Abstract
Objectives
To evaluate the relationship among dysnatraemia at hospital presentation and duration of admission, risk of intensive care unit (ICU) admission and all-cause mortality and to assess the underlying pathophysiological mechanism of hyponatraemia in patients with COVID-19. Our hypothesis is that both hyponatraemia and hypernatraemia at presentation are associated with adverse outcomes.
Design
Observational study.
Setting
Secondary care; 11 Dutch hospitals (2 university and 9 general hospitals).
Participants
An analysis was performed within the retrospective multicentre cohort study COVIDPredict. 7811 patients were included (60% men, 40% women) between 24 February 2020 and 9 August 2022. Patients who were ≥18 years with PCR-confirmed COVID-19 or CT with COVID-19 reporting and data system score≥4 and alternative diagnosis were included. Patients were excluded when serum sodium levels at presentation were not registered in the database or when they had been transferred from another participating hospital.
Outcome measures
We studied demographics, medical history, symptoms and outcomes. Patients were stratified according to serum sodium concentration and urinary sodium excretion.
Results
Hyponatraemia was present in 2677 (34.2%) patients and hypernatraemia in 126 (1.6%) patients. Patients with hyponatraemia presented more frequently with diarrhoea, lower blood pressure and tachycardia. Hyponatraemia was, despite a higher risk for ICU admission (OR 1.27 (1.11–1.46; p<0.001)), not associated with mortality or the risk for intubation. Patients with hypernatraemia had higher mortality rates (OR 2.25 (1.49–3.41; p<0.001)) and were at risk for ICU admission (OR 2.89 (1.83–4.58)) and intubation (OR 2.95 (1.83–4.74)).
Conclusions
Hypernatraemia at presentation was associated with adverse outcomes in patients with COVID-19. Hypovolaemic hyponatraemia was found to be the most common aetiology of hyponatraemia. Hyponatraemia of unknown aetiology was associated with a higher risk for ICU admission and intubation and longer duration of admission.
Keywords: internal medicine, COVID-19, infectious diseases
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study includes over 7000 patients from different COVID-19 waves and from multiple hospitals, resulting in a heterogenous patient population.
This study relates the different presumed aetiologies to clinical outcomes.
A relative low number of urinary samples was available for patients with hyponatraemia.
Different treatment options that became available for COVID-19 during the ongoing pandemic were not taken into account in this study, which may have influenced the outcome of patients.
Introduction
SARS-CoV-2, a strain of the coronavirus family, has caused a global pandemic since February 2020. By 19 October 2022, there had been over 621 million reported cases and 2.9 million deaths attributed to COVID-19, which is caused by SARS-CoV-2 infection. Respiratory failure resulting from acute respiratory distress syndrome is the leading cause of death associated with SARS-CoV-2 infection.1–3
Common signs and symptoms of COVID-19 infection vary widely, but fever, cough and dyspnoea are frequently present. Other less frequent symptoms include anosmia, nausea, vomiting, diarrhoea and general illness.1 In addition to these clinical symptoms, certain laboratory markers can indicate COVID-19. Elevated lactate dehydrogenase (LDH) levels and lymphopaenia are commonly observed.4 5 Furthermore, electrolyte imbalances such as hypocalcaemia, hypokalaemia and dysnatraemia (hyponatraemia or hypernatraemia) are often present in patients with COVID-19 on hospital admission.4 6 Hyponatraemia, in particular, has been reported in 7%–64% of COVID-19 cases,7–11 compared with 20%–30% in all hospitalised patients.12 It has been demonstrated that critically ill patients with COVID-19 more frequently develop hyponatraemia during the first 72 hours of admission.13 Hyponatraemia is also frequently present in other infectious diseases, such as pneumonia, tuberculosis, meningitis, HIV infection, malaria and leishmaniasis and has been linked to negative outcomes in these diseases and in COVID-19.7 8 11 14–17 On the other hand, hypernatraemia is less common, occurring in less than 10% of the general population and in up to 38% of patients in intensive care units (ICUs). Hypernatraemia is also associated with adverse clinical outcomes.9 16 18–20
The aetiology of hyponatraemia in infectious diseases, including COVID-19, can broadly be categorised into two groups based on urinary sodium excretion (USE). Low USE (<30 mmol/L) indicates an activation of the renin–angiotensin system (RAAS), for example, due to hypovolaemia resulting from inadequate dietary intake, vomiting or diarrhoea. Conversely, high USE suggests RAAS inactivation, which could occur in patients with syndrome of inappropriate antidiuretic hormone secretion (SIADH) and in patients with critical illness-related corticoid deficiency, although diuretic usage can affect diagnostic accuracy.21 22 In other infectious diseases, antidiuretic hormone (ADH) release has been linked to secretion of inflammatory marker interleukin 6.23 Interleukin 6 is also enhanced in patients with COVID-19 and is targeted by off-label administration of interleukin 6 inhibitors, such as tocilizumab and sarilumab.3 24 Both aetiologies (hypovolaemic hyponatraemia and inadequate ADH secretion) have been proposed to contribute to hyponatraemia in COVID-19, although the exact mechanism is still unclear. Hypernatraemia primarily occurs due to insufficient water intake, often caused by hypothalamic thirst centre dysfunction or limited access to fluid intake. It can also result from diabetes insipidus, a condition characterised by ADH deficiency or resistance.25
Previous studies have associated both hyponatraemia and hypernatraemia with worse clinical outcomes in patients with COVID-19 during the early stages of the pandemic.7 8 11 15 17 19 However, most of these studies were conducted before interleukin 6 inhibitors were administered and before the registration of SARS-CoV-2 vaccines.3 7 11 19 26–29 Additionally, they lacked data on clinical parameters at presentation and how they differed between patients with or without dysnatraemia, making it difficult to determine the underlying cause of the hyponatraemia and to relate this cause to clinical outcomes.16 27 30–32
This study reports the incidence rates of hyponatraemia and hypernatraemia on admission in patients with COVID-19 from a large multicentre cohort study in the Netherlands, encompassing multiple COVID-19 waves. We hypothesise that both hyponatraemia and hypernatraemia can predict adverse outcomes, including ICU admission, the need for invasive ventilation and mortality rates among hospitalised patients with COVID-19. Furthermore, we seek to investigate potential pathophysiological mechanisms underlying these conditions based on clinical features and laboratory values at presentation.
Methods
Patient recruitment
We used data from the ongoing retrospective multicentre COVIDPredict Clinical Course Cohort, containing over 10 000 patients with COVID-19, recruited between 24 February 2020 and 9 August 2022 in 11 Dutch hospitals (2 university and 9 general hospitals). Inclusion criteria for the database required patients to be 18 years or older and either had a positive PCR test for SARS-CoV-2 or had a COVID-19 reporting data system (CO-RADS) score of 4 (indicating abnormalities suspicious for COVID-19) or 5 (indicating typical COVID-19) on thoracic CT scan in the absence of an alternative diagnosis.33 A waiver for the use of hospital data was obtained from the medical ethical committees of the participating centres (Amsterdam UMC; 20.131) to use the hospital data. Patients were given the opportunity to opt-out. To avoid duplicate entries, patients transferred from one participating hospital to another were excluded, resulting in a total 297 of exclusions.
Study design
The included patients were categorised into three groups based on their serum sodium concentration on admission to the participating hospital. The serum sodium concentration was adjusted for serum glucose concentration, whenever available, following the method described by Hillier et al.34 The sodium concentrations were stratified as follows: ‘normonatremia’ (corrected serum sodium concentration (Na) 135–145 mmol/L), hyponatraemia (corrected serum sodium concentration (Na)≤134 mmol/L), further subcategorised as ‘mild’ (corrected serum sodium concentration Na 131–134 mmol/L), ‘moderate’ (corrected serum sodium concentration Na 126–130 mmol/L) and ‘severe’ (corrected serum sodium concentration Na≤125 mmol/L) (online supplemental information). ‘Hypernatraemia’ referred to corrected serum sodium concentration Na≥146 mmol/L. Throughout the text, serum sodium concentrations and sodium groups refer to the corrected sodium values unless otherwise specified.
bmjopen-2023-075232supp001.pdf (379.1KB, pdf)
Demographic information such as ethnicity, sex at birth and age, as well as comorbidities categorised according to predetermined groups (additional information in the online supplemental information), home medication and presenting signs, and symptoms were compared between the groups and between normonatremia and different severity categories of hyponatraemia (online supplemental information). Serum concentrations of creatinine, urea, C reactive protein (CRP) and LDH were measured at the time of first presentation in the participating hospital. The estimated glomerular filtration rate (eGFR) was calculated using the 2021 Chronic Kidney Disease Epidemiology Collaboration formula based on serum creatinine levels.35 The Modified Early Warning Score (MEWS) and Quick Sequential Organ Failure Assessment (qSOFA) were calculated based on clinical values obtained at presentation.
The following clinical outcome measures were compared between the groups and across different severity categories: duration of hospitalisation, admission to ICU, invasive ventilation, duration of ICU admission, discharge alive, death and the administration of tocilizumab, sarilumab or anakinra. Additionally, the incidence of complications was compared between the groups.
Statistical analysis
All data were analysed using SPSS V.27. Comparisons were conducted among hypernatraemia, normonatremia and hyponatraemia (main text) and between the normonatremia, mild, moderate and severe hyponatraemia groups (online supplemental information). Baseline numerical data were presented as median and IQR, and the Kruskal-Wallis test was used for analysis when the data were not normally distributed. For normally distributed data, baseline numerical data were presented as mean and SD, and one-way Analysis of Variance (ANOVA) was employed for analysis. Baseline categorical data were displayed as absolute number and percentage of patients with the specific condition, and the χ2 test was used for analysis.
Outcome data (risk for ICU admission, intubation, mortality rates, use of tocilizumab, sarilumab, or anakinra, and complications) were assessed using a binary logistic regression model. The ORs were calculated and adjusted for age, sex assigned at birth (categorised as male or female based on genotype and internal and external anatomy at birth), a history of chronic kidney disease, and a history of hypertension. The duration of hospital and ICU admission was evaluated using a Kruskal-Wallis test. Survival analysis over a 6-week period from hospital admission was conducted using Cox proportional hazard regression analysis to estimate cumulative mortality rates and cumulative rates for being discharged alive for patients with and without dysnatraemia. The HRs were adjusted for age, sex assigned at birth, a history of chronic kidney disease and a history of hypertension.
A p value of ≤0.05 was considered statistically significant for all statistical tests. Patients who did not have data available for the specific variable being tested were excluded from the corresponding analysis.
Patients and public involvement
This study was largely conducted during the first waves of the COVID-19 pandemic. As a result, it was not feasible to directly involve patients in the design of the study. Patients received information about the CovidPredict database via pamphlets and verbal communication. Additionally, information was available on the websites of participating hospitals and through various media channels. Patients are referred to our website www.covidpredict.org for details regarding the study design and dissemination plans.
Results
Incidence of dysnatraemia at presentation
At the time of 9 August 2022, the database contained a total of 11 382 records. Serum sodium concentrations at admission were available for 8278 (73%) admissions from 7811 patients (170 duplicate entries due to readmission and 297 patients had been transferred from or previously admitted to another participating hospital and transfer records were therefore excluded). Patients were included based on two criteria: a positive result for SARS-CoV-2 PCR (6673 patients) and or a CO-RADS Score 4 or 5 in the absence of an alternative diagnosis (1138 patients). In cases where patients were readmitted, the admission with the abnormal sodium level at presentation (in case of hyponatraemia or hypernatraemia) or the first admission (in case sodium concentrations were normal for both presentations) was included in the analysis.
Of the 7811 included patients with COVID-19, 2677 (34.3%) presented with hyponatraemia (corrected blood serum Na≤134 mmol/L) and 126 (1.6%) presented with hypernatraemia (corrected blood serum Na≥146 mmol/L). Among the patients presenting with hyponatraemia, 1957 (25.1%) presented with blood serum Na ranging 131–134 mmol/L (considered ‘mild’), 582 (7.5%) presented with blood serum Na ranging 126–130 mmol/L (considered ‘moderate’) and 138 (1.8%) with blood serum Na≤125 mmol/L (considered ‘severe’) (see online supplemental figure 1). A total of 1888 patients were included after the start of the SARS-CoV-19 vaccination campaign in the Netherlands on 6 January 2021, of whom 445 were vaccinated (319 had received 2 or more doses). A total of 6186 patients (79.2%) started having symptoms prior to the 7th week of 2021, when the initial SARS-CoV-2 variants were most prevalent. In total, 800 patients (10.2%) developed symptoms from 7th to 25th week of 2021, when Alpha variants dominated in the Netherlands. A total of 700 patients (9.0%) started having symptoms when Delta variants dominated (26th to 51st week of 2021) and 122 patients (1.6%) when the Omicron variants dominated (after the 52nd week of 2021).36
Patient characteristics of patients presenting with dysnatraemia
Table 1 shows the characteristics of patients with hyponatraemia and hypernatraemia compared with patients presenting with normal sodium concentrations at presentation. Both hyponatraemia and hypernatraemia occurred more often in men than in women (table 1), except for ‘severe’ hyponatraemia (online supplemental table 1). The mean age of patients with and without hyponatraemia differed slightly, with patients presenting with ‘moderate’ or ‘severe’ hyponatraemia being significantly older (median age 68.1 and 70.6 years, respectively). Patients with hypernatraemia were also older, with a mean age of 72.5 years. The body mass index (BMI) of patients presenting with hyponatraemia tended to be slightly lower compared with those with normal sodium levels and was also lower in patients presenting with hypernatraemia. Abnormal sodium levels at presentation were associated with chronic kidney disease. Patients with hyponatraemia, particularly those with severe hyponatraemia, more frequently had a history of hypertension, but this difference was not statistically significant for the subgroup of patients who did not use diuretics (36.4% (normonatremia) versus 39.1% (hyponatraemia); p=0.003; determined by a χ2 test). The presence of hyponatraemia or hypernatraemia was not associated with a history of chronic heart, pulmonary or liver disease (refer to online supplemental table 2 for definitions). Regarding medication use, the use of thiazide diuretics was higher in patients with hyponatraemia (table 1), but the overall use of diuretics or the use of loop diuretics did not differ between the groups. Similarly, the use of selective serotonin (and noradrenalin) reuptake inhibitors did not show significant differences between the groups. The use of immunosuppressives was more common in patients presenting with hyponatraemia as compared with those with normal sodium concentration at presentation.
Table 1.
Comparison of patient characteristics between COVID-19 patients with hyponatraemia, normonatremia and hypernatraemia
| Hyponatraemia Na≤134 mmol/L N=2677 |
Normonatremia Na 136–145 mmol/L N=5008 |
Hypernatraemia Na≥146 mmol/L N=126 |
|
| Sex assigned at birth (N (%)) | Male 1673 (62.5%) Female 1003 (37.5%) P=0.002 |
Male 2946 (58.8%) Female 2060 (41.2%) |
Male 84 (66.7%) Female 42 (33.3%) |
| Age (median age in years (IQR)) | N=2675 67.0 (58.0–77.0) P<0.001 |
N=5008 66.1 (55.0–76.0) |
N=126 72.5 (62.8–80.3) P<0.001 |
| BMI (median BMI in kg/m2 (IQR)) | N=1740 27.2 (24.2–31.1) P=0.009 |
N=3374 27.7 (24.6–31.6) |
N=91 25.0 (22.2–29.1) P<0.001 |
| Order ‘do not intubate’ (N (%)) | 440/1442 (30.5%) | 796/2469 (32.2%) | 39/77 (50.6%) P=0.004 |
| Chronic cardiac disease (N (%)) | 760/2666 (28.5%) P=0.07 |
1334/4982 (26.8%) | 42/123 (34.1%) P=0.07 |
| Hypertension (N (%)) | 1055/2374 (44.4%) P=0.002 |
1889/4586 (41.2%) | 64/120 (53.3%) |
| Chronic pulmonary disease (N (%)) | 466/2662 (17.5%) P=0.75 |
844/4979 (17.0%) | 19/122 (15.6%) P=0.75 |
| Chronic kidney disease (N (%)) | 329/2379 (13.8%) P<0.001 |
491/4587 (10.7%) | 26/121 (21.5%) P<0.001 |
| Moderate to severe liver disease (N (%)) | 30/2662 (1.1%) P=0.46 |
50/4972 (1.0%) | 0/123 (0.0%) P=0.46 |
| Diabetes (N (%)) | 664/2662 (24.9%) P=0.39 |
1261/4972 (25.4%) | 38/125 (30.4%) P=0.39 |
| Immunosuppressives (N (%)) | 192/2283 (8.4%) P=0.002 |
295/4445 (6.6%) | 2/118 (1.7%) |
| Thiazide diuretics (N (%)) | 258/2671 (9.7%) P=0.015 |
394/4994 (7.9%) | 7/125 (5.6%) |
| Loop diuretics (N (%)) | 187/2671 (7.0%) P=0.22 |
389/4994 (7.8%) | 13/125 (10.4%) P=0.22 |
| SSRIs/SNRIs (N (%)) | 78/2671 (2.9%) P=0.69 |
164/4994 (3.3%) | 4/125 (3.2%) P=0.69 |
Significance was assessed using a Kruskal-Wallis test with post hoc correction (for numerical data; non-normally distributed) or χ2 test (for categorical data). P values for all groups indicate the adjusted significance after post hoc correction when compared with the normonatremia group. When no p value was provided, there was no significant difference compared with the normonatremia group. Subgroup analyses for hyponatraemia are provided in the online supplemental information.
%, percentage of patients in this group with indicated characteristic; BMI, body mass index; SNRI, selective serotonin and noradrenalin reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
Signs and symptoms of patients presenting with dysnatraemia
Patients with hyponatraemia more frequently presented with diarrhoea and anosmia compared with patients without hyponatraemia (table 2 and online supplemental table 2). The presence of vomiting or nausea as presenting symptoms was not associated with hyponatraemia. In the hypernatraemia group, confusion was more frequently observed compared with patients with normal sodium levels. A prolonged capillary refill time of ≥3 s, which may indicate dehydration, was more often present in the hypernatraemia group. Patients with hypernatraemia also had a slightly higher heart rate. Hyponatraemia was associated with a slightly higher heart rate and a slightly lower systolic blood pressure, although these differences were not clinically significant. Both patients with hypernatraemia and hyponatraemia had a lower eGFR, with a more pronounced effect observed in the hypernatraemia group (table 2). A lower eGFR was associated with slightly higher mortality rates (unadjusted HR 1.008, 95% CI 1.007 to 1.008; p=0.001, analysed using a Cox proportional hazard regression analysis), regardless of sodium levels at presentation or exclusion of patients with chronic kidney disease. Enhanced blood urea concentration was only associated with hypernatraemia.
Table 2.
Comparison of signs and symptoms at presentation between COVID-19 patients with hyponatraemia, normonatremia and hypernatraemia
| Signs and symptoms | Hyponatraemia Na≤134 mmol/L N=2677 |
Normonatremia Na 136–145 mmol/L N=5008 |
Hypernatraemia Na≥146 mmol/L N=126 |
| Nausea/vomiting (N (%)) | 679/2273 (29.9%) P=0.04 |
1150/4129 (27.9%) | 16/83 (19.3%) P=0.04 |
| Diarrhoea (N (%)) | 804/2298 (35.0%) P<0.001 |
1146/4157 (27.6%) | 15/82 (18.3%) |
| Anosmia (N (%)) | 244/1904 (12.8%) P=0.002 |
352/3330 (10.6%) | 1/66 (1.5%) |
| Confusion (N (%)) | 311/2319 (13.4%) | 651/4381 (14.9%) | 45/105 (42.9%) P<0.001 |
| Seizures (N (%)) | 10/1977 (0.5%) P=0.20 |
31/3452 (0.9%) | 0/80 (0.0%) P=0.20 |
| FiO2 (median fraction (IQR)) | N=1159 0.36 (0.28–0.50) P=0.05 |
N=2084 0.36 (0.28–0.50) |
N=67 0.44 (0.30–0.80) P=0.05 |
| SBP (mean SBP in mm Hg (SD)) | N=2648 132 (± 22) P<0.001 |
N=4971 135 (±23) |
N=120 135 (± 25) P=1.00 |
| HR (mean HR in BPM (SD)) | N=2661 92 (±18) P=0.003 |
N=4965 91 (±20) |
N=123 95 (±25) P=0.034 |
| Capillary refill≥3 s (N (%)) | 81/863 (9.4%) | 93/1369 (6.8%) | 6/33 (18.2%) P=0.008 |
| Blood urea level (median level n mmol/L (IQR)) | N=2549 6.3 (4.5–9.3) P=0.87 |
N=4776 6.2 (4.5–9.2) |
N=115 12.6 (7.9–25.3) P<0.001 |
| eGFR rate using 2021 CKD-epi creatinine equation in (median clearance in mL/min/1.73 m3 (IQR)) | N=2656 64 (45–90) P<0.001 |
N=4983 68 (46–94) |
N=125 41 (24–71) P<0.001 |
| CT severity score (mean score (SD)) | N=909 12.4 (±5.5) P=0.58 |
N=1401 12.1 (±5.6) |
N=30 14.5 (±7.2) P=0.06 |
| Blood CRP level (median level in mg/L (IQR)) | N=2646 93.1 (49.0–154) P<0.001 |
N=4939 70.8 (28.0–131) |
N=123 75.0 (29.0–148) P=1.00 |
| Blood LDH level (median level in U/L (IQR)) | N=2238 349 (268–471) P<0.001 |
N=4226 323 (247–426) |
N=89 363 (255–447) P=0.52 |
| MEWS (median score (IQR)) | N=2337 3.0 (2.0–4.0) P<0.001 |
N=4055 3.0 (2.0–4.0) |
N=103 4.0 (2.0–5.0) P<0.001 |
| qSOFA (median score (IQR)) | N=2373 1.0 (0.0–1.0) P=1.00 |
N=4131 1.0 (0.0–1.0) |
N=104 1.0 (1.0–1.0) P<0.001 |
Significance was assessed using a Kruskal-Wallis test with post hoc correction (for numerical data; non-normally distributed), one-way ANOVA (for numerical data; normally distributed) or χ2 test (for categorical data). P values for all groups indicate significance when compared with the normonatremia group. When no p value was provided, there was no significant difference to the normonatremia group. Subgroup analyses for hyponatraemia are provided in the online supplemental information.
%, percentage of patients in this group with indicated characteristic; BPM, beats per minute; CKD-epi, Chronic Kidney Disease Epidemiology Collaboration; CRP, C reactive protein; eGFR, estimated glomerular filtration rate; HR, heart rate; LDH, lactate dehydrogenase; MEWS, Modified Early Warning Score; qSOFA, Quick Sequential Organ Failure Assessment; SBP, systolic blood pressure.
Patients with hyponatraemia had higher blood CRP and LDH concentrations compared with those with normal sodium levels (table 2). However, the fraction of supplemented oxygen (FiO2) and CT severity scores did not differ significantly between the groups. The clinical score systems MEWS and qSOFA37 (table 2) also showed significant differences between the groups, but these differences were not clinically relevant.
Furthermore, patients with hyponatraemia had a slightly longer duration of complaints compared with those with normonatremia (8.8 days for hyponatraemia vs 8.6 days for normonatremia; p=0.010; assessed using a Kruskal-Wallis test), although this difference was not clinically relevant.
Clinical outcomes in patients presenting with dysnatraemia
Hypernatraemia was associated with higher mortality rates or palliative discharge rates compared with the normonatremia and hyponatraemia groups (table 3, figure 1, and online supplemental figure 2). Additionally, patients with hypernatraemia had a higher risk of ICU admission and invasive ventilation. However, hyponatraemia was not associated with increased mortality or palliative discharge rates (table 3). Although there was a trend towards increased mortality in patients with severe hyponatraemia, these results did not reach statistical significance due to the low number of patients that presented with sodium levels ≤125 mmol/L (online supplemental table 4). After excluding patients with a ‘do not intubate’ order, hyponatraemia was associated with a higher likelihood of ICU admission, but not with the need for invasive ventilation. Of all hyponatraemic patients admitted to the ICU (n=486), 62 (12.8%) did not receive any form of ventilatory support ((non-)invasive ventilation or high flow nasal therapy). This percentage was similar (10.5%; p=0.403) among patients with normonatremia admitted to the ICU. The duration of ICU admission was similar for patients with hyponatraemia, normonatremia and hypernatraemia (table 3). Based on the additional details provided in online supplemental table 5, patients with the order ‘do not intubate’ are considered frailer and thus had limited life expectancy.
Table 3.
Comparison of clinical outcomes between COVID-19 patients with hyponatraemia, normonatremia and hypernatraemia
| Outcome | Hyponatraemia Na≤134 mmol/L N=2677 |
Normonatremia Na 136–145 mmol/L N=5008 |
Hypernatraemia Na≥146 mmol/L N=126 |
| Duration of admission (median days (IQR)) | N=2372 7 (4–16) P<0.001 |
N=4116 7 (3–14) |
N=103 8 (4–15) P=0.998 |
| Death or palliative discharge (N (%)) | 405/2360 (17.2%) AOR 1.04 (0.91–1.20) P=0.56 |
729/4568 (16.0%) | 42/119 (35.3%) AOR 2.25 (1.49–3.41) P<0.001 |
| ICU admission (N (%)), ‘do not intubate’ excluded |
439/1923 (22.8%) AOR 1.27 (1.11–1.46) P<0.001 |
710/3778 (18.8%) | 32/80 (40.0%) AOR 2.89 (1.83–4.58) P<0.001 |
| Duration of ICU admission (days (IQR)) ‘do not intubate’ excluded |
N=299 8 (3–19) P=0.356 |
N=437 10 (4–19) |
N=25 11 (3.5–19) P=0.356 |
| Invasive ventilation (N (%)), ‘do not intubate’ excluded |
352/1889 (18.6%) AOR 1.12 (0.97–1.30) P=0.121 |
623/3706 (16.8%) | 29/77 (37.7%) AOR 2.95 (1.83–4.74) P<0.001 |
| Discharge alive within 42 days; N indicating the number of non-censored cases | N=1527 AHR 0.96 (0.90–1.02) P=0.15 |
N=2747 | N=52 AHR 0.78 (0.59–1.03) P=0.08 |
| Use of tocilizumab, sarilumab or anakinra (N (%)) | 134/688 (19.5%) AOR 1.256 (0.984–1.604) P=0.068 |
199/1245 (16.0%) | 3/34 (8.8%) AOR 0.550 (0.165–1.830) P=0.330 |
| Complications |
Na≤134 mmol/L
N=2677 |
Na 136–145 mmol/L
N=5008 |
Na≥146 mmol/L
N=126 |
| Bacterial pneumonia (N (%)) | 289/2212 (13.1%) AOR 1.12 (0.96–1.31) P=0.14 |
501/4307 (11.6%) | 18/109 (16.5%) AOR 1.44 (0.85–2.40) P=0.17 |
| Aspergillosis pneumonia (N (%)) | 67/1915 (3.5%) AOR 1.44 (1.03–1.99) P=0.031 |
83/3442 (2.4%) | 5/90 (5.6%) AOR 2.26 (0.89–5.74) P=0.084 |
| ARDS (N (%)) | 224/2223 (10.1%) AOR 1.08 (0.91–1.29) P=0.377 |
404/4323 (9.3%) | 17/110 (15.5%) AOR 1.78 (1.05–3.04) P=0.033 |
| Treatment for septic shock (N (%))* | 94/2153 (4.4%) AOR 1.33 (1.01–1.74) P=0.04 |
135/4175 (3.2%) | 12/109 (11.0%) AOR 3.37 (1.80–6.33) P<0.001 |
| Congestive heart failure (N (%)) | 64/2235 (2.9%) AOR 0.95 (0.70–1.29) P=0.73 |
125/4352 (2.9%) | 2/111 (1.8%) AOR 0.48 (0.12–1.96) P=0.31 |
| Physical decline (N (%)) | 576/2116 (27.2%) AOR 1.22 (1.08–1.38) P<0.001 |
950/4126 (23.0%) | 30/106 (28.3%) AOR 1.18 (0.77–1.82) P=0.44 |
| Delirium (N (%)) | 237/2136 (11.1%) AOR 0.99 (0.83–1.17) P=0.88 |
451/4146 (10.5%) | 27/107 (25.7%) AOR 2.25 (1.42–3.56) P<0.001 |
Significance was assessed using a Cox proportional hazard model at the mean of the covariates (discharge alive) or logistic regression (all other values). P values for all groups indicate significance when compared with the normonatremia group.
*Treatment for septic shock was defined as the need for vasopressors in order to maintain mean arterial blood pressure>65 mm Hg and blood lactate level <2 mmol/L, in the absence of other causes including hypovolaemia.
AHR, adjusted HR; HR adjusted for sex assigned at birth, age, a history of chronic kidney disease and a history of hypertension; AOR, adjusted OR; OR adjusted for sex assigned at birth, age, a history of chronic kidney disease and a history of hypertension; ARDS, acute respiratory distress syndrome; ICU, intensive care unit.
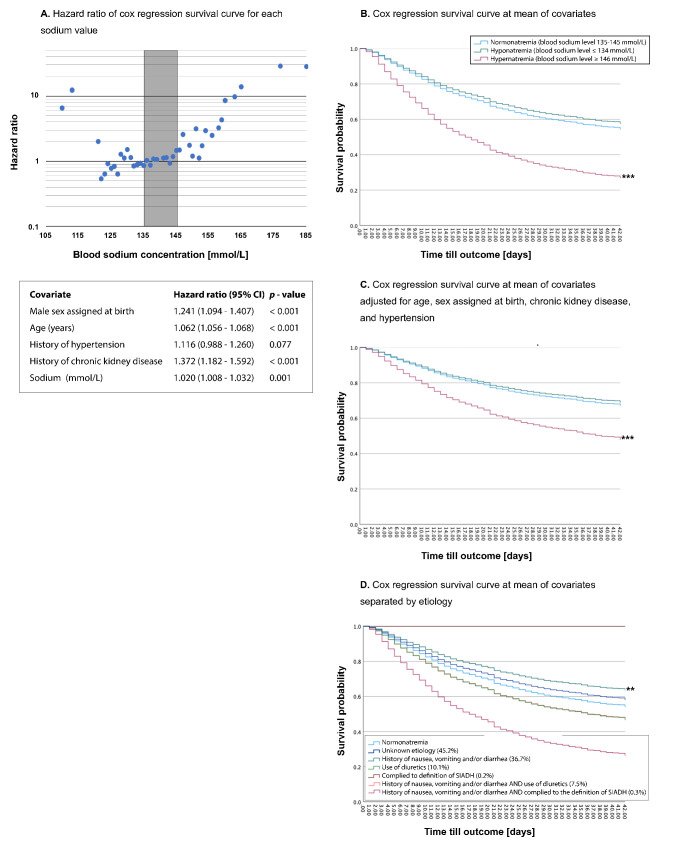
Figure 1.
HRs of Cox proportional survival curves for survival probability for each sodium value adjusted for age, sex assigned at birth, a history of chronic kidney disease and a history of hypertension. The grey area indicates the normonatremia. Table shows HRs for covariates and sodium as a continuous variable (A). Cox proportional survival curves at the mean of covariates for (B) unadjusted 6-week mortality stratified by normonatremia, hyponatraemia and hypernatraemia, (C) 6-week mortality adjusted for age, sex assigned at birth, a history of chronic kidney disease and a history of hypertension stratified in normonatremia, hyponatraemia and hypernatraemia, (D) unadjusted 6-week mortality stratified by aetiology. **Indicates a p value<0.01. ***Indicates a p value<0.001.
Hyponatraemia corrected for glucose was used for all statistical testing. However, as some other studies used uncorrected hyponatraemia,30 38 we also examined the association of uncorrected hyponatraemia with different outcomes. Without correction for serum glucose concentration, hyponatraemia was still associated with a slightly higher rate of ICU admission (adjusted OR (AOR) 1.40 (1.23–1.60); p<0.001) and with the need for intubation (AOR 1.26 (1.10–1.46); p=0.001), but not with death or palliative discharge rates (AOR 1.11 (0.97–1.28); p=0.13).
Despite the correlation with ICU admission in patients with hyponatraemia, the duration of admission was not significantly longer in this group. Similar outcomes were observed for patients with confirmed COVID-19 (SARS-CoV-2 PCR positive; 6673 patients) only, although in this subgroup, the higher risk for ICU admission for patients with hyponatraemia no longer reached statistical significance.
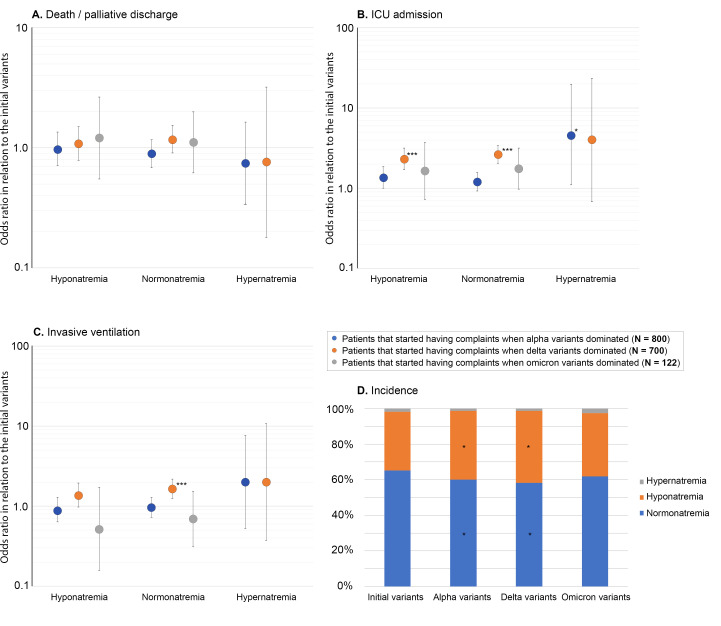
As the COVID-19 pandemic progressed, the incidence of adverse outcomes was significantly higher for patients with normonatremia and hyponatraemia at presentation that started having complaints when Delta variants dominated as compared with those admitted during the earlier COVID-19 waves when the initial variants dominated (figure 2). The use of tocilizumab, sarilumab (interleukin 6 receptor agonists) and anakinra (interleukin 1 receptor agonist) did not differ between the groups. The administration of COVID-19 vaccination was not reported frequently enough to draw conclusions about its possible effects on outcome measures.
Figure 2.
OR for adverse outcomes (death/palliative discharge (A), intensive care unit admission (B), invasive ventilation (C)) for each SARS-CoV-2 variant compared with patients in that started having symptoms when the initial variants for patients with hyponatraemia, hypernatraemia or normonatremia at admission. ***Indicates a p value<0.001 for the OR as calculated by binary logistic regression. (D) Incidence of hyponatraemia, normonatremia and hypernatraemia for each variant, *Indicates a p value<0.05 as compared with the first quartile for the χ2 statistic with Bonferroni post hoc correction.
Complications associated with hyponatraemia on admission
After adjusting for sex assigned at birth, age, a history of chronic kidney disease and hypertension, the course of disease of patients with hyponatraemia was more often complicated by an aspergillosis pneumonia (almost exclusively in patients that needed invasive ventilation and more frequently in patients treated with dexamethasone, antibiotics, tocilizumab, sarilumab or anakinra) and physical decline (the latter was scored when explicitly documented in the patients’ medical records, when the patient suffered from ‘ICU acquired weakness’ or when the patient was referred for medical rehabilitation).
Patients with hypernatraemia, on the other hand, were more likely to experience acute respiratory distress syndrome and receive treatment for septic shock (defined as the need for vasopressors in order to maintain mean arterial blood pressure>65 mm Hg and blood lactate level>2 mmol/L, in the absence of other causes including hypovolaemia). They also had a higher incidence of delirium. It should be noted that excessive fluid resuscitation for the management of hyponatraemia or hypernatraemia could potentially lead to congestive heart failure, but the occurrence of this complication was rare and did not occur more frequently in patients with abnormal sodium values at presentation.
USE related to patients’ characteristics and outcomes
USE was measured in 185 (6.9%) patients with hyponatraemia of whom 145 (78%) did not use diuretics. Among these patients, there were 48 with ‘mild’, 67 with ‘moderate’ and 30 with ‘severe’ hyponatraemia. The range of USE was 5.0–239 mmol/L, with a median of 30.0 mmol/L. Urinary osmolarity (UOL) was measured in 81 (3.0%) patients who did not use diuretics, including 26 with ‘mild’, 37 with ‘moderate’ and 18 with ‘severe’ hyponatraemia. The range of UOL values was 8–1007 mOsmol/kg, with a median of 496 mOsmol/kg. Among patients in whom both USE and UOL were measured, 12 patients (15% of the total) met the definition of SIADH (USE≥30 mmol/L and UOL≥100 mOsmol/kg in the absence of diuretic use and signs of hypovolaemia (systolic blood pressure<90 mm Hg or heart rate≥100 beats per minute)).
Patients were divided into two groups based on USE. Out of urinary sodium measurements, 72 patients (49.7%) had low USE (<30 mmol/L), indicating activation of the RAAS, while 73 patients (50.3%) had high USE (≥30 mmol/L), indicating inactivation of the RAAS (online supplemental table 6). A low USE was associated with higher levels of CRP (111 (52.5–163) mmol/L vs 70 (35.0–154) mmol/L; p=0.028) and LDH (351 (270–491) U/L vs 273 (227- 434) U/L; p=0.021) at presentation (online supplemental table 6), but was not associated with symptoms such as nausea/vomiting or clinical signs of hypovolaemia, such as tachycardia or hypotension. There were no significant differences in outcome measures, such as duration of admission, ICU admission or death/palliative discharge, between patients with a low and high USE.
Aetiology related to outcomes
Among the patients who presented with hyponatraemia, 983 patients (36.7%) reported a history of gastrointestinal symptoms, such as nausea, vomiting or diarrhoea, and did not use diuretics or met the criteria for SIADH. The prevalence of gastrointestinal symptoms was highest when Alpha variants dominated (online supplemental table 7). A total of 271 patients (10.1%) used diuretics in the absence of gastrointestinal symptoms and this percentage was higher for patients that started having symptoms during the Omicron wave (online supplemental table 7). In total, 12 (0.5%) patients who did not use diuretics complied to the definition of SIADH, of whom 5 also had gastrointestinal symptoms. All patients who complied to the definition of SIADH started having symptoms when the initial COVID-19 variants dominated (online supplemental table 7). Another group of 201 patients (7.5%) had a history of nausea, vomiting or diarrhoea and used diuretics. However, the largest portion of patients (1210 patients, 45.2%) had an unknown aetiology for hyponatraemia, as they did not have a history of gastrointestinal symptoms, did not use diuretics and did not meet the criteria for SIADH.
Figure 1D illustrates a Cox proportional hazard curve, with separate lines representing each proposed aetiology. It was observed that patients with a history of gastrointestinal symptoms had lower mortality rates compared with those with normal sodium levels (unadjusted HR 0.739, 95% CI 0.611 to 0.894; p=0.002), despite higher CRP (mean 95 mg/L, IQR 47.5–151) and LDH levels (mean 350 U/L, IQR 271–470) compared with normonatremia (p<0.001; assessed using a Kruskal-Wallis test). Patients with hyponatraemia of unknown aetiology had a higher risk of ICU admission (unadjusted OR 1.299, 95% CI 1.091 to 1.549; p=0.003; linear regression) and were at risk for intubation (unadjusted OR 1.313, 95% CI 1.109 to 1.554; p=0.002; linear regression), which was in line with higher CRP levels (mean 98 mg/L, IQR 53–166) and LDH levels (mean 353 U/L, IQR 270–479) in this group compared with normonatremia (p<0.001; assessed using a Kruskal-Wallis test). However, the duration of ICU admission did not differ significantly among the different groups. It was found that patients with hyponatraemia of unknown aetiology had a slightly longer duration of hospital admission (8 days, IQR 4–17) compared with other groups (p=0.005; assessed using the Kruskal-Wallis test).
Discussion
This large multicentre observational cohort study examined 7811 patients with COVID-19 over an extended period and multiple phases of the COVID-pandemic. We found that hyponatraemia was highly prevalent but not associated with higher mortality rates. Although less prevalent, hypernatraemia was associated with a threefold to fourfold increased risk of worse outcomes, including increased risk of ICU admission, intubation and mortality. Hyponatraemia was also associated with a higher risk for ICU admission, but not for intubation.
Patients with hyponatraemia experienced more complications such as aspergillosis pneumonia and physical decline, while those with hypernatraemia were more prone to sepsis and delirium. Similar to previous studies, hyponatraemia and hypernatraemia were more prevalent in men than in women, in elderly patients, those with chronic kidney disease and a lower BMI.9 15 17 26 28–30 38 In contrast to others, we did not find an association between hyponatraemia and diabetes, which possibly relates to the fact that we corrected sodium levels for serum glucose.9 15 17 26 30 Among patients with COVID-19, hyponatraemia appeared to have multiple aetiologies, but hypovolaemic hyponatraemia was found to be predominant.
The incidence of hyponatraemia among patients with COVID-19 in this study was 34.3%, which is higher than the pooled prevalence of hyponatraemia in previous systematic reviews which included studies conducted during the earlier COVID-19 waves, which was 24%–25.8%.7 11 However, it aligns with Tezcan et al,32 Voets et al 39 and Sarvazad et al 31 who reported rates of 34%, 35.8% and 38%, respectively (the latter study included only patients without underlying disease), although even higher incidences have been reported.10 28 40–42 The incidence of hyponatraemia in COVID-19 was also found to be higher compared with hyponatraemia in other types of pneumonia: 5.4%–28%.9 13 14 39 43 Hyponatraemia is most common in pneumonias caused by viral pathogens (eg, rhinovirus, respiratory syncytial virus, (para)influenza virus and adenovirus) with an incidence reported of 17.6%, as compared with 13.8% in patients with bacterial pneumonias.43 Patients presenting with hyponatraemia in this study were significantly older compared with patients with normonatremia, potentially due to age-related tubular atrophy and subsequent decreased urine concentrating capacity and sodium reabsorption.44 The fact that previous studies have identified various other underlying conditions as risk factors for hyponatraemia, including cardiac,17 pulmonary17 and liver diseases17 possibly relates to the older age of patients with hyponatraemia included (median age was 67 years in our study vs a mean age of 74.3 years in Chan et al 17 and a median age of 70 years in Ruiz-Sánchez et al.38).
Hypernatraemia is less common among patients with COVID-19 compared with other pneumonias. We found an incidence of 1.6% among patients with COVID-19. This number is lower than the incidences reported in previous studies (2.9%–38%)16 39 and lower than the incidence of hypernatraemia (5.3%) reported in patients with a community acquired pneumonia.45 Patients with hypernatraemia were found to be older than patients with normonatremia or hyponatraemia. These age differences were in line with the expected age-related impairment of the thirst mechanism and potential barriers to accessible fluids (eg, due to immobilisation or dementia), which could contribute to inadequate fluid intake with subsequent development of hypernatraemia.25
Hyponatraemia in infectious diseases can have multiple aetiologies, of which SIADH, hypovolaemia and the use of diuretics are the most common, but critical illness-related corticoid insufficiency is also reported.14 22 In this study, we showed that multiple aetiologies seem to play a role in patients with COVID-19. Among patients with hyponatraemia, a higher incidence of diarrhoea and anosmia was observed. These symptoms could contribute to decreased appetite and subsequently lower dietary intake. Clinical investigations revealed an increased heart rate and slightly decreased systolic blood pressure, which suggests a possible hypovolaemic state as an underlying cause for hyponatraemia. Correspondingly, eGFR was lower in this group, despite comparable blood urea levels, which have been employed by others as measure to differentiate euvolaemic from hypovolaemic hyponatraemia.29 This hypovolaemia could result from both reduced dietary intake and dehydration due to diarrhoea. The low median USE (30 mmol/L) in a proportion of patients also points to extrarenal sodium loss and a hypovolaemic status.46 However, due to the limited number of patients with USE measurements, these findings should be interpreted as supportive rather than definitive evidence.
Moreover, patients presenting with hyponatraemia had higher serum concentrations of LDH and CRP. A relationship between serum CRP and sodium concentration has been observed in other infectious diseases and has also been demonstrated in patients with COVID-19.17 28 41 This phenomenon has been attributed to release of cytokines such as interleukin 6 and interleukin 1β,47 which can affect the secretion of ADH and potentially contribute to the development of SIADH.23 48 In patients with COVID-19, elevated levels of interleukin 6 and interleukin 1β have been noted.30 49 50 Furthermore, a negative correlation between interleukin 6 and sodium levels has been demonstrated, implying a similar mechanism in the development of hyponatraemia.3 29 It is important to note that although administration of interleukin 6 receptor antagonists (tocilizumab and sarilumab) and interleukin 1 receptor antagonist (anakinra) was similar between groups, this observation does not undermine the afore-mentioned hypothesis, as these agents were administered based on indirect markers of interleukin release such as disease severity and CRP levels. Additionally, most patients in the study were included before registration of these agents for COVID-19 treatment, and the sample sizes of the groups might have been too small to draw definitive conclusions on the relationship between cytokine levels and hyponatraemia in patients with COVID-19.
Contrary to previous studies and in contrast to patients with community acquired pneumonia, we did not find SIADH as a frequent cause of hyponatraemia in patients with COVID-19.8 11 30 51 In our study, only a small proportion of USE+UOL samples complied with the definition of SIADH, and a correlation between low USE and serum CRP concentration was found, which is in contrast to the theory that interleukin 6 induces ADH release (online supplemental table 6). The overall incidence of SIADH in our study suggests that SIADH is a less frequent cause of hyponatraemia among patients with COVID-19, compared with hyponatraemia in patients with other pneumonias. This is possibly because COVID-19 more often causes diarrhoea, thereby also leading to other causes of hyponatraemia. Frontera et al 30 reported a prevalence of 36% of SIADH among patients with COVID-19 that presented with a serum sodium level≤120 mmol/L. However, in our study population, less than 1% presented with a sodium level this low, and mild and severe hyponatraemia differ in pathophysiology. Previous studies that identified SIADH as a frequent underlying mechanism of hyponatraemia in patients with COVID-19 based their information mostly on case reports, which likely focused on more severe cases.11 The fact that in our study, urinary investigation was not performed in all patients with hyponatraemia may suggest that hyponatraemia was not persistent or was otherwise not found to be severe enough to do so. This could also contribute to the lower incidence of confirmed SIADH cases in our study.
The association between thiazide diuretics and hyponatraemia is well-established. Thiazide diuretics are known to increase the risk of developing hyponatraemia due to their effects on renal sodium and water excretion.52 Therefore, it is not surprising that patients with hyponatraemia more frequently used thiazide diuretics. The use of immunosuppressive medications, such as glucocorticoids, was also related to hyponatraemia. Glucocorticoids can potentially affect the body’s water and electrolyte imbalance, including sodium levels. The development of iatrogenic adrenal insufficiency, resulting from the (prior) prescription of steroids, can contribute to relative glucocorticoid efficiency and potentially lead to hyponatraemia.53 54
We did not find a significant association between hyponatraemia and the risk of mortality or intubation, although ICU admission rates were higher in the hyponatraemia group. These results are in line with Machiraju et al,41 who also demonstrated a higher need for ICU admission in patients with COVID-19 presenting with hyponatraemia but could not relate hyponatraemia to mortality nor the length of hospital stay. Consistent with our results, Tzoulis et al 29 found no significant association between hyponatraemia and mortality but did relate hyponatraemia to invasive ventilation and the length of hospital admission. The higher serum CRP and LDH concentrations in hyponatraemic patients in our study indicate that these patients might be more ill compared with those with normal sodium levels, which is not in line with the similar mortality rates.55 56 Moreover, 13% of all patients admitted to the ICU did not receive any form of ventilatory support, suggesting that there were reasons other than respiratory failure for ICU admission. The fact that this percentage was similar among patients with normonatremia suggests that hyponatraemia was not a frequent reason for ICU admission. We speculate that dehydration accompanied by hyponatraemia, along with elevated LDH and CRP levels were reasons for hospital admission. However, other pathophysiologic mechanisms leading to worse outcomes were absent in these patients, favouring a relatively good outcome.
Our findings are in contrast with previous studies, in which the presence of hyponatraemia at presentation was independently associated with disease severity and prolonged hospital stay17 43 and was thought to be an independent predictor of hospital mortality.7 8 11 15 17 These studies suggest that hyponatraemia, especially when not corrected for serum glucose concentration,57 is a significant factor in determining the prognosis of patients. The observed trend towards increased mortality in patients with severe hyponatraemia was also demonstrated by Ruiz-Sánchez et al.,38 Chan et al 17 and Frontera et al. 30 However, the latter study obtained statistically significant results with a lower number of patients (36 out of 4645, representing 1% of the population, stratified as having severe hyponatraemia based on sodium levels≤120 mmol/L) compared with 1.8% in our study.
There are several potential explanations for the difference in outcomes between our study and previous studies. First, previous studies only included patients that were admitted during 2020 and the spring of 2021, the beginning of the COVID-19 pandemic.7 8 11 15 16 In large previous studies, mortality rates between 22.6% and 28.9% have been reported.9 57 In contrast, our study included patients from the beginning of the COVID-19 pandemic until August 2022 and the overall mortality in our study was 16.7% (despite an increased risk for ICU admission and intubation for hyponatraemic patients that started having complaints when the Delta variant dominated). These differences in outcomes are likely attributed to increased knowledge about the disease, the development of new treatments such as dexamethasone and tocilizumab and the commencement of widespread vaccination campaigns starting in January 2021. It is important to note that a study by Chan et al 17 included patients from late 2021 and early 2022 and still found an association between hyponatraemia and adverse outcomes. However, these results may not be directly comparable to our study due to potential differences in vaccine efficacy and COVID-19 policies between Hong Kong and Western countries.58 These variations in patient cohorts and treatment strategies could influence outcomes and thus could lead to different results as compared with other studies. We speculate that the absence of a higher risk of adverse outcomes in patients with COVID-19 presenting with hyponatraemia, contrary to previous studies, could be partly attributed to the overall decrease in mortality as the pandemic progressed.
Second, previous studies examined uncorrected sodium concentration at presentation as a prognostic factor and found increased mortality rates in patients with hyponatraemia.10 11 15 26 28 30 32 38 59 However, other studies that corrected for serum glucose concentration when these exceeded 10 mmol/L, found no significant association between hyponatraemia and mortality.29 Hirsch et al 57 demonstrated that the association between hyponatraemia and mortality was only evident prior to correction for serum glucose concentration, and the association disappeared after correcting for glucose levels. These findings are similar to studies conducted outside the context of COVID-19.60 In our study, uncorrected hyponatraemia was associated with an elevated risk of ICU admission and intubation, whereas corrected hyponatraemia did not show an association between hyponatraemia and intubation. This suggests that a similar effect related to the correction of sodium levels for glucose concentration could explain the discrepancies between our study and previous studies.30 38
The association between ICU admission and hyponatraemia was most pronounced in patients with a hyponatraemia of unknown aetiology. However, it is important to consider that this group may include mild presentations of SIADH due to the limited number of urinary samples available. These findings align with the higher CRP and LDH levels observed in this group. Patients who had a history of gastrointestinal symptoms had a lower risk of ICU admission, despite having higher levels of CRP and LDH levels. The higher CRP and LDH levels in this group could not be related to the SARS-CoV-2 variants, as the highest CRP levels were observed in patients that developed symptoms during a period in which the Delta variant dominated. Notably, this group also had the lowest prevalence of gastrointestinal symptoms (data not shown). We suggest that the prevalence of SIADH in our study group was very low for two reasons. First, we included patients during later COVID-19 waves (when Alpha, Delta and Omicron variants dominated), whereas patients with hyponatraemia due to SIADH that was severe enough to perform urinary analysis presented mostly during the period where initial variants dominated. This could have resulted in a lower prevalence than studies that only included patients during the first COVID-19 wave. Second, SIADH can only be diagnosed based on USE and UOL, but only a limited number of urinary samples were available, so we were not able to provide a precise estimate.
In contrast to the findings in patients with hyponatraemia, our study revealed a significant association between hypernatraemia and adverse outcomes such as ICU admission, intubation and death. While there were no significant differences in serum CRP and LDH concentration, as well as CT severity scores at admission, between hypernatraemic and normonatremic patients, higher MEWS and qSOFA scores indicated that a greater extent of lung tissue in hypernatraemic patients. Furthermore, elevated serum urea concentration, lower eGFR and a prolonged capillary refill time suggested dehydration in this group of patients. These findings collectively point towards a more severely ill patient population, which could account for the worse clinical outcomes observed. The association between hypernatraemia and worse clinical outcomes has been previously documented in COVID-1915 19 and other type of pneumonias.45
Our study on hyponatraemia in COVID-19 is characterised by its large size, including over 7000 patients from various hospitals across the Netherlands. A notable strength of our study lies in the inclusion of patients from different waves of the COVID-19 and from multiple hospitals, both university and general. This approach resulted in a diverse patient population, making our findings applicable to the current situation. Furthermore, our study benefitted from the availability of a large amount of clinical data being available for each patient. This allowed us to analyse the associations we discovered in conjunction with relevant patient background details. For instance, we had access to vital signs recorded at admission, providing us with a more comprehensive understanding of the patients’ condition on admission compared with previous studies.30 38 Consequently, we were able to offer more substantiated insights into the presumed underlying aetiology and how the different aetiologies were related to clinical outcomes.
This study has several limitations that should be acknowledged. First, the availability of urinary samples of patients with hyponatraemia (185 out of the total) limits the generalisability of our findings. Additionally, information on the duration of hyponatraemia in participating patients was not provided. Exploring these aspects would have been valuable, as a previous study by de La Flor et al 61 demonstrated that persistent hyponatraemia (72–96 hours after admission) was associated with higher mortality in patients with COVID-19. Second, the variability in treatment protocols among the participating hospital may have influenced the outcome of patients in our study. Lastly, we were unable to study specific treatment options for hyponatraemia in patients.
Our results suggest that while hyponatraemia is commonly observed among patients with COVID-19, it is not associated with adverse clinical outcome. However, the presence of hypernatraemia should be of concern to clinicians, as it is indicative of a poorer prognosis. To enhance our understanding of the aetiology of hyponatraemia in COVID-19, future studies should focus on monitoring the clinical course of hyponatraemia during hospitalisation, documenting the duration of hyponatraemia and recording the treatment administered. It is crucial to obtain urinary samples from all patients presenting with COVID-19 and hyponatraemia to further elucidate the underlying causes. Moreover, further research is warranted to investigate the incidence and potential mechanisms of SIADH in relation to disease severity and inflammation. More specifically, studies examining the relationship with interleukin 6 would be valuable, given that the interleukin 6 antagonist tocilizumab is used in the treatment of patients with moderate to severe COVID-19.
Conclusion
Hyponatraemia is a common electrolyte disorder found in one third of patients hospitalised with COVID-19. Several risk factors have been identified, including male sex assigned at birth, a slightly lower BMI, pre-existing conditions such as chronic kidney disease, hypertension, as well as the use of certain medications such as the use of thiazide diuretics and immunosuppressives. We found that hyponatraemia was not associated with a higher need for invasive ventilation nor with mortality. In contrast, hypernatraemia was associated with worse outcomes as compared with normonatremia. Regarding the underlying pathophysiological mechanisms, hypovolaemic hyponatraemia appeared to be the predominant mechanism in patients with COVID-19. Other causes of hyponatraemia, such as SIADH, were less commonly observed in our study population.
Supplementary Material
Acknowledgments
We want to acknowledge the contribution of the CovidPredict working group, including the clinicians that contributed to data collection and the students responsible for data entry, and we would like to acknowledge E Martens for his help with the analysis.
Footnotes
Collaborators: Steering committee: Martijn Beudel, neurologist, Amsterdam UMC; Brent Appelman, MD, PhD student, Amsterdam UMC; Renée Douma, internist–infectiologist, Flevo Hospital; Daisy Rusch, epidemiologist, Martini Hospital; Suat Simsek, internist–endocrinologist, Noordwest Hospitals; Professor Kees Brinkman, internist–infectiologist, Onze Lieve Vrouwen Gasthuis; Niels Gritters van den Oever, intensivist, Treant Hospitals; Professor Joop van den Bergh, internist–endocrinologist, Viecuri Medical Centre; Hazra Moeniralam, internist–intensivist–endocrinologist, St. Antonius Hospital; Neyma Bokhizzou, internist, BovenIJ Hospital. Martijn de Kruif, pulmonologist, Zuyderland Medical Centre; Helen Leavis, internist–immunologist, University Medical Centre Utrecht. Research during the project, many professionals became directly involved in the project, including: Dan Piña-Fuentes, MD, postdoctoral researcher, Amsterdam UMC. Rajat Thomas, data scientist, Amsterdam UMC. Derk Arts, CEO Castor EDC. Nick Nurmohamed MD, PhD student, Amsterdam UMC. Rens Reeskamp, MD, PhD student, Amsterdam UMC. Shi Hu MSc, PhD student, University of Amsterdam. Willem Herter, BSc, director, Pacmed. Bas Vonk, MSc, software engineer, Pacmed. Ricardo Lopes, PhD student, Amsterdam UMC. Lucas Ramos, data scientist, Amsterdam UMC. Deborah Huberts, BSc, student technical medicine, University of Twente. Marije Wolfers, data scientist, Amsterdam UMC. Michiel Schinkel, PhD student, Amsterdam UMC. Sander de Kuij, data scientist, Maastricht UMC+. Tom Dormans, intensive care specialist, Zuyderland Medical Centre. Support staff: Amsterdam UMC: Sophie Noordzij, Sibbeliene van den Bosch, Benthe verhoef, Michele Methorst, Shahan Darwesh, Tijn van Egmond, Gulsum Nasim, Hamza Ali, Lars de Boer, Myrthe Nagel, Maud Koenis, Agnetha Bijlsma, Britt Balvers, Isabella Ghauharali, Jelle de Jongh, Maira Emanuel, Nisrine Aynaou, Rosemarie de Ridder, Insaff Darraz, Oumaima Darraz, Alanar Cinar, Jesse Roosen, Larissa Heideman, Asabi Leliveld, Dana Ruijter, Neeltje Rosenberg, Djoeke Woutman, Tom Vermeulen, Gwendolyn Telting, Sam de Joode, Willem Berger, Thomas Ludden, Özgü varan, Soesja Pinto, Sientje Sluis, Bibi Kuiper, Tim van der Putten, Dominique Bosje, Mahunda Sinyangwe, Roos van Rhijn, Koen Kruif. Flevo Hospital: Lianne de Haan, Miriam den Heijer, Koen de Kruijf, Sam Hofhuis, Asabi Leliveld, Tom Vermeulen, Gwendolyn Telting, Sam de Joode, Lars de Boer, Rosemarie de Ridder, Susan van der Lei, Pien van Paassen, Emil ter Veer, MD, Krijna Opschoor, Camille Breukhoven, Florine Jiwa, Peter de Gooyer, Jan Vrijdag, Stans van Gelder, Anne Pannekoek, Laura Dommershuijzen. Viecuri Medical Centre: Estelle Adang, Maud van Maren, Isabel Koop, Bart Sanders, Joukje Wanten, Dax Trommelen, Fleur Smeets, Kyra Heuvelings, Maud Cox, Milou Rademaekers, Vivian Hendrikx, Ilona van Rooij—Tieleman, Wendy Heuts. Noordwest Hospitals: Nelly Wijdenes, Laura Oudeman, Evelien Brans, Karin Slot, Kirsten Boerma-Argelo, Wilma Kok, Lieke Harmsen, Ingrid Boerema. Maastricht UMC+: Anita Vinke, Anne Pirson, Anne Raafs, Anne Smaal, Bibiane Pop, Babette Verkouteren, Carmen Waterink, Casper Vrij, Daphne van der Willik, Deborah Hubers, Esther Lems, Frederic Reitsma, Imogene Pieters, Jade Logger, Laura Sijben, Maikel Peeters, Mariël Teunissen, Marije Blok-Hoos, Marjolein Ketels, Michiel Henkens, Mireille Spanjers, Monique Jacob-Dols, Patrickde Hoogt, Rik Houben, Robert-Jan Goldhoorn, Roos van Gorp, Wouter Hinsenveld, Josien Jansen.
Contributors: LdH, MtW and RD conceptualised and designed the study and were responsible for the planning, conduct, data analysis and interpretation. LdH drafted the article, supervised by MtW and RD. Figures and tables were designed by LdH. LdH, MtW, RD, MB, RHGOE, BA, EKH-H, DR, NCGvdO, SS, NP, JPvdB, CEW, MDdK, TD, HM, NB and KB were responsible for the inclusion of patients and data entry in the COVID-PREDICT database in their respective centres on behalf of the COVID-PREDICT study group. MB, RHGOE, BA, EKH-H, DR, NCGvdO, SS, NP, JPvdB, CEW, MDdK, TD, HM, NB and KB critically revised the manuscript and online supplemental material. All authors provided final approval of the manuscript and accepted responsibility for the integrity and accuracy of the work. They also ensued that any inquiries regarding the work’s integrity or accuracy would be thoroughly investigated and resolved. LdH, MtW and RD are the guarantors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
The Dutch COVID-PREDICT study group:
Martijn Beudel, Brent Appelman, Renée Douma, Daisy Rusch, Suat Simsek, Kees Brinkman, Niels Gritters van den Oever, Joop van den Bergh, Hazra Moeniralam, Neyma Bokhizzou, Martijn de Kruif, Helen Leavis, Dan Piña-Fuentes, Rajat Thomas, Derk Arts, Nick Nurmohamed, Rens Reeskamp, Shi Hu, Willem Herter, Bas Vonk, Ricardo Lopes, Lucas Ramos, Deborah Huberts, Marije Wolfers, Michiel Schinkel, Sander de Kuij, Tom Dormans, Sophie Noordzij, Sibbeliene van den Bosch, Benthe Verhoef, Michele Methorst, Shahan Darwesh, Tijn van Egmond, Gulsum Nasim, Hamza Ali, Lars de Boer, Myrthe Nagel, Maud Koenis, Agnetha Bijlsma, Britt Balvers, Isabella Ghauharali, Jelle de Jongh, Maira Emanuel, Nisrine Aynaou, Rosemarie de Ridder, Insaff Darraz, Oumaima Darraz, Alanar Cinar, Jesse Roosen, Larissa Heideman, Asabi Leliveld, Dana Ruijter, Neeltje Rosenberg, Djoeke Woutman, Tom Vermeulen, Sam de Joode, Willem Berger, Thomas Ludden, Ozgu Varan, Soesja Pinto, Sientje Sluis, Bibi Kuiper, Tim van der Putten, Dominique Bosje, Mahunda Sinyangwe, Roos van Rhijn, Koen Kruif, Lianne de Haan, Miriam den Heijer, Koen de Kruijf, Sam Hofhuis, Asabi Leliveld, Tom Vermeulen, Gwendolyn Telting, Lars de Boer, Rosemarie de Ridder, Susan van der Lei, Pien van Paassen, Emil ter Veer, Krijna Opschoor, Camille Breukhoven, Florine Jiwa, Peter de Gooyer, Jan Vrijdag, Stans van Gelder, Anne Pannekoek, Laura Dommershuijzen, Estelle Adang, Maud van Maren, Isabel Koop, Bart Sanders, Joukje Wanten, Dax Trommelen, Fleur Smeets, Kyra Heuvelings, Maud Cox, Milou Rademaekers, Vivian Hendrikx, Ilona van Rooij, Wendy Heuts, Laura Oudeman, Evelien Brans, Karin Slot, Kirsten Boerma-Argelo, Wilma Kok, Lieke Harmsen, Ingrid Boerema, Anne Pirson, Anne Raafs, Anne Smaal, Bibiane Pop, Babette Verkouteren, Carmen Waterink, Casper Vrij, Daphne van der Willik, Deborah Hubers, Esther Lems, Frederic Reitsma, Imogene Pieters, Jade Logger, Laura Sijben, Maikel Peeters, Mariël Teunissen, Marije Blok-Hoos, Marjolein Ketels, Michiel Henkens, Mireille Spanjers, Monique Jacob-Dols, Patrick de Hoogt, Rik Houben, Robert-Jan Goldhoorn, Roos van Gorp, Wouter Hinsenveld, and Josien Jansen
Data availability statement
No data are available. Not all patients provided active informed consent, and therefore sharing data is not possible.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Amsterdam UMC; 20.131. Participants gave informed consent to participate in the study before taking part.
References
- 1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Horby P, Lim WS, et al. , RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 2021;384:693–704. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berni A, Malandrino D, Parenti G, et al. Hyponatremia, IL-6, and SARS-Cov-2 (COVID-19) infection: may all fit together J Endocrinol Invest 2020;43:1137–9. 10.1007/s40618-020-01301-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu Y, Hou B, Liu J, et al. n.d. Risk factors associated with long-term hospitalization in patients with COVID-19: a single-centered, retrospective study. Front Med;7. 10.3389/fmed.2020.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. COVID-PREDICT-werkgroep . Klinisch Beloop Van COVID-19 in Nederland. NTVG 2021;165. [PubMed] [Google Scholar]
- 6. Malieckal DA, Uppal NN, Ng JH, et al. Electrolyte abnormalities in patients hospitalized with COVID-19. Clin Kidney J 2021;14:1704–7. 10.1093/ckj/sfab060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akbar MR, Pranata R, Wibowo A, et al. n.d. The prognostic value of hyponatremia for predicting poor outcome in patients with COVID-19: a systematic review and meta-analysis. Front Med;8. 10.3389/fmed.2021.666949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ayus JC, Kalantar-Zadeh K, Tantisattamo E, et al. Is Hyponatremia a novel marker of inflammation in patients with COVID 19? Nephrol dial transplant 2023 Nephrology Dialysis Transplantation 2023;38:1921–4. 10.1093/ndt/gfad111 [DOI] [PubMed] [Google Scholar]
- 9. Liu D, Mowrey W, Fisher M, et al. Associations of dysnatremia with COVID-19 status and mortality. Kidney360 2022;3:1323–31. 10.34067/KID.0001062022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Islam MK, Hasan P, Sharif MM, et al. Hyponatremia in COVID-19 patients: experience from Bangladesh. Health Sci Rep 2022;5:e565. 10.1002/hsr2.565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khidir RJY, Ibrahim BAY, Adam MHM, et al. Prevalence and outcomes of hyponatremia among COVID-19 patients: a systematic review and meta-analysis. Int J Health Sci (Qassim) 2022;16:69–84. [PMC free article] [PubMed] [Google Scholar]
- 12. Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med 2006;119:S30–5. 10.1016/j.amjmed.2006.05.005 [DOI] [PubMed] [Google Scholar]
- 13. Gustafson BD, Zhao Y, Milkovits AE, et al. Incidence of hyponatremia among critically ill patients with and without COVID-19 infection at a community teaching hospital. J Intensive Care Med 2023;38:911–6. 10.1177/08850666231170760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liamis G, Milionis HJ, Elisaf M. Hyponatremia in patients with infectious diseases. J Infect 2011;63:327–35. 10.1016/j.jinf.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 15. Królicka A, Letachowicz K, Adamik B, et al. Dysnatremia in COVID-19 patients-an analysis of the COLOS study. J Clin Med 2023;12:2802. 10.3390/jcm12082802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tzoulis P, Grossman AB, Baldeweg SE, et al. MANAGEMENT OF ENDOCRINE DISEASE: dysnatraemia in COVID-19: prevalence, prognostic impact, pathophysiology, and management. Eur J Endocrinol 2021;185:R103–11. 10.1530/EJE-21-0281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan GCK, Wong CK, So BYF, et al. n.d. Epidemiology and outcomes of hyponatremia in patients with COVID-19-A territory-wide study in Hong Kong. Front Med;9. 10.3389/fmed.2022.1096165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Genovesi S, Regolisti G, Rebora P, et al. Negative prognostic impact of electrolyte disorders in patients hospitalized for COVID-19 in a large multicenter study. J Nephrol 2023;36:621–6. 10.1007/s40620-022-01429-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shrestha AB, Sapkota UH, Shrestha S, et al. Association of hypernatremia with outcomes of covid-19 patients: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 2022;101:e32535. 10.1097/MD.0000000000032535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sabaghian T, Honarvar M, Safavi-Naini SAA, et al. Effect of electrolyte imbalance on mortality and late acute kidney injury in hospitalized COVID-19 patients. Iran J Kidney Dis 2022;16:228–37. [PubMed] [Google Scholar]
- 21. Rondon-Berrios H, Agaba EI, Tzamaloukas AH. Hyponatremia: pathophysiology, classification, manifestations and management. Int Urol Nephrol 2014;46:2153–65. 10.1007/s11255-014-0839-2 [DOI] [PubMed] [Google Scholar]
- 22. Honore PM, Redant S, Preseau T, et al. Understanding the underlying mechanisms of hyponatremia in coronavirus disease 2019 is critical since treatment varies based on etiology: let us not forget critical illness-related corticosteroid insufficiency as the treatment is very different and often lifesaving Crit Care Med 2021;49:e724–5. 10.1097/CCM.0000000000005006 [DOI] [PubMed] [Google Scholar]
- 23. Hodax JK, Bialo SR, Yalcindag A. SIADH in systemic JIA resolving after treatment with an IL-6 inhibitor. Pediatrics 2018;141. 10.1542/peds.2016-4174 [DOI] [PubMed] [Google Scholar]
- 24. Salama C, Mohan SV. Tocilizumab in patients hospitalized with COVID-19 pneumonia. reply. N Engl J Med 2021;384:1473–4. 10.1056/NEJMc2100217 [DOI] [PubMed] [Google Scholar]
- 25. Kugler JP, Hustead T. Hyponatremia and hypernatremia in the elderly. Am Fam Physician 2000;61:3623–30. [PubMed] [Google Scholar]
- 26. Hu W, Lv X, Li C, et al. Disorders of sodium balance and its clinical implications in COVID-19 patients: a multicenter retrospective study. Intern Emerg Med 2021;16:853–62. 10.1007/s11739-020-02515-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martino M, Falcioni P, Giancola G, et al. Sodium alterations impair the prognosis of hospitalized patients with COVID-19 pneumonia. Endocr Connect 2021;10:1344–51. 10.1530/EC-21-0411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ayus JC, Negri AL, Moritz ML, et al. n.d. Hyponatremia, inflammation at admission, and mortality in hospitalized COVID-19 patients: a prospective cohort study. Front Med;8. 10.3389/fmed.2021.748364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tzoulis P, Waung JA, Bagkeris E, et al. Dysnatremia is a predictor for morbidity and mortality in hospitalized patients with COVID-19. J Clin Endocrinol Metab 2021;106:1637–48. 10.1210/clinem/dgab107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frontera JA, Valdes E, Huang J, et al. Prevalence and impact of hyponatremia in patients with coronavirus disease 2019 in New York City. Crit Care Med 2020;48:e1211–7. 10.1097/CCM.0000000000004605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sarvazad H, Cahngaripour SH, Eskandari Roozbahani N, et al. Evaluation of electrolyte status of sodium, potassium and magnesium, and fasting blood sugar at the initial admission of individuals with COVID-19 without underlying disease in Golestan hospital, Kermanshah. New Microbes New Infect 2020;38:100807. 10.1016/j.nmni.2020.100807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tezcan ME, Dogan Gokce G, Sen N, et al. Baseline electrolyte abnormalities would be related to poor prognosis in hospitalized coronavirus disease 2019 patients. New Microbes New Infect 2020;37:100753. 10.1016/j.nmni.2020.100753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prokop M, van Everdingen W, van Rees Vellinga T, et al. CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluation. Radiology 2020;296:E97–104. 10.1148/radiol.2020201473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hillier TA, Abbott RD, Barrett EJ. Hyponatremia: evaluating the correction factor for hyperglycemia. Am J Med 1999;106:399–403. 10.1016/s0002-9343(99)00055-8 [DOI] [PubMed] [Google Scholar]
- 35. Inker LA, Eneanya ND, Coresh J, et al. New Creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 2021;385:1737–49. 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Environment NIfPHat . Variants of the Coronavirus SARS-Cov-2. 2023. Available: https://wwwrivmnl
- 37. van der Woude SW, van Doormaal FF, Hutten BA, et al. Classifying sepsis patients in the emergency department using SIRS, qSOFA or MEWS. Neth J Med 2018;76:158–66. [PubMed] [Google Scholar]
- 38. Ruiz-Sánchez JG, Núñez-Gil IJ, Cuesta M, et al. Prognostic impact of hyponatremia and hypernatremia in COVID-19 pneumonia. A HOPE-COVID-19 (health outcome predictive evaluation for COVID-19) Registry analysis. Front Endocrinol (Lausanne) 2020;11:599255. 10.3389/fendo.2020.599255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Voets PJ, Frölke SC, Vogtländer NP, et al. COVID-19 and dysnatremia: a comparison between COVID-19 and non-COVID-19 respiratory illness. SAGE Open Med 2021;9:20503121211027778. 10.1177/20503121211027778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taci Hoca N, Berktaş BM. Baseline electrolyte disorders predict disease severity and mortality in patients with COVID-19. Medicine (Baltimore) 2022;101:e32397. 10.1097/MD.0000000000032397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Machiraju PK, Alex NMSafinaaz et al. Hyponatremia in coronavirus disease-19 patients: a retrospective analysis. Can J Kidney Health Dis 2021;8:20543581211067069. 10.1177/20543581211067069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sjöström A, Rysz S, Sjöström H, et al. Electrolyte and acid-base imbalance in severe COVID-19. Endocr Connect 2021;10:805–14. 10.1530/EC-21-0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Królicka AL, Kruczkowska A, Krajewska M, et al. Hyponatremia in infectious diseases-A literature review. Int J Environ Res Public Health 2020;17:5320. 10.3390/ijerph17155320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chang-Panesso M. Acute kidney injury and aging. Pediatr Nephrol 2021;36:2997–3006. 10.1007/s00467-020-04849-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tokgöz Akyil F, Akyil M, Çoban Ağca M, et al. Hyponatremia prolongs hospital stay and hypernatremia better predicts mortality than hyponatremia in hospitalized patients with community-acquired pneumonia. Tuberk Toraks 2019;67:239–47. 10.5578/tt.68779 [DOI] [PubMed] [Google Scholar]
- 46. Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Intensive Care Med 2014;40:320–31. 10.1007/s00134-014-3210-2 [DOI] [PubMed] [Google Scholar]
- 47. Park SJ, Shin JI. Inflammation and hyponatremia: an Underrecognized condition Korean J Pediatr 2013;56:519. 10.3345/kjp.2013.56.12.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nogueira GM, Silva NLOR, Moura AF, et al. Acute kidney injury and electrolyte disorders in COVID-19. World J Virol 2022;11:283–92. 10.5501/wjv.v11.i5.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leaf DE, Gupta S, Wang W. Tocilizumab in COVID-19. N Engl J Med 2021;384:86–7. 10.1056/NEJMc2032911 [DOI] [PubMed] [Google Scholar]
- 50. Soy M, Keser G, Atagündüz P, et al. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol 2020;39:2085–94. 10.1007/s10067-020-05190-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cuesta M, Slattery D, Goulden EL, et al. Hyponatraemia in patients with community-acquired pneumonia; prevalence and aetiology, and natural history of SIAD. Clin Endocrinol (Oxf) 2019;90:744–52. 10.1111/cen.13937 [DOI] [PubMed] [Google Scholar]
- 52. Filippone EJ, Ruzieh M, Foy A. Thiazide-associated hyponatremia: clinical manifestations and pathophysiology. Am J Kidney Dis 2020;75:256–64. 10.1053/j.ajkd.2019.07.011 [DOI] [PubMed] [Google Scholar]
- 53. Garrahy A, Thompson CJ. Hyponatremia and glucocorticoid deficiency. Front Horm Res 2019;52:80–92. 10.1159/000493239 [DOI] [PubMed] [Google Scholar]
- 54. Rodríguez Virgili J, Cabal García AA. Iatrogenic adrenal insufficiency. Semergen 2012;38:468–71. 10.1016/j.semerg.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 55. Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol 2020;55:327–31. 10.1097/RLI.0000000000000672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Salvatore C, Roberta F, Angela de L, et al. Clinical and laboratory data, radiological structured report findings and quantitative evaluation of lung involvement on baseline chest CT in COVID-19 patients to predict prognosis. Radiol Med 2021;126:29–39. 10.1007/s11547-020-01293-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hirsch JS, Uppal NN, Sharma P, et al. Prevalence and outcomes of hyponatremia and hypernatremia in patients hospitalized with COVID-19. Nephrol Dial Transplant 2021;36:1135–8. 10.1093/ndt/gfab067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Graña C, Ghosn L, Evrenoglou T, et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst Rev 2022;12:CD015477. 10.1002/14651858.CD015477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Atila C, Sailer CO, Bassetti S, et al. Prevalence and outcome of dysnatremia in patients with COVID-19 compared to controls. Eur J Endocrinol 2021;184:409–18. 10.1530/EJE-20-1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med 2009;122:857–65. 10.1016/j.amjmed.2009.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de La Flor JC, Gomez-Berrocal A, Marschall A, et al. The impact of the correction of hyponatremia during hospital admission on the prognosis of SARS-Cov-2 infection. Med Clin (Barc) 2022;159:12–8. 10.1016/j.medcli.2021.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-075232supp001.pdf (379.1KB, pdf)
Data Availability Statement
No data are available. Not all patients provided active informed consent, and therefore sharing data is not possible.