Abstract
Signal degradation impacts all communications. Although acyl-homoserine lactone (acyl-HSL) quorum-sensing signals are known to be degraded by defined laboratory cultures, little is known about their stability in nature. Here, we show that acyl-HSLs are biodegraded in soils sampled from diverse U.S. sites and by termite hindgut contents. When amended to samples at physiologically relevant concentrations, 14C-labeled acyl-HSLs were mineralized to 14CO2 rapidly and, at most sites examined, without lag. A lag-free turf soil activity was characterized in further detail. Heating or irradiation of the soil prior to the addition of radiolabel abolished mineralization, whereas protein synthesis inhibitors did not. Mineralization exhibited an apparent Km of 1.5 μM acyl-HSL, ca. 1,000-fold lower than that reported for a purified acyl-HSL lactonase. Under optimal conditions, acyl-HSL degradation proceeded at a rate of 13.4 nmol · h−1 · g of fresh weight soil−1. Bioassays established that the final extent of signal inactivation was greater than for its full conversion to CO2 but that the two processes were well coupled kinetically. A most probable number of 4.6 × 105 cells · g of turf soil−1 degraded physiologically relevant amounts of hexanoyl-[1-14C]HSL to 14CO2. It would take chemical lactonolysis months to match the level of signal decay achieved in days by the observed biological activity. Rapid decay might serve either to quiet signal cross talk that might otherwise occur between spatially separated microbial aggregates or as a full system reset. Depending on the context, biological signal decay might either promote or complicate cellular communications and the accuracy of population density-based controls on gene expression in species-rich ecosystems.
Over a 30-year period, it has become apparent that a diversity of Proteobacteria employ acyl-homoserine lactones (acyl-HSLs) as dedicated signal molecules in quorum-sensing controlled gene expression (15, 20, 22, 43, 58, 66). Among these are strains isolated from soil belonging to the genera Agrobacterium (23, 72), Burkholderia (35), Chromobacterium (6, 39), Pseudomonas (24, 67, 68), Ralstonia (17), Rhizobium and other related genera involved in legume symbioses (5, 48, 53, 56), Rhodobacter (49, 55), and Serratia (1, 8, 51). These soil bacteria can use quorum sensing to regulate the production of biologically active secondary metabolites in soils, such as cyanide (6, 50), phenazines (18, 47, 57, 68), prodigiosin (26, 60), violacein (39), and carbapenems (41). Quorum sensing by soil bacteria can benefit agriculture: the acyl-HSL-controlled production of phenazines and other antifungal metabolites by certain pseudomonads is now well established to underlie their biocontrol activities (25, 38, 59, 67). Other quorum-sensing species, such as certain species of Agrobacterium, Burkholderia, Erwinia, Pseudomonas, and Ralstonia, are known to use quorum-sensing mechanisms during plant pathogenesis (48). Acyl-HSL regulation can also control the production of compounds that alter the properties of soils and soil aggregates. Serratia and Pseudomonas species are known to regulate the production of diverse surfactants by using acyl-HSL signaling (10, 26, 35), and exopolysaccharide production is also known to be quorum controlled in several bacterial species (63).
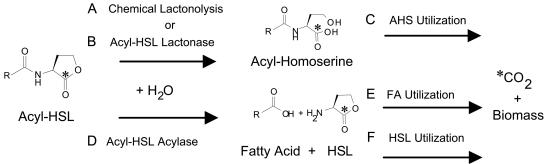
Over the past 4 years, research has documented a diversity of soil microbes capable of rapidly biodegrading acyl-HSLs (13, 27, 37, 42, 44, 62) by cleaving either the amide or lactone bonds of these molecules (32). The two routes by which acyl-HSLs are known to be degraded are shown in Fig. 1. The potent negative effects of enzyme-based acyl-HSL degradation on signal accumulations and quorum sensing have been demonstrated during pure culture laboratory studies (13, 37, 50, 71). Such effects have also been examined in simple synthetic communities by using defined cocultures (44, 62), laboratory soil microcosms seeded with recombinant strains (42), and transgenic plants expressing bacterial proteins (12). However, the stability of acyl-HSLs in natural environments over short and long periods is poorly understood, and we are not aware of any studies demonstrating signal decay in naturally occurring microbial communities.
FIG. 1.
Pathways of acyl-HSL degradation. Acyl-HSLs are known to be inactivated by hydrolysis at either the lactone ring or the acyl-amide linkage. Lactone hydrolysis occurs chemically as a function of increased pH (A) (54) or due to the activity of acyl-HSL lactonases produced by strains of Agrobacterium, Arthrobacter, Bacillus, and Klebsiella (B) (12, 44, 71). (C) The resultant acyl-homoserine (AHS) hydrolysis product is known to be utilized by Arthrobacter strain VAI-A (16). (D) Amide hydrolysis is known to be catalyzed by acyl-HSL acylases produced by Pseudomonas, Ralstonia, and Variovorax species (27, 33, 37). (E) The fatty acid (FA) released is utilized as an energy source by the strains producing the acylase enzyme. (F) The HSL released by the acylase can be utilized as a N source by Variovorax and Arthrobacter in a process that involves the mineralization of the lactone ring (16, 33) and as an energy source by several Arthrobacter and Burkholderia strains (69).
Do bacteria actually express signal-degrading activities in bulk soils in nature, and can they act on physiologically relevant concentrations of these molecules in the field? What is the biochemical stability of acyl-HSLs in soils? Here we have begun to examine such issues. We have taken the approach of synthesizing radiolabeled acyl-HSLs to examine their fate when amended at low concentrations, relevant to quorum sensing, to buffered bulk soil slurries and other samples.
MATERIALS AND METHODS
Site descriptions.
Sites sampled during the course of this study included soils from the suburban Caltech campus in Pasadena, Calif., agricultural soils from U.S. Department of Agriculture (USDA) plots in Pullman, Wash., and from National Science Foundation (NSF) Long-Term Ecological Research plots near Michigan State University's Kellogg Biological Station, Hickory Corners, Mich., and the hindgut contents of a damp-wood termite, Zootermopsis.
The activity best characterized in this study was present in bulk soils sampled from well-watered and well-fertilized turf grass in front of Beckman Auditorium on the Caltech campus. Bulk soil samples from this and the other Caltech sites were collected from the upper 5 cm. Other Caltech sites examined included the soil collected from under the canopies of coastal redwood (Sequoia sempervirens), olive (Olea europaea), rose (Rosa hybrid tea), and wisteria (Wisteria sinensis) and from surface sediments from a shallow, lily-laden pond.
Samples from USDA agricultural plots in Pullman, Wash., were collected by Linda Thomashow and included bulk soils collected from fields of winter wheat and spring wheat. Spring wheat plots exhibiting Take-All (a fungal pathogen)-suppressive and nonsuppressive activities were examined. Take-All suppression is known to be mediated by acyl-HSL quorum sensing-regulated antibiotic production by biocontrol pseudomonads (59, 67, 68).
The descriptions of sites and soils from Kellogg Biological Station's NSF Long-Term Ecological Research (KBS-LTER) plots are available online at http://lter.kbs.msu.edu/ExperimentalDesign.html. KBS-LTER samples were taken from the main experimental agricultural sites as well as from successional and other forest sites. At the main site, annual crops consist of a rotation of corn, soybean, and wheat. Samples from two distinct subplots were examined: treatment 1 (r1) and treatment 4 (r1). The former was chisel-plowed and received standard levels of common chemical inputs. The latter was an organic-based (no chemical inputs at any time) system planted with a winter leguminous cover crop and receiving additional postplanting cultivation to control weeds. Other LTER sites examined included perennial systems planted with alfalfa (treatment 6, r1) and poplar trees (treatment 5, r1). Samples from the never-plowed treatment 8 site, which is 200 m south of the others and serves as the surrogate native soil for soil organic matter studies, were examined. In the successional and other forest plots, samples were taken from three sites: a 40- to 60-year-old successional forest, which was formerly an agricultural field, a 40- to 60-year-old conifer plantation, and a late successional forest that has never been cut.
Analysis of Caltech campus soils and termite hindgut homogenates commenced within ca. 1 h of collection. Soils from the USDA and KBS-LTER plots were collected in April and May 2004, respectively, delivered via overnight mail, and analyzed immediately upon receipt. For the case of the soils collected from KBS-LTER plots, samples were maintained on ice from the time of collection until analyses commenced. For all soils, samples were passed through a 2-mm-pore-size sieve after collection and before further analysis. The water content of each soil was determined in triplicate, as was the pH by using the CaCl2 method (4) with a soil-to-solution ratio of 1:2.5.
Specimens of Zootermopsis were collected from decaying Ponderosa pine logs located near the Chilao Flats campground in the Angeles National Forest of southern California and maintained in the laboratory in plastic containers on a ponderosa diet. Ten guts were extracted and homogenized in 10 ml of a buffered salt solution (pH 7.0) in a ground-glass homogenizer and then equally distributed between two reaction tubes.
Synthesis and purification of 14C-labeled acyl-HSLs.
Escherichia coli BL21(DE3) expressing recombinant EsaI (65), the Pantoea stewartii acyl-HSL synthase, was obtained from S. Beck von Bodman and used to generate oxohexanoyl-l-[1-14C]HSL and hexanoyl-l-[1-14C]HSL from l-[1-14C]methionine as previously described (33). Briefly, cells were grown in 18-mm-diameter tubes in 5 ml of lysogeny broth containing ampicillin (400 μg · ml−1). Isopropyl-β-thiogalactoside (1 mM) was added after 2 h of growth at 37°C. Cells were then harvested by centrifugation when the culture had reached an optical density (at 600 nm) of 1.0. The cells were resuspended in 2 ml of ampicillin-containing minimal medium (65) to which 2 μCi of l-[1-14C]methionine (55 mCi · mmol−1; American Radiolabeled Chemicals, Inc., St. Louis, Mo.) was added and incubated for an additional 5 h. After the cells were removed by centrifugation, the culture fluid was extracted with 2 equal volumes of acetic acid-acidified (0.01% vol/vol) ethyl acetate (54). After evaporating away the ethyl acetate to dryness, the residue was dissolved in 500 μl of 50% methanol (balance water) and loaded onto a reversed-phase high-performance liquid chromatography (HPLC) system (Ultrasphere ODS Hypersil 5 μm, 125- by 4.6-mm column; Beckman Coulter System Gold). The HPLC system included an in-line β-particle detector (β-RAM; IN/US Systems). The identity of HPLC-purified acyl-HSLs was confirmed by using liquid chromatography and atmospheric chemical ionization mass spectrometry (27). The EsaI acyl-HSL synthase, when expressed in Escherichia coli, catalyzed the synthesis of nearly equimolar amounts of oxohexanoyl-HSL and hexanoyl-HSL. The specific activities of the purified [14C]acyl-HSLs were determined by using a combination of bioassay quantification methods (54) and quench-corrected liquid scintillation counting (Beckman Coulter LS 6500). Authentic acyl-HSL standards were obtained from Sigma or were a generous gift from Bernhard Hauer. Acyl-HSLs were stored in acetic acid-acidified ethyl acetate at −20°C until use.
Mineralization of 14C-labeled acyl-HSLs and quantification of 14CO2.
Purified oxohexanoyl-l-[1-14C]HSL and hexanoyl-l-[1-14C]HSL were used as substrates in 250 soil slurry reactions. The [14C]acyl-HSL substrate was dispensed from an acetic acid-acidified ethyl acetate solution into a sterile, dry 18-mm-diameter tube. The ethyl acetate was evaporated away to dryness. Five milliliters of sterile buffer of the appropriate pH was dispensed into each tube. Immediately after this, the amount of radioactivity was measured. Two hundred milligrams of fresh soil was added to each tube. Soil slurries were incubated under once flowthrough aeration; 14CO2 released from the radiolabeled substrate was collected and quantified by bubbling the reactor outgas through a triple tandem array of phenylethylamine-alkaline traps (3, 33) replaced at every time point. The rates and yields of 14CO2 emissions from the soil slurries incubated were determined by using liquid scintillation counting of radioactivity collected in the CO2 traps as previously described (3, 33). Acidification of the reaction fluid was performed at the end of the experiment and was not found to release further radioactivity. Assays under each set of conditions were performed at least in duplicate and at 21°C unless otherwise noted.
The CO2 traps at no less than seven time points were collected during the first 2 h of incubation for each of the 250 soil slurry reactions comprising this study (e.g., Fig. 2A). Mineralization by 200 reactions was further monitored for several days. All slurries described in this study contained 200 mg of fresh weight soil in 5 ml of a buffered salt solution at a pH as noted. For sterilization controls, soils were either autoclaved for 40 min at 120°C or were γ-irradiated (Mark I-68A 137Cs irradiator), receiving 1.80 × 106 rad.
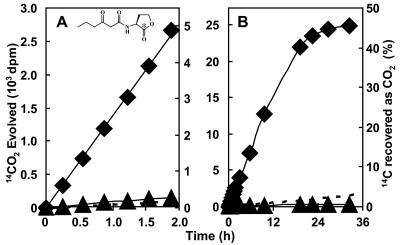
FIG. 2.
Acyl-HSL mineralization by untreated and heat-inactivated turf soil. The left and right y axes describe each data point as disintegrations per minute and percent recovery, respectively. Dashed lines represent the theoretical amount of 14CO2 that would be released if the initial degradation step was not biological and governed by a rate-limiting, abiotic hydrolysis of the acyl-HSL lactone ring, with subsequent biological mineralization of the resultant acyl-homoserine product (16, 54, 70). (A) Release of 14CO2 over the initial 2 h of incubation of a representative soil slurry with oxohexanoyl-l-[1-14C]HSL at pH 5.5. ⧫, untreated turf soil; ▴, autoclaved turf soil. An influence similar to that of the autoclaved soil on degradation activity was observed when the soil had been γ-irradiated (data not plotted). (B) Mineralization by the same samples over a 34-h incubation period.
When noted, the protein synthesis inhibitors chloramphenicol or cycloheximide were amended to slurries (100 μg · ml−1, final concentration) prior to the addition of radiolabel. However, whether such treatments were effective in inhibiting protein synthesis was not determined, and thus the results must be interpreted with some uncertainty. The interpretation of inhibition controls (e.g., examining rates of [3H]leucine or [35S]methionine incorporation into new proteins) would be complicated in samples such as soils because of their great species richness. Whereas a 99% inhibition (or similar lack thereof) of protein synthesis might be interpreted in a clear-cut manner during a pure-culture study, such would be difficult to interpret in a sample containing thousands of different species. The organisms responsible for signal decay might be among the dozens to hundreds of species accounting for the remaining 1%.
Interpretation of acyl-HSL degradation rates from CO2 release.
Since only one carbon position in the acyl-HSL molecules synthesized and used in these analyses contained radiolabel, the specific activity of the 14CO2 that was released was equivalent to the experimentally determined specific activity of the parent [14C]acyl-HSL substrate. The recovery of each mole of 14CO2 would (at a minimum) be interpreted as reflecting the inactivation of at least an equimolar amount of acyl-HSL signal. Signal inactivation occurring in soil slurries might have been judged to be more extensive (see below) if any radiolabel had been incorporated into cell material or other biodegradation products, but no attempts were made in this study to perform a complete 14C inventory.
Determination of optimal conditions for acyl-HSL degradation.
The pH optimum was determined at an incubation temperature of 21°C in reaction vessels containing 200 mg of soil and buffered with 5 ml of 20 mM sodium phosphate at the desired pH. The temperature optimum for acyl-HSL degradation was determined at an incubation pH of 6.0. The temperature variation was measured by monitoring a thermometer inserted into control reaction vessels and found to be ≤1°C. The influence of initial acyl-HSL substrate concentration on signal decay rates was determined at an incubation pH of 6.0 and a temperature of 21°C. Radiolabeled acyl-HSLs were diluted with their corresponding, unlabeled acyl-HSL to a specific activity of 0.35 mCi · mmol−1 for experiments involving elevated concentrations of acyl-HSL. Origin software (OriginLab) was used for kinetic line fitting and for determining Michaelis-Menten characteristics.
Bioassay determination of acyl-HSL inactivation rates in turf soil.
Concurrent with measuring the rates of mineralization of oxohexanoyl-l-[1-14C]HSL ([14C]3OC6HSL) in turf soil, the rates of its disappearance were measured by using the bacterial luciferase assay strain (54). For each reaction, 10 g of the same freshly collected turf soil that had been sieved through 2-mm-diameter pores used in the mineralization assays was added to 500 ml of sterile sodium phosphate buffer (20 mM; pH 6.0) in a 2-liter Fernbach flask. For control reactions, either no soil was added or the soil-buffer slurry was autoclaved for 40 min at 120°C and cooled to room temperature prior to initiation of the assay. The reaction mixtures were amended with nonradioactive 3OC6HSL to a final concentration of 1 μM and stirred gently with magnetic stirring bars at 21°C. At each time point, triplicate 1-ml samples were taken from the flasks for subsequent bioassay analysis (45). The overall volume loss due to sampling over the course of the experiment was less than 10% of the initial total volume. Kinetic data were fitted with Microsoft Excel to zero-order or first-order half-life decay kinetics.
Sorption controls were performed specifically to examine the possibility that any observed acyl-HSL disappearance as measured by bioassays might reflect reduced bioavailability or extractability rather than inactivation per se. Purified oxohexanoyl-l-[1-14C]HSL was added to 18-mm-diameter reaction vessels. After the ethyl acetate was evaporated, 5 ml of sodium phosphate buffer (20 mM; pH 6.0) was added to the tube. Samples were taken immediately to measure the initial radioactivity by use of a scintillation counter. To this reaction, 200 mg of autoclaved soil was added to the buffer, and the reaction tube was shaken for 30 min. After this, the soil was separated from the fluid by centrifugation at 13,500 rpm with an Eppendorf model 5415C centrifuge for 15 min. The fluid was extracted with acidified ethyl acetate, and the radioactivity in the various fractions was determined. Under these precise incubation conditions, the sorption of 1 μM 3OC6HSL to the turf soil was found to be experimentally insignificant.
MPN of soil microbes mineralizing 14C-labeled acyl-HSLs.
For most probable number (MPN) enumeration of acyl-HSL-mineralizing microbes, a suspension of freshly collected turf-grass soil was serially diluted into a MES (morpholineethanesulfonic acid)-buffered (10 mM; pH 5.5) growth medium containing 50 mg of yeast extract liter−1, 50 μM glucose, and 50 μM succinate. Two-milliliter aliquots from each dilution were transferred to 18-mm-diameter tubes containing 102 ± 2 nM (final concentration) radiolabeled hexanoyl-l-[1-14C]HSL, and tubes were closed with butyl rubber stoppers. Tubes in the series contained from 2 × 10−1 to 2 × 10−10 g of fresh soil. The dilution series from the soil sample were performed in triplicate and were allowed to incubate for 18 days prior to analysis of the 14CO2 released. Cultures were considered positive for acyl-HSL mineralization if 14CO2 recovery was greater than 25% of that of the initial substrate and were scored with an MPN table. The MPN values derived here would be abundance underestimates if the microbes responsible for the high rates of acyl-HSL mineralization observed in soil slurries were either unable to use the energy substrates provided or otherwise unable to thrive in the cultivation medium, which is consistent with what is known regarding many microbial populations (31).
RESULTS
Acyl-HSL mineralization by bulk soil slurries.
Upon the introduction of acyl-[1-14C]HSLs into the vast majority of the ca. 250 soil slurry reactions performed in this study, 14CO2 was released at a linear rate and without any evident lag. Samples from two sites mineralized the radiolabel after a lag of several hours (discussed below). A representative curve of the initial degradation kinetics is presented in Fig. 2A. It has previously been shown that the biological degradation of acyl-homoserines by soil Arthrobacter isolate VAI-A (which does not utilize acyl-HSLs) is limited by, and thus does not supersede in rate, the half-life kinetics of the alkaline chemical hydrolysis of the acyl-HSL lactone bond (16). The acyl-HSL degradation that was observed far outpaced that which would have occurred if the initial acyl-HSL inactivation event had been chemical and not biochemical, followed by a biological decomposition of the corresponding acyl-homoserine hydrolysis product (Fig. 2A and B, dashed lines). After the initial, linear kinetic phase, mineralization was observed to decelerate to a plateau in all ca. 200 reactions monitored for extended time periods (e.g., Fig. 2B). By the time of plateau, at least 33% of the acyl-HSL radiolabel had been recovered as 14CO2. To confirm that an enzymatic (either abiontic or cell-associated) activity was responsible for acyl-HSL mineralization, soil was autoclaved and cooled prior to the addition of radiolabel (Fig. 1A and B) or was γ-irradiated (data not shown). Release of label as 14CO2 was markedly diminished by such treatments, decreasing release rates by over 99%, suggesting that the activity was catalyzed by free enzymes or microorganisms.
Soils and pond sediments ranging in pH from 5.1 to 7.3 were sampled from six sites across the Caltech campus and were screened for acyl-HSL mineralization. On a weight-for-weight basis, the initial rates of degradation between any of two of these sites differed by no greater than a factor of 5 (data not shown); the fastest degradation was observed in samples collected from a turf-grass soil, which was chosen for characterization in detail. Soil from this site had a pH of 6.6 at the time of collection.
Soils from USDA agricultural plots and KBS-LTER plots were obtained and screened for acyl-HSL mineralization as well (Table 1). Most of the sites were observed to exhibit significant mineralization activities without lag. One soil sample, the corn-soybean-wheat rotation soil with standard chemical inputs from KBS-LTER, and the termite gut homogenates exhibited 4- and 6-h lags, respectively. These were the only lags in mineralization observed during the course of this study. From both samples, mineralization proceeded at a linear rate after the lag. Although the reasons are not clear, the results suggest that signal decay might actually be induced by the exogenous acyl-HSL at those sites. The total 14C recovered as CO2 was 64% ± 7% for the soils and 36% ± 1% for the termite guts.
TABLE 1.
Initial rates of release of 14CO2 from hexanoyl-l-[1-14C]HSL by soil communities and other environmental samples
| Sample type | Sample pH | Hexanoyl-HSL mineralization at pH 6.0 and 32°C (nmol · h−1 · g of fresh weight−1)a |
|---|---|---|
| Turf soil (Calif.) | 6.6 | 13.4 ± 0.9 |
| Spring wheat soil, Take-All suppressive (Wash.) | 4.8 | 4.4 ± 0.5 |
| Spring wheat soil, nonsuppressive (Wash.) | 4.9 | 4.9 ± 0.8 |
| Winter wheat soil (Wash.) | 5.4 | 4.9 ± 0.4 |
| Corn-soybean-wheat rotation soil, standard chemical inputs (Mich.) | 5.8 | ≤0.1b |
| Corn-soybean-wheat rotation soil, organic inputs only (Mich.) | 5.7 | 2.2 ± 0.1 |
| Poplar soil (Mich.) | 6.0 | 2.4 ± 0.3 |
| Alfalfa soil (Mich.) | 6.0 | 3.7 ± 0.2 |
| Surrogate native soil (Mich.) | 4.8 | 2.5 ± 0.4 |
| Late successional forest soil (Mich.) | 4.8 | 2.5 ± 0.1 |
| Early successional forest soil (Mich.) | 4.8 | 2.2 ± 0.3 |
| Conifer plantation soil (Mich.) | 4.3 | 2.1 ± 0.1 |
| Zootermopsis termite guts (Calif.) | ∼7 | ≤0.1c |
Rates represent linear kinetics observed over the initial 2 h of incubation with 10.3 ± 0.5 μM [14C]C6HSL at 32°C and pH 6.0, conditions found to be optimal for C6HSL mineralization by Caltech turf soil. Reactions were performed at least in duplicate.
After a 4-h lag, mineralization proceeded at a linear rate of 2.0 ± 0.1 nmol·h−1·g of fresh weight−1.
After a 6-h lag, mineralization proceeded at a linear rate of 10.1 ± 0.3 nmol·h−1·g of fresh weight−1.
Influence of pH and temperature on quorum signal degradation.
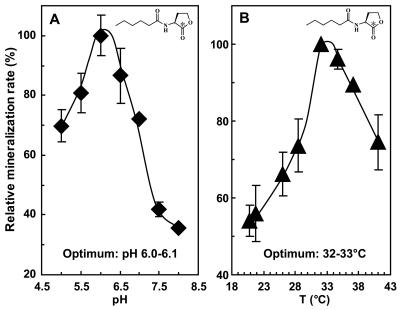
The optimal incubation pH for hexanoyl-HSL mineralization by turf soil (original pH, 6.6) was determined to be 6.0 (Fig. 3A); the slowest 14CO2-release rates were observed at pH values of ≥7.5, conditions under which the relative contribution of chemical acyl-HSL inactivation events would be expected to increase substantially (70). The optimal incubation temperature for mineralization was determined to be 32°C (Fig. 3B), with significant mineralization occurring at the full range of temperatures tested between 21 and 42°C.
FIG. 3.
The influence of incubation pH and temperature on hexanoyl-HSL mineralization. (A) When incubated at 21°C, the maximum rate of mineralization was observed at a pH of 6.0. (B) When incubated at pH 6.0, the maximum rate was observed at 32°C. Symbols and error bars denote the average ± standard deviation of rates over the initial 2 h of incubation determined from duplicate reactions.
Degradation kinetics of two acyl-HSL signals.
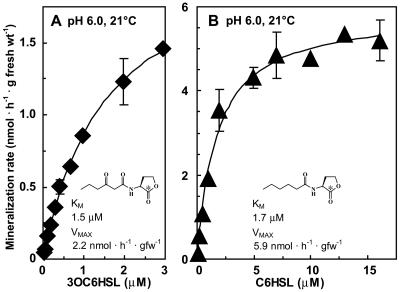
The acyl side chains of known, naturally occurring acyl-HSLs range in length from 4 to 16 carbons, may contain a degree of unsaturation, and may be either unmodified or modified with a keto or hydroxyl functional group at carbon position 3 (21). Past laboratory studies have shown that the nature of the side chain can impart markedly different in vitro chemical and biochemical stabilities (27, 33, 70). We performed a limited examination of the influence of acyl side chain structure on acyl-HSL stability by comparing the rates at which hexanoyl-HSL and oxohexanoyl-HSL were degraded by turf-grass soil. Hexanoyl-HSL was observed to be degraded more rapidly than oxohexanoyl-HSL at all substrate concentrations tested. Varying the initial concentrations of both acyl-HSLs markedly influenced their rates of degradation with apparent Michaelis-Menten kinetics (Fig. 4A and B). Although hexanoyl-HSL was degraded at nearly three times the rate of oxohexanoyl-HSL, the activities for these acyl-HSLs essentially shared equivalent apparent Km half-saturation constants, 1.7 and 1.5 μM acyl-HSL. At the deduced temperature and pH incubation optima of 32°C and pH 6.0, the maximum acyl-HSL degradation rate observed during the course of these studies was 13.4 ± 0.9 nmol of hexanoyl-HSL degraded · h−1 · g of fresh weight soil−1 using 10 μM acyl-HSL as initial substrate (n = 3).
FIG. 4.
Acyl-HSL mineralization by soil exhibits apparent Michaelis-Menten saturation kinetics. The influence of the initial concentration of oxohexanoyl-HSL (A) or hexanoyl-HSL (B) on acyl-HSL degradation kinetics at 21°C and pH 6.0. Symbols and error bars denote the average ± standard deviation of rates over the initial 2 h of incubation determined from duplicate reactions.
Influence of protein synthesis inhibitors on acyl-HSL mineralization.
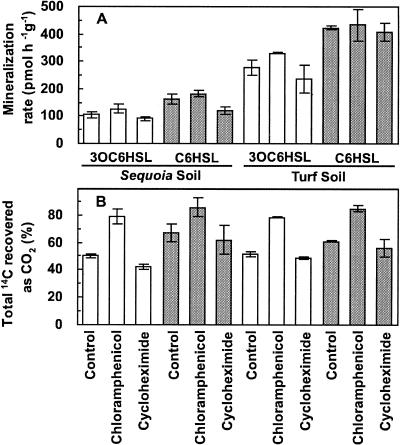
That 14CO2 was released without lag from most of the soils examined suggested that the biochemical machinery involved in acyl-HSL mineralization was already in place at the time those soils had been collected, i.e., that the addition of radiolabel had not served to induce bacterial or fungal microbiota in those particular samples to express the activity during the course of the ex situ assay. To explore this issue further, the prokaryotic and eukaryotic protein synthesis inhibitors chloramphenicol and cycloheximide were amended to slurries prior to the addition of radiolabeled oxohexanoyl-HSL or hexanoyl-HSL. Addition of the inhibitors did not abolish the activity. The initial 14CO2 release rates from chloramphenicol- or cycloheximide-treated slurries were statistically equal between the controls and the antibiotic-treated reactions (Student's t test; P > 0.01), irrespective of the two soil types and two acyl-HSLs examined (Fig. 5A). This observation is consistent with the conclusion that the degradation potential observed in the Sequoia and turf soil slurries was an endogenous feature of the soils at the time of their collection. However, whether the inhibitors had actually influenced the target populations as intended was not confirmed (for rationale, see Materials and Methods).
FIG. 5.
The influence of bacterial and eukaryotic protein synthesis inhibitors on acyl-HSL mineralization by Sequoia and turf soils. Incubation was at 21°C and a pH of 6.0 with an initial concentration of 293 ± 27 nM acyl-HSL. (A) Rates ovter the initial 2 h of incubation of acyl-HSL mineralization. (B) Final recoveries of acyl-HSL radiolabel as 14CO2 after extended incubation.
In striking contrast, the final extent of degradation was markedly influenced by the addition of one but not the other of the two inhibitors (Fig. 5B). Recoveries of 14CO2 from radiolabeled substrates were significantly stimulated in all reactions to which the bacterial protein synthesis inhibitor chloramphenicol had been added (i.e., Sequoia redwood or turf soils amended with either [14C]3OC6HSL or [14C]C6HSL) relative to the control and cycloheximide treatments, which had statistically equal recoveries (Student's t test; P > 0.01). This result is not inconsistent with the possibility that bacteria, when treated with chloramphenicol, were redirecting ca. one-fifth of the original acyl-HSL-derived carbon that would normally have been routed to de novo protein synthesis pathways to their preexisting mineralization apparatus. Alternatively, a chloramphenicol-resistant subpopulation with acyl-HSL mineralization capacity might have become free to thrive on this substrate in the absence of competition, thus contributing to the higher recoveries. Such issues are now being investigated further.
Comparison of acyl-HSL disappearance with CO2 product appearance in turf soil.
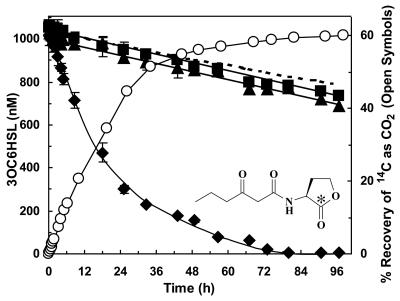
That chloramphenicol treatments (see above) stimulated the final extent of radiolabel recovery as 14CO2 suggests that there could be another major product(s) generated during acyl-HSL degradation. To examine this possibility further, the rate and extent of disappearance (i.e., inactivation) of biologically active 3OC6HSL was compared concurrently with its recovery as 14CO2. In reactions containing 1 μM initial 3OC6HSL, turf soil was observed to inactivate the signal molecule with kinetics similar to and to be well coupled with its conversion to 14CO2, albeit at three- to fourfold greater an initial rate (Fig. 6). Heat inactivation of the soil-buffer mixture prior to the addition of the signal or no addition of soil to the mixture resulted in dramatically slower rates of signal inactivation (Fig. 6), well modeled by the reported chemical half-life of this molecule at the given incubation pH (54). Curiously, there was a remarkable difference observed between the extent of 3OC6HSL inactivation and the extent of its conversion to 14CO2. By hour 96, the amount of biologically active acyl-HSL had decreased to below the 20 picomolar detection limit (i.e., after acyl-HSL extraction, concentration, determination, and subsequent back calculation). By extrapolation, it would take ca. 3 months for chemical inactivation alone to achieve similar results under similar incubation conditions. In contrast to the essential totality with which biological action had inactivated the signal molecule, only 60% was recovered as 14CO2 (Fig. 6). This result provides further (albeit indirect and circumstantial) evidence to suggest that substantial portions of acyl-HSL molecules, when supplied in small amounts that are physiologically relevant to quorum sensing, might be being converted into cell material by bacterial populations active in the soil.
FIG. 6.
Acyl-HSL disappearance versus 14CO2 release. Shown are acyl-HSL disappearance from freshly collected soil (⧫), heat-killed soil (▴), and soil-free reactions (▪) and the release of 14CO2 (○) from [14C]oxohexanoyl-HSL in samples of the same freshly collected soil. Reactions were performed at pH 6.0 and 21°C using an initial concentration of 1 μM oxohexanoyl-HSL. The viable soil exhibited initial rates of 3OC6HSL degradation of 2.1 nmol · h−1 · g of fresh weight−1 (⧫; i.e., by bioassay) and 0.5 nmol · h−1 · g of fresh weight−1 (○; i.e., by 14CO2 release). Dashed lines represent the theoretical chemical degradation of the starting acyl-HSL as a function of its reported half-life, 240 h. Over the full 96-h incubation period, acyl-HSL decrease from both the heat-killed soil (▴) and soil-free controls (▪) exhibited first-order exponential decay kinetics, with an apparent half-life of ∼185 h. The endogenous 3OC6HSL content of the soil at the time of its collection was ≤20 pM, the bioassay detection limit after acyl-HSL extraction and concentration.
Monitoring for substrate acyl-HSL disappearance versus the appearance of an intermediate or final product, such as CO2, can be complicated by issues of sorption, i.e., if the signal molecule was being tightly bound to soil particles and unavailable for assay rather than being inactivated. The issue of sorption was examined directly in heat-inactivated soil. For this specific line of experiments (i.e., incubation pH and temperature and acyl-HSL:soil:buffer ratio), 3OC6HSL was not observed to sorb to soil strongly (≤6% sorbed) and thus remained in solution for accurate concentration determinations via bioassay. Similarly, the acidification of reaction mixtures at the end of soil slurry experiments (i.e., to convert particulate or dissolved [14C]carbonates to gaseous 14CO2) did not serve to further stimulate the recovery of radiolabel, suggesting that the differences in extent of acyl-HSL disappearance versus its recovery as CO2 were real and reflected the formation of at least one other major product, perhaps biomass.
MPN of acyl-HSL-mineralizing microbes.
Since the apparent Km for acyl-HSL degradation by soils was ca. 1,000-fold lower than (i.e., remarkably superior to) the Km determined for the purified acyl-HSL lactonase from Bacillus cereus (64), and because of the strong circumstantial evidence (see above) that suggests that bacteria might be converting a significant fraction of acyl-HSL decay products into cell material, we wished to begin to examine which not-yet-cultivated organisms capable of metabolizing oligotrophic amounts of carbon might be responsible for the observed soil degradation activities. An MPN enumeration of turf soil microbes capable of mineralizing low concentrations of hexanoyl-HSL was performed. After 18 days of incubation, an MPN of 2.3 × 107 heterotrophic cells · g of fresh weight soil−1 was observed to develop in the enrichments. Two percent of the cells recovered in dilution tubes (MPN = 4.6 × 105 cells · g of fresh weight soil−1) released ≥25% of the initial 100 nM hexanoyl-[1-14C]HSL radiolabel as 14CO2 over the same period. Attempts are currently under way to isolate and study the most abundant of the organisms utilizing oligotrophic amounts of acyl-HSLs from these enrichments.
DISCUSSION
The results of this study show that acyl-HSL-inactivating microbes are indeed active in the environment and that physiologically relevant concentrations of quorum signals are subject to a rapid biodegradation in bulk soils. Moreover, the apparent Km of the degradation activity for acyl-HSL quorum signals in the soil is ca. 1,000-fold lower than that of a purified acyl-HSL-degrading enzyme from Bacillus cereus (64), suggesting that acyl-HSL-degrading organisms currently available in culture may not be representative sources of the observed soil activity.
Possible impact of signal decay on quorum sensing over long and short timescales.
As introduced previously (33), quorum-sensing systems cannot be expected to function as monitors of population density over the long term if the signal molecules employed were inherently stable. The population sizes of quorum-sensing bacteria in nature are likely often in flux. A nascent quorum-sensing population or single cell should begin its growth with a slate clean of signals for the regulatory circuit to function properly, i.e., as a reflection of population density. That is, local quorum signal concentrations should illuminate current events as opposed to being an historical record of microbial societies long since vanished. Biological signal decay accomplished an effective “system reset” in days that would have taken chemical inactivation to accomplish over a long season (Fig. 2 and 6). This clearly would considerably shorten the timescale required for reequilibration of a site after the dissipation of an active quorum-sensing population.
However, rapid signal decay might also challenge quorum-regulated activities in nature, a possibility strongly supported by recent studies performed in defined multiculture sand mesocosms (42) as well as other studies on “quorum quenching” (11-14, 34, 37, 44, 50, 64). The maximum biological degradation rate observed in this study, 13.4 ± 0.9 nmol of C6HSL · h−1 · g of fresh weight soil−1, is ca. 450-fold greater than the initial chemical degradation rate expected under incubation at similar pH, i.e., as predicted by the equation acyl-HSL t1/2 = 10[7 − pH] days (54). For instance, the acyl-HSL chemical half-life is ca. 60 h in a turf soil at pH 6.6. Clearly, acyl-HSL degradation can greatly outmatch chemical inactivation events in these and similar soils (Table 1). Would such high rates be expected to have a meaningful impact on the short-term accumulation of acyl-HSLs by either growing or resting populations of quorum-sensing bacteria in the environment? We have observed that the phytopathogen Pantoea stewartii and the opportunistic pathogen Pseudomonas aeruginosa synthesize acyl-HSLs at rates no greater than 10−18 moles · cell−1 · h−1, i.e., when grown optimally in vitro (unpublished data). Although acyl-HSL synthesis rates are not readily accessible from other published reports, this rate appears to be reasonably representative of the observed upper limits for a variety of quorum-sensing bacteria (13, 27, 37, 50, 71). Moreover, many known acyl-HSL-producing bacteria require a local accumulation of acyl-HSLs to reach (depending on the species in question) levels from 5 nM to 2 μM for a quorum response to occur in vitro (19, 45, 63), whereas the biological activity revealed here can consume acyl-HSLs to levels of less than 20 pM, i.e., >100-fold less than is sensed by the keenest of the known quorum-sensing bacteria. Assuming a well-mixed system, acyl-HSL-producing bacteria could be considerably challenged to outpace biological acyl-HSL degradation, becoming hampered in their ability to engage in cell-cell communications until reaching population densities of greater than 109 or 1010 cells · g of soil−1. Such high population densities would indeed be quite a high cell load for a single species living in a soil, as soils typically contain <1011 total microbial cells · g of soil−1, representing thousands of different species.
However, soils are not well-mixed systems, and their water content can be extremely variable. Bacteria typically grow as microcolonies in soils. The results of this study do not indicate whether cell-associated or abiontic signal hydrolases infiltrate acyl-HSL-producing aggregates. Little is known of the natural distribution of quorum-sensing bacteria in soils. However, infiltration of acyl-HSL-producing aggregates by signal-degrading organisms or enzymes might not be absolutely necessary for this activity to have an impact on quorum-regulated events. Quorum signals are known to diffuse readily through biomass (28, 46), and signal binding by LuxR-type transcriptional activators is known to be a reversible process in some but not other quorum-sensing bacteria (61). Strong signal decay and signal sinks directly adjacent to acyl-HSL-producing populations have previously been modeled and predicted to influence signal accumulations within biofilms, increasing the effective population size required to engage in quorum sensing (7).
Signal degradation could serve to insulate microbial aggregates.
The possibility that beneficial or deleterious cross talk between spatially separated microbial populations (even those belonging to different species) might occur in nature has previously been suggested (2, 36, 40, 47). The proximity of acyl-HSL mineralization activities to nascent or well-developed aggregates of quorum-sensing bacteria in soil is not yet known, but signal degradation might be expected to serve to insulate (for better or worse) such populations from signal molecules produced by cells elsewhere, disrupting both intra- and interspecies communications that might otherwise occur between spatially separated microcolonies. Disruption of quorum sensing by signal-degrading bacteria has already been demonstrated to occur in simple plant mesocosms seeded with defined microbial cocultures left unmixed after inoculation (42). Insulation of microbial aggregates from extraneous signals thus could preserve and even accentuate the spatial and chemical heterogeneity and the biological microstructure of complex microbial ecosystems. The quorum-sensing systems of soil bacteria likely have evolved and continue to evolve in the context of a significant challenge by signal decay (32) and may already be tuned to meet it. Clearly, biological signal degradation should be taken into account during the design and interpretation of studies aimed at reaching a better understanding of microbial cell-cell communications and quorum sensing-controlled processes in species-rich environments, such as soils.
Future directions.
A recent review has raised the teleological question of why and for what intended evolutionary function have enzymes capable of signal decay evolved (52). Certainly, approaching with clarity the design and interpretation of experiments aimed at revealing “why” an activity occurs is significantly more challenging than establishing that or how it occurs (30). This challenge notwithstanding, we hope to continue to better answer some of the provocative questions that have been raised about biological acyl-HSL degradation. It is not at all beyond reason that abiontic (no longer cell-associated) soil hydrolases of broad substrate specificity might be responsible for several of the activities revealed in this study (9, 29), especially those present in samples wherein linear kinetics commenced without lag and appeared to be insensitive to protein synthesis inhibitors. While the activity of abiontic enzymes would yield the same expected potential impacts on quorum-sensing populations, it would be difficult to infer that the activities of such enzymes necessarily benefit the cells that had at one time produced them. The activity could essentially be accidental.
However, this is not to say that the enzymes are certainly abiontic. In samples from two sites examined (Table 1), signal decay progressed only after an initial lag of several hours and was not likely incidental. If de novo protein synthesis was required in those samples, then abiontic enzymes alone would not likely account for signal decay. Signal decay might actually be a direct response to, i.e., induced by exposure to, acyl-HSLs at those sites, suggesting that the activity is regulated and presumably of some benefit to the cells in being so. Finally, the results of the MPN cultivation experiments performed in this study suggest that it may be achievable to isolate pure cultures of microbes catalyzing the low Km activities revealed in this study, cells perhaps capable of reaping some definite and definable nutritional reward during their metabolism of trace levels of an intriguing class of biological molecules. Their cultivation and identification would open the door to future experimental lines, including the use of oligonucleotide-based fluorescence in situ hybridization techniques to map the distribution of signal-producing and -degrading microbes in soils and other environments.
Acknowledgments
This research was supported by grants from the USDA (CSREES 2001-01242) and by a generous gift from William Davidow and family. The Long-Term Ecological Research Program at Michigan State University's Kellogg Biological Station is supported by the NSF and the Michigan Agricultural Experiment Station.
We thank D. Newman for liquid scintillation counter usage; L. Thomashow for providing agricultural soil samples from Pullman, Wash.; A. Corbin for providing KBS-LTER soil samples; and J. Hering, A. Kappler, E. R. Leadbetter, J. Suflita, our laboratory colleagues, and several anonymous reviewers of this and prior work for their insightful comments and suggestions.
Footnotes
This paper is dedicated to John A. Breznak in celebration of his 60th birthday and his 30 years as a research leader.
REFERENCES
- 1.Ang, S., Y. T. Horng, J. C. Shu, P. C. Soo, J. H. Liu, W. C. Yi, H. C. Lai, K. T. Luh, S. W. Ho, and S. Swift. 2001. The role of RsmA in the regulation of swarming motility in Serratia marcescens. J. Biomed. Sci. 8:160-169. [DOI] [PubMed] [Google Scholar]
- 2.Blosser, R. S., and K. M. Gray. 2000. Extraction of violacein from Chromobacterium violaceum provides a new quantitative bioassay for N-acyl homoserine lactone autoinducers. J. Microbiol. Methods 40:47-55. [DOI] [PubMed] [Google Scholar]
- 3.Brune, A., E. Miambi, and J. A. Breznak. 1995. Roles of oxygen and the intestinal microflora in the metabolism of lignin-derived phenylpropanoids and other monoaromatic compounds by termites. Appl. Environ. Microbiol. 61:2688-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter, M. R. (ed.). 2000. Soil sampling and methods of analysis. CRC Press, Boca Raton, Fla.
- 5.Cha, C., P. Gao, Y. C. Chen, P. D. Shaw, and S. K. Farrand. 1998. Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Mol. Plant-Microbe Interact. 11:1119-1129. [DOI] [PubMed] [Google Scholar]
- 6.Chernin, L. S., M. K. Winson, J. M. Thompson, S. Haran, B. W. Bycroft, I. Chet, P. Williams, and G. S. Stewart. 1998. Chitinolytic activity in Chromobacterium violaceum: substrate analysis and regulation by quorum sensing. J. Bacteriol. 180:4435-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chopp, D. L., M. J. Kirisits, B. Moran, and M. R. Parsek. 2003. The dependence of quorum sensing on the depth of a growing biofilm. Bull. Math. Biol. 65:1053-1079. [DOI] [PubMed] [Google Scholar]
- 8.Christensen, A. B., K. Riedel, L. Eberl, L. R. Flodgaard, S. Molin, L. Gram, and M. Givskov. 2003. Quorum-sensing-directed protein expression in Serratia proteamaculans B5a. Microbiology 149:471-483. [DOI] [PubMed] [Google Scholar]
- 9.Conrad, R. 1996. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, N2O, NO). Microbiol. Rev. 60:609-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Kievit, T., P. C. Seed, J. Nezezon, L. Passador, and B. H. Iglewski. 1999. RsaL, a novel repressor of virulence gene expression in Pseudomonas aeruginosa. J. Bacteriol. 181:2175-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong, Y.-H., A. R. Gusti, Q. Zhang, J.-L. Xu, and L.-H. Zhang. 2002. Identification of quorum-quenching N-acyl homoserine lactonases from Bacillus species. Appl. Environ. Microbiol. 68:1754-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong, Y.-H., L.-H. Wang, J.-L. Xu, H.-B. Zhang, X.-F. Zhang, and L.-H. Zhang. 2001. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 411:813-817. [DOI] [PubMed] [Google Scholar]
- 13.Dong, Y.-H., J.-L. Xu, X.-Z. Li, and L.-H. Zhang. 2000. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 97:3526-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong, Y.-H., X.-F. Zhang, J.-L. Xu, and L.-H. Zhang. 2004. Insecticidal Bacillus thuringiensis silences Erwinia carotovora virulence by a new form of microbial antagonism, signal interference. Appl. Environ. Microbiol. 70:954-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eberhard, A., A. L. Burlingame, C. Eberhard, G. L. Kenyon, K. H. Nealson, and N. J. Oppenheimer. 1981. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry 20:2444-2449. [DOI] [PubMed] [Google Scholar]
- 16.Flagan, S., W.-K. Ching, and J. R. Leadbetter. 2003. Arthrobacter strain VAI-A utilizes acyl-homoserine lactone inactivation products and stimulates quorum signal biodegradation by Variovorax paradoxus. Appl. Environ. Microbiol. 69:909-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flavier, A. B., L. M. Ganova-Raeva, M. A. Schell, and T. P. Denny. 1997. Hierarchical autoinduction in Ralstonia solanacearum: control of acyl-homoserine lactone production by a novel autoregulatory system responsive to 3-hydroxypalmitic acid methyl ester. J. Bacteriol. 179:7089-7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fray, R. G., J. P. Throup, M. Daykin, A. Wallace, P. Williams, G. S. Stewart, and D. Grierson. 1999. Plants genetically modified to produce N-acylhomoserine lactones communicate with bacteria. Nat. Biotechnol. 17:1017-1020. [DOI] [PubMed] [Google Scholar]
- 19.Fuqua, C., M. Burbea, and S. C. Winans. 1995. Activity of the Agrobacterium Ti plasmid conjugal transfer regulator TraR is inhibited by the product of the traM gene. J. Bacteriol. 177:1367-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuqua, C., and E. P. Greenberg. 2002. Listening in on bacteria: acyl-homoserine lactone signalling. Nat. Rev. Mol. Cell Biol. 3:685-695. [DOI] [PubMed] [Google Scholar]
- 21.Fuqua, C., and E. P. Greenberg. 1998. Self perception in bacteria: quorum sensing with acylated homoserine lactones. Curr. Opin. Microbiol. 1:183-189. [DOI] [PubMed] [Google Scholar]
- 22.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 23.Fuqua, W. C., and S. C. Winans. 1994. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J. Bacteriol. 176:2796-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray, K. M., L. Passador, B. H. Iglewski, and E. P. Greenberg. 1994. Interchangeability and specificity of components from the quorum-sensing regulatory systems of Vibrio fischeri and Pseudomonas aeruginosa. J. Bacteriol. 176:3076-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamdan, H., D. M. Weller, and L. S. Thomashow. 1991. Relative importance of fluorescent siderophores and other factors in biological control of Gaeumannomyces graminis var. tritici by Pseudomonas fluorescens 2-79 and M4-80R. Appl. Environ. Microbiol. 57:3270-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horng, Y. T., S. C. Deng, M. Daykin, P. C. Soo, J. R. Wei, K. T. Luh, S. W. Ho, S. Swift, H. C. Lai, and P. Williams. 2002. The LuxR family protein SpnR functions as a negative regulator of N-acylhomoserine lactone-dependent quorum sensing in Serratia marcescens. Mol. Microbiol. 45:1655-1671. [DOI] [PubMed] [Google Scholar]
- 27.Huang, J. J., J.-I. Han, L.-H. Zhang, and J. R. Leadbetter. 2003. Utilization of acyl-homoserine lactone quorum signals for growth by a soil pseudomonad and Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 69:5941-5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan, H. B., and E. P. Greenberg. 1985. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J. Bacteriol. 163:1210-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klose, S., and M. A. Tabatabai. 2002. Response of amidohydrolases in soils to chloroform fumigation. J. Plant Nutr. Soil Sci. 165:125-132. [Google Scholar]
- 30.Lander, A. D. 2004. A calculus of purpose. PLoS Biol. 2:712-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leadbetter, J. R. 2003. Cultivation of recalcitrant microbes: cells are alive, well, and revealing their secrets in the 21st century laboratory. Curr. Opin. Microbiol. 6:274-281. [DOI] [PubMed] [Google Scholar]
- 32.Leadbetter, J. R. 2001. News and views: plant microbiology—quieting the raucous crowd. Nature 411:748-749. [DOI] [PubMed] [Google Scholar]
- 33.Leadbetter, J. R., and E. P. Greenberg. 2000. Metabolism of acyl-homoserine lactone quorum-sensing signals by Variovorax paradoxus. J. Bacteriol. 182:6921-6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee, S. J., S. Y. Park, J. J. Lee, D. Y. Yum, B. T. Koo, and J. K. Lee. 2002. Genes encoding the N-acyl homoserine lactone-degrading enzyme are widespread in many subspecies of Bacillus thuringiensis. Appl. Environ. Microbiol. 68:3919-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewenza, S., B. Conway, E. P. Greenberg, and P. A. Sokol. 1999. Quorum sensing in Burkholderia cepacia: identification of the LuxRI homologs CepRI. J. Bacteriol. 181:748-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewenza, S., M. B. Visser, and P. A. Sokol. 2002. Interspecies communication between Burkholderia cepacia and Pseudomonas aeruginosa. Can. J. Microbiol. 48:707-716. [DOI] [PubMed] [Google Scholar]
- 37.Lin, Y.-H., J.-L. Xu, J. Hu, L.-H. Wang, S. L. Ong, J. R. Leadbetter, and L.-H. Zhang. 2003. Acyl-homoserine lactone acylase from Ralstonia str. XJ12B represents a novel and potent class of quorum quenching enzymes. Mol. Microbiol. 47:849-860. [DOI] [PubMed] [Google Scholar]
- 38.Mazzola, M., R. J. Cook, L. S. Thomashow, D. M. Weller, and L. S. Pierson. 1992. Contribution of phenazine antibiotic biosynthesis to the ecological competence of fluorescent pseudomonads in soil habitats. Appl. Environ. Microbiol. 58:2616-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McClean, K. H., M. K. Winson, L. Fish, A. Taylor, S. R. Chhabra, M. Camara, M. Daykin, J. H. Lamb, S. Swift, B. W. Bycroft, G. S. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703-3711. [DOI] [PubMed] [Google Scholar]
- 40.McDougald, D., S. Srinivasan, S. A. Rice, and S. Kjelleberg. 2003. Signal-mediated cross-talk regulates stress adaptation in Vibrio species. Microbiology 149:1923-1933. [DOI] [PubMed] [Google Scholar]
- 41.McGowan, S. J., M. T. Holden, B. W. Bycroft, and G. P. Salmond. 1999. Molecular genetics of carbapenem antibiotic biosynthesis. Antonie Leeuwenhoek 75:135-141. [DOI] [PubMed] [Google Scholar]
- 42.Molina, L., F. Constantinescu, L. Michel, C. Reimmann, B. Duffy, and G. Defago. 2003. Degradation of pathogen quorum-sensing molecules by soil bacteria: a preventive and curative biological control mechanism. FEMS Microbiol. Ecol. 45:71-81. [DOI] [PubMed] [Google Scholar]
- 43.Nealson, K. H., T. Platt, and J. W. Hastings. 1970. Cellular control of the synthesis and activity of the bacterial luminescence system. J. Bacteriol. 104:313-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park, S.-Y., S.-J. Lee, T.-K. Oh, J.-W. Oh, B.-T. Koo, D.-Y. Yum, and J.-K. Lee. 2003. AhlD, an N-acylhomoserine lactonase in Arthrobacter sp., and predicted homologues in other bacteria. Microbiology 149:1541-1550. [DOI] [PubMed] [Google Scholar]
- 45.Pearson, J. P., L. Passador, B. H. Iglewski, and E. P. Greenberg. 1995. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:1490-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearson, J. P., C. Van Delden, and B. H. Iglewski. 1999. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 181:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pierson, E. A., D. W. Wood, J. A. Cannon, F. M. Blachere, and L. S. Pierson. 1998. Interpopulation signaling via N-acyl-homoserine lactones among bacteria in the wheat rhizosphere. Mol. Plant-Microbe Interact. 11:1078-1084. [Google Scholar]
- 48.Pierson, L. S., D. W. Wood, and E. A. Pierson. 1998. Homoserine lactone-mediated gene regulation in plant-associated bacteria. Annu. Rev. Phytopathol. 36:207-225. [DOI] [PubMed] [Google Scholar]
- 49.Puskas, A., E. P. Greenberg, S. Kaplan, and A. L. Schaefer. 1997. A quorum-sensing system in the free-living photosynthetic bacterium Rhodobacter sphaeroides. J. Bacteriol. 179:7530-7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reimmann, C., N. Ginet, L. Michel, C. Keel, P. Michaux, V. Krishnapillai, M. Zala, K. Heurlier, K. Triandafillu, H. Harms, G. Defago, and D. Haas. 2002. Genetically programmed autoinducer destruction reduces virulence gene expression and swarming motility in Pseudomonas aeruginosa PAO1. Microbiology 148:923-932. [DOI] [PubMed] [Google Scholar]
- 51.Riedel, K., T. Ohnesorg, K. A. Krogfelt, T. S. Hansen, K. Omori, M. Givskov, and L. Eberl. 2001. N-acyl-l-homoserine lactone-mediated regulation of the lip secretion system in Serratia liquefaciens MG1. J. Bacteriol. 183:1805-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roche, D. M., J. T. Byers, D. S. Smith, F. G. Glansdorp, D. R. Spring, and M. Welch. 2004. Communications blackout? Do N-acylhomoserine-lactone-degrading enzymes have any role in quorum sensing? Microbiology 150:2023-2028. [DOI] [PubMed] [Google Scholar]
- 53.Rodelas, B., J. K. Lithgow, F. Wisniewski-Dye, A. Hardman, A. Wilkinson, A. Economou, P. Williams, and J. A. Downie. 1999. Analysis of quorum-sensing-dependent control of rhizosphere-expressed (rhi) genes in Rhizobium leguminosarum bv. viciae. J. Bacteriol. 181:3816-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schaefer, A. L., B. L. Hanzelka, M. R. Parsek, and E. P. Greenberg. 2000. Detection, purification and structural elucidation of acylhomoserine lactone inducer of Vibrio fischeri luminescence and other related molecules. Methods Enzymol. 305:288-301. [DOI] [PubMed] [Google Scholar]
- 55.Schaefer, A. L., T. A. Taylor, J. T. Beatty, and E. P. Greenberg. 2002. Long-chain acyl-homoserine lactone quorum-sensing regulation of Rhodobacter capsulatus gene transfer agent production. J. Bacteriol. 184:6515-6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schripsema, J., K. E. E. de Rudder, T. B. van Vliet, P. P. Lankhorst, E. de Vroom, J. W. Kijne, and A. A. N. van Brussel. 1996. Bacteriocin small of Rhizobium leguminosarum belongs to the class of N-acyl-l-homoserine lactone molecules, known as autoinducers and as quorum sensing co-transcription factors. J. Bacteriol. 178:366-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stead, P., B. A. Rudd, H. Bradshaw, D. Noble, and M. J. Dawson. 1996. Induction of phenazine biosynthesis in cultures of Pseudomonas aeruginosa by l-N-(3-oxohexanoyl)homoserine lactone. FEMS Microbiol. Lett. 140:15-22. [DOI] [PubMed] [Google Scholar]
- 58.Swift, S., J. A. Downie, N. A. Whitehead, A. M. L. Barnard, G. P. C. Salmond, and P. Williams. 2001. Quorum sensing as a population-density-dependent determinant of bacterial physiology. Adv. Microb. Physiol. 45:199-270. [DOI] [PubMed] [Google Scholar]
- 59.Thomashow, L. S., and D. M. Weller. 1988. Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. J. Bacteriol. 170:3499-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomson, N. R., M. A. Crow, S. J. McGowan, A. Cox, and G. P. Salmond. 2000. Biosynthesis of carbapenem antibiotic and prodigiosin pigment in Serratia is under quorum sensing control. Mol. Microbiol. 36:539-556. [DOI] [PubMed] [Google Scholar]
- 61.Urbanowski, M. L., C. P. Lostroh, and E. P. Greenberg. 2004. Reversible acyl-homoserine lactone binding to purified Vibrio fischeri LuxR protein. J. Bacteriol. 186:631-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uroz, S., C. D'Angelo-Picard, A. Carlier, M. Elasri, C. Sicot, A. Petit, P. Oger, D. Faure, and Y. Dessaux. 2003. Novel bacteria degrading N-acylhomoserine lactones and their use as quenchers of quorum-sensing-regulated functions of plant-pathogenic bacteria. Microbiology 149:1981-1989. [DOI] [PubMed] [Google Scholar]
- 63.von Bodman, S. B., D. R. Majerczak, and D. L. Coplin. 1998. A negative regulator mediates quorum-sensing control of exopolysaccharide production in Pantoea stewartii subsp. stewartii. Proc. Natl. Acad. Sci. USA 95:7687-7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang, L. H., L. X. Weng, Y. H. Dong, and L. H. Zhang. 2004. Specificity and enzyme kinetics of the quorum-quenching N-acyl homoserine lactone lactonase. J. Biol. Chem. 279:13645-13651. [DOI] [PubMed] [Google Scholar]
- 65.Watson, W. T., F. V. Murphy, T. A. Gould, P. Jambeck, D. L. Val, J. E. Cronan, S. B. von Bodman, and M. E. A. Churchill. 2001. Crystallization and rhenium MAD phasing of the acyl-homoserine lactone synthase EsaI. Acta Crystallogr. Sect. D 57:1945-1949. [DOI] [PubMed] [Google Scholar]
- 66.Winans, S. C., and B. L. Bassler. 2002. Mob psychology. J. Bacteriol. 184:873-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wood, D. W., F. Gong, M. M. Daykin, P. Williams, and L. S. Pierson III. 1997. N-acyl-homoserine lactone-mediated regulation of phenazine gene expression by Pseudomonas aureofaciens 30-84 in the wheat rhizosphere. J. Bacteriol. 179:7663-7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wood, D. W., and L. S. Pierson III. 1996. The phzI gene of Pseudomonas aureofaciens 30-84 is responsible for the production of a diffusible signal required for phenazine antibiotic production. Gene 168:49-53. [DOI] [PubMed] [Google Scholar]
- 69.Yang, W.-W., and J. R. Leadbetter. Unpublished data.
- 70.Yates, E. A., B. Philipp, C. Buckley, S. Atkinson, S. R. Chhabra, R. E. Sockett, M. Goldner, Y. Dessaux, M. Camara, H. Smith, and P. Williams. 2002. N-acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infect. Immun. 70:5635-5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang, H.-B., L.-H. Wang, and L.-H. Zhang. 2002. Genetic control of quorum-sensing signal turnover in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 99:4638-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang, L., P. J. Murphy, A. Kerr, and M. E. Tate. 1993. Agrobacterium conjugation and gene regulation by N-acyl-L-homoserine lactones. Nature 362:446-448. [DOI] [PubMed] [Google Scholar]