Abstract
Decentralized clinical trials (DCTs) leverage digital technologies to reduce dependency on study sites and intermediaries. DCT should be balanced with accessibility and data reliability while meeting regulatory requirements. Here, we conducted a pilot study for functional constipation symptoms to investigate the feasibility of DCT. The study was an open, fully remote, randomized clinical trial in participants who had functional constipation symptoms. Electronic consent was obtained remotely, and study volunteers were screened through web‐based questionnaires. Subjects were randomized to either receive Lactobacillus and vitamin C supplements or vitamin C alone in a 1:1 ratio, which were delivered directly to subjects. Subjects kept track of bowel diaries daily during the 1‐week baseline and 2‐week treatment period using mobile applications. Bowel symptoms and the validity of the records were descriptively evaluated. A total of 30 subjects were randomized and completed the study. A total of 26.7% of subjects resided outside of the metropolitan area. Two‐week Lactobacillus treatments increased the number of defecations (+0.80 vs. +0.46 times per week) and decreased the defecation time (−3.94 h vs. −1.62 h) compared to the comparator group. Overall, 67.1% of bowel diary records were completed in accordance with the schedule whereas 32.9% were not. Implementation of DCTs can facilitate geographic accessibility but should be guaranteed for data reliability. Prompt detection of errors and response using objective metrics would be required.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Decentralized clinical trials (DCTs) leverage digital technologies to reduce dependency on study sites and intermediaries. DCTs should be balanced with accessibility and data reliability while meeting regulatory requirements.
WHAT QUESTION DID THIS STUDY ADDRESS?
Are the outcomes in a fully DCT in participants with functional constipation symptoms comparable to traditional clinical trials and reliable in data collection and the reporting process?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
In this feasibility study, participants from distant areas could save at least 2 h of travel time to visit the trial site. Collected data from DCTs were clinically relevant. However, retrospective or prospective recording of data which could compromise data reliability were also observed.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The findings suggest that DCTs can facilitate geographic accessibility but should be guaranteed for data reliability based on the prompt detection of errors and response using objective metrics.
INTRODUCTION
Accessible clinical trials can diversify the study population. The representativeness of trial participants to the whole population has often been criticized, 1 , 2 , 3 which has become more emphasized during the coronavirus disease 19 (COVID‐19) pandemic. 4 Geography is a critical barrier to trial access. 5 In a previous study, 50% of patients with cancer were willing to participate in a clinical trial when offered, but actual participation was less than 8%. 2 , 6 This was partly attributed to inaccessible trial sites, which were routinely selected as large‐scale hospitals in conventional clinical trials. 2 Unequal access to clinical trials can be a potential source of biases in treatment response and outcomes. 3 , 7
Decentralized clinical trials (DCTs) are expected to improve access to clinical trials. 8 Although the definition of DCT varies, 9 , 10 DCT is often characterized by reduced dependency on trial sites and study intermediaries. 11 The Decentralized Trial in Atrial Fibrillation Patients (DeTAP) trial in the pandemic period is a practical example, where patients were recruited through social media and overall study procedures were conducted using mobile applications. 12 The foremost advantages using the DCT approach in the DeTAP trial were rapid recruitment and high engagement of the participants. 12
DCT is not an all‐or‐nothing approach; adoption of DCT elements should be justified. 13 Researchers have noted that several DCT elements cannot fully substitute for face‐to‐face procedures, and the data collection process must be reliable for clinical decisions. 14 Trial participants can perfunctorily give consent without proper understanding of the contents in remote settings, 15 which can expose the participants to protocol deviations and unexpected risks. Therefore, it has been recommended that the rationale for adopting DCT elements should be clearly justified at the early stage of a DCT. 10 , 13
Despite the higher accessibility that DCTs can provide, data quality and integrity are subject to increased risk. 16 , 17 Regulatory agencies particularly emphasized DCT as a novel trial design that requires robust measures to ensure data integrity. 17 Data integrity risks can either exist in study procedures (e.g., unchecked drug compliance) or in trial monitoring (e.g., unverified source data verification), all of which require the corresponding mitigation plans. 18 However, as implementation of DCT is influenced by the specific local regulations and patient population, identifying and tackling the “real‐world” risk in feasibility studies are of importance. 12
In a previous study, we developed a blockchain‐based electronic consent application named METORY and evaluated the application in a DCT scenario. 15 , 19 We integrated an electronic diary module into METORY to manage DCT procedures in a single application. The application can keep logs for the study procedures, and investigators can analyze the adherence to study procedures with the log. We did not strictly restrict the participants' action for study procedures; for example, we allowed a participant to enter the study data retrospectively. Such minimal restrictions can enable the analysis of real‐world behaviors of the participants and were used to evaluate the participants' understanding of informed consent. 15
To investigate the feasibility of DCT, we conducted a pilot clinical trial in participants with functional constipation symptoms. We designed a full DCT with “low‐risk” alternatives (e.g., drug to dietary supplements and patients to participants with mild symptoms) to evaluate practical feasibility while reducing risks to participants. Functional constipation was considered a suitable target disease for this pilot study, as clinical evaluation of the treatments is relatively insufficient 20 and patient‐reported symptoms are important efficacy end points. We evaluated the data reliability of DCT in two aspects: whether the effect size and variability were comparable to traditional clinical trials, and whether there were potential sources of error in the data collection and reporting process.
METHODS
Participants
Adult subjects aged between 19 and 64 years who had at least one of the following functional constipation symptoms fulfilled for the past 2 weeks were enrolled (relaxed Rome III criteria for functional constipation 21 ): straining with defecation more than 25% of the time; hard or lumpy stools more than 25% of the time; sensation of anorectal obstruction more than 25% of the time; sensation of incomplete evacuation more than 25% of the time; manual maneuvers necessary to facilitate defecation more than 25% of the time; and less than three bowel movements per week.
Subjects who were pregnant, lactating, or had hypersensitivity to supplements were excluded from the study. Subjects should sincerely keep track of medication and bowel diaries during the study period and were not allowed to take other Lactobacillus or vitamin C‐containing supplements except for the study supplements. Subjects were recruited from the recruitment website of the Seoul National University Hospital Clinical Trials Center. Subjects could either be referred to the website by physicians or voluntarily visit it on the internet. Participants were assessed for eligibility through self‐reported, web‐based questionnaires for functional constipation symptoms.
Written consent forms were obtained from all subjects by the METORY platform prior to any study‐related procedures (Figure S1). METORY was a Bring Your Own Device application that was deployed on the participants' personal devices. The platform was available on the Android operating system. There was no minimum screen size requirement for the application. Data were encrypted during transfer between investigators and participants. Subjects were identified by a mobile authentication system integrated in the electronic consent system. 19 Subjects were instructed for the study procedures by the delegated investigators via the teleconference system. After subjects agreed to participate in the study, subjects electronically signed the informed consent form, and the signed copy was recorded onto the blockchain platform. An electronic copy of the informed consent form was provided to the subjects. After giving consent to the study, subjects completed the web‐based questionnaires, and the questionnaires were assessed for eligibility by the delegated investigators. The study was approved by the Institutional Review Board of Seoul National University Hospital and conducted in accordance with the Declaration of Helsinki (ClinicalTrials.gov registration no.: NCT05520073).
Study design
The study was an open‐labeled, fully remote, randomized clinical trial. Subjects were randomized to receive either treatment (Lactobacillus and vitamin C supplements) or placebo (vitamin C supplements alone) at a 1:1 ratio. Treatments were delivered directly to the subjects' home as the treatments were stable at ambient temperature for 24 months. Because the formulation of the two supplements was apparently different (pack and tablet), blinding was not possible. Subjects were informed of the allocated treatments after randomization. All trial‐related procedures were conducted outside of the trial site.
The study schedule consisted of 1‐week baseline and 2‐week treatment periods. During the baseline period, subjects should keep track of bowel diaries using the mobile application. After the baseline period, subjects took the allocated treatments (i.e., Lactobacillus + vitamin C or only vitamin C) on their own daily for 2 weeks. During the treatment periods, subjects should keep track of both bowel diaries and medication diaries.
Treatment adherence was monitored through semiquantitative urine vitamin C measurement using home‐based urine strips for vitamin C (Self‐Stik Vita‐Check, Chungdo Pharmaceuticals, Chuncheon‐si, Korea). The test kits were directly delivered to the subjects in a dedicated container. The operation of the kit was similar to routine dipstick test kits (dip and observe). The urine kit provided grade 4, semiquantitative urine vitamin C concentrations (negative, 10, 20, and 30 mg/dL), and participants reported the results in text using the mobile application. Additionally, participants were required to take a picture of the specimen and send the stored pictures to investigators for double‐check. The urine strip test was validated and cleared through the US Food and Drug Administration (FDA) 510(k) process. Urine samples and test kits were discarded by participants after the test. Urine vitamin C measurements were performed five times (at day 1 pre‐ and postdose, days 5, 9, and 13).
Exploratory fecal microbiome assessment was performed in subjects who agreed to give stool samples using validated stool collection kits (Gut Inside, CJBioscience). 22 , 23 Stool collection kits were delivered directly to the subjects by the central laboratory through a courier. The participants used a sterile spoon from the kit to collect the stool sample, and the stool was mixed with reagents in the sample tube. Samples were then delivered to the central laboratory, and the results were sent to the investigators. Detailed procedures for fecal microbiome analysis were described elsewhere. 23
After completing the study schedule, subjects submitted the patient experience questionnaire regarding participation in a DCT in an anonymous manner. Subjects could freely comment on the positive and negative aspects of DCT based on their experience (Figure 1, Figure S2).
FIGURE 1.
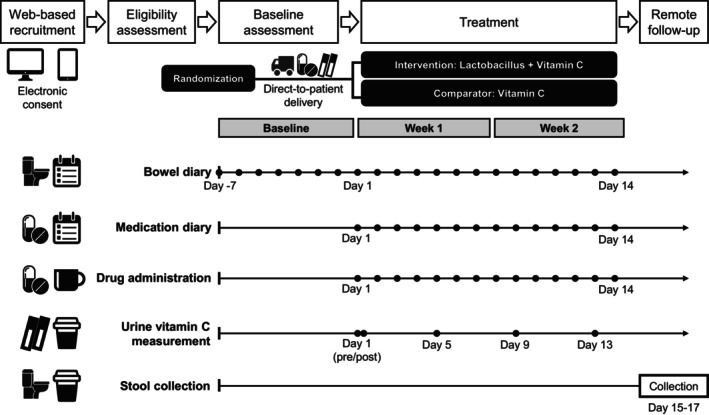
Schematic representation of the study design.
Outcomes
Bowel symptoms were assessed weekly (i.e., baseline, week 1, and week 2) based on the records in electronic bowel diaries. The following bowel symptoms were evaluated: the total number of defecations, use of laxatives, stool consistency measured by Bristol Stool Chart, defecation time, the number of events for straining, sensation of incomplete evacuation, sensation of anorectal obstruction/blockage, abdominal pain/discomfort, and manual maneuvers to facilitate.
Exploratory analysis of the fecal microbiome was performed using the gut microbiome index (GMI). 23 The index quantifies the similarity of the microbiome profile to that of healthy Koreans and represents the probability of being a “healthy” microbiome state. Details on the index were described in another article. 23
Evaluation of decentralized elements
To evaluate the quality of the data collection process, the timestamps of the electronic diary records were analyzed. We categorized the records as either “correct” or “incorrect.” Correct records were defined as those performed on the scheduled date, whereas incorrect records were not. Similarly, procedural adherence was assessed based on the records reported by the participants as follows: correct records were defined as those where the subject performed and reported the home‐based urine test on the scheduled date, meeting the predose or postdose conditions.
Assessment of direct‐to‐patient procedures included shipping of the investigational products (IPs) and test kits by investigators and stool collection kits by the contracted laboratory. The procedures were evaluated by the proportion of subjects who received IPs, who received wrong IPs, and the elapsed time to receive IPs. The fecal microbiome analysis procedure was evaluated by the proportion of subjects who gave consent and the number of valid samples. All records were evaluated individually by study day, and the proportion of each category was calculated. In addition, the travel time between the trial site and the local distribution center near the participants' homes was estimated and summarized by location.
Patient opinions on the positive and negative aspects of participating in the study were grouped by the similarity of the contents, and representative comments were quoted.
Statistical analysis
Given the exploratory nature of the study, the sample size was determined empirically, and statistical testing was not performed. Continuous variables were summarized descriptively with the mean and standard deviation unless the median and range were appropriate considering the distribution of data. Categorical variables were summarized by the proportion of each category in a subject, and the mean and standard deviation of the proportions were calculated. All statistical procedures were performed using R version 4.1.0. (R Foundation for Statistical Computing).
RESULTS
Subject disposition and demographics
A total of 30 subjects were randomized and completed the study. There were more female participants (76.7%) than male participants (23.3%). The gender distribution between the two treatment groups was not remarkably different. Participants ranged in all age groups between 20 and 65, giving a peak enrollment at the age group of 30–40 years (53.3%). Participants from Seoul, where the study center was located, accounted for the largest proportion (43.3%), whereas those outside of the metropolitan area accounted for 26.7%. On average, travel time to the trial site was 1.4 h, and participants residing outside of the metropolitan area (Seoul and Gyeonggi‐do) could save more than 2 h for visiting the trial site (Figure S3, Table 1). The study started in early September (September 1, 2022) and completed enrollment in mid‐October (October 19, 2022). Given a total of a 3‐week run‐in and intervention period, the recruitment was completed within a month.
TABLE 1.
Demographics of the subjects.
| Lactobacillus + vitamin C (n = 15) | Vitamin C (n = 15) | Total (N = 30) | |
|---|---|---|---|
| Sex | |||
| Male | 3 (20.0) | 4 (26.7) | 7 (23.3) |
| Female | 12 (80.0) | 11 (73.3) | 23 (76.7) |
| Age group, years | |||
| 20–29 | 2 (13.3) | 2 (6.7) | |
| 30–39 | 4 (26.7) | 12 (80.0) | 16 (53.3) |
| 40–49 | 5 (33.3) | 1 (6.7) | 6 (20.0) |
| 50–59 | 2 (13.3) | 2 (13.3) | 4 (13.3) |
| 60–64 | 2 (13.3) | 2 (6.7) | |
| Location (mean travel time) a | |||
| Seoul (0.8 h) | 7 (46.7) | 6 (40.0) | 13 (43.3) |
| Gyeonggi‐do (1.2 h) | 6 (40.0) | 3 (20.0) | 9 (30.0) |
| Sejong (2.6 h) | 1 (6.7) | 2 (13.3) | 3 (10.0) |
| Gangwon‐do (2.1 h) | 2 (13.3) | 2 (6.7) | |
| Chungcheongbuk‐do (2.1 h) | 2 (13.3) | 2 (6.7) | |
| Gyeongsangnam‐do (4.2 h) | 1 (6.7) | 1 (3.3) | |
Note: The number of subjects (proportion, %) are presented.
Average one‐way travel time between the trial site and the local distribution center of the participants residing in the location.
Efficacy outcomes
After the 2‐week Lactobacillus treatment, the number of defecations was slightly increased (+0.80 vs. +0.46 times per week; Figure 2a), whereas the defecation time was decreased (−3.94 h vs. −1.62 h; Figure 2c). The S tool consistency was not remarkably changed after treatment (Figure 2b). Compared to the results in a traditional clinical trial with Lactobacillus supplements, 24 the overall trend of changes was comparable (Figure 2a,b). Other functional constipation symptoms were mildly alleviated after Lactobacillus treatments except for the sensation of anorectal obstruction/blocking. However, similar trends were also observed in the comparator group, and no remarkable difference between the two groups was observed. The use of laxatives and manual maneuvers to facilitate defecation were not observed during the study period (Figures S4 and S5; Table 2; Tables S1 and S2). In the exploratory analysis of the fecal microbiome, GMI was higher in the Lactobacillus treatment group (55.0) than in the comparator group (41.6) after 2 weeks of treatment, but this difference was not decisive due to the small sample size (Figure 2d).
FIGURE 2.
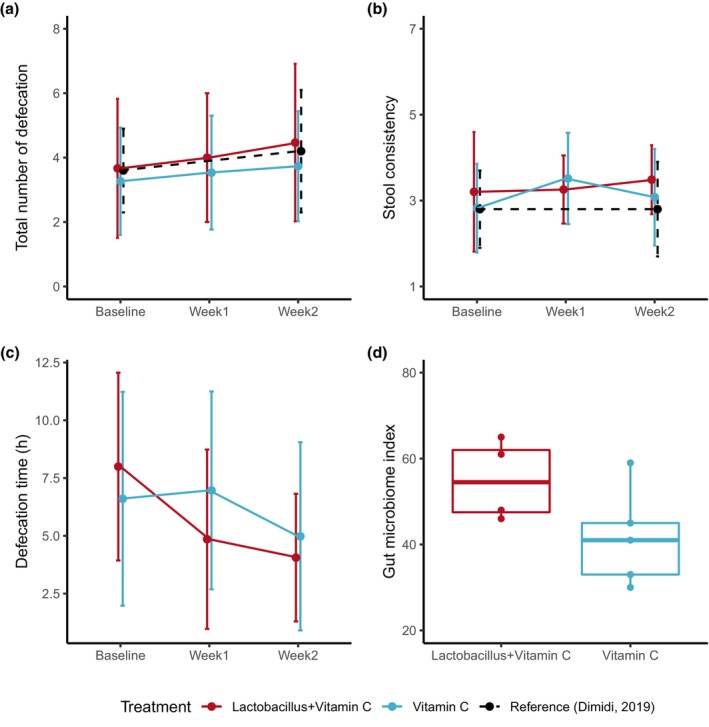
Summary of the efficacy parameters. The total number of defecations (a), stool consistency (b), defecation time (c), and gut microbiome index (index for the diversity of microflora) after 2 weeks of administration of the supplements (d). Dots and error bars in the scatter plots represent the mean and standard deviation, respectively. Black dashed lines denote the efficacy results from a traditional clinical trial (Dimidi et al. 24 ).
TABLE 2.
Summary of the efficacy outcomes.
| Lactobacillus + vitamin C (n = 15) | Vitamin C (n = 15) | |
|---|---|---|
| Total number of defecation (#/week) | ||
| Baseline | 3.67 (2.16) | 3.27 (1.67) |
| Week 1 | 4.00 (2.00) | 3.53 (1.77) |
| Week 2 | 4.47 (2.45) | 3.73 (1.71) |
| Use of laxatives (#/week) | ||
| Baseline | 0 | 0 |
| Week 1 | 0 | 0 |
| Week 2 | 0 | 0 |
| Stool consistency | ||
| Baseline | 3.20 (1.39) | 2.82 (1.03) |
| Week 1 | 3.26 (0.79) | 3.51 (1.06) |
| Week 2 | 3.49 (0.80) | 3.08 (1.12) |
| Defecation time (h) | ||
| Baseline | 8.00 (4.06) | 6.60 (4.63) |
| Week 1 | 4.85 (3.88) | 6.97 (4.28) |
| Week 2 | 4.06 (2.76) | 4.98 (4.07) |
| Straining (#/week) | ||
| Baseline | 1.53 (1.60) | 1.73 (1.58) |
| Week 1 | 1.33 (1.50) | 1.60 (0.99) |
| Week 2 | 0.93 (1.03) | 1.00 (1.13) |
| Sensation of incomplete evacuation (#/week) | ||
| Baseline | 1.67 (1.99) | 1.13 (1.36) |
| Week 1 | 1.47 (1.81) | 1.47 (2.29) |
| Week 2 | 1.80 (1.82) | 1.73 (1.98) |
| Sensation of anorectal obstruction/blockage (#/week) | ||
| Baseline | 0.60 (1.12) | 0.40 (0.74) |
| Week 1 | 0.60 (0.91) | 0.33 (0.62) |
| Week 2 | 0.93 (1.53) | 0.27 (1.03) |
| Abdominal pain/discomfort (#/week) | ||
| Baseline | 1.13 (1.46) | 2.00 (1.25) |
| Week 1 | 0.87 (1.06) | 1.20 (1.26) |
| Week 2 | 0.53 (0.83) | 1.00 (1.25) |
| Manual maneuvers to facilitate (#/week) | ||
| Baseline | 0 | 0 |
| Week 1 | 0 | 0 |
| Week 2 | 0 | 0 |
Note: Mean (standard deviation) are presented.
Abbreviation: #, the number of events.
Procedural adherence to decentralized elements
Overall, 67.1% of bowel diary records were completed as scheduled, and 32.9% were not (Figure 3a; Table 3). Similarly, 63.8% of medication diary records were completed as scheduled, whereas 36.2% were not (Figure 3b, Table 3). Incorrect records included retrospective/prospective entries and missing records. A total of 76.9% of urine vitamin C tests were correctly performed and recorded, whereas 23.3% were not. There were no undetectable vitamin C concentration results after treatment. However, predose detection and incorrectly performed tests (e.g., not performed on the scheduled day; missing either pre‐ or postdose tests) were observed (Figure S6, Table 3). All subjects received the allocated treatments correctly with a mean delivery time of 21.3 h. A total of nine subjects agreed to give stool samples, and all samples were valid for fecal microbiome analysis (Table 3).
FIGURE 3.
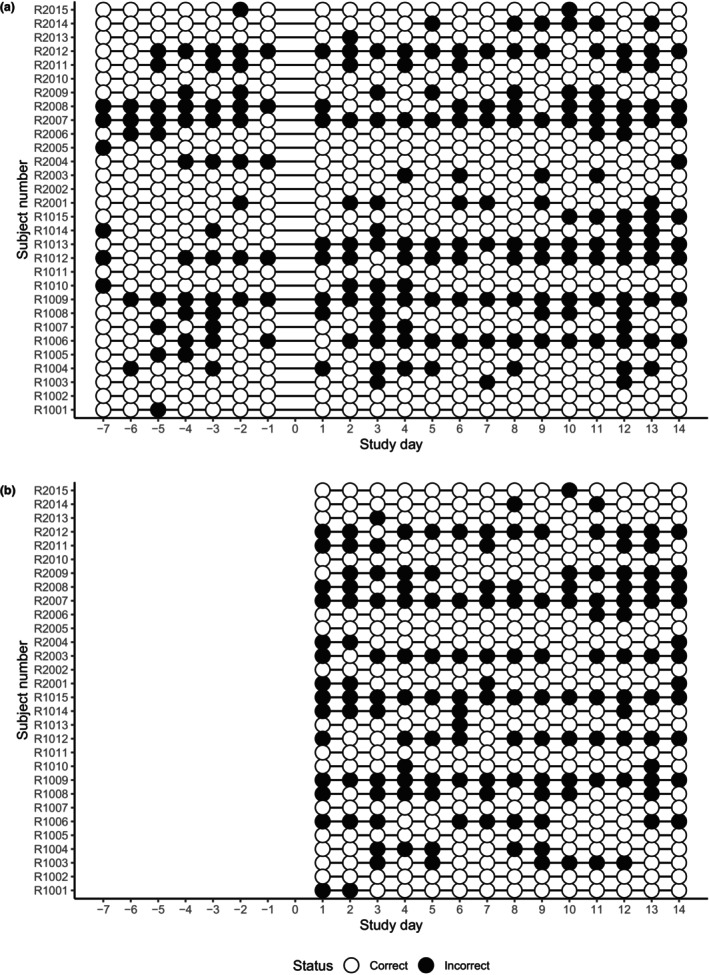
Evaluation of the electronic diary records. Bowel diary (a) and medication diary (b). Incorrect records included retrospective/prospective entries and missing records.
TABLE 3.
Evaluation of the decentralized elements.
| Lactobacillus + vitamin C (n = 15) | Vitamin C (n = 15) | Total (N = 30) | |
|---|---|---|---|
| Bowel diary records (%) a | |||
| Correct | 65.7 (31.1) | 68.6 (30.9) | 67.1 (30.5) |
| Incorrect | 34.3 (31.1) | 31.4 (30.9) | 32.9 (30.5) |
| Medication diary records (%) a | |||
| Correct | 63.3 (36.1) | 64.3 (35.3) | 63.8 (35.1) |
| Incorrect | 36.7 (36.1) | 35.7 (35.3) | 36.2 (35.1) |
| Urine vitamin C test records (%) b | |||
| Correct | 77.1 (17.9) | 76.2 (19.2) | 76.7 (18.3) |
| Incorrect | 22.9 (17.9) | 23.8 (19.2) | 23.3 (18.3) |
| Direct‐to‐patient shipping procedures | |||
| Who received the IP (n, %) | 15 [100.0] | 15 [100.0] | 30 [100.0] |
| Who received the incorrect IP (n, %) | 0 | 0 | 0 |
| Time to receive IP (h) | 21.5 (2.2) | 21.1 (2.0) | 21.3 (2.1) |
| Who agreed to collect stool (n, %) | 4 [26.7] | 5 [33.3] | 9 [30.0] |
| Number of valid stool samples c (n, %) | 4 [26.7] | 5 [33.3] | 9 [30.0] |
Note: Proportions of each category in subjects are summarized by mean (standard deviation) for the bowel diary, medication diary, and urine vitamin C test records. Time to receive IP was also summarized by mean (standard deviation). The other records are summarized by the number of subjects [percentages].
Abbreviation: IP, investigational product.
“Correct” records were defined as those performed on the scheduled date, whereas incorrect records were not.
“Correct” records were defined as those where the subject performed and reported the home‐based urine test on the scheduled date, meeting the predose or postdose conditions.
Predose sample was not obtained due to logistical issue in the local laboratory.
Patient experience
Subjects commonly commented that the home‐based procedures were convenient and lessened the burden of participation. (e.g., “As all procedures were performed online, it was comfortable that we don't have to visit the study center.”). Several subjects commented that they participated in a clinical trial for the first time and that the overall experience was satisfactory (e.g., “It was the first time to participate in a clinical trial. I feel comfortable, as there were no restrictions on time and place. Remote consent process was also comfortable.”).
The user interface of the mobile application complained, in particular, with notification of the study procedures (e.g., “I had to check the study schedules in the informed consent form during the study. It was not convenient and user interface was not intuitive.”). Several subjects complained that the response to inquiries was not always prompt or found difficulty in contacting investigators using other routes when a system error occurred. Subjects also commented that they felt insufficiently notified of some study procedures (e.g., “It was difficult to solve application errors or where to contact.”; e.g., “I want to know how the results of urine strip test were and why I should do such tests.”; Table 4).
TABLE 4.
Selected quotes of the comments from the participants.
| Positive aspects |
| “As all procedures were performed online, it was comfortable that we don’t have to visit the study center.” |
| “It was easy to participate as I don’t have to visit the study center and just use the mobile application. I feel threshold for participation was lowered. In addition, I was less embarrassed because I could do urine strip test by myself.” |
| “It was the first time to participate in a clinical trial. I feel comfortable as there was no restrictions on time and place. Remote consent process was also comfortable.” |
| Negative aspects or comments |
| “I found difficulty in entering the records as the scheduled date was not displayed but only study day (1d, 2d). I had to calculate the dates and sometimes entered the records in another date.” |
| “I had to check the study schedules in the informed consent form during the study. It was not convenient and user interface was not intuitive.” |
| “Notifications on 1:1 inquiry were not working well and keyboards were overlaid on the window where I write inquiries. Sometimes I could not enter inquires or inquiries were duplicated.” |
| “Feedbacks on the complaints and application errors were not smooth.” |
| “It was difficult to find how to solve application errors or where to contact.” |
| “I was not sufficiently notified of the stool collection procedures.” |
| “I want to know how the results of urine strip test were and why I should do such tests.” |
DISCUSSION
One of the major advantages of DCT lies in patient recruitment and retention. Price et al. 25 revealed that DCTs recovered faster from the unexpected decline in recruitment due to the COVID‐19 pandemic than traditional clinical trials. The distance to trial sites has been a huge hurdle that discourages patient participation, which is considerably overcome in the patient‐centered model in DCTs. 26 Participants in our trial similarly commented that the DCT approach removed the hurdle for trial participation and that trial procedures in the home were more comfortable than those in conventional trial sites.
We found that DCTs could accelerate recruitment in clinical trials. Despite a small number of subjects and a relatively simple design, rapid recruitment within a month and the retention of subjects (no dropouts) were notable. Although an apple‐to‐apple comparison of recruitment between different trials is extremely difficult, we found that a similar study with probiotics in healthy volunteers (40 subjects, 4‐week intervention, and 2‐week follow‐up period) in South Korea required a year (study start: May 2012, primary completion: July 2013, ClinicalTrials.gov identifier: NCT01651741).
Another consideration for recruitment is geographic accessibility. In our previous study, we found that ~70% of clinical trials in South Korea were conducted in the metropolitan area (Seoul and Gyeonggi‐do). 27 Similarly, most participants (76.3%) are from the metropolitan area in this study. However, the proportion of participants from other regions was increased. The regions previously accounted for only 5% of total clinical trials in South Korea, 27 but they accounted for 26.7% of total subjects in this study. As participants in the regions took greater than 2 h to visit the trial site, they could benefit from the DCT.
The DCT design would be suitable for chronic diseases that require everyday monitoring and treatment. Functional constipation, for example, is managed through everyday lifestyle modifications and pharmacological therapy. 20 In addition, keeping track of bowel diaries is required for treatment and can be preferably done with electronic diaries. 28 The considerations comprehensively support the DCT design, as it enables study monitoring with little cost and can reflect real‐world clinical practices. In the same vein, DCT approaches have been observed in trials for atrial fibrillation, 12 Parkinson's disease, 29 and type 2 diabetes, 30 all of which satisfy the aforementioned features. The efficacy results in our study were comparable to those in the traditional clinical trial, suggesting the feasibility of a DCT design in functional constipation.
It is noteworthy that recruitment and data collection processes can be influenced by various unexpected factors, such as device heterogeneity. 29 Li et al. 29 found that the device type of mobile phones (Android vs. iOS) substantially affected the data sharing patterns (e.g., providing barometer data) of participants in a study with 10,000 participants. In another study, the layout of the recruitment website influenced participant engagement and interest in the study. 31 We also noted that more missing records were observed in the medication diary than in the bowel diary, which was conducted in the same mobile application. The findings imply that the software interface should be carefully designed to provide reliable data collection. This issue emphasizes the necessity of early patient engagement in designing a DCT. 9 , 10 , 13
We noted that trial participants were occasionally not informed of the study procedures properly and did not follow the instructions. Retrospective records of bowel and medication diaries were found in 15.5%–24.0% of the total records, and prospective records were found in 6.2%–9.0%. The results are alarming in that adherence to study procedures would be compromised and affect the study outcomes. In addition, most participants did not perform urine vitamin C measurements as per the study protocol (e.g., missing postdose measurement), indicating the importance of monitoring adherence to study procedures. Participants also complained that checking study procedures on their own was difficult and demanded automatic alarm systems. A similar phenomenon was found in our previous research, where procedural adherences fell to 59.0% when protocol amendments were notified and consented remotely. 15 The overall findings strongly suggest systematic approaches to monitor trial procedures and can benefit from artificial intelligence‐based analytics. 32
Direct‐to‐patient procedures are often confronted by regulatory requirements. Direct‐to‐patient shipping of investigational medicinal products is not allowed in several jurisdictions, and regulatory requirements are highly varied among countries. 33 Therefore, we utilized low‐risk alternatives to direct‐to‐patient shipping of IP using marketed dietary supplements. In addition, test kits for urine vitamin C and fecal microbiome analysis were also directly supplied to subjects. These two tests represented home‐based tests that were expected to be implemented in DCTs. In urine vitamin C testing, participants were involved in the whole process of the test (sample collection to reporting). In contrast, participants were only involved in sample collection in stool tests. We found that direct‐to‐patient shipping procedures are suitable to DCT designs, but detailed management also needs to be warranted as guided in Good Clinical Practice (e.g., identification of the recipients, and storage and disposal of the products). We also demonstrated that self‐stool collection procedures could yield analyzable microbiome data and could be utilized in further trials. 34
Of note, regulatory guidance for DCTs has recently been published. The guidance from the FDA provides a detailed description of the oversight of study personnel outside of trial sites. 35 The guidance delineates criteria to distinguish sub‐investigators from personnel in routine healthcare facilities. Because the utilization of local healthcare facilities or laboratories is essential in DCTs, the guidance could help in implementing such trials with less ambiguity.
Our study had several major limitations. The small number of participants and short study duration limited the generalizability of the results. The low‐risk alternatives cannot fully reflect the characteristics of further DCTs in patients. Lack of direct comparison with traditional clinical trial designs significantly limits the interpretation of the results. A single‐center trial design limits the application of the results to larger trials in which multiple stakeholders can participate. Nonetheless, to the best of our knowledge, this study is the first to comprehensively involve multiple DCT elements that meet regulatory requirements in Korea. Further investigations for various DCT scenarios will warrant appropriate implementation of DCT in Korea.
In conclusion, the implementation of DCTs can facilitate geographic accessibility but should be guaranteed for data reliability. Prompt detection of errors and response using objective metrics would be required.
AUTHOR CONTRIBUTIONS
K.Y.H., W.K.C., J.P., S.L., M.‐G.K., J.O., and K.‐S.Y. wrote manuscript. K.Y.H., W.K.C., J.P., J.O., and K.‐S.Y. designed research. K.Y.H., W.K.C., and J.P. performed research. K.Y.H., and K.‐S.Y. analyzed data. S.L. and M.‐G.K. contributed new analytical tools.
FUNDING INFORMATION
This research was supported by a grant (22113MFDS497) from Ministry of Food and Drug Safety in 2023.
CONFLICT OF INTEREST STATEMENT
The authors declared no competing interests for this work.
Supporting information
Data S1
ACKNOWLEDGMENTS
This research was supported by a grant (22113MFDS497) from Ministry of Food and Drug Safety in 2023. The study was planned and conducted as a part of a project for DCTs driven by “Advanced Regulatory Innovation for Clinical Trials Transformation (ARICTT),” a multistakeholder collaboration group for clinical trial regulations in Korea.
Huh KY, Chung WK, Park J, et al. Feasibility study for a fully decentralized clinical trial in participants with functional constipation symptoms. Clin Transl Sci. 2023;16:2177‐2188. doi: 10.1111/cts.13617
REFERENCES
- 1. Knepper TC, McLeod HL. When will clinical trials finally reflect diversity? Nature. 2018;557(7704):157‐159. doi: 10.1038/d41586-018-05049-5 [DOI] [PubMed] [Google Scholar]
- 2. Woodcock J, Araojo R, Thompson T, Puckrein GA. Integrating research into community practice—toward increased diversity in clinical trials. N Engl J Med. 2021;385(15):1351‐1353. doi: 10.1056/NEJMp2107331 [DOI] [PubMed] [Google Scholar]
- 3. Mohan SV, Freedman J. A review of the evolving landscape of inclusive research and improved clinical trial access. Clin Pharmacol Ther. 2023;113(3):518‐527. doi: 10.1002/cpt.2832 [DOI] [PubMed] [Google Scholar]
- 4. Chaudhry MS, Spahn J, Patel S, et al. Myths about diversity in clinical trials reduce return on investment for industry. Nat Med. 2022;28(8):1520‐1522. doi: 10.1038/s41591-022-01858-4 [DOI] [PubMed] [Google Scholar]
- 5. Clougherty JE, Kinnee EJ, Cardet JC, et al. Geography, generalisability, and susceptibility in clinical trials. Lancet Respir Med. 2021;9(4):330‐332. doi: 10.1016/s2213-2600(21)00046-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Unger JM, Hershman DL, Till C, et al. "when offered to participate": a systematic review and meta‐analysis of patient agreement to participate in cancer clinical trials. J Natl Cancer Inst. 2021;113(3):244‐257. doi: 10.1093/jnci/djaa155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Unger JM, Moseley A, Symington B, Chavez‐MacGregor M, Ramsey SD, Hershman DL. Geographic distribution and survival outcomes for rural patients with cancer treated in clinical trials. JAMA Netw Open. 2018;1(4):e181235. doi: 10.1001/jamanetworkopen.2018.1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goodson N, Wicks P, Morgan J, Hashem L, Callinan S, Reites J. Opportunities and counterintuitive challenges for decentralized clinical trials to broaden participant inclusion. NPJ Digit Med. 2022;5(1):58. doi: 10.1038/s41746-022-00603-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alemayehu D, Hemmings R, Natarajan K, Roychoudhury S. Perspectives on virtual (remote) clinical trials as the "new Normal" to accelerate drug development. Clin Pharmacol Ther. 2022;111(2):373‐381. doi: 10.1002/cpt.2248 [DOI] [PubMed] [Google Scholar]
- 10. de Jong AJ, van Rijssel TI, Zuidgeest MGP, et al. Opportunities and challenges for decentralized clinical trials: European Regulators' perspective. Clin Pharmacol Ther. 2022;112(2):344‐352. doi: 10.1002/cpt.2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khozin S, Coravos A. Decentralized trials in the age of real‐world evidence and inclusivity in clinical investigations. Clin Pharmacol Ther. 2019;106(1):25‐27. doi: 10.1002/cpt.1441 [DOI] [PubMed] [Google Scholar]
- 12. Sarraju A, Seninger C, Parameswaran V, et al. Pandemic‐proof recruitment and engagement in a fully decentralized trial in atrial fibrillation patients (DeTAP). NPJ Digit Med. 2022;5(1):80. doi: 10.1038/s41746-022-00622-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Apostolaros M, Babaian D, Corneli A, et al. Legal, regulatory, and practical issues to consider when adopting decentralized clinical trials: recommendations from the clinical trials transformation initiative. Ther Innov Regul Sci. 2020;54(4):779‐787. doi: 10.1007/s43441-019-00006-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Rijssel TI, de Jong AJ, Santa‐Ana‐Tellez Y, Boeckhout M, Zuidgeest M, van Thiel GJ. Ethics review of decentralized clinical trials (DCTs): results of a mock ethics review. Drug Discov Today. 2022;27(10):103326. doi: 10.1016/j.drudis.2022.07.011 [DOI] [PubMed] [Google Scholar]
- 15. Huh KY, Moon SJ, Jeong SU, et al. Evaluation of a blockchain‐based dynamic consent platform (METORY) in a decentralized and multicenter clinical trial using virtual drugs. Clin Transl Sci. 2022;15(5):1257‐1268. doi: 10.1111/cts.13246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moore J, Goodson N, Wicks P, Reites J. What role can decentralized trial designs play to improve rare disease studies? Orphanet J Rare Dis. 2022;17(1):240‐244. doi: 10.1186/s13023-022-02388-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khin NA, Grandinetti C, Dixey H, et al. Tackling challenging data integrity topics in 2020: update on good clinical practice perspectives from the US FDA and MHRA UK. Clin Pharmacol Ther. 2022;112(1):31‐43. doi: 10.1002/cpt.2386 [DOI] [PubMed] [Google Scholar]
- 18. van Koningsbruggen‐Rietschel S, Dunlevy F, Bulteel V, et al. Protecting clinical trials in cystic fibrosis during the SARS‐CoV‐2 pandemic: risks and mitigation measures. Trials. 2021;22(1):578. doi: 10.1186/s13063-021-05457-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huh KY, Jeong SU, Moon SJ, et al. METORY: development of a demand‐driven blockchain‐based dynamic consent platform tailored for clinical trials. Front Med. 2022;9:837197. doi: 10.3389/fmed.2022.837197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vriesman MH, Koppen IJN, Camilleri M, Di Lorenzo C, Benninga MA. Management of functional constipation in children and adults. Nat Rev Gastroenterol Hepatol. 2020;17(1):21‐39. doi: 10.1038/s41575-019-0222-y [DOI] [PubMed] [Google Scholar]
- 21. Shih DQ, Kwan LY. All roads Lead to Rome: update on Rome III criteria and new treatment options. Gastroenterol Rep. 2007;1(2):56‐65. [PMC free article] [PubMed] [Google Scholar]
- 22. Lee SH, Yoon SH, Jung Y, et al. Emotional well‐being and gut microbiome profiles by enterotype. Sci Rep. 2020;10(1):20736. doi: 10.1038/s41598-020-77673-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oh HS, Min U, Jang H, et al. Proposal of a health gut microbiome index based on a meta‐analysis of Korean and global population datasets. J Microbiol. 2022;60(5):533‐549. doi: 10.1007/s12275-022-1526-0 [DOI] [PubMed] [Google Scholar]
- 24. Dimidi E, Zdanaviciene A, Christodoulides S, et al. Randomised clinical trial: Bifidobacterium lactis NCC2818 probiotic vs placebo, and impact on gut transit time, symptoms, and gut microbiology in chronic constipation. Aliment Pharmacol Ther. 2019;49(3):251‐264. doi: 10.1111/apt.15073 [DOI] [PubMed] [Google Scholar]
- 25. Price J, Goodson N, Warren EJ, Wicks P, Reites J. Resilient design: decentralized trials recovered faster from the impact of COVID‐19 than traditional site‐based designs. Expert Rev Med Devices. 2021;18(sup1):1‐4. doi: 10.1080/17434440.2021.2014818 [DOI] [PubMed] [Google Scholar]
- 26. Banks MA. Core concept: In the wake of COVID‐19, decentralized clinical trials move to center stage. Proc Natl Acad Sci USA. 2021;118(47):e2119097118. doi: 10.1073/pnas.2119097118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huh KY, Yu KS, Lim HS, Kim H. Trends of clinical trials from 2017 to 2019 in Korea: an integrated analysis based on the Ministry of Food and Drug Safety (MFDS) and the clinical research information service (CRIS) registries. Transl Clin Pharmacol. 2021;29(4):186‐196. doi: 10.12793/tcp.2021.29.e24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koppen IJN, Saps M, Lavigne JV, et al. Recommendations for pharmacological clinical trials in children with functional constipation: the Rome foundation pediatric subcommittee on clinical trials. Neurogastroenterol Motil. 2018;30(4):e13294. doi: 10.1111/nmo.13294 [DOI] [PubMed] [Google Scholar]
- 29. Li SX, Halabi R, Selvarajan R, et al. Recruitment and retention in remote research: learnings from a large, decentralized real‐world study. JMIR Form Res. 2022;6(11):e40765. doi: 10.2196/40765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ali Z, Valk TJ, Bjerre‐Christensen T, et al. Exploring decentralized glucose and Behaviometric monitoring of persons with type 2 diabetes in the setting of a clinical trial. J Diabetes Sci Technol. 2023;17(1):117‐124. doi: 10.1177/19322968211045656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miller HN, Plante TB, Gleason KT, et al. A/B design testing of a clinical trial recruitment website: a pilot study to enhance the enrollment of older adults. Contemp Clin Trials. 2021;111:106598. doi: 10.1016/j.cct.2021.106598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomas KA, Kidziński Ł. Artificial intelligence can improve patients' experience in decentralized clinical trials. Nat Med. 2022;28(12):2462‐2463. doi: 10.1038/s41591-022-02034-4 [DOI] [PubMed] [Google Scholar]
- 33. Malone M, Ferguson P, Rogers A, Mackenzie IS, Rorie DA, MacDonald TM. When innovation outpaces regulations: the legal challenges for direct‐to‐patient supply of investigational medicinal products. Br J Clin Pharmacol. 2022;88(3):1115‐1142. doi: 10.1111/bcp.15040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Frias JP, Lee ML, Carter MM, et al. A microbiome‐targeting fiber‐enriched nutritional formula is well tolerated and improves quality of life and hemoglobin A1c in type 2 diabetes: a double‐blind, randomized, Placebo‐Controlled Trial. Diabetes Obes Metab. 2023;25(5):1203‐1212. doi: 10.1111/dom.14967 [DOI] [PubMed] [Google Scholar]
- 35. The United States Food and Drug Administration . Guidance for Industry, Investigators, and Other Stakeholders: Decentralized Clinical Trials for Drugs, Biological Products, and Devices. Updated May 1, 2023. Accessed July 16, 2023. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/decentralized‐clinical‐trials‐drugs‐biological‐products‐and‐devices
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


