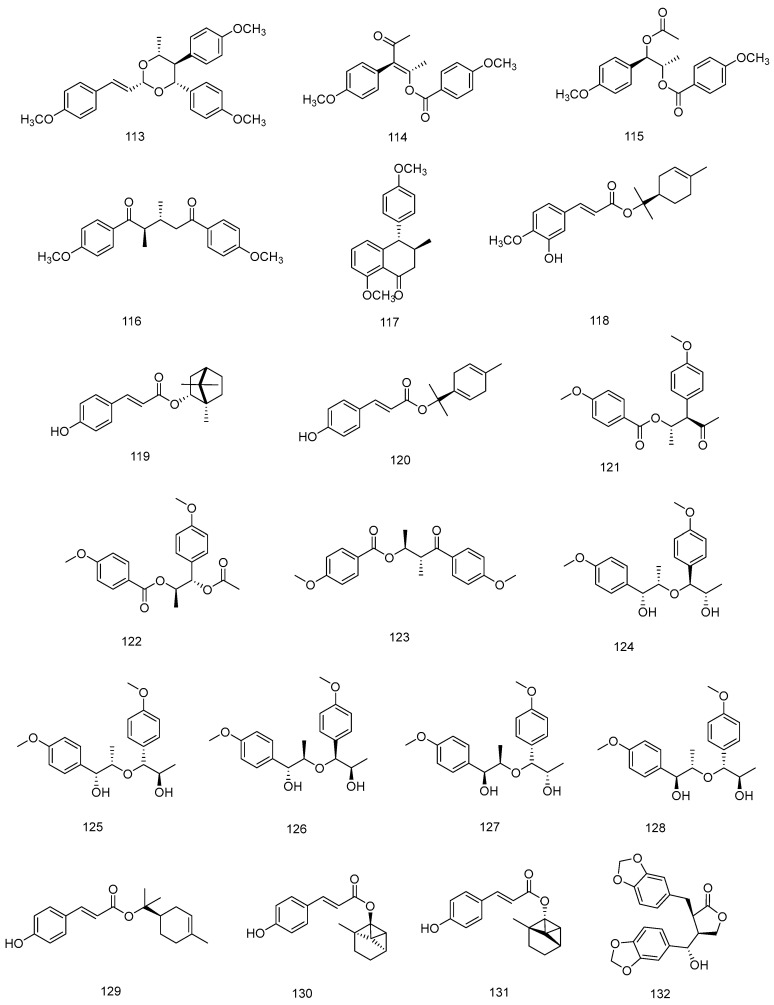
In 1998, Sy and Brown isolated novel lignans from anise leaves (98–102 and 105–107) [66], and later these compounds were also found in anise fruits [67,69]. The compounds verimol A (98) and harmandianone (92) showed concentration-dependent significant inhibition of lipopolysaccharide-induced tumour necrosis factor production in RAW264.7 cells [69]. Compounds 96, 97, 103, 104, 108, 111 and 112 are known lignan-like compounds that are found in anise [67,73]. In 2022 [33], six new lignans and phenylpropanoid derivatives, illiciumiones AF (114–118) were isolated from the anise fruits. Although relatively few lignans have been found in anise so far, most of them are novel compounds. The phenylpropanoids isolated from Illicium verum are shown in Table 3, and chemical structure is shown in Figure 2, Figure 3 and Figure 4.
Table 3.
The phenylpropanoids isolated from Illicium verum.
| No. | Chemical Constituents | Parts | References |
|---|---|---|---|
| 28 | 1-(4′-methoxyphenyl)-(1S,2R)-propan-1-ol 2-O-β-D-glucopyranoside | Fruit | [61] |
| 29 | 1-(4′-methoxyphenyl)-(1R,2S)-propan-1-ol 2-O-β-D-glucopyranoside | Fruit | [61] |
| 30 | 1-(4′-methoxyphenyl)-(1S,2S)-propan-1-ol 2-O-β-D-glucopyranoside | Fruit | [61] |
| 31 | 1-(4′-methoxyphenyl)-(1R,2R)-propan-1-ol 2-O-β-D-glucopyranoside | Fruit | [61] |
| 32 | (E)-1,2-dimethoxy-4-propenyl benzene | Root | [56] |
| 33 | anisoxide | Root | [63,64] |
| 34 | (2R)-2-hydroxy-1-(4-methoxyphenyl)-1-propane | Fruit | [67] |
| 35 | 3-methylbut-2-enyl-4-hydroxybenzoate | Fruit | [33] |
| 36 | 4-hydroxy-3-(3-methyl-2-buten-1-yl) benzaldehyde | Fruit | [33] |
| 37 | (E)-methyl p-coumarate | Fruit | [33] |
| 38 | 4-methoxy-2-(E)-propenylphenyl-β-D-glucopyranoside | Fruit | [60] |
| 39 | verimol I | Fruit | [66] |
| 40 | 3-(p-methoxyphenyl)-2-propen-1-ol | Fruit | [66] |
| 41 | 3-(4-methoxyphenyl)-2-(E)-propenoic-acid | Fruit | [67] |
| 42 | 1-methoxyphenyl)-4-propenylbenzene | Fruit | [67] |
| 43 | trans-(3,5-dimethoxyphenyl)-propene | Fruit | [67] |
| 44 | trans-p-coumary aldehyde | Fruit | [69] |
| 45 | E-4-methoxycinnamaldehyde | Fruit | [69] |
| 46 | methoxyeugenol | Root | [56] |
| 47 | eugenol | Root | [56] |
| 48 | (Z)-4-hydroxycinnamyl alcohol 4-O-β-D-glucopyranoside | Fruit | [71] |
| 49 | (E)-4-hydroxycinnamyl alcohol 4-O-β-D-glucopyranoside | Fruit | [71] |
| 50 | 1-(4′-methoxyphenyl)-(1R,2S and 1S,2R)-propanediol | Fruit | [61] |
| 51 | 1-(4′-methoxyphenyl)-(1R,2R and 1S,2S)-propanediol | Fruit | [61] |
| 52 | verimol J | Fruit | [66] |
| 53 | 1-(4′-methoxyphenyl)-1,2,3-trihydroxypropane | Fruit | [65] |
| 54 | (1R,2S)-1-(4′-methoxyphenyl)-propanediol | Fruit | [60] |
| 55 | 1′-(1-methoxyphenyl)-1′-propanol | Fruit | [70] |
| 56 | (1R,2R)-1-(4-methoxyphenyl) propane-1,2-diol | Fruit | [67] |
| 57 | (1S,2R)-1-(4-methoxyphenyl) propane-1,2-diol | Fruit | [67] |
| 58 | (±) -(1R,2R)-2-hydroxy-1-(4-methoxyphenyl) propyl acetate | Fruit | [67] |
| 59 | (±) -(1R,2R)-1-hydroxy-1-(4-methoxyphenyl) propyan-2-yl-acetate | Fruit | [67] |
| 60 | 1-(4-methoxyphenyl)-1-propanol | Fruit | [67] |
| 61 | (1S,2S)-1-(4-methoxyphenyl)-1,2,3-propanetriol | Fruit | [67] |
| 62 | (1R,2R)-1-(4-methoxyphenyl)-1,2,3-propanetriol | Fruit | [67] |
| 63 | 4-(4-methoxyphenyl)-2,2,5-trimethyl-1,3-dioxolane | Fruit | [67] |
| 64 | 2-acetoxy-1-(p-methoxyphenyl) propsn-1-ol | Fruit | [69] |
| 65 | 1-acetoxy-1-(p-methoxyphenyl) propan-2-ol | Fruit | [69] |
| 66 | 1-methoxy-1-(4-methoxyphenyl)-2-propanol | Fruit | [69] |
| 67 | 1-(4′-methoxyphenyl)-(1R,2S)-propane diol | Fruit | [69] |
| 68 | thero-anethole glycol | Fruit | [67] |
| 69 | orythro-anetholeglycol | Fruit | [67] |
| 70 | (1S,2R)-1-ethoxy-1-(4-methoxyphenyl) propan-2-ol | Fruit | [33] |
| 71 | (1R,2R)-1-(4-methoxyphenyl) propane-1,2-diol | Fruit | [74] |
| 72 | (1′R,2′S)-anethole glycol 2′-O-β-D-glucopyranoside | Fruit | [71] |
| 73 | (1′S,2′R)-anethole glycol 2′-O-β-D-glucopyranoside | Fruit | [71] |
| 74 | (1′R,2′R)-anethole glycol 2′-O-β-D-glucopyranoside | Fruit | [71] |
| 75 | (1′S,2′S)-anethole glycol 2′-O-β-D-glucopyranoside, | Fruit | [71] |
| 76 | an equivalent mixture of two stereoisomeric erythro-1′-(4-hy- droxyphenyl) propane-1′,2′-diol 4-O-β-D-glucopyranosides |
Fruit | [71] |
| 77 | (1′R,2′S)-1′-(4-hydroxyphenyl) propane-1′,2′-diol 2′-O-β-D-glucopyranoside | Fruit | [71] |
| 78 | (1′S,2′R)-1′-(4-hydroxyphenyl) propane-1′,2′-diol 2′-O-β-D-glucopyranoside | Fruit | [71] |
| 79 | (1′R,2′R)-1′-(4-hydroxyphenyl) propane-1′,2′-diol 2′-O-β-D-glucopyranoside | Fruit | [71] |
| 80 | (1′S,2′S)-1′-(4-hydroxyphenyl) propane-1′,2′-diol 2′-O-β-D-glucopyranoside | Fruit | [71] |
| 81 | (1′R,2′R)-guaiacyl glycerol | Fruit | [71] |
| 82 | (1′R,2′R)-guaiacyl glycerol 4-O-β-D-glucopyranoside | Fruit | [71] |
| 83 | (1′R,2′R)-guaiacyl glycerol 3′-O-β-D-glucopyranoside | Fruit | [71] |
| 84 | (1′S,2′R)-guaiacyl glycerol 3′-O-β-D-glucopyranoside | Fruit | [71] |
| 85 | (1′R,2′R)-4-O-methylguaiacyl glycerol 3′-O-β-D-glucopyranoside | Fruit | [71] |
| 86 | 4-O-methylguaiacyl glycerol 2′-O-β-D-glucopyranoside | Fruit | [71] |
| 87 | (7S,8S)-7-(4-((3-methylbut-2-en-1-yl) oxy) phenyl) propane-7,8-diol | Fruit | [74] |
| 88 | (7R,8R)-7-(4-((3-methylbut-2-en-1-yl) oxy) phenyl) propane-7,8-diol | Fruit | [74] |
| 89 | (E)-2-(prop-1-enyl)-5-methoxyphenol-1-O-α-L-rhamnopyranosyl-(1→6)-O-β-D-glucopyranoside | Fruit | [65,66] |
| 90 | (1S,2R)-1-ethoxy-1-(4-methoxyphenyl) propan-2-ol | Fruit | [74] |
| 91 | (E)-1,4-bis (4-methoxyphenyl) but-3-en-2-one | Fruit | [72] |
| 92 | harmandianone | Fruit | [69] |
| 93 | verimol K | Fruit | [66] |
| 94 | (E)-1,2-bis(4-methoxyphenyl) ethene | Fruit | [69] |
| 95 | 1-biphenyl-2,2′-dimethoxy-5,5′-di-2-propenyl | Fruit | [67] |
| 96 | anemonenorin B | Fruit | [33] |
| 97 | verimol O | Fruit | [67] |
| 98 | verimol A | Leaf | [66] |
| 99 | verimol B | Leaf | [66] |
| 100 | verimol D | Leaf | [66] |
| 101 | verimol E | Leaf | [66] |
| 102 | verimol F | Leaf | [66] |
| 103 | verimol L | Fruit | [67] |
| 104 | verimol M | Fruit | [67] |
| 105 | verimol G | Leaf | [66] |
| 106 | verimol H | Leaf | [66] |
| 107 | verimol C | Leaf | [66] |
| 108 | secoisolariciresinol | Fruit | [67] |
| 109 | 4-((4-(4-hydroxy-3-methoxybenzyl) tetrahydrofuran-3-yl) methyl)-2-methylphenol | Leaf | [66] |
| 110 | anemonenorin A | Fruit | [33] |
| 111 | xanthiumnolic C | Fruit | [73] |
| 112 | icariside E4 | Fruit | [73] |
| 113 | illiciumiones A | Fruit | [33] |
| 114 | illiciumiones B | Fruit | [33] |
| 115 | illiciumiones C | Fruit | [33] |
| 116 | illiciumiones D | Fruit | [33] |
| 117 | illiciumiones E | Fruit | [33] |
| 118 | illiciumiones F | Fruit | [33] |
| 119 | (−)-bornyl p-coumarate | Fruit | [33] |
| 120 | (R)-2-(4-methylcyclohex-3-en-1-yl) propan-2-yl (E)-3-(4-hydroxyphenyl) acrylate | Fruit | [33] |
| 121 | (2α,3β)-3-(4-methoxyphenyl)-4-oxopentan-2-yl 4-methoxybenzoate | Fruit | [74] |
| 122 | (7α,8α)-7-acetoxy-7-(4-methoxyphenyl) propan-8-yl-4-methoxybenzoate | Fruit | [74] |
| 123 | (8α,9β)-1-(4-methoxyphenyl)-8,9-methyl-9-methoxybenzoate | Fruit | [74] |
| 124 | verimol O | Fruit | [74] |
| 125 | verimol P | Fruit | [74] |
| 126 | verimol Q | Fruit | [74] |
| 127 | verimol R | Fruit | [74] |
| 128 | verimol S | Fruit | [74] |
| 129 | (R)-2-(4-methylcyclohex-3-en-1-yl) propan-2-yl (E)-3-(4-hydroxyphenyl) acrylate | Fruit | [74] |
| 130 | (-)-bornyl p-coumarate | Fruit | [74] |
| 131 | (+)-bornyl p-coumarate | Fruit | [74] |
| 132 | (7′S)-parabenzlactone | Fruit | [74] |
Figure 2.
Chemical structures of compounds 28–91 from Illicium verum.
Figure 3.
Chemical structures of compounds 92–112 from Illicium verum.
Figure 4.
Chemical structures of compounds 113–132 from Illicium verum.
4.4. Organic Acids and Phenols
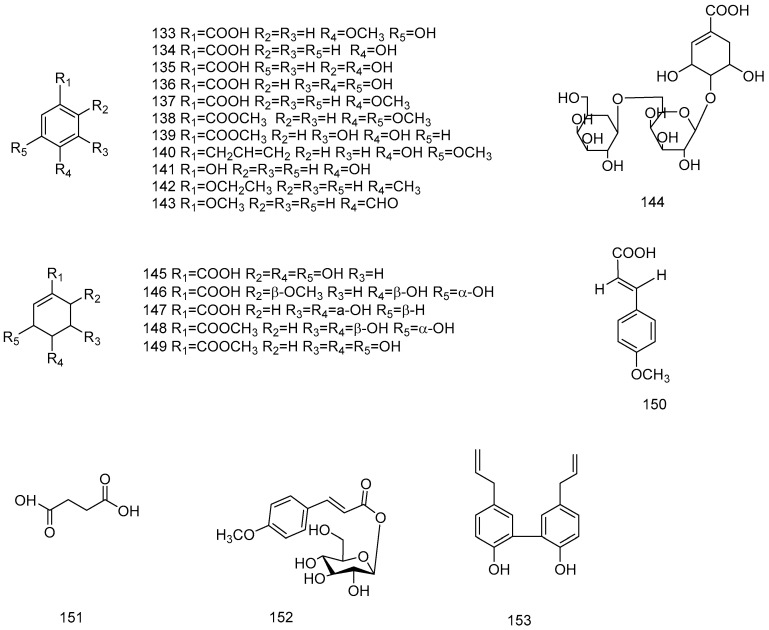
Organic acids and phenols in star anise include aliphatic organic acids (144–149, 151) [58,60,69,73] and aromatic organic acids (133–143, 150, 152, 153) [60,70,73]. All 20 organic acids are derived from the fruits of star anise. The composition of organic acids in star anise fruits is complex, and shikimic acid is the most important component. The organic acids and phenols isolated from Illicium verum are shown in Table 4, and chemical structure is shown in Figure 5.
Table 4.
The organic acids and phenols isolated from Illicium verum.
| No. | Chemical Constituents | Parts | References |
|---|---|---|---|
| 133 | 3-hydroxy-4-methoxy benzoic acid | Fruit | [60] |
| 134 | p-hydroxy benzoic acid | Fruit | [60] |
| 135 | 2,4-dihydroxy benzoic acid | Fruit | [60] |
| 136 | gallic acid | Fruit | [60] |
| 137 | p-methoxy benzoic acid | Fruit | [60] |
| 138 | 3,4-dimethoxy-benzoic acid | Fruit | [58] |
| 139 | qrotocatecheuic acid | Fruit | [58] |
| 140 | engemol | Fruit | [58] |
| 141 | 1,4-benzenediol | Fruit | [70] |
| 142 | 1-ethoxy-4-methylbenzene | Fruit | [70] |
| 143 | 1-methoxy benzaldehyde | Fruit | [70] |
| 144 | shikimic acid-3-O-β-D-mannopyranose (1″-6′)-β-D-mannopyranose | Fruit | [58] |
| 145 | iso-shikimic acid | Fruit | [58] |
| 146 | (3R,4R,6S)-3,4,6-trihydroxycyclohex-1-enecarboxylic acid | Fruit | [60] |
| 147 | shikimic acid | Fruit | [67] |
| 148 | shikimic acid methyl ester | Fruit | [58] |
| 149 | methyl shikimate | Fruit | [73] |
| 150 | 4-methoxy cinnamic acid | Fruit | [60] |
| 151 | succinic acid | Fruit | [69,75,76] |
| 152 | 9-O-β-D-glucopyranosyl-4-methoxy-cinnamic acid | Fruit | [73] |
| 153 | Magnolol | Fruit | [58] |
Figure 5.
Chemical structures of compounds 133–153 from Illicium verum.
Shikimic Acid
Shikimic acid is an aliphatic organic acid that was first isolated in 1885 from parts of the Japanese tree shikimino-ki (I. anisatum) [75,76] and later found to be present in the fruits of star anise. The extraction of shikimic acid from star anise was initiated following a global outbreak of avian influenza. Oseltamivir phosphate is the key component of Tamiflu and the only specific agent that has been recommended by the International Health Organization for the synthesis of antibodies against influenza A (H5N1). Shikimic acid is commonly used as the starting material for the industrial synthesis of oseltamivirphosphate [77]. In addition, shikimic acid has analgesic, antioxidant, anticoagulant, anti-inflammatory and antithrombotic activity as well as neuroprotective properties, and it is a precursor of antibacterial and anticancer agents [78].
Tamiflu played an important role in the fight against avian influenza attacks in 2005, and in October of that year, the World Health Organization and other international agencies recommended stockpiling the drug in preparation for a possible pandemic. At that time, the vast majority (90%) of the star anise produced in China was used by Roche to produce Tamiflu. Roche published the active ingredient of Tamiflu to be shikimic acid that was extracted from star anise and revealed a road map for the synthesis of Tamiflu, see Figure 6 below. So far, the presence of shikimic acid has been reported from I. dunrlianum Tutch, I. henryi, I. temstroemioides, I. lanceolatum, I. simonsi and other anise plants of the same genus [79,80,81,82]. In 2009, LC–UV and LC–MS methods were developed for the quantitative and qualitative analysis of shikimic acid. Nine different species of Illicium and 173 various plant extracts were tested for the presence of shikimic acid. The Illicium Linn plant had the highest content of shikimic acid, with some plants containing nearly 25 wt% shikimic acid in their dried fruits. Although shikimic acid exists in a large number of illicium plants and microorganisms, star anise is still the main raw material for its production worldwide due to its easy availability, abundant production and economic benefits [83]. Currently, the worldwide source of shikimic acid is star anise plants grown in China.
Figure 6.
Roche’s published method for the synthesis of Tamiflu using shikimic acid [84].
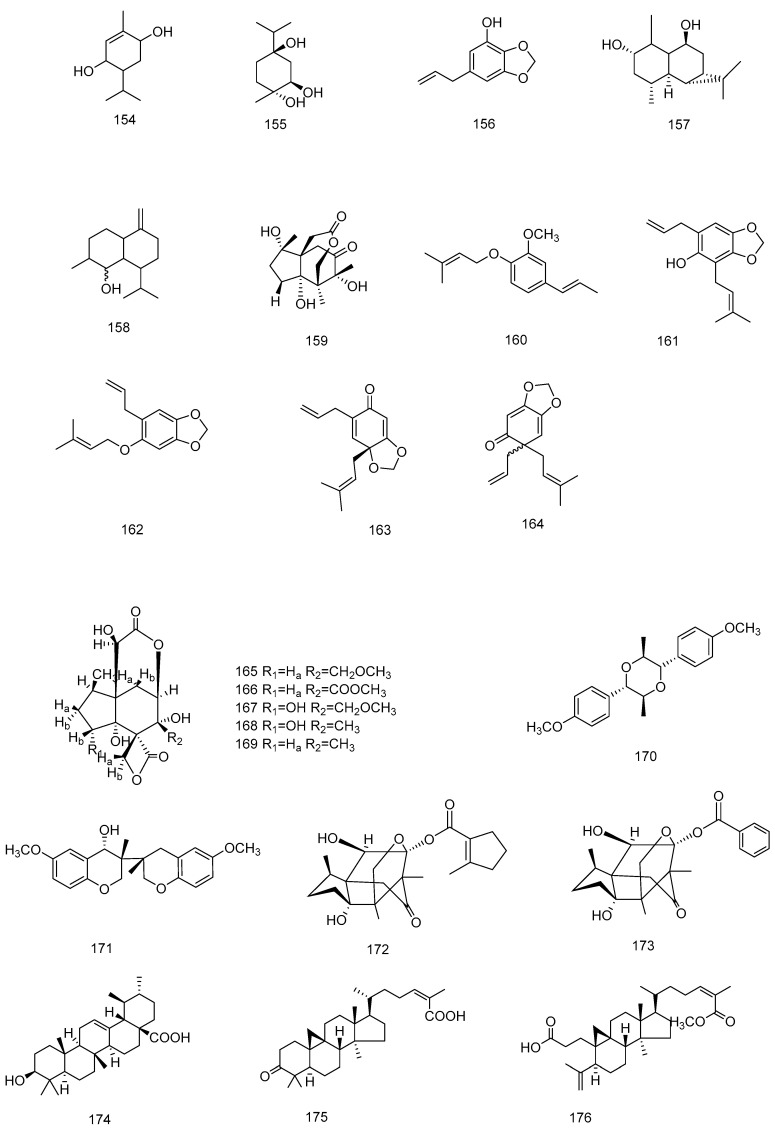
4.5. Terpenoids
A total of 23 terpenoids, including monoterpenes (154–156) [13,70,85], sesquiterpenes (157–169) [41,57,67,86], diterpenes (170–173) [67] and triterpenes (174–176), are currently [13,56,70], obtained from star anise. These include 2α,9β-dihydroxy-14 (10→1)-olivane-1(10)-ene) (157) [86] from the bottom of the anise distillation container, (E)-1-[(3-methylbut-2-enyl) oxy]-2-methoxy-4-(prop-1-enyl) benzene (160) [13] from the roots of anise and 11-O-denbenzoyl-11α-O-2-methylcyclopent-1-enecarboxyl tashironin A (172) [13], which are the new terpenoid compounds found. Among the family of Illiciaceae, the fruits of Illicium anisatum L. (shikimi in Japanese) are well known as neurotoxic and contain the convulsants anisatin and neoanisatin. However, these ingredients were not found in star anise until the 1990s. Seco-prezizaane-type sesquiterpenes have neurotoxicity [87] as well as anti-coxsackievirus B3 [88], anti-hepatitis B virus, neurotrophic [89] and other activities. These types of compounds are extremely rare in other plants but are characteristic of the star anise genus [90].
In 1996 [41], three new neurotoxic components, veranisatins A (165), B (166) and C (167), were isolated and identified from star anise, and preliminary pharmacological studies were carried out. The veranisatins led to convulsions and had lethal toxicities in mice at a dose of 3 mg/kg (p.o.), and at lower doses they caused hypothermia. Veranisatin A was tested for other pharmacological activities such as locomotor activity, as well as its effect as an analgesic. Veranisatin A decreased the locomotion enhanced by methamphetamine at oral doses of 0.1 mg/kg, and it demonstrated an analgesic effect on acetic acid-induced writhing and tail pressure pain in mice. 3,4-seco-(24Z)-cycloart-4(28),24-diene-3,26-dioic acid, 26-methyl ester (176) was found in both the roots and leaves of anise, and it as well as (-)-illicinole-A possessed moderate anti-HIV activities, with EC50 values of 16.0 and 5.1 μM and SI values of 18.2 and 15.6, respectively. When extracting and separating the chemical components of SAO after its hydrodistillation, the products were placed into a distillation kettle for single separations. Generally, the bottom liquid from the kettle was discarded as waste, but some groups analyzed the remaining residues in order to obtain a new olivetane-type sesquiterpene, 2α,9β-dihydroxy-14 (10→1) -olivane-1(10)-ene (1) (157), which was presumed to be from star anise. The terpenoids isolated from Illicium verum are shown in Table 5, and chemical structure is shown in Figure 7.
Table 5.
The terpenoids isolated from Illicium verum.
| No. | Chemical Constituents | Parts | References |
|---|---|---|---|
| 154 | 3,6-two hydroxy-1-menthene | End of distillation liquid | [85,86] |
| 155 | 1R,3R,4R-trihydroxy-menthene | Fruit | [70] |
| 156 | 3-hydroxy-4,5-methylenedioxyallyl-benzene | Root | [13] |
| 157 | 2α,9β-dihydroxy-14 (10→1)-olivane-1(10)-ene | End of distillation liquid | [86] |
| 158 | naphthalenol, decahydro-2-methyl-5-methylene-8(1-methylethyl) | Fruit | [67] |
| 159 | 1α-hydroxy-3-deoxypseudoanisatin | Fruit | [57] |
| 160 | (E)-1-[(3-methylbut-2-enyl)oxy]-2-methoxy-4-(prop-1-enyl) benzene | Root | [13] |
| 161 | 4-allyl-2-(3-methylbut-2-enyl)-1,6-methylenedioxybenzene-3-ol | Root | [13] |
| 162 | illicinole | Root | [13] |
| 163 | (-)-illicinone-A | Root | [13] |
| 164 | 4-allyl-4-(3-methylbut-2-enyl)-1,2-methylenedioxycyclohexa-2,6-dien-5-one | Root | [13] |
| 165 | veranisatin A | Fruit | [41] |
| 166 | veranisatin B | Fruit | [41] |
| 167 | veranisatin C | Fruit | [41] |
| 168 | anisatin | Fruit | [41] |
| 169 | neoanisatin | Fruit | [41] |
| 170 | vermiol N | Fruit | [67] |
| 171 | (3R,4R,3′R,4′R)-6,6′-dimethoxy-3,4,3′,4′-tetrahydro-2H,2′H- [3,3′] bichromenyl-4,4′-diol | Fruit | [67] |
| 172 | 11-O-denbenzoyl-11α-O-2-methylcyclopent-1-enecarboxyltashironin | Root | [13] |
| 173 | tashironin | Root | [13] |
| 174 | ursolic acid | Root | [56,91] |
| 175 | schizandronic acid | Leaf | [91] |
| 176 | 3,4-seco-(24Z)-cycloart-4(28),24-diene-3,26-dioic | Root, Leaf | [13] |
Figure 7.
Chemical structures of compounds 154–176 from Illicium verum.
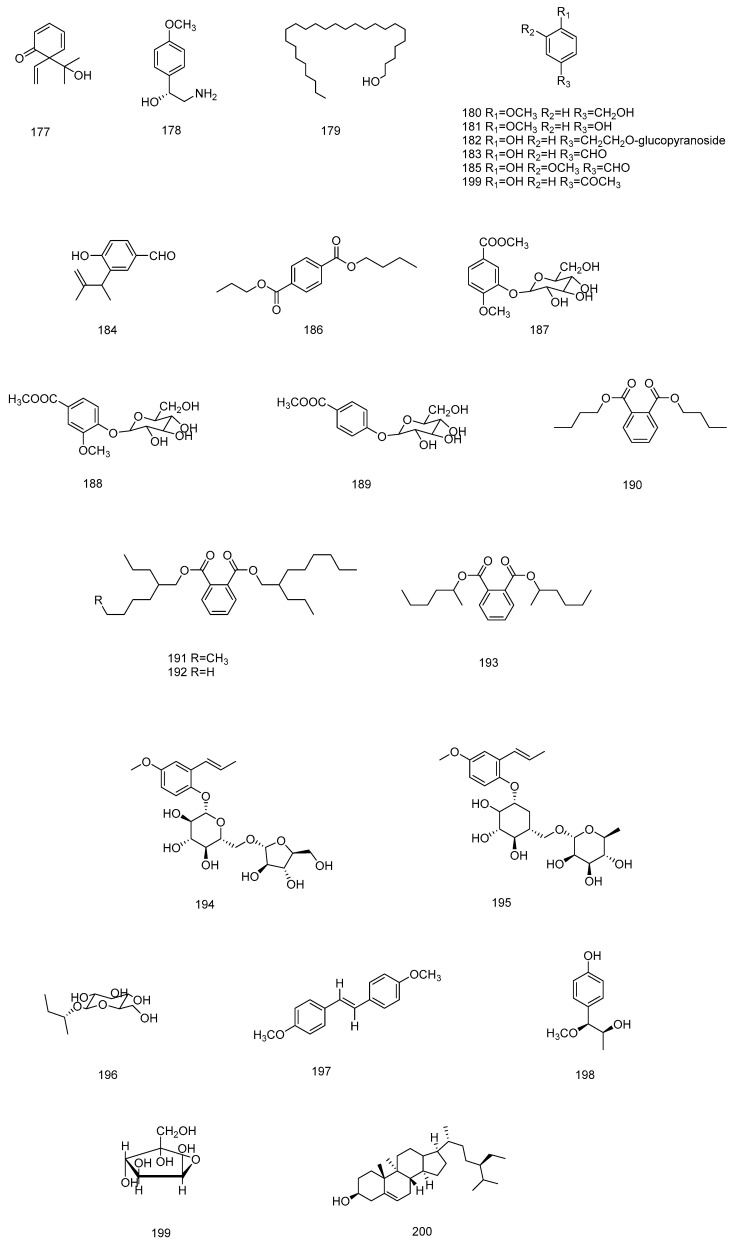
4.6. Other Constituents
Some alcohols (177–180), phenols (181, 182), aldehydes (183–185), esters (186–193), phenolic glycosides (194, 195) and other compounds (196–201) with irregular chemical structures have also been isolated from star anise. The other constituents isolated from Illicium verum are shown in Table 6, and chemical structure is shown in Figure 8.
Table 6.
The other constituents isolated from Illicium verum.
| No. | Chemical Constituents | Parts | References |
|---|---|---|---|
| 177 | (1′R,2′R)-anethole glycol | Fruit | [67] |
| 178 | (S)-2-amino-1-(4-methoxyphenyl) ethanol | Fruit | [68] |
| 179 | 1-hexaco sanol | Root | [56] |
| 180 | 4-methoxybenzenethanol | Fruit | [68] |
| 181 | 4-methoxyphenol | Fruit | [68] |
| 182 | 4-hydroxy-phenethylalcohol-O-β-D-glucopyranoside | Fruit | [72] |
| 183 | 4-hydroxybenzaldehyde | Fruit | [68] |
| 184 | 4-hydroxy-3-(3-methylbut-3-en-2-yl) benzaldehyde | Root | [56] |
| 185 | vanillin | Fruit | [67] |
| 186 | dibutyl terephthalate | Fruit | [58] |
| 187 | 4-methoxy-3-O-β-D-glucopyranosyloxy benzoic acid methyl ester | Fruit | [59] |
| 188 | 3-methoxyl-4-O-β-D-glucopyranosyloxy benzoic acid methyl ester | Fruit | [59] |
| 189 | 4-O-β-D-glucopyranosyloxy benzoic acid methyl ester | Fruit | [59] |
| 190 | dibutyl phthalate | Fruit | [74] |
| 191 | bis(2-propyloctyl) phthalate | Fruit | [70] |
| 192 | bis(2-propylheptyl) phthalate | Fruit | [70] |
| 193 | bis(1-methylpentyl) ester | Fruit | [70] |
| 194 | (E)-4-methoxy-2-(1′-propen-1′-yl)-phenol-1-O-α-L-arabinofuranosyl-(1′′′→6′′)-β-D-glucopyranoside | Fruit | [73] |
| 195 | (E)-4-methoxy-2-(1′-propen-1′-yl)-phenol-1-O-α-L-rhamnopyranosyl-(1′′′→6′′)-β-D-glucopyranoside | Fruit | [73] |
| 196 | (R)-sec-butyl-β-D- glucopyranoside | Fruit | [65] |
| 197 | (E)-1,2-bis(4-methoxyphenyl) ethene | Fruit | [60] |
| 198 | thero-4-hydroxyphenylpropyan-7,8-diol 7-O-methyl ether | Fruit | [68] |
| 199 | P-hydroxyacetophenone | Fruit | [68] |
| 200 | fructose | Fruit | [58] |
| 201 | β-sitosterol | Root, Fruit | [56,70] |
Figure 8.
Chemical structures of compounds 177–200 from Illicium verum.
5. Modern and Traditional Uses of Star Anise
In the Song Dynasty (AD960–AD1279), star anise was widely produced in the region around Jiang in Guangxi, but at that time, people only knew that star anise was used as a condiment, not for medicinal purposes. Zhou Qufei wrote in his “Ling Nan Dai Da” (AD1178) that star anise should only be used as a soup blend and not as a medicine. By the Ming Dynasty (AD1368–AD1644), people not only knew that they could use star anise as a spice for seasoning but also that it could be used as a medicine. The use of star anise for a medicinal puposes was first published in the Collected Essentials of Species of Materia. (AD1505): “It is the size of a copper coin with 8 prongs and a seed inside each prong, and is used a lot in Chinese medicine”. The Compendium of Materia Medica (AD1578) recorded the morphological characteristics of star anise as split into eight petals, one petal and one nucleus, yellow-brown and sweet. This shows that star anise has been used in China for at least four hundred years. Modern monographs such as “Genuine and well-reputed medicinal materials in Guangxi”, “A list of plants in Nanning Arboretum, Guangxi” and “Modern Chinese Materia Medica” have all recorded that star anise has pharmacological effects such as elevating white blood cells while offering anti-bronchospasm and antibacterial properties. The compositions of 14 ancient classical formulas of star anise and 44 modern proprietary Chinese medicines using these plants are listed in Table 7 and Table 8.
Table 7.
The prescriptions of modern Chinese patent medicines (https://www.yaozh.com/, accessed on 15 October 2021).
| Prescription Name | Main Composition | Clinical Uses | Prescription Sources |
|---|---|---|---|
| Jiebei Zhike Qutan Pian | Platycodon grandiflorum. (Jacq.) A. DC. (225 g)/Polygala tenuifolia Willd (44.12 g)/Fritillaria cirrhosa D. Don (150 g)/eucalyptus oil (2 g)/Star anise oil (2 g)/Glycyrrhiza uralensis Fisch. (25 g)/ammonium chloride (100 g)/crospovidone (10 g)/sodium starch glycolate (12 g) | Lung heat cleaning, cough-reducing and phlegm-reducing | Guo Jia Zhong Cheng Yao Biao Zhun Hui Bian (2002) |
| Beishen Pingchuan Jiaonang | Herba Ardisiae Japonicae (550 g)/Ephedra sinica Stapf (500 g)/Illicium verum Hook. F (180 g)/Fritillaria thunbergii Miq. (400 g)/Pseudostellaria heterophylla (Miq.) Pax ex Paxet Hoffm. (300 g)/Siraitia grosuenorii., (Swingle) C. Jeffreyex A. M. Lu et Z. Y. Zhang (265 g)/Gekko gecko Linnaeus (100 g)/Glycyrrhiza uralensis Fisch. (200 g)/starch (17 g) | Warming lungs to eliminate cold, anti-cough and anti-asthmatic | Guo Jia Zhong Cheng Yao Biao Zhun Hui Bian (2002) |
| Jiuji Xingjun Jiaonang |
borneol (21.74 g)/Scutellaria baicalensis Georgi (21.41 g)/Dryopteris crassirhizoma Nakai (21.41 g)/Mentha haplocalyx Briq (27.34 g)/Asarum heterotropoides Fr. Schmidt (10.54 g)/Angelica dahurica (Fisch.ex Hoffm) Benth. et Hook (10.54 g)/Gleditsia sinensis Lam. (3.62 g)/Atractylodes lancea (Thunb.) DC. (10.54 g)/Nardostachys jatamansi DC (21.41 g)/theophylline (20.42 g)/realgar (6.92 g)/cinnabaris (8.23 g)/Cinnamomum cassia Presl (28.32 g)/Alpinia officinarum Hance (10.54 g)/Illicium verum Hook. F. (21.41 g)/Eugenia caryophyllata Thunb. (10.54 g)/Aucklandia lappa Decne. (21.41 g)/Citrus reticulata Blanco (21.41 g)/Glycyrrhiza uralensis Fisch. (32.38 g) | Eliminating stagnated food, relieving pain and diarrhea | Guo Jia Zhong Cheng Yao Biao Zhun Hui Bian (2002) |
| Jiuji Xingjun San | Cinnamomum camphora (L.) Presl (66 g)/Scutellaria baicalensis Georgi (64.5 g)/Dryopteris crassirhizoma Nakai (64.5 g)/Mentha haplocalyx Briq (82.5 g)/Asarum heterotropoides Fr. Schmidt (32.3 g)/Angelica dahurica (Fisch.ex Hoffm.) Benth. et Hook (32.3 g)/Gleditsia sinensis Lam. (11 g)/Atractylodes lancea. (Thunb.) DC. (32.3 g)/Nardostachys jatamansi DC. (64.5 g)/tea (62 g)/realgar (21 g)/cinnabaris (25 g)/Cinnamomum cassia Presl (86 g)/Alpinia officinarum Hance. (32.3 g)/Illicium verum Hook. F. (64.5 g)/Eugenia caryophyllata Thunb. (32.3 g)/Aucklandia lappa Decne. (64.5 g)/Citrus reticulata Blanco (64.5 g)/Glycyrrhiza uralensis Fisch. (98 g) | Eliminating stagnated food, relieving pain and diarrhea | Guo Jia Zhong Cheng Yao Biao Zhun Hui Bian (2002) |
| Xiao’er Kesouning Tangjiang | Tolu Bals (6 g)/Extractum Scillae. (2.4 g)/star anise oil (0.04 mL)/Mentha haplocalyx Briq. (0.15 g)/benzoic acid (1 g)/sucrose (510 g)/talc (6 g) | Expelling phlegm and arrest coughing | Guo Jia Zhong Cheng Yao Biao Zhun Hui Bian (2002) |
| Fufang Yatongning Chaji | Pinus massoniana Lamb. (120 g)/Zanthoxylum schinifolium Sieb. et Zucc. (90 g)/Cinnamomum camphora (L.) Presl. (22 g)/Eugenia caryophyllata Thunb. (15 g)/Mentha haplocalyx Briq (13 g)/Schizonepeta tenuifolia Briq. (10 g)/Piper longum L. (10 g)/Artemisia scoparia Waldst.et Kit. (10 g)/Glycyrrhiza uralensis Fisch. (10 g)/Illicium verum Hook. F. (10 g) | Detumescence and pain relief | Guo Jia Zhong Cheng Yao Biao Zhun Hui Bian (2002) |
| Shuwei Yaojiu | Osmunda japonica Thunb. (100 g)/Amomum tsao-ko Crevost et Lemaire. (10 g)/Cinnamomum cassia Presl (10 g)/Glycyrrhiza uralensis Fisch./Illicium verum Hook. F. (20 g) | Warming spleen and stomach for dispelling cold, regulating vital energy and alleviating pain | Guo Jia Zhong Cheng Yao Biao Zhun Hui Bian (2002) |
| Guixiang Qushu San | Mentha haplocalyx Briq (60 g)/Cinnamomum camphora (L.) Presl (100 g)/Eugenia caryophyllata Thunb. (7.5 g)/Cinnamomum cassia Presl (7.5 g)/Illicium verum Hook. F.(7 g)/Glycyrrhiza uralensis Fisch. (150 g)/Carmine (4.8 g)/Starch(520 g)/Dextrin(46 g) | Fragrant herbs repelling foulness, dispelling cold and relieving heat, relieving heat and providing energy | Guo Jia Zhong Cheng Yao Biao Zhun Hui Bian (2002) |
| LonghuRendan | Mentha haplocalyx Briq (40 g)/Cinnamomum camphora (L.) Presl (30 g)/Eugenia caryophyllata Thunb. (25 g)/Amomum villosum Lour. (25 g)/Illicium verum Hook. F. (15 g)/Cinnamomum cassia Presl (40 g), Piper nigrum L. (15 g)/Aucklandia lappa Decne. (15 g)/Zingiber officinale Rosc. (25 g)/Acacia catechu. (L. f.) Willd. (200 g)/Glycyrrhiza uralensis Fisch. (364.1 g)/glutinous rice flour (180 g)/sodium benzoate (5 g) | Inducing resuscitation, eliminating turbid and summer heat, warming middle energizer to arrest vomiting | Guo Jia Zhong Cheng Yao Biao Zhun Hui Bian (2002) |
| Hantongle Refudai | Aconitum carmichaelii Debx. (120 g)/Aconitum kusnezoffii Reichb. (120 g)/Ephedra sinica Stapf. (120 g)/Angelica sinensis (Oliv.) Diels (247 g)/Euodia rutaecarpa (Juss.) Benth. (498 g)/Atractylodes lancea (Thunb.) DC. (247 g)/Illicium verum Hook. F. (80 g)/Kaempferia galanga Linn. (100 g)/Mentha haplocalyx Briq. (52 g)/Camphor (34 g)/Cinnamomum camphora (L.) Presl. (34 g)/methyl salicylate (51 g)/iron dust (31,106 g)/aluminum (552 g)/vermiculite (8818 g)/sodium chloride (1066 g)/cuprous chloride (10,066 g) | Relieving rheumatism and cold, relaxing the muscles and stimulating blood circulation | Guo Jia Zhong Cheng Yao Biao Zhun Hui Bian (2002) |
| Shexiang Zhuanggu Babugao | Moschus berezovskii Flerov. (0.63 g)/leopard bone (0.6 g)/Aconitum carmichaelii Debx. (158 g)/Aconitum kusnezoffii Reichb. (158 g)/Ephedra sinica Stapf (158 g)/Angelica sinensis (Oliv.) Diels (325 g)/Kaempferia galanga L. (132 g)/Angelica dahurica (Fisch.ex Hoffm.) Benth. et Hook. (200 g)/Atractylodes lancea (Thunb.) DC (325 g)/Illicium verum Hook. F. (105 g)/Zingiber officinale Rosc. (446 g)/Cinnamomum camphora (L.) Presl (80 g)/Mentha haplocalyx Briq (105 g)/castor oil (50 g)/chondroitin sulfate sodium (3 g)/methyl salicylate (67 g)/gelatin (80 g)/diphenhydramine hydrochloride (16 g)/sodium polyacrylate (273 g)/vinyl alcohol (50 g)/carboxymethylcellulose sodium (45 g)/gelatin (91 g)/kaolin (364 g)/glycerol (1365 g)/sorbitol (500 g) | Relieving rheumatism and cold, promoting blood circulation to relieve pain | Guo Jia Zhong Cheng Yao Biao Zhun Hui Bian (2002) |
| SijiYou | Pinus tabuliformis Carr. (10 g)/peppermint oil (430 g)/Cinnamomum cassia Presl (2.5 g)/star anise oil (17.5 g)/methyl salicylate (490 g)/camphor (15 g)/methyl salicylate (490 g) | Qufeng and exicting | Wei Sheng Bu Zhun Zhong Yao Cheng Fang Zhi Ji (1994) |
| Qixiang Zhitong Wan | Vladimiria souliei (Franch.) Ling (160 g)/Aucklandia lappa Decne. (20 g)/Aquilaria sinensis (Lour.) Gilg (20 g)/Dalbergia odorifera T. Chen (80 g)/Foeniculum vulgare Mill. (80 g)/Illicium verum Hook. F. (80 g)/Eugenia caryophyllata Thunb. (80 g)/Boswellia carterii Birdw. (80 g)/Pogostemon cablin (Blanco) Benth. (80 g) | Warming spleen and stomach for dispelling cold; promoting Qi circulation to stop pain | Wei Sheng Bu Zhun Zhong Yao Cheng Fang Zhi Ji (1990) |
| Fengshang Zhitong Gao | Cinnamomum cassia Presl (45 g)/Illicium verum Hook. F. (24 g)/Eugenia caryophyllata Thunb. (12 g)/Xanthium sibiricum Patr. (30 g)/Cinnamomum japonicum Sieb. (C.chekiangense Nakai) (17 g)/Ricinus communis L. (90 g)/Nardostachys jatamansi DC. (17 g)/Arisaema erubescens (Wall.) Schott (90 g)/Angelica dahurica (Fisch.ex Hoffm.) Benth. Et Hook. (30 g)/Piper kadsura (Choisy)Ohwi. (90 g)/Rheum palmatum L. (30 g)/Pinellia ternate (Thunb.) Breit. (90 g)/Notopterygium incisum Ting ex H. T. Chang (60 g)/Aconitum carmichaelii Debx. (90 g)/Angelica pubescens Maxim.f. biserrata Shan et Yuan (60 g)/Aconitum kusnezoffii Reichb. (90 g)/Asarum heterotropoides Fr. Schmidt. (30 g)/Siegesbeckia orientalis L. (90 g)/Cinnamomum cassia Pres (90 g)/Angelica sinensis (Oliv.) Diels (30 g)/Ephedra sinica Stapf. (90 g) | Stimulating the circulation of blood and causing the muscles and joints to relax, promoting the circulation of blood to relieve pain | Wei Sheng Bu Zhun Zhong Yao Cheng Fang Zhi Ji (1990) |
| Liuwei Wan | Aconitum carmichaelii Debx. (180 g)/Aconitum kusnezoffii Reichb. (180 g)/Ephedra sinica Stapf (180 g)/Atractylodes lancea (Thunb.) DC. (360 g)/Angelica sinensis (Oliv.) Diels (360 g)/Angelica dahurica (Fisch.ex Hoffm.) Benth. et Hook (229 g)/Zingiber officinale Rosc. (485 g)/Kaempferia galanga L. (146 g)/Illicium verum Hook. F. (114 g)/Mentha haplocalyx Briq (80 g)/Cinnamomum camphora (L.) Presl (60 g)/camphor (40 g)/methyl salicylate (60 g) | Nourishing the liver and kidneys | Wei Sheng Bu Zhun Zhong Yao Cheng Fang Zhi Ji (1990) |
| Shixiang Nuanqi Gao | Illicium verum Hook. F. (120 g)/Foeniculum vulgare Mill. (120 g)/Lindera aggregate (Sims)Kos-term. (120 g)/Cyperus rotundus L. (120 g)/Angelica sinensis (Oliv.) Diels (120 g)/Angelica dahurica (Fisch.ex Hoffm.) Benth. et Hook (120 g)/Eugenia caryophyllata Thunb. (30 g)/Cinnamomum cassia Presl (30 g)/Aquilaria sinensis (Lour.) Gilg (30 g)/Boswellia carterii Birdw. (30 g)/Commiphora myrrha Engl. (30 g)/Aucklandia lappa Decne. (30 g) | Warming the spleen and stomach for dispelling cold, stopping pain | Wei Sheng Bu Zhun Zhong Yao Cheng Fang Zhi Ji (1991) |
| Sanceng Huixiang Wan |
llicium verum Hook. F. (200 g)/Melia toosendan Sieb. et Zucc. (200 g)/Aucklandia lappa Decne. (200 g)/Poria cocos (Schw.) Wolf (800 g)/Glehnia littoralis Fr. Schmidtex Miq. (200 g)/Piperlongum L. (200 g)/Areca catechu L. (100 g)/Aconitum carmichaelii Debx. (100 g) | Warming meridians to dissipate cold, promoting Qi circulation to stop pain | Wei Sheng Bu Zhun Zhong Yao Cheng Fang Zhi Ji (1991) |
| Bajiao Huixiang Shui | star anise oil (20 mL)/ethanol (570 mL) | Flavor correction, Qufeng | Wei Sheng Bu Zhun Zhong Yao Cheng Fang Zhi Ji (1993) |
| Shangshi qutong Gao | Aconitum carmichaelii Debx. (180 g)/Aconitum kusnezoffii Reichb.(180 g)/Ephedra sinica Stapf (180 g)/Atractylodes lancea (Thunb.) DC. (360 g)/Angelica sinensis (Oliv.) Diels (360 g)/Angelica dahurica (Fisch. ex Hoffm.) Benth. et Hook (229 g)/Zingiber officinale Rosc. (485 g)/Kaempferia galanga L. (146 g)/Illicium verum Hook. F. (114 g)/Mentha haplocalyx Briq (80 g)/Cinnamomum camphora (L.) Presl (60 g)/camphor (40 g)/methyl salicylate (60 g) | Treating rheumatism, stopping pain | Wei Sheng Bu Zhun Zhong Yao Cheng Fang Zhi Ji (1993) |
| Suhe Wan |
Liquidambar orientalis Mill. (10 g)/Eugenia caryophyllata Thunb. (120 g)/Styrax tonkinensis (Pierre) Craib ex Hart. (20 g)/Boswellia carterii Birdw. (10 g)/Aucklandia lappa Decne. (20 g)/Santalum album L. (20 g)/Illicium verum Hook. F./Cyperus rotundus L. (20 g)/Atractylodes macrocephala Koidz. (20 g)/Terminalia chebula Retz. (20 g)/Piperlongum L. (20 g)/cinnabaris (10 g)/Cinnamomum camphora (L.) Presl (10 g) |
Dispelling the wind, analgesia, eliminating phlegm | Wei Sheng Bu Zhun Zhong Yao Cheng Fang Zhi Ji (1997) |
| Yulong You | Zingiber officinale Rosc. (48 g)/Paeonia lactiflora Pall. (48 g)/Arisaema erubescens (Wall.) Schott (16 g)/Aconitum kusnezoffii Reichb. (16 g)/Aconitum carmichaelii Debx. (16 g)/Aconitum carmichaelii Debx. (16 g)/Boswellia carterii Birdw. (10.7 g)/Angelica dahurica (Fisch.ex Hoffm.) Benth. et Hook (8 g)/Clematis chinensis Osbeck(8 g)/Asarum heterotropoides Fr. Schmidt (6.4 g)/Rhus chinensis Mill. (2.7 g)/Mentha haplocalyx Briq. (56 mL)/ambrum (36 g)/Mentha haplocalyx Briq (20 g)/Ocimum gratissimum L. (5.6 mL)/Cinnamomum cassia Presl (3.6 mL)/star anise oil (3.6 mL)/methyl salicylate (56 mL)/camphor (56 g) | Qufeng, removing blood stasis, relieving pain | Wei Sheng Bu Zhun Zhong Yao Cheng Fang Zhi Ji (1997) |
| Chanling Wan | Panax ginseng C. A. Mey. (90 g)/Atractylodes macrocephala Koidz. (15 g)/Angelica sinensis (Oliv.) Diels (15 g)/Ligusticum chuanxiong Hort. (90 g)/Atractylodes lancea (Thunb.) DC. (240 g)/Polygonum multiflorum Thunb. (15 g)/Schizonepeta tenuisfolia Briq. (90 g)/Saposhnikovia divaricate (Turcz) Schischk. (90 g)/Ephedra sinica Stapf (90 g)/Aconitum carmichaelii Debx. (90 g)/Aconitum kusnezoffii Reichb. (90 g)/Angelica dahurica (Fisch.ex Hoffm.) Benth. et Hook (90 g)/Asarum heterotropoides Fr. Schmidt. (15 g)/Illicium verum Hook. F. (90 g)/Aucklandia lappa Decne. (15 g)/Anemone raddeana Regel (15 g)/Platycodon grandiflorum (Jacq.) A. DC. (90 g)/Daemonorops draco Bl. (15 g)/Glycyrrhiza uralensis Fisch. (60 g) | Reinforcing Qi and nourishing blood, dissipating the wind and relieving pain | Wei Sheng Bu Zhun Zhong Yao Cheng Fang Zhi Ji (1995) |
| Hupo Zhitong Gao | Kaempferia galanga L (140 g)/Acorus tatarinowii Schott (70 g)/Coptis chinensis Franch. (42 g)/Strychnos nux-vomica L. (140)/Mylabris phalerata Pallas (2.8 g)/Clematis chinensis Osbeck (280 g)/Arisaema erubescens (Wall.) Schott (105 g)/Bufo bufo gargarizans Cantor (5.7 g)/Ambrum (20.6 g)/Ocimum gratissimum L. (9.8 g)/Mentha haplocalyx Briq (22 g)/star anise oil (14.7 g)/Cinnamomum tamala (Bauch.-Ham.) Nees et Eberm (7.4 g)/Cinnamomum camphora (L.) Presl (14.7 g)/camphor (14.7 g) | Blood activating and phlegm resolving, eliminating the mass and relieving swelling, dredging collaterals and relieving pain | Wei Sheng Bu Zhun Zhong Yao Cheng Fang Zhi Ji |
| Ruihua You | Mentha haplocalyx Briq (320 g)/methyl salicylate (230 g)/star anise oil (10 g)/camphor (70 g)/Eucalyptus globulus Labill. (70 g) | Qufeng and relieving pain, relieving itching | Wei Sheng Bu Zhun Zhong Yao Cheng Fang Zhi Ji (1997) |
| Nuanqi Gao | Angelica sinensis (Oliv.) Diels (80 g)/Angelica dahurica (Fisch.ex Hoffm.) Benth. et Hook (80 g)/Lindera aggregate (Sims) Kos-term. (80 g)/Foeniculum vulgare Mill. (80 g)/Illicium verum Hook. F. (80 g)/Aucklandia lappa Decne. (40 g)/Cyperus rotundus L. (80 g)/Boswellia carterii Birdw. (20 g)/Eugenia caryophyllata Thunb. (20 g)/Commiphora myrrha Engl. (20 g)/Cinnamomum cassia Presl (20 g)/Aquilaria sinensis (Lour.) Gilg (20 g)/Moschus berezovskii Flerov. (3 g) | Dispelling cold, promoting Qi circulation to stop pain | Wei Sheng Bu Zhun Zhong Yao Cheng Fang Zhi Ji (1998) |
| Yaoshen Gao | Cistanche deserticola Y.C. Ma/Illicium verum Hook. F./Rehmanniae radix praeparata/Psoralea corylifolia L./Epimedium brevicornu Maxim./Cnidium monnieri (L.) Cuss./Achyranthes bidentata Bl./Dipsacus asper Wall. ex Henry/Glycyrrhiza uralensis Fisch./Eucommia ulmoides Oliv./Cuscuta australis R.Br./Lycium barbarum L./Plantago asiatica L./Foeniculum vulgare Mill./Aconitum carmichaelii Debx./Schisandra chinensis (Turcz.) Baill./Boswellia carterii Birdw./Commiphora myrrha Engl./Eugenia caryophyllata Thunb./Cynomorium songaricum Rupr./Camphor/Cinnamomum camphora (L.) Presl/Mentha haplocalyx Briq/Cinnamomum cassia Presl/methyl salicylate/Liquidambar formosana Hance/diphenhydramine hydrochloride | Warming kidneys and enhancing Yang, strengthening tendons and bones, Qufeng and relieving pain | Wei Sheng Bu Zhun Zhong Yao Cheng Fang Zhi Ji (1998) |
| Huixiang Juhe Wan |
Oeniculum vulgare Mill. (40 g)/Illicium verum Hook. F. (40 g)/Citrus reticulata Blanco (40 g)/Litchi chinensis Sonn. (80 g)/Psoralea corylifolia L. (20 g)/Cinnamomum cassia Presl (16 g)/Melia toosendan Sieb. et Zucc. (80 g)/Corydalis yanhusuo W.T. Wang (40 g)/Curcuma phaeocaulis VaL (20 g)/Aucklandia lappa Decne. (20 g)/Cyperus rotundus L. (40 g)/Citrus reticulata Blanco (40 g)/Laminariajaponica Aresch. (40 g)/Areca catechu L. (40 g)/Boswellia carterii Birdw. (20 g)/Prunus persica (L.) Batsch (16 g)/Manis (20 g) | Dispersing cold, conducting Qi, detumescence and pain relief | Pharmacopoeia of the People’s Republic of China (2020) |
| Qianlietong Pian | Receptaculum Fici Pumilae (400 g)/Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao (464 g)/Plantago asiatica L. (264 g)/phellodendron amurense Rupr. (336 g)/Anemone raddeana Regel (336 g)/Taraxacum mongolicum Hand. -Mazz. (336 g)/Lycopus lucidus Turcz. var. hirtus Regel (336 g)/Ambrum (75 g)/star anise oil (1.7 mL)/cinnamon oil (0.88 mL) | Clearing heat and promoting diuresis, transforming stasis and dissipating binds | Pharmacopoeia of the People’s Republic of China (2020) |
| Suoyang Gujing Wan | Cynomorium songaricum Rupr. (20 g)/Cistanche deserticola Y.C. Ma (25 g)/Morinda officinalis How (30 g)/Psoralea corylifolia L. (25 g)/Cuscuta australis R. Br. (20 g)/Eucommia ulmoides Oliv. (25 g)/Illicium verum Hook. F. (25 g)/Allium tuberosum Rott L. ex Spreng. (20 g)/Euryale ferox Salisb. (20 g)/Nelumbo nucifera Gaertn. (20 g)/Nelumbo nucifera Gaertn. (25 g)/Ostrea gigas Thunberg (20 g)/dragonsbones (20 g)/Cornu Cervi Degelatinatum (2 g)/Rehmanniaglutinosa (Gaetn.) Libosch. ex Fisch. et Mey. (56 g)/Cornus officinalis Sieb. et Zucc. (17 g)/Paeonia suffruticosa Andr. (11 g)/Dioscorea opposita Thunb. (56 g)/Poria cocos (Schw.) Wolf (11 g)/Alisma orientale (Sam.) Juzep. (11 g)/Anemarrhena asphodeloides Bge. (4 g)/Phellodendron chinense Schneid. (4 g)/Achyranthes bidentata Bl. (20 g)/haltum (25 g) | Warming the kidneys and stopping emission | Pharmacopoeia of the People’s Republic of China (2020) |
| Nuanqi Gao | Angelica sinensis (Oliv.) Diels (80 g)/Angelica dahurica (Fisch.ex Hoffm.) Benth. et Hook (80 g)/Lindera aggregate (Sims) Kos-term. (80 g)/Foeniculum vulgare Mill. (80 g)/Illicium verum Hook. F. (80 g)/Aucklandia lappa Decne. (40 g)/Cyperus rotundus L. (80 g)/Boswellia carterii Birdw. (20 g)/Eugenia caryophyllata Thunb. (220 g)/Commiphora myrrha Engl. (20 g)/Cinnamomum cassia Presl (20 g)/quilaria sinensis (Lour.) Gilg (20 g)/Moschus berezovskii Flerov (3 g) | Dispelling cold, promoting Qi circulation to stop pain | Pharmacopoeia of the People’s Republic of China (2020) |
| Qianlietong Shuan | Ficus pumila Linn./Astragalus membranaceus (Fisch.) Bge.var.mongholicus (Bge.) Hsiao/Plantago asiatica L./Phellodendron chinense Schneid./Anemone raddeana Regel/Taraxacum mongolicum Hand. -Mazz./Lycopus lucidus Turcz. var. hirtus Regel/star anise oil/cinnamon oil/ambrum | Clearing away the heat-evil, expelling superficial evils, clearing heat and promoting diuresis, promoting the circulation of Qi and blood, anti-inflammatory and analgesic effects, dispelling stasis and freeing strangury | Xin Yao Zhuan Zheng Biao Zhun (2002) |
| SiJi PingAn You | Mentha haplocalyx Briq/Pogostemon cablin (Blanco) Benth./Citrus reticulata Blanco/Mentha haplocalyx Briq./camphor/Cinnamomum cassia Presl/Glycyrrhiza uralensis Fisch./Gardenia jasminoides Ellis/Cinnamomum camphora (L.) Presl/Borneo/Angelica sinensis (Oliv.) Diels/Illicium verum Hook. F./Ligusticum chuanxiong Hort./Eugenia caryophyllata Thunb./clove oil/Aucklandia lappa Decne./Cinnamomum cassia Presl/Daemonorops draco Bl. | Dispelling cold and relieving the heat | Xin Yao Zhuan Zheng Biao Zhun (2002) |
| Jiuxiang Zhitong Wan | Vladimiria souliei (Franch.) Ling (160 g)/Aucklandia lappa Decne. (20 g)/Aquilaria sinensis (Lour.) Gilg (20 g)/Dalbergia odorifera T. Chen (80 g)/Foeniculum vulgare Mill. (80 g)/Illicium verum Hook. F. (80 g)/Eugenia caryophyllata Thunb. (80 g)/Boswellia carterii Birdw. (80 g)/Pogostemon cablin (Blanco) Benth. (80 g) | Dispelling cold, promoting Qi circulation to stop pain | Pharmacopoeia of the People’s Republic of China (2015) |
| Mentha Laryngitic Tablets | caramel/star anise oil/Eucalyptus globulus Labill./chlorobutanol/sodium benzoate/Mentha haplocalyx Briq | Algefacient, antiseptic, antalgic | Pharmacopoeia of the People’s Republic of China (1963) |
| Ganlanjing Keli | Canarium album Raeusch./Pinelliae rhizome praeparatum cum zingibere et al. umine/Perilla frutescens (L.) Britt./Prunus armeniaca L.var.ansu Maxim./Alpinia officinarum Hance/Crataegus pinnatifida Bge. var. major N. E. Br./Foeniculum vulgare Mill./Zanthoxylum schinifolium Sieb. et Zucc./Poncirus trifoliata (Linn.) Raf./Glycyrrhiza uralensis Fisch./Citrus reticulata Blanco/Cyperus rotundus L./Magnolia officinalis Rehd. et Wils./Mentha haplocalyx Briq/Amomum villosum Lour./Cinnamomum tamala (Bauch-Ham.) Nees et Eberm/Eugenia caryophyllata Thunb./Illicium verum Hook. F. | Appetizing, promoting digestion to eliminate stagnation, driving away summer heat, antidiarrheic, giving appetite | Xin Bian Guo Jia Zhong Cheng Yao (2002) |
| Hantong leYunji | Aconitum carmichaelii Debx. (2.2 mg)/Aconitum kusnezoffii Reichb. (2.2 g)/Ephedra sinica Stapf. (2.2 g)/Angelica sinensis (Oliv.) Diels (4.5 g)/Euodia rutaecarpa (Juss.) Benth. (9.1 mL)/Atractylodes lancea (Thunb.) DC. (4.5 mg)/Illicium verum Hook. F. (1.5 mg)/Kaempferia galanga Linn. (1.8 mg)/Mentha haplocalyx Briq (0.95 mg)/camphor (0.62 mg)/Cinnamomum camphora (L.) Presl (0.62 mg)/methyl salicylate (0.93 mL/g) | Expelling wind and clearing away cold, relaxing the muscles and stimulating blood circulation | Xin Bian Guo Jia Zhong Cheng Yao (2002) |
| Jiuxiang Zhitong Wan | Vladimiria souliei (Franch.) Ling (160 g)/Eugenia caryophyllata Thunb. (20 g)/Aquilaria sinensis (Lour.) Gilg (20 g)/Dalbergia odorifera T. Chen (80 g)/Foeniculum vulgare Mill. (80 g)/Illicium verum Hook. F. (80 g)/Eugenia caryophyllata Thunb (80 g)/Boswellia carterii Birdw. (80 g)/Pogostemon cablin (Blanco) Benth. (80 g) | Dispelling cold, promoting Qi circulation to stop pain | Pharmacopoeia of the People’s Republic of China (2020) |
Table 8.
Prescriptions of traditional Chinese patent medicine.
| Prescription Name | Main Composition | Traditional Uses | Prescription Sources |
|---|---|---|---|
| Huixiang Wan | Illicium verum Hook. F. (25 g)/Melia toosendan Sieb.et Zucc. (5 g)/Angelica pubescens Maxim.f. biserrata Shan et Yuan (25 g)/Glycyrrhiza uralensis Fisch. (25 g)/Eriocaulon buergerianum Koern. (25 g)/matcha (50 g)/Atractylodes lancea (Thunb.) DC (50 g) | Tetanus | Pu Ji Fang (AD1390) |
| Huixiang Cangzhu Wan | Atractylodes lancea (Thunb.) DC. (500 g)/Psoralea corylifolia L. (100 g)/Illicium verum Hook. F. (100 g)/Morinda officinalis How (100 g)/pomoea cairica (Linn.) Sweet (100 g) | Swelling and prolapse of one of the scrota, twinge | Pu Ji Fang (AD1390) |
| Chenxiang Jiji Wan | Citrus aurantium L. (150 g)/Melia toosendan Sieb. et Zucc. (150 g)/Morinda officinalis How (150 g)/Allium tuberosum Rottl. ex Spreng (150 g)/Illicium verum Hook. F. (150 g)/Poria cocos (Fr.) Wolf. (150 g)/Aucklandia lappa Decne. (50 g)/Aquilaria sinensis (Lour) Gilg (50 g)/Moschus berezovskii Flerov (100 g)/halitum (50 g)/Equus caballus orientalis Noack (1 g) | Reglution of Qi, afrodyn | Pu Ji Fang (AD1390) |
| Baishai Dan | Illicium verum Hook. F. (100 g)/Aconitum carmichaelii Debx. (100 g)/Rhizoma Areactylodis Lanceae (100 g)/Rehmannia glutinosa (Gdertn) Iibosch. ex Fisch. et Mey. (150 g)/Poria cocos (Fr.) Wolf. (100 g)/Dioscorea opposita Thunb. (100 g) | Enriching muscles, bones, arteries and veins, breaking obscure complexion, resolving visceral accumulation, reconciling viscera, getting rid of heart Qi, night sweat and wind disease, blacken hair and beard | Qi Fang Lei Bian (AD1644~1911) |
| Chenxiang Wan | Aquilaria sinensis (Lour.) Gilg (15 g)/Aucklandia lappa Decne. (15 g)/Symplocos paniculata (Thunb.) Miq. (15 g)/Juglans regia L. (15 g)/Eugenia caryophyllata Thunb. (15 g)/Lycium barbarum L. (15 g)/Illicium verum Hook. F. (15 g)/Buthus martensii Karsch (25 g)/Foeniculum vulgare Mill. (25 g)/Melia toosendan Sieb. et Zucc. (25 g)/Trigonella foenum-graecum L. (25 g)/Psoralea corylifolia L. (25 g)/Manis (15 g)/Cuscuta australis R. Br. (25 g)/Dipsacus fullonum L. (25 g)/Polygala tenuifolia Willd. (25 g)/Allium tuberosum Rottl. ex Spreng (25 g)/Nelumbo nucifera Gaertn. (15 g)/Morinda officinalis How (25 g)/Dioscorea opposita Thunb. (25 g)/Cornus officinalis Sieb. et Zucc. (25 g)/Dioscorea opposita Thunb. (25 g)/Epimedium sagittatum (Sieb. et Zucc.) Maxim. (10 g)/Citrus reticulata Blanco (15 g)/Citrus reticulata Blanco (15 g)/Poria cocos (Fr.) Wolf. (25 g) | Enhancing memory ability, reinforcing the five viscera, enriching muscles and bones, improving eyesight, consolidating the teeth | Yi Fang Lei Ju (AD1443~1447) |
| Da Huixiang Wan | Crataegus pinnatifida Bge. var. major N. E. Br. (200 g)/Citrus reticulata Blanco (100 g)/Foeniculum vulgare Mill. (100 g)/Gardenia jasminoides Ellis (100 g)/Bupleurum chinense DC. (50 g)/Paeonia suffruticosa Andr. (50 g)/Prunus persica (L.) Batsch (50 g)/Illicium verum Hook. F. (50 g)/Euodia rutaecarpa (Juss.) Benth. (25 g) | Head pain | XingYuan (AD1586) |
| Erxiang Wuzi Sanzhu Wan | Illicium verum Hook. F. (25 g)/Inula helenium L. (25 g)/Areca catechu L. (50 g)/Melia toosendan Sieb. et Zucc. (150 g)/Cyperus rotundus L. (50 g)/Raphanus sativus L. (100 g)/Ipomoea cairica (Linn.) Sweet (150 g)/Euodia rutaecarpa (Juss.) Benth. (150 g)/Zanthoxylum ailanthoides (150 g)/Cornus officinalis Sieb. et Zucc. (150 g) | Lumbago | Yong Lei Qian Fang (AD1331) |
| Guyuan Wan | Illicium verum Hook. F. (30 g)/Rubus chingii Hu (30 g)/Foeniculum vulgare Mill. (30 g)/Poria cocos (Fr.) Wolf. (30 g)/Cyathula officinalis Kuan (30 g)/Magnetitum (30 g)/Apatite (30 g)/Psoralea corylifoliaL. (30 g)/Aconitum carmichaeli Debx.(30 g)/Schisandra chinensis (Turcz.) Baill. (60 g)/Cuscuta australis R. Br. (60 g)/Cervus nippon Temminck (75 g)/Cistanche salsa (C. A. Mey.) G. Beck (75 g)/Plantago asiatica L. (15 g)/Moschus berezovskii Flerov (1.5 g)/stalactitum (45 g)/Aconitum carmichaelii Debx. (45 g)/Aconitum carmichaelii Debx. (45 g)/Cinnamomum cassia Presl (45 g)/Morinda officinalis How (45 g) | Deficiency/weakness of primordial Qi | Zhu Shi Ji Yan Fang (AD1127~1279) |
| Huichun Wan | Poria coco s (Schw.) Wolf (30 g)/Atractylodes macrocephala Koidz. (30 g)/Crataegus pinnatifida Bge. var. major N. E. Br. (30 g)/Citrus aurantium L. (24 g)/Illicium verum Hook. F. (30 g)/Cornus (30 g)/Citrus reticulata Blanco (90 g)/Litchi chinensis Sonn. (30 g) | Hernia | She Sheng Zhong Miao Fang (AD1550) |
| Huixiang Jupi Jiu | Illicium verum Hook. F. (50 g)/Citrus reticulata Blanco (100 g)/Amomum kravanh Pierre ex Gagnep. (25 g) | Lower abdominal pain, chest and abdominal pain | Yi Tong (AD1556) |
| Huixiang San | Aucklandia costus Falc. (50 g)/Camellia sinensis (L.) O. Ktze. (50 g)/Illicium verum Hook. F. (25 g)/Boswellia carterii Birdw. (25 g)/Panax ginseng C. A. Mey. (25 g)/Melia toosendan Sieb. et Zucc. (125 g)/Glycyrrhiza uralensis Fisch. (75 g)/Anemarrhena asphodeloides Bge. (75 g)/Foeniculum vulgare Mill. (75 g)/Fritillaria spp. (75 g)/Aquilaria sinensis (Lour.) Gilg (10 g)/Styrax tonkinensis (Pierre) Craib ex Hart. (12.5 g) | Toothache | Yi Fang Lei Ju (AD1443~1447) |
| Huixiang Yin | Illicium verum Hook. F./Cissusrepens Lamk. | Hernia | Zhu Shi Ji Yan Fang (AD1127~1279) |
| Wuxian Zhushen Dan | Illicium verum Hook. F. (2.4 g)/Psoralea corylifolia L. (2.4 g)/Eucommia ulmoides Oliv. (2.4 g)/halitum (2.4 g)/Cistanche deserticola Y.C. Ma | Lumbago | Fu Shou Jing Fang (AD1530) |
Star anise is considered 95% edible and 5% medicinal. Star anise is widely used in some areas in Indonesia as a condiment for spicy cuisine. For instance, Aceh curry, Rendang Minang, Javanese cuisines and Balinese cuisines employ star anise. In northern China, star anise is one of the most popular seasonings, and it can be used directly in a variety of food preparations such as stewing, boiling, pickling, brining and soaking, or it can be processed into a five-spice seasoning powder [92]. Star anise extracts have the advantages of being natural, non-toxic and harmless, and they are usually used in the food industry to produce various food flavors. Star anise is also used in the daily chemical industry as a fragrant agent for perfumes, soaps, toothpastes and other cosmetics-associated products. It has certain developmental prospects as an essential oil fungicide, and it can be used as an antiseptic preservative for agricultural uses [93,94].
6. Quality Control
6.1. Studies on the quality standards of I. verum Hook. F.
Several new plants of the genus Illicium Linn have been discovered since 1982. In 2000, based on the study of more than 10,000 specimens of the genus I. Linn obtained from 120 herbarium collections in 18 countries and regions, Qi et al. proposed three new combinations. They confirmed that there were 34 species, 3 subspecies and 1 variety of plants of the Illicium L. worldwide. Among them, there were 9 species and 1 subspecies in the Illicium group and 25 species, 2 subspecies and 1 variety in the I. verum group. They also suggested that some species were morphologically difficult to distinguish [95,96]. I. verum is a medicinal as well as an edible plant and it has a wide distribution and grows in many places. It has a range of uses, and the quality standard of its medicinal composition has been constantly improved. The 2000 version of the Pharmacopoeia of the People’s Republic of China records the main identification methods of star anise. These are typically associated with a color reaction of its phloroglucinol to hydrochloric acid and its characteristics on TLC when compared with a standard star anise reference material. The volatility of its oil should not be less than 4.0% mL/g. In the 2005 version of the Pharmacopoeia of the People’s Republic of China, ultraviolet and visible spectrophotometry with a maximum absorption wavelength of 259 nm was added. The 2015 version of the Pharmacopoeia added that the trans-anisidine content should not be less than 4.0%. According to the latest 2020 edition of the Chinese Pharmacopoeia, the identification of star anise is mainly through trait identification, color reaction of resorcinol hydrochloric acid, and other methods that include a combination of the above methods [91].
In 2012 [97], Yuan et al. used TLC and HPLC analysis to establish quality control for star anise medicinal herbs. The extracted total and acid-insoluble ash products of star anise from 10 different origins were determined. At the same time, the active ingredients, shikimic acid and kaempferol, were identified in thin layers, and an HPLC quantitative study of the former was carried out. Their results provided a reference standard for the quality of star anise. The amounts of water- and alcohol-soluble extracts should not be less than 21.0 and 16.0%, respectively. In addition, the total and acid-insoluble ash content should not exceed 4.0 and 0.2%, respectively. The shikimic acid content should also not be less than 1.0%. This is used as a reference basis for formulating quantitative analysis standards for star anise medicinal materials. The methods used in this study were simple and accurate and were an effective control for the quality of star anise medicinal materials.
6.2. Extraction and Separation Methods
SAO is the main effective component of star anise, and anethole is the main volatile component of this oil. These two components are also the main standards for the quality control of I. verum. Therefore, optimizing their extraction is essential for ensuring the efficacy of this medical product. The extraction methods of SAO include TSD, Soxhlet extraction (SE), ultrasonic-assisted extraction (UE), SCFE, hydrodistillation (HD) and a combination microwave-assisted Soxhlet extraction method (MAEE). The extraction solvent generally includes absolute ethanol, petroleum ether and ethyl acetate. Different extraction solvents will not only change the composition of the extracts obtained but they can also alter the quality of the SAO as well as its physical properties. A ratio of solvent to sample of 17 mL/g, an extraction time of 16 min and a microwave power 505 Watts were found to be the optimal extraction conditions for using MAEE. The highest yield of SAO obtained was 24.98%, which was much higher than that from the SD method (7.17%) [35,98,99].
The conditions for TSD were optimized and it was concluded that the fruits must be fragmented to small size of less than 425 μm using a knife mill. For HD, the conditions reported as ideal were granulometry <425 μm, 8% mass, 1 h and a water volume of 200 mL. This process provided an SAO yield of 10.2% [99]. The conditions for UE were optimized as a solid-to-liquid ratio of 1:15 (g/mL), crushing particle size of 60 mesh and an ultrasonic time of 30 min [100]. Under the optional extract conditions, the extraction rate of SAO was determined to be 28.438 ± 0.11%. The optimum conditions for supercritical fluid CO2 extraction were extraction time of 1 h at a temperature of 40 °C and pressure of 250 bar when the yield of SAO was 14.5% [9]. The latest experiments compared SE, UE and DS methods, and the extraction rates of the different solvents used were ranked as ethanol > petroleum ether > ethyl acetate. Extracting the SAO using the different methods showed that the extraction yields were SE > UE > SD. The comparison of common extraction methods of star anise extract is shown in Table 9.
Table 9.
A comparison of the common extraction methods used for obtaining star anise extracts.
| Extraction Methods | Optimal Extraction Conditions | Extraction Rates (Retain Two Significant Digits) |
Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Steam distillation (SD) | fruit size: 425 μm granulometry < 0.425 mm 8% mass 1 h water volume of 200 mL |
10% | Simple equipment, convenient operation and low cost | Time-consuming and low oil yield | [99] |
| Ultrasonic-assisted extraction (UE) | solid-to-liquid ratio of 1:15 (g/mL) crushing particle size of 60 mesh ultrasonic time of 30 min |
28% | Stable temperature, simple operation, short extraction time | Difficult and costly equipment manufacture | [100] |
| Supercritical fluid CO2 extraction (SCFE) | 1 h extraction temperature 40 °C extraction pressure 250 bar |
15% | Low temperature extraction No solvent residue |
Strict requirements for equipment and materials | [9] |
| Microwave-assisted Soxhlet extraction (MAEE) | ratio of solvent to sample 17 mL/g extraction time 16 min microwave power 505 W |
25% | Safe and environmentally friendly High extraction rate Short time |
High operational requirements | [35,98,99] |
7. Conclusions
In this review, we have provided a critical analysis of the botanical properties, traditional uses, phytochemistry, pharmacology, quality control and toxicology of Illicium verum, which is used as a food and pharmaceutical product worldwide. Modern pharmacological studies have shown the plant components to have antioxidant, antibacterial, anti-inflammatory, insecticidal and antiviral effects. With an improvement in extraction techniques and the progressive advancement in pharmacological research, some success has been achieved in determining the chemical composition and pharmacological effects of this widely used plant.
Acknowledgments
We are grateful to Dev Sooranna of Imperial College London for editing the manuscript.
Author Contributions
Q.Z.; writing—original draft preparation, J.Y.; writing—review and editing, C.L., W.Z. and Y.H. contributed to the conception and design of this article and interpreting the relevant literature. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was financially supported by the National Natural Science Foundation of China (Grant 82060700), the Major Science and Technology Projects of Guangxi Province (2022JBGS042) and the Self-Financing Research Project from the Guangxi Provincial Administration of Traditional Chinese Medicine (GXZYZ20210223).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Wu J., Ba D., Liu D., Hou Y., Chen K. Chinese Medical Encyclopedia. Peking Union Medical College Press; Beijing, China: 2012. pp. 318–320. [Google Scholar]
- 2.Bukar B.B., Dayom D.W., Uguru M.O. The Growing Economic Importance of Medicinal Plants and the Need For Developing Countries to Harness from it: A Mini Review. IOSR J. Pharm. 2016;6:42–52. [Google Scholar]
- 3.Yang D., Zhou S., Huang B. Textual research on the original plants of “Huixiang”. Lishizhen Med. Mater. Med. Res. 2018;29:2664–2666. [Google Scholar]
- 4.Wu Z., Raven P. Flora of China. 30th ed. Science Press; Beijing, China: 1994. pp. 227–228. [Google Scholar]
- 5.Wang Y., Ding H., Xu X., Dan C., Wang Z., Liu L. Antibacterial Effect of Plant Essential Oil on Bacillus subtilis. J. Chin. Inst. Food Sci. Technol. 2019;19:72–78. [Google Scholar]
- 6.Wu L.M., Lu N.H. Preliminary Studies on Antifungal Activity of Illicium verum. J. Henan Agric. Sci. 2008;37:80–82. [Google Scholar]
- 7.Dzamic A., Sokovic M., Ristic M.S., Grijic-Jovanovic S., Vukojevic J., Marin P.D. Chemical composition and antifungal activity of Illicium verum and Eugenia caryophyllata essential oils. Chem. Nat. Compd. 2009;45:259–261. doi: 10.1007/s10600-009-9283-4. [DOI] [Google Scholar]
- 8.Huang Y., Zhao J., Zhou L., Wang J., Gong Y., Chen X., Guo Z., Wang Q., Jiang W. Antifungal Activity of the Essential Oil of Illicium verum Fruit and Its Main Component trans-Anethole. Molecules. 2010;15:7558–7569. doi: 10.3390/molecules15117558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y., Wang Y., Kong W., Yang S., Luo J., Yang M. Illicium verum essential oil, a potential natural fumigant in preservation of lotus seeds from fungal contamination. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. 2020;141:111347. doi: 10.1016/j.fct.2020.111347. [DOI] [PubMed] [Google Scholar]
- 10.Singh G., Maurya S., Delampasona M.P., Catalan C. Chemical constituents, antimicrobial investigations and antioxidative potential of volatile oil and acetone extract of star anise fruits. J. Sci. Food Agric. 2006;86:111–121. doi: 10.1002/jsfa.2277. [DOI] [Google Scholar]
- 11.Yang J.F., Yang C.H., Chang H.W., Yang C.S., Wang S.M., Hsieh M.C., Chuang L.Y., Yang J.F., Yang C.H., Chang H.W., et al. Chemical composition and antibacterial activities of Illicium verum against antibiotic-resistant pathogens. J. Med. Food. 2010;13:1254–1262. doi: 10.1089/jmf.2010.1086. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim M.K., Mattar Z.A., Abdel-Khalek H.H., Azzam Y.M. Evaluation of antibacterial efficacy of anise wastes against some multidrug resistant bacterial isolates. J. Radiat. Res. Appl. Sci. 2016;10:34–43. doi: 10.1016/j.jrras.2016.11.002. [DOI] [Google Scholar]
- 13.Song W.Y., Ma Y.B., Bai X., Zhang X.M., Gu Q., Zheng Y.T., Zhou J., Chen J.J. Two new compounds and anti-HIV active constituents from Illicium verum. Planta Med. 2007;73:372–375. doi: 10.1055/s-2007-967162. [DOI] [PubMed] [Google Scholar]
- 14.Astani A., Reichling J., Schnitzler P. Screening for Antiviral Activities of Isolated Compounds from Essential Oils. Evidence-Based Complement. Altern. Med. 2009;2:288–289. doi: 10.1093/ecam/nep187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch C., Reichling J., Schneele J., Schnitzler P. Inhibitory effect of essential oils against herpes simplex virus type 2. Phytomed.-Tub. -Gustav Fisch. Verl. 2008;15:71–78. doi: 10.1016/j.phymed.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Liu M., Yu Q., Xiao H., Yi Y., Cheng H., Putra D.F., Huang Y., Zhang Q., Li P. Antiviral activity of Illicium verum Hook. F. extracts against grouper iridovirus infection. J. Fish Dis. 2020;43:531–540. doi: 10.1111/jfd.13146. [DOI] [PubMed] [Google Scholar]
- 17.Graça Miguel M. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules. 2010;15:9252–9287. doi: 10.3390/molecules15129252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auddy B., Ferreira M., Blasina F., Lafon L., Arredondo F., Dajas F., Tripathi P.C., Seal T., Mukherjee B. Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J. Ethnopharmacol. 2003;84:131–138. doi: 10.1016/S0378-8741(02)00322-7. [DOI] [PubMed] [Google Scholar]
- 19.Narayanasamy B., Jeyakumar N., Balasubramanian D. Effect of Star Anise as a Natural Antioxidant Additive on the Oxidation Stability of Lemon Grass Oil. Waste Biomass Valorization. 2020;15:2983–2997. doi: 10.1007/s12649-020-01218-8. [DOI] [Google Scholar]
- 20.Dinesha R., Thammannagowda S.S., Shwetha K.L., Msl P., Srinivas L. The antioxidant and DNA protectant activities of Star Anise (Illicium verum) aqueous extracts. AkiNik Publ. 2014;2:98–103. [Google Scholar]
- 21.Yang C.H. Investigation of the antioxidant activity of Illicium verum extracts. J. Med. Plant Res. 2012;6:314–324. [Google Scholar]
- 22.Wong Y., Lee P., Nurdiyana W. Extraction and Antioxidative Activity of Essential Oil From Star Anise (Illicium verum) Orient. J. Chem. 2014;30:1159–1171. doi: 10.13005/ojc/300329. [DOI] [Google Scholar]
- 23.Madhusudhanan N., Ignatus V., Lakshmi T. Antioxidant activity of berberine on benzo (A) pyrene induced experimentallung carcinogenesis in mice. J. Chem. Pharm. Res. 2013;5:194–200. [Google Scholar]
- 24.Du Z., Huang L., Hao E., Xie Y., Hou X. The anti-inflammatory and analgesic effects of star anise, an aromatic herb in South China. Acta Hortic. 2016;11:151–160. [Google Scholar]
- 25.Assiry A.A., Karobari M.I., Bhavikatti S.K., Marya A. Crossover Analysis of the Astringent, Antimicrobial, and Anti-inflammatory Effects of Illicium verum/Star Anise in the Oral Cavity. BioMed Res. Int. 2021;2021:5510174. doi: 10.1155/2021/5510174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandhrakanthan M., Handunnetti S.M., Gas P., Chelvendran S. Evaluation of anti-inflammatory activity of Alpinia calcarata leaf extracts: An in vitro study. ResearchGate. 2021;12:278. [Google Scholar]
- 27.Tuseef H., Raza M.L., Assad T. Comparative Evaluation of Analgesic, Antipyretic & Anti-Inflammatory Effects of Various Extracts of Dried Fruit of Illicium verum Hook.f (Star anise) in Rodents. Walailak J. Sci. Technol. 2021;18:9456. [Google Scholar]
- 28.Xu H., Zhao S., Zhuo J., Ding J., Yun X. Studies on insecticidal activity of the anise oil and analysis of its components. J. Plant Prot. 1996;10:338–342. [Google Scholar]
- 29.Kim J., Jang M., Shin E., Kim J., Lee S.H., Park C.G. Fumigant and contact toxicity of 22 wooden essential oils and their major components against Drosophila suzukii (Diptera: Drosophilidae) Pestic. Biochem. Physiol. 2016;10:35–43. doi: 10.1016/j.pestbp.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z., Xiang C., Lu J., Chen Y., Wang X., Zhou G. Effects of star anise Illicium verum extracts on feeding and ovipositional selectivity of white-backed rice planthopper Sogatella furcifera and their insecticidal activities. J. Plant Prot. 2021;48:907–913. [Google Scholar]
- 31.Lee J.O., Park M.A., Yoon C.S., Na J.H., Han J. Characterization and Preservation Performance of Multilayer Film with Insect Repellent and Antimicrobial Activities for Sliced Wheat Bread Packaging. J. Food Ence. 2019;11:3194–3203. doi: 10.1111/1750-3841.14823. [DOI] [PubMed] [Google Scholar]
- 32.Choi I., Kim S., Lee J.S., Chang Y., Na J.H., Han J. Analysis of the insect-repelling mechanism of star anise extract and its major active compounds against Plodia interpunctella. Food Sci. Biotechnol. 2022;31:451–462. doi: 10.1007/s10068-022-01053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li W., Wu Z., Xia Y., Tan J., Zhao H., Chen S., Li Y., Tang H., Wang G., Zhang Y. Antiviral and Antioxidant Components from the Fruits of Illicium verum Hook. F. (Chinese Star Anise) J. Agric. Food Chem. 2022;70:3697–3707. doi: 10.1021/acs.jafc.1c08376. [DOI] [PubMed] [Google Scholar]
- 34.Chouksey D., Upmanyu N., Pawar R.S. Central nervous system activity of Illicium verum fruit extracts. Asian Pac. J. Trop. Med. 2013;6:869–875. doi: 10.1016/S1995-7645(13)60155-8. [DOI] [PubMed] [Google Scholar]
- 35.Xie Y., Deng J.G., Huang L.Z., He Y.P., Wu Q.L., Wei L.Y., Li Y.J. Comparison of Illicium verum Pharmacodynamic Effect of Different processing Products of Trans-anethone Content as well as Warming Yang for Dispelling Cold and Analgesic Effect. Sci. Technol. Eng. 2016;16:6. [Google Scholar]
- 36.He J.H., Yao L., Chang Z. Anti-fatigue effects of Fructus Anisi Stellati extract. J. Clin. Rehabil. Tissue Eng. Res. 2011;15:4. [Google Scholar]
- 37.Divya L., Nisha K. Shah, I.B.O. Experimental and computational evaluation of Illicium verum as a novel eco-friendly corrosion inhibitor for aluminium. Mater. Corros. 2018;69:125–139. [Google Scholar]
- 38.Díaz A., Vargas-Perez I., Aguilar-Cruz L., Calva-Rodríguez R., Treviño S., Venegas B., Contreras-Mora I.R. A mixture of chamomile and star anise has anti-motility and antidiarrheal activities in mice. Rev. Bras. Farmacogn. 2014;24:419–424. doi: 10.1016/j.bjp.2014.07.016. [DOI] [Google Scholar]
- 39.Al-Qasmi N., Almughem F.A., Jarallah S.J., Almaabadi A. Efficient Green Synthesis of (Fe3O4) and (NiFe2O4) Nanoparticles Using Star Anise (Illicium verum) Extract and Their Biomedical Activity against Some Cancer Cells. Materials. 2022;15:4832. doi: 10.3390/ma15144832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu X., Tao M., Meng K. First reported poisoning due to excessive consumption of star anise. Chin. J. Sch. Dr. 1992;6:7. [Google Scholar]
- 41.Nakamura T., Okuyama E., Yamazaki M. Neurotropic Components from Star Anise (Illicium verum Hook, fil.) Chem. Pharm. Bull. 1996;44:1908–1944. doi: 10.1248/cpb.44.1908. [DOI] [PubMed] [Google Scholar]
- 42.Russell M.G., Held S.K., Katherine F. A case of infantile star anise toxicity. Pediatr. Emerg. Care. 2012;28:284–285. doi: 10.1097/PEC.0b013e3182495ba7. [DOI] [PubMed] [Google Scholar]
- 43.Perret C. Phenytoin: Neurological disorders in an infant: Case report. React. Wkly. 2013;20:1435. [Google Scholar]
- 44.Campos-Estrada C., Vozmediano A., Beltrán D., Irarrazabal D., Escobar M., Cavieres M.F. Neurotoxicological and chemical characterization of aqueous extracts of star anise (Illicium verum Hook f.): A preliminary study. Toxicol. Lett. 2016;259:138. doi: 10.1016/j.toxlet.2016.07.356. [DOI] [Google Scholar]
- 45.Ize-Ludlow D. Chemical composition of Chinese star anise (Illicium verum) and neurotoxicity in infants. JAMA J. Am. Med. Assoc. 2004;291:562–563. doi: 10.1001/jama.291.5.562. [DOI] [PubMed] [Google Scholar]
- 46.Yan J.H., Xiao X.X., Huang K.L. Component analysis of volatile oil from Illicium verum Hook. F. J. Cent. South Univ. 2002;9:173–176. doi: 10.1007/s11771-002-0021-3. [DOI] [Google Scholar]
- 47.Peng Y.H., Yu K., Lu Y.D., Liu M., Jiang L.J. Bioactivity and Chemical Compositions of Essential Oil Extracted from Illicium verum against Mosquitoes. Adv. Mater. Res. 2014;11:164–167. doi: 10.4028/www.scientific.net/AMR.864-867.164. [DOI] [Google Scholar]
- 48.Wang Q., Jiang L., Wen Q.B., Yang D.P., Mo L.H., Jiang X.Y. Analysis of the chemical composition of star anise oil by GC-MS. Sci. Technol. Food Ind. 2006;27:3. [Google Scholar]
- 49.Guo Y., Lei Y.G., Liao J.H., Chen Q.P., Qin L.Y. Study on Gas Chromatography-Mass spectrometry analysis of star anise oil by Subcritical C02 fluid extraction from Illicium verum Hook. F. Lishizhen Med. Mater. Med. Res. 2008;19:803–806. [Google Scholar]
- 50.Luís Â., Sousa S., Wackerlig J., Dobusch D., Duarte A.P., Pereira L., Domingues F. Star anise (Illicium verum Hook. F.) essential oil: Antioxidant properties and antibacterial activity against Acinetobacter baumannii. Flavour Fragr. J. 2019;33:260–270. doi: 10.1002/ffj.3498. [DOI] [Google Scholar]
- 51.Jiang C.Y., Wang C., Xu N.X., Fujita T., Murata Y., Kumamoto E. 1,8-and 1,4-cineole enhance spontaneous excitatory transmission by activating different types of transient receptor potential channels in the rat spinal substantia gelatinosa. J. Neurochem. Off. J. Int. Soc. Neurochem. 2016;136:764–777. doi: 10.1111/jnc.13433. [DOI] [PubMed] [Google Scholar]
- 52.Tiwari M., Kakkar P. Plant derived antioxidants—Geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol. Vitr. 2009;23:295–301. doi: 10.1016/j.tiv.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 53.Aly S.E., Sabry B.A., Shaheen M.S., Hathout A.S. Assessment of antimycotoxigenic and antioxidant activity of star anise (Illicium verum) in vitro. J. Saudi Soc. Agric. Sci. 2016;15:1097–1108. doi: 10.1016/j.jssas.2014.05.003. [DOI] [Google Scholar]
- 54.Zhang G., Yuan C., Sun Y. Effect of Selective Encapsulation of Hydroxypropyl-β-cyclodextrin on Components and Antibacterial Properties of Star Anise Essential Oil. Molecules. 2018;23:1126. doi: 10.3390/molecules23051126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J., Liu H., Zhao J., Gao H., Zhou L., Liu Z., Chen Y., Sui P. Antimicrobial ands Antioxidant Activities of the Root Bark Essential Oil of Periploca sepium and Its Main Component 2-Hydroxy-4-methoxybenzaldehyde. Molecules. 2010;15:5807–5817. doi: 10.3390/molecules15085807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song W.Y., Ma Y.B., Chen J.J., Zhou J., Jiang Z.Y., Chang X.L., Zhang X.M. Study on the chemical composition of star anise root. Chin. Herb. Med. 2009;40:1216–1219. [Google Scholar]
- 57.Yuan J.Q., Zhou X.L., Wang S., Liao J.H., Ke C.Q., Yang X.Z. Chemical constituents from Illicium verum. Chin. Tradit. Pat. Med. 2010;32:4. [Google Scholar]
- 58.Zhu S.J. Constituents of the Water Soluble Fractions of 75% Ethanol Extract of Illicium verum Hook. F. Guangxi Normal University; Guilin, China: 2011. [Google Scholar]
- 59.Shang Y.L., Shi R.J. Phenolic glycosides from the fruits of Illicium verum. Chin. Tradit. Pat. Med. 2016;38:4. [Google Scholar]
- 60.Liang D., Wu C., Zi Z., Chen R., He Y. A new flavane acid from the fruits of Illicium verum. Nat. Prod. Res. 2016;30:1585–1590. doi: 10.1080/14786419.2015.1120726. [DOI] [PubMed] [Google Scholar]
- 61.Lee S.W., Li G., Lee K.S., Jung J.S., Son J.K. Preventive Agents against Sepsis and New Phenylpropanoid Glucosides from the Fruits of Illicium verum. Planta Med. 2003;69:861–864. doi: 10.1055/s-2003-43210. [DOI] [PubMed] [Google Scholar]
- 62.Zhu Q.H. Star anise—Five of Ten Notes on Guixiang. Red Beans. 2018;6:3. [Google Scholar]
- 63.Okely H.M., Grundon M.F. Anisoxide: An artefact from star anise oil formed by Claisen rearrangement. J. Chem. Soc. D Chem. Commun. 1971:1157–1158. doi: 10.1039/c2971001157b. [DOI] [Google Scholar]
- 64.Okely H.M., Grundon M.F. Thermal rearrangement of 1-(3-methylbut-2-enyloxy)-4-prop-1-enylbenzene (feniculin): A new synthesis of anisoxide and re-investigation of the constituents of star anise oil. Chem. Inf. 1981;12:897–899. doi: 10.1039/p19810000897. [DOI] [Google Scholar]
- 65.Lee S.W., Li G., Lee K.S., Song D.K., Son J.K. A new phenylpropanoid glucoside from the fruits of Illicium verum. Arch. Pharmacal Res. 2003;26:591–593. doi: 10.1007/BF02976705. [DOI] [PubMed] [Google Scholar]
- 66.Sy L., Brown G. Novel phenylpropanoids and lignans from Illicium verum. J. Nat. Prod. 1998;61:987–992. doi: 10.1021/np9800553. [DOI] [PubMed] [Google Scholar]
- 67.Du R.N. Chemical Constituents of Two Illicium Plants and Artemisia Carvifolia. Kunming Medical University; Kunming, China: 2018. [Google Scholar]
- 68.Chen J.W. Study on the Chemical Constituents from the CH2CL2 Fraction of Illicium verum Hook. F. and Their TNF-α Inhibitory Activities. YanBian University; Yanbian, China: 2019. [Google Scholar]
- 69.Chen Z.Z. Chemical Constituents of the Ethyl Acetate Extract from the Fruits of Illicium verum Hook. F. Guangxi Normal University; Guilin, China: 2012. [Google Scholar]
- 70.Shuai P. Study on Chemical Constituents from the Petroleum ether and EtOAc Fractions of Illicium verum Hook. F. and Their TNF-α inhibtory Activities. Yanbian University; Yanbian, China: 2019. [Google Scholar]
- 71.Ishikawa T., Fujimatu E., Kitajima J. Water-soluble constituents of anise: New glucosides of anethole glycol and its related compounds. Chem. Pharm. Bull. 2002;50:1460–1466. doi: 10.1248/cpb.50.1460. [DOI] [PubMed] [Google Scholar]
- 72.Lin K., Xie J.Z., Zhang S.M., Hui X., Huang D.P., Wei Y.L. A new benzaldehyde from residue left over rectification of star anise oil. Chin. Tradit. Herb. Drugs. 2015;46:3. [Google Scholar]
- 73.Li S.N., Sun J.F., Wang J.M., Jin L., Zong T.Q., Zhou W., Li G. Two new phenolic glycosides from the fruits of Illicium verum. J. Asian Nat. Prod. Res. 2022;24:34–38. doi: 10.1080/10286020.2021.1871606. [DOI] [PubMed] [Google Scholar]
- 74.Si C. Master’s Degree. Jinan University; Jinan, China: 2021. Study on the Chemical Constituents of Illicium verum Hook. F. [Google Scholar]
- 75.Eijkman J.F. Sur les principes constituants de l’Illicium religiosum (Sieb.) (Shikimi-no-ki en japonais) Recl. Des. Trav. Chim. Des. Pays.-Bas. 1885;4:32–54. doi: 10.1002/recl.18850040202. [DOI] [Google Scholar]
- 76.Edmonds M., Payne R. Isolation of Shikimic Acid from Star Aniseed. J. Chem. Educ. 2005;82:599. doi: 10.1021/ed082p599. [DOI] [Google Scholar]
- 77.Bian Q.Q., Hong W., Ying L., Liu J.Q. HPLC determines the content of shikimic acid in different plants. China J. Chin. Mater. Med. 2007;32:1485–1487. [Google Scholar]
- 78.Estevez A.M., Estevez R.J. A Short Overview on the Medicinal Chemistry of (—)-Shikimic Acid. Mini Rev. Med. Chem. 2012;12:1443–1454. doi: 10.2174/138955712803832735. [DOI] [PubMed] [Google Scholar]
- 79.Lei D., Huang J.M., Yang C.P., Mang C.Y. Study on the chemical composition of pachyderm star anise peel. Chin. Pharm. J. 2006;6:411–413. [Google Scholar]
- 80.Zhang J.W. Study on the chemical composition of safflower star anise stems and leaves. China J. Chin. Mater. Med. 1989;1:36–37+63. [Google Scholar]
- 81.Jin Y., Li Q.H. Research Development on the Chemical Constituents of Illicium Plants. J. Honghe Univ. 2006;5:7–11. [Google Scholar]
- 82.Ye F.Y., Xie Y.G., Yan Z., Jie R., Xing W., Jin H.Z., Yan S.K. Chemical Constituents of Branches and Leaves of Illicium wardii A. C. Smith. Nat. Prod. Res. Dev. 2015;27:604–608+625. [Google Scholar]
- 83.Qiu M., Ma J.L., Zhang R.Q., Lu S.Z., Na L. The Progress in Research on Shikimic Acid. Guangxi For. Sci. 2009;38:45–47. [Google Scholar]
- 84.Sagandira C.R., Mathe F.M., Guyo U., Watts P. The evolution of Tamiflu synthesis, 20 years on: Advent of enabling technologies the last piece of the puzzle? Tetrahedron. 2020;76:131440. doi: 10.1016/j.tet.2020.131440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang D.P., Xie J.Z., Kui L., Zhang S.M., Huang L.L., Chen J.H., Liang B.X. The Separation, Purification and Identification of Residue of Star Anise Rectifying Tank Bottom. Guangdong Chem. Ind. 2019;46:54–55. [Google Scholar]
- 86.Kui L., Xie J.Z., Huang D.P., Li Q., Zhang S.M., Hui X., Huang L.L., Ling Q.J., Liang B.X. A new olive alkyl sesquiterpene from residue left over rectification of star anise oil. Chin. Tradit. Pat. Med. 2020;42:1072–1074. [Google Scholar]
- 87.Schmidt T.J., Okuyama E., Fronczek F.R. The molecular structure of 2alpha-hydroxyneoanisatin and structure-activity relationships among convulsant sesquiterpenes of the seco-prezizaane and picrotoxane types. Bioorg. Med. Chem. 1999;7:2857–2865. doi: 10.1016/S0968-0896(99)00240-0. [DOI] [PubMed] [Google Scholar]
- 88.Bai J., Chen H., Fang Z.F., Yu S.S., Wang W.J., Liu Y., Ma S.G., Li Y., Qu J., Xu S. Sesquiterpenes from the roots of Illicium dunnianum. Phytochemistry. 2012;80:137–147. doi: 10.1016/j.phytochem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 89.Liu J., Jiang Z., Zhang Q., Shi Y., Ma Y., Xie M., Zhang X., Chen J. Henrylactones A–E and Anti-HBV Constituents fromIllicium henryi. Planta Med. 2010;76:152–158. doi: 10.1055/s-0029-1186037. [DOI] [PubMed] [Google Scholar]
- 90.Liu J., Liu F., Zhang N., Wang Y., Yang L., Bi Y., Zhang Y., Liu M. Two new sesquiterpene lactones from the fruits of Illicium jiadifengpi. Nat. Prod. Res. 2016;30:322–326. doi: 10.1080/14786419.2015.1058793. [DOI] [PubMed] [Google Scholar]
- 91.Sy L.K., Brown G.D. A seco-cycloartane from Illicium verum. Phytochemistry. 1998;48:1169–1171. [Google Scholar]
- 92.Puspita D., Ardhiawati E., Desi D. Formulation and antibacterial test of star anise extract (Illicium verum Hook. F) as a hand sanitizer. Biolink (J. Biol. Lingkung. Ind. Kesehat.) 2020;7:90–96. [Google Scholar]
- 93.Quan M.P. Research Progress on Application of Star Anise Extract in Food Industry. China Condiment. 2016;41:4. [Google Scholar]
- 94.Huang K., Li G., An J., Li K. Developing Situation of Processing and Utilization Industry of Characteristic Resources of Star Anise(Illicium verum) Biomass Chem. Eng. 2020;54:7 [Google Scholar]
- 95.Qi L. Taxonomic notes on the genus Illicium Linn. J. Syst. Evol. 2000;38:167–181. [Google Scholar]
- 96.Qi L. Medicinal plant resources of lllicium L. Chin. Tradit. Herb. Drugs. 2002;33:81–84. [Google Scholar]
- 97.Yuan J.Q., Yang W., Zhou Y.Q., Zhou X.L., Shuo W., Zhang R.T., Bi C.Y., Qin Y.N., Liao J.H. Study on quality specification of Illiciun verum Hook. F. China J. Tradit. Chin. Med. Pharm. 2012;27:199–201. [Google Scholar]
- 98.Cai M., Guo X., Liang H., Sun P. Microwave-assisted extraction and antioxidant activity of star anise oil from Illicium verum Hook. F. Int. J. Food Sci. Technol. 2013;48:2324–2330. doi: 10.1111/ijfs.12221. [DOI] [Google Scholar]
- 99.Destro B.G.I., Jorge R.M.M., Mathias A.L. Optimization of high-concentration trans-anethole production through hydrodistillation of star anise. Braz. J. Chem. Eng. 2019;36:83. doi: 10.1590/0104-6632.20190362s20180203. [DOI] [Google Scholar]
- 100.Xie Z., Chen L., Zhang W., Chandler W. The Ultrasonic-assisted Extraction Technology and Antioxidation of Star Anise Volatile OiI. China Condiment. 2018;43:124–128. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.