Abstract
Single amino acid (AA) supplementations in foods are increasing, however their potential nutritional and physiological impacts are not fully understood. This study examined the effects of l-lysine (Lys) supplementation on protein quality of diets, serum AA concentrations and associations between the ratio of supplemental Lys to dietary protein (X) with body weight gain (BWG) in Sprague–Dawley male rats. Rats were fed one of 10 diets containing either 7% or 20% casein and supplemented with 0% (Control), 1.5%, 3%, 6% Lys or 6% Lys + 3% l-arginine (Arg) (8 rats/diet group) for 1 week. Lys supplementation reduced the protein quality of the casein-based diets (p < 0.01). BWG was reduced by supplemental Lys when X > 0.18. Free Lys supplementation dose-dependently increased serum Lys levels (p < 0.01), while increased protein-bound Lys (1.4% vs 0.52%) had little effect on serum Lys (p > 0.05). In the 7% casein diets, ≥ 1.5% supplemental Lys reduced serum alanine, asparagine, glycine, isoleucine, leucine, serine, tyrosine, valine, carnitine, ornithine, and increased urea. Supplementation of ≥ 3% Lys additionally reduced tryptophan and increased histidine, methionine and α-aminoadipic acid (α-AAA) compared to the Control (p < 0.05). In the 20% casein diets, addition of ≥ 1.5% Lys reduced serum asparagine and threonine, and ≥ 3% Lys reduced leucine, proline, tryptophan, valine, and ornithine, and 6% Lys reduced carnitine, and increased histidine, methionine, and α-AAA. Overall, this study showed that free Lys supplementation in a Lys-sufficient diet reduced the protein quality of the diets and modified the serum concentrations of many amino acids. Excess free Lys intake adversely affected growth and utilization of nutrients due to AA imbalance or antagonism. Overall lower protein intake increases susceptibility to the adverse effects of Lys supplementation.
Subject terms: Biochemistry, Physiology
Introduction
l-Lysine (Lys) is an essential amino acid (EAA) in mammals, and must be obtained from foods or dietary supplements since it cannot be synthesized in the body1. Lys is usually low in cereal-based food products2, but rich in animal food sources such as lean beef, chicken, pork, and shellfish1. The Lys requirement in healthy adults is 30 mg/kg BW/day3, and Lys intake from Western diets is about 40–180 mg/kg BW/day4. The average Lys intake of Americans is 75.3 mg/kg BW/day5, which is over two times the daily requirement. Besides its nutritional roles, Lys has garnered popularity as a supplement in foods and drinks for its purported functional properties.
Lys has been used as a common workout supplement because of its potential roles in energy production and stimulation of muscle growth. Lys is a precursor of carnitine that is involved in mitochondrial β-oxidation to convert fatty acids into energy in mammals6. Moreover, Lys was shown to reduce protein degradation7 and stimulate protein synthesis8,9. Lys stimulated growth hormone release when infused intravenously or administered orally10,11. It is believed that supplementation with Lys before strength training workouts could increase exercise-induced growth hormone release, improving athletic performance by increasing muscle mass and strength12,13.
Lys supplement may prevent the recurrence of certain viral infections such as herpes simplex, shingles, and human papilloma virus, and improve the associated symptoms14–16. Lys and l-arginine (Arg) share the same Lys/Arg transporters and therefore compete for uptake into cells. Excess Lys can decrease intracellular Arg levels. Replication of certain viruses requires higher Arg and lower Lys. Increasing cellular Lys concentrations disrupts the metabolic balance between Lys and Arg, and inhibits viral replication17.
Addition of Lys to foods containing high starch and low protein such as fabricated snack products reduces formation of acrylamide via Maillard reaction when processed at high temperature18. Asparagine content in these foods was positively associated with acrylamide formation in a dose-dependent manner19. When Lys was added prior to frying, the formation of acrylamide was eliminated20,21. Kim et al. showed that pre-treatment of snack food products with 3% Lys prior to frying reduced acrylamide formation by 80%22. It was postulated that the ε-NH2 group of Lys reacts with acrylamide to form a Lys-acrylamide adduct in a 2:1 ratio, thereby eliminating the formation of acrylamide21,23. Lys is currently allowed to be added in fabricated snack foods up to a level of 1.2% to reduce the formation of acrylamide.
Nutritional quality of a protein in diets is assessed by comparing the ratios of its EAA content to a reference protein or their requirements in humans or animals and corrected with protein digestibility of the diet24. The lowest EAA ratio in a protein is defined as the amino acid score. The EAA that has the lowest ratio in a diet is the first limiting EAA, and it limits the utilization of other EAA and determines the quality of the protein in the diet. Excess Lys supplementation could alter the ratios of the EAA and affect protein quality of the diets, and result in amino acid imbalance thereby adversely affecting physiological functions25–27. Our previous studies showed that the effects of Lys supplementation were modified by protein content in diets. The rats fed lower dietary protein were less tolerant to Lys supplementation and showed adverse effects such as decreased food intake and retarded growth28,29. However, the mechanism(s) involved, and the tolerable upper levels of Lys supplementation remain to be determined.
The nutritional safety and health effects of supplemental Lys intake are not fully understood. In this study, using rats as a model, we examined, (a) the effects of Lys supplementation in the context of a low or normal protein diet on protein digestibility and quality of the diets, (b) the effects of dietary protein-bound vs. free Lys on serum free amino acids and metabolites, (c) the association of the ratios of supplemental Lys to protein content in diets (X) with protein quality and body weight gain (BWG), and (d) the maximum level of supplemental Lys in terms of its ratio to dietary protein content that has no significant effects on BWG.
Results
EAA content and limiting EAA scores in diets
Dietary EAA content and scores are shown in Table 1. l-methionine (Met) + l-cysteine (Cys) were the limiting EAA in both the 7% and 20% casein diets and their amino acid scores were 0.71 and 0.69, respectively. Lys supplementation dose-dependently reduced the limiting EAA (Met + Cys) scores in the diets, and the extent of reduction by the same amount of supplemental Lys was higher in the 7% casein than in the 20% casein diet (Table 1). Supplementation with 1.5%, 3%, 6% Lys or 6% Lys + 3% Arg in 7% casein diets reduced the limiting EAA scores by 18%, 32%, 56% and 68% compared to the Control, respectively. The corresponding values for diets with 20% casein were 9%, 16%, 33% and 43% (Table 1).
Table 1.
Dietary essential amino acid (EAA) content and ratios.
| mg/g protein | Rat EAA req* | 7% casein | 20% casein | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1.5% Lys | 3% Lys | 6% Lys | 6%Lys + 3%Arg | 0 | 1.5% Lys | 3% Lys | 6% Lys | 6%Lys + 3%Arg | ||
| Arginine | 29 | 32.2 | 25.8 | 22.5 | 15.7 | 151.7 | 34.3 | 31.3 | 29.9 | 24.4 | 107.7 |
| Histidine | 19 | 29.0 | 23.3 | 20.4 | 14.1 | 10.1 | 27.6 | 25.3 | 23.9 | 19.7 | 16.9 |
| Isoleucine | 41 | 51.5 | 41.8 | 36.5 | 25.1 | 18.0 | 49.5 | 45.1 | 42.4 | 35.0 | 29.9 |
| Leucine | 71 | 98.2 | 78.6 | 66.5 | 47.1 | 34.0 | 92.3 | 84.3 | 79.7 | 65.7 | 55.8 |
| Lysine | 61 | 83.7 | 199.0 | 289.7 | 371.0 | 255.2 | 78.8 | 126.5 | 170.3 | 225.0 | 191.1 |
| Met + Cys | 65 | 46.7 | 38.1 | 31.1 | 20.4 | 14.9 | 45.1 | 41.2 | 37.8 | 29.9 | 25.6 |
| Phe + Tyr | 68 | 80.5 | 65.1 | 56.9 | 39.2 | 28.6 | 95.7 | 85.8 | 82.4 | 68.5 | 58.1 |
| Threonine | 41 | 43.5 | 34.4 | 29.0 | 20.4 | 14.3 | 46.2 | 37.2 | 35.0 | 28.7 | 24.3 |
| Tryptophan | 13 | 14.5 | 9.8 | 9.7 | 6.3 | 5.3 | 15.2 | 11.4 | 11.0 | 10.6 | 8.1 |
| Valine | 49 | 66.0 | 52.8 | 45.1 | 31.4 | 22.8 | 62.5 | 57.5 | 53.9 | 44.5 | 37.6 |
| EAA scores | |||||||||||
| Arginine | 1.11 | 0.89 | 0.78 | 0.54 | 5.23 | 1.18 | 1.08 | 1.03 | 0.84 | 3.71 | |
| Histidine | 1.55 | 1.25 | 1.09 | 0.76 | 0.54 | 1.48 | 1.36 | 1.28 | 1.05 | 0.90 | |
| Isoleucine | 1.25 | 1.01 | 0.88 | 0.61 | 0.44 | 1.20 | 1.09 | 1.02 | 0.85 | 0.72 | |
| Leucine | 1.38 | 1.10 | 0.93 | 0.66 | 0.48 | 1.29 | 1.18 | 1.12 | 0.92 | 0.78 | |
| Lysine | 1.37 | 3.24 | 4.72 | 6.05 | 4.16 | 1.29 | 2.06 | 2.78 | 3.67 | 3.12 | |
| Met + Cys | 0.71 | 0.58 (− 18%)** | 0.48 (− 32%) | 0.31 (− 56%) | 0.23 (− 68%) | 0.69 | 0.63 (− 9%) | 0.58 (− 16%) | 0.46 (− 33%) | 0.39 (− 43%) | |
| Phe + Tyr | 1.18 | 0.96 | 0.84 | 0.58 | 0.42 | 1.41 | 1.26 | 1.21 | 1.01 | 0.85 | |
| Threonine | 1.05 | 0.83 | 0.70 | 0.49 | 0.35 | 1.12 | 0.90 | 0.85 | 0.69 | 0.59 | |
| Tryptophan | 1.11 | 0.76 | 0.74 | 0.48 | 0.41 | 1.17 | 0.88 | 0.85 | 0.82 | 0.62 | |
| Valine | 1.34 | 1.07 | 0.91 | 0.64 | 0.46 | 1.27 | 1.17 | 1.09 | 0.90 | 0.76 | |
*Rat EAA requirement30.
**Percentage reduction of the limiting EAA scores compared to respective Control.
Fecal true protein digestibility (TPD) and PDCAAS
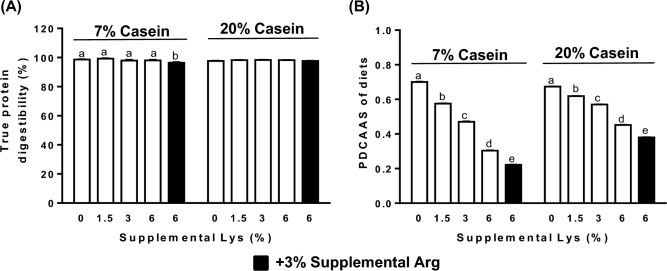
Lys supplementation in both the 7% and 20% casein diets did not affect the fecal protein digestibility in the rats (p > 0.05). However, addition of 3% Arg along with 6% Lys in the 7% casein diet significantly lowered the fecal protein digestibility (p < 0.05, Fig. 1A). PDCAAS of the diets containing either 7% or 20% casein were dose-dependently lowered by increasing levels of supplemental Lys compared to their respective Controls (p < 0.05, Fig. 1B). Addition of 3% Arg in the 7% or 20% casein diets further lowered their PDCAAS over that of the 6% Lys alone (p < 0.05).
Figure 1.
Fecal true protein digestibility (A), and protein digestibility-corrected amino acid scores (PDCAAS) (B) of the diets containing either 7% or 20% casein and supplemented with 0 (Control), 1.5%, 3%, 6% Lys (open bar) or 6% Lys + 3% Arg (filled bar). Values are means ± SEM, n = 8. Means with different letters in the same dietary casein content (7% or 20%) differ, p < 0.05.
Associations of ratios of supplemental Lys to dietary protein content with protein quality and BWG
The crude protein content and the ratios of supplemental Lys to the crude protein in diets (X) increased with Lys supplementation compared to the Controls. Addition of 3% Arg further increased crude protein content in the diets (Supplementary Table 2).
The protein quality of the diets, as measured by PDCAAS, were inversely correlated with X (Y = -0.7196*X + 0.6388, r = − 0.99, p < 0.0001, Fig. 2A). X showed a two-phase linear regression with BWG (Line 1: Y = 0.095*X + 1.008, r = 0.92; Line 2: Y = − 5.564*X + 2.026, r = − 0.94, p < 0.01, Fig. 2B). The inflection point for the two-phase linear regression with BWG occurred at 0.18, with 95% confidence intervals of (0.14, 0.32). When X > 0.18, the BWG was markedly reduced. Based on the regression equation Y = − 0.7196*X + 0.6388, the corresponding PDCAAS in the diets that would not result in a significant reduction in BWG was estimated to be 0.51.
Figure 2.
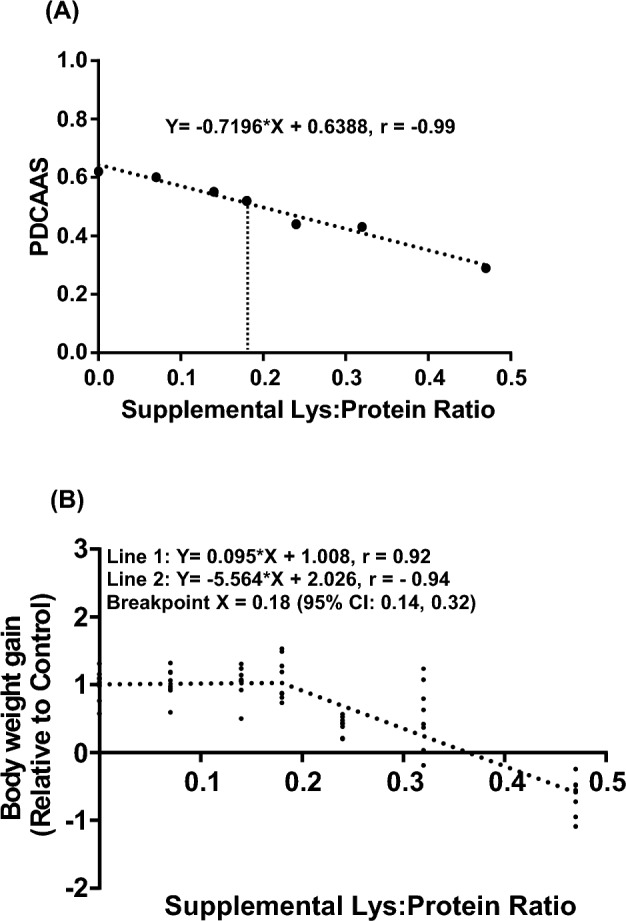
Correlations of the ratios of supplemental Lys to crude protein content (X) in diets with protein digestibility-corrected amino acid scores (PDCAAS) (A) and body weight gain (B) in the rats fed diets containing either 7% or 20% casein supplemented with 0, 1.5%, 3%, 6% Lys or 6%Lys + 3% Arg for 1 week. Values are means ± SEM, n = 8.
Serum Lys and Arg levels
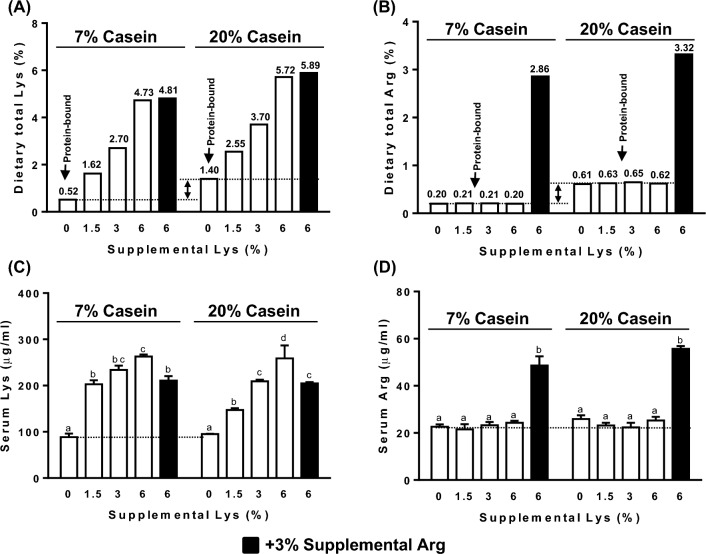
Lys supplementation in the diets dose-dependently increased serum levels of Lys compared to the Controls (p < 0.01, Fig. 3C). Addition of 3% Arg to the diets containing 7% or 20% casein supplemented with 6% Lys significantly increased serum Arg (p < 0.01, Fig. 3D), and lowered serum Lys to levels similar to those in the rats fed 1.5% or 3% supplemental Lys, respectively (Fig. 3C). Serum Lys (Fig. 3C) and Arg (Fig. 3D) concentrations in the rats fed different levels of protein-bound Lys (i.e. 0.52% in 7% casein diet vs 1.40% in 20% casein diet, Fig. 3A) or Arg (0.20% in 7% casein diet vs 0.61% in 20% casein diet, Fig. 3B) did not differ. The same levels of Lys supplementations at 1.5% or 3% resulted in higher retention of serum Lys in rats fed the 7% casein diet compared with rats fed the 20% casein diet (Fig. 3C).
Figure 3.
Protein-bound and supplemental free lysine (Lys) (A) and arginine (Arg) (B) content in the diets containing 7% or 20% casein supplemented with 0, 1.5%, 3%, 6% Lys (open bar) or 6% Lys + 3% Arg (filled bar). Serum free Lys (C), Arg (D) concentrations in the rats fed diets containing 7% or 20% casein supplemented with 0, 1.5%, 3%, 6% Lys (open bars) or 6% Lys + 3% Arg (filled bars) for 1 week. Values are mean ± SEM, n = 8. Means with different letters in the same dietary casein content (7% or 20%) differ, p < 0.05.
Other serum amino acids and metabolites
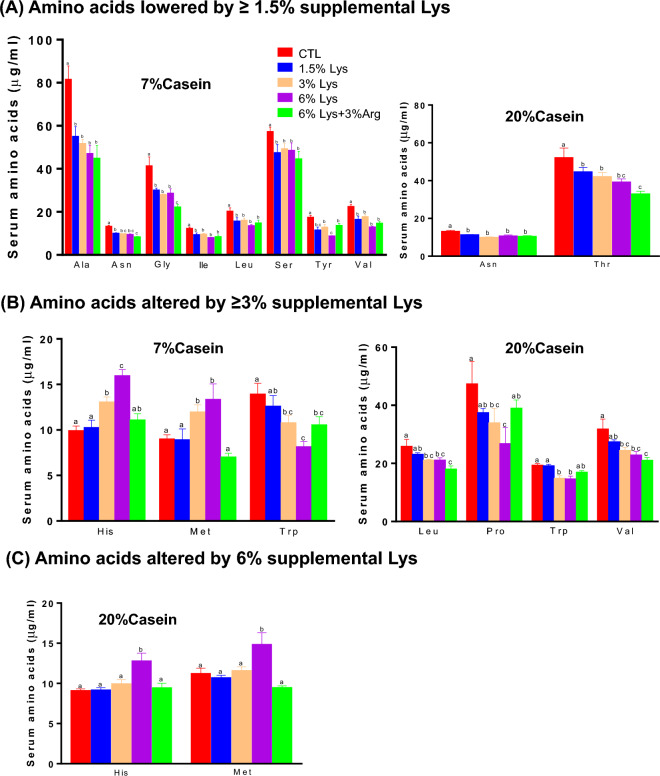
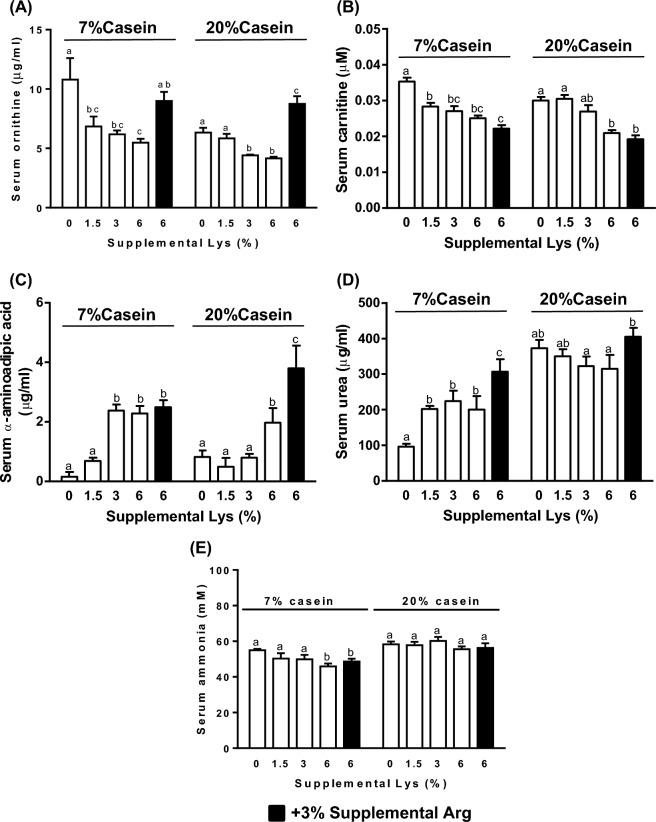
The effects of supplemental Lys on serum Gly, Thr, Tyr, α-aminoadipic acid (α-AAA), carnitine, and urea levels were modified by dietary protein content (p < 0.05). In the 7% casein diets, supplementation with ≥ 1.5% Lys lowered serum Ala, Asn, Gly, Ile, Leu, Ser, Tyr, Val (Fig. 4A), carnitine (Fig. 5A), and ornithine (Fig. 5B), and increased urea (Fig. 5D), and ≥ 3% Lys further reduced Trp, and increased His, Met (Fig. 4B) and α -AAA (Fig. 5C) compared to the Controls (p < 0.01). Addition of 3% Arg along with 6% Lys increased serum Tyr (Fig. 4A), ornithine (Fig. 5A) and urea (Fig. 5D) levels (p < 0.05) and lowered His and Met concentrations (p < 0.01, Fig. 4B) compared to the 6% Lys alone. Serum ammonia levels were lower in rats fed the 7% casein diet with either 6% Lys or 6% Lys + 3% Arg supplementations compared with the Control (p < 0.05, Fig. 5E).
Figure 4.
Serum concentrations of amino acids significantly altered by ≥ 1.5% (A), ≥ 3% (B) or 6% (C) supplemental Lys in male Sprague–Dawley rats fed diets containing either 7% or 20% casein and supplemented with 0, 1.5%, 3%, 6% Lys or 6% Lys + 3% Arg for 1 week. Values are mean ± SEM, n = 8. Means with different letters differ, p < 0.05.
Figure 5.
Serum ornithine (A), carnitine (B), α-aminoadipic acid (C), urea (D) and ammonia (E) concentrations in the male Sprague–Dawley rats fed diets containing either 7% or 20% casein and supplemented with 0, 1.5%, 3%, 6%Lys (open bars) or 6% Lys + 3% Arg (filled bars) for 1 week. Values are means ± SEM, n = 8. Means with different letters in the same dietary casein content (7% or 20%) differ, p < 0.05.
In the 20% casein diets, ≥ 1.5% supplemental Lys reduced serum Asn and Thr levels (Fig. 4A), and ≥ 3% Lys additionally lowered serum Leu, Pro, Trp, Val (Fig. 4B), and ornithine (Fig. 5A) compared to the Controls. Serum His and Met (Fig. 4C) and α-AAA (Fig. 5C) were increased, whereas carnitine was reduced (Fig. 5B) by 6% supplemental Lys (p < 0.01). Addition of 3% Arg with 6% Lys significantly reduced His and Met levels (Fig. 4C), while it increased ornithine, α-AAA and urea levels compared to the 6% Lys alone (Fig. 5A,C and D, p < 0.01).
Discussion
This study assessed the effect of Lys supplementation above Lys intakes needed to meet requirements. The Lys ratios in comparison with requirements in rats in the 7% and 20% casein-based diets used in the present study were 1.37 and 1.29, indicating that Lys was sufficient to meet the requirement of growing rats30. The sulfur-containing amino acids, Met and Cys, had the lowest ratios compared to other EAA, and were the limiting amino acids, even though the 7% or 20% casein diets were supplemented with 0.105% or 0.3% l-cystine, respectively.
Supplementation with increasing amounts of Lys in the Lys-sufficient casein diets dose-dependently lowered the protein quality as evidenced by reduced limiting amino acid (Met + Cys) scores. The extent of reduction in the limiting EAA scores and protein quality by the same amount of supplemental Lys was greater when overall protein in the diets was lower. This suggests that dietary protein content modifies the effects of free Lys supplementation and low protein intake may exacerbate the effects of Lys supplementation. The upper tolerant levels of Lys supplementation may be dependent on dietary protein intake and determined by the ratio of supplemental free Lys to the amounts of protein consumed. This is supported by the strong inverse correlation among the ratios of supplemental Lys to dietary protein content and protein quality of the diets and BWG.
The present study showed that supplemental free Lys led to higher serum concentrations of Lys compared to similar levels of protein-bound Lys. This suggests higher intestinal absorption rates of free Lys as serum free AA concentration is indicative of absorption and availability31. Free Lys was absorbed more rapidly than protein-bound Lys in pigs32. Similar observations have been reported in studies on other AA in other species33–35. The efficiency of AA absorption was improved when both protein-bound and free AA were included in the diet31. The form in which AA is consumed affects its immediate metabolic fate and retention by the body in humans36. Protein-bound AA are believed to have much less effect on their serum levels as protein intake stimulates protein synthesis36–38.
Supplemental Lys reduced serum ornithine levels but had no significant effect on serum Arg and citrulline levels. This suggests that arginase, responsible for the conversion of Arg to ornithine, might have been inhibited by supplemental Lys. This is consistent with our proteomic analysis results showing decreased arginase-1 protein abundance in the livers of rats fed a 7% casein diet with 1.5% supplemental Lys compared to the Control (Supplementary Fig. 1 and Table 3). Furthermore, Lys is a competitive inhibitor of arginase39, and therefore addition of 3% Arg along with 6% Lys eliminated the inhibitory action of supplemental Lys on serum ornithine concentrations. Meanwhile, addition of 3% Arg also attenuated the increase in serum Lys concentrations in response to Lys supplementation. Similar effects of Arg on serum Lys have been observed in other species40–42. This effect may be explained by decreased absorption of Lys as a consequence of competitive inhibition of membrane transport between Lys and Arg in the intestinal mucosa43 and renal tubule44,45.
Effects of Lys supplements on blood levels of Arg and Arg metabolites may vary depending on the paths and doses of Lys administration as well as levels of dietary protein intake. Intravenous infusion of Lys monohydrochloride in human subjects increased plasma Arg, ornithine, blood ammonia, and urinary homocitrulline levels, which appears to reflect inhibited arginase activity and conversion of ornithine to citrulline via ornithine transcarbamylase. There was little change in plasma urea and citrulline46. Our study showed that serum urea but not citrulline and ammonia levels were increased in rats fed the 7% casein diets by supplemented Lys, but no changes were observed in rats fed 20% casein diets.
Supplemental Lys at ≥ 3% in 7% casein or at 6% in 20% casein diets markedly reduced BWG and food intake as reported previously28. Supplemental Lys at ≥ 1.5% markedly lowered serum branched chain amino acids (BCAA) (Ile, Leu, Val) and 5 non-EAA (Ala, Asn, Gly, Ser and Tyr) concentrations in the rats fed the 7% casein diet, and Asn and Thr in rats fed the 20% casein diet compared to the Controls. Supplemental Lys at ≥ 3% in 7% casein or 6% in 20% casein diets markedly increased serum His and Met levels. Addition of Arg fully or partially reversed the effects of supplemental Lys on food intake, BWG, serum Lys, Tyr, His and Met, indicating antagonism between Lys and Arg. Arg supplementation also partially attenuated the growth retardation induced by 4.3% Lys supplementation in rats47.
In addition to Lys antagonism on Arg, some effects of Lys supplementation may be attributed to other mechanisms such as inhibition of AA absorption and metabolism, resulting in AA imbalance. In pigs, feeding diets with 3.45% supplemental Lys in the background of marginal Arg lowered both BWG and feed intake, and increased free Lys and α-AAA levels in plasma, liver, kidney, and muscle tissues compared to the basal diet. However, the tissue levels of arginase and ornithine transcarbamoylase were unchanged by supplemental Lys48. Increased dietary Lys levels reduced two of the three plasma BCAA (Ile, and Val) and Thr concentrations in growing barrows49, or plasma Thr, Tyr and some non-EAA in finishing pigs50. The mRNA abundances of cationic AA transporters in the small intestine of pigs were shown to be lowered by high dietary Lys levels51, which may play a role in mediating the reduction of neutral AA such as Ala, Gly, Leu, and Ile through suppression of intestinal absorption.
Dietary amino acid imbalance, particularly deficiencies in essential amino acids, can trigger cellular stress response pathways such as the integrated stress response and the amino acid response52. Activation of these pathways leads to a reduction in protein synthesis and cell growth as a means of conserving energy and resources during periods of amino acid scarcity. The general control nonderepressible-2 kinase is a sensor for amino acid imbalance in activating the integrated stress response leading to inhibition of protein synthesis and extended longevity53–55. Target of Rapamycin (TOR) kinase, a central regulator of cell growth and metabolism, is tightly regulated by amino acid levels52. When amino acid imbalance occurs, TOR kinase activity can be inhibited, which can further induce autophagy, a cellular process that helps remove damaged cellular components, contributing to cellular maintenance and overall health. Furthermore, reduced TOR activity has been associated with increased lifespan in various organisms56,57, suggesting a potential link between amino acid imbalance-induced TOR inhibition and longevity.
α-AAA, structurally similar to glutamine, is an intermediate of Lys metabolism, and competitively inhibits the activity of glutamine synthetase (Glns), γ-glutamylcysteine synthetase and the high–affinity glutamate/aspartate transporter58. Our previous study showed that dietary Lys supplementation reduced serum glutamine levels and Glns abundance in skeletal muscle of rats fed a 7% casein diet28. The present study showed that supplementation of ≥ 3% Lys in 7% casein or 6% Lys in 20% casein markedly increased serum α-AAA. This suggests that supplemental Lys may have a dual effect on Glns, reducing its protein abundance along with inhibition of its activity through increased α-AAA. α-AAA is an emerging predictor of the risk for development of diabetes in both normoglycemic and high CVD risk individuals59,60. The baseline α-AAA circulating level was associated with future risk of type-2 diabetes and was increased up to 12 years before the onset of diabetes and the development of other known risk markers. α-AAA was also associated with obesity and metabolic syndrome60–62. α-AAA may modulate insulin secretion and plasma glucose levels60,63, impair insulin signalling, result in abnormal gluconeogenesis61 and reduce high-density lipoprotein cholesterol level62.
Dietary supplementation with Lys significantly lowered serum carnitine concentrations and this effect was dependent on the protein content of the diets. All levels of Lys supplementation in 7% casein lowered serum carnitine levels, however, only the highest level of Lys (6%) added in the 20% casein diet had this effect. Excess dietary Lys was shown to reduce plasma carnitine concentrations, but increase the concentrations of free trimethyllysine (TML), a carnitine precursor, in skeletal muscle and plasma of rats64. Further research in pigs suggested that the potential mechanism underlying this effect of Lys may involve downregulation of the mRNA expression of muscle TML dioxygenase, a rate-limiting enzyme responsible for conversion of TML to γ-butyrobetaine (BB). Impaired conversion of TML to BB resulted in increased TML concentrations and decreased BB in muscles, reducing serum carnitine levels as BB is the precursor of carnitine biosynthesis65.
In conclusion, this study showed that addition of Lys in Lys-sufficient diets reduced the limiting EAA (Met + Cys) scores and protein quality in a dose-dependent manner when dietary protein was adequate (20% casein) or low (7% casein). The percentage of reduction by the same amount of Lys supplementation was greater when protein was low. The ratios of supplemental free Lys to dietary protein content were inversely correlated with protein quality of diets, BWG and utilization of nutrients. Dietary protein-bound and free Lys and Arg differentially affect blood circulating Lys and Arg levels. Compared with protein-bound Lys, similar levels of supplemental free Lys markedly increased serum Lys. Moreover, circulating amino acid profiles and many of their metabolites were significantly altered. Overall, these results suggest that Lys supplementation adversely affected protein quality of diets and resulted in growth retardation, and low protein intakes exaggerate the adverse effects from Lys supplementation. Further investigation is warranted to explore the potential long-term impact of Lys and Arg supplementation on the regulation of circulating sulfur amino acid concentrations and its relationship to longevity.
Materials and methods
Animals and diets
The animal experimental protocol (No. 2014-018) was approved by the Health Canada Animal Care Committee, and all animal handling and care followed the guidelines of Canadian Council for Animal Care. The reporting in this paper followed the recommendations in the ARRIVE guidelines66. Male SAS Sprague Dawley rats (Strain Code: 400) at 9 weeks of age from Charles River (St. Constant, Quebec, Canada) were housed individually and placed on a 12:12 h light:dark cycle, with free access to food and water. After acclimation for 1 week on a 20% casein diet, the rats were assigned to 10 groups (8 rats/group) based on their body weights to ensure that the mean initial body weights were similar among groups. The sample size calculation was conducted based on BWG with a 25% standard deviation to detect a 35% reduction. The power of the experiment was set to 80%. A minimum sample size of 8 was considered necessary. The study is a two-way randomization trial with 80 rats. The grouped rats were randomly assigned to one of the 10 experimental diets, which contained either 7% or 20% casein and were supplemented with 0 (Control), 1.5%, 3%, 6% of Lys or 6% Lys + 3% Arg for 1 week. The rats in cages were randomly located on the rack. The diets were formulated following the AIN-93G recommendations for rodents67, and changes in casein and Lys or Arg contents were at the expense of cornstarch or sucrose, respectively. The digestible energy content of the diets containing the same levels of casein (7% or 20%) were equivalent. Titanium dioxide (TiO2) at a level of 0.3% was added to all diets as an indigestible marker (Supplementary Table 1). Food consumption and body weight were measured at the end of the study. On the day before necropsy, all rats were moved to metabolic cages with food for collection of urine and feces. After a 4-h fast, rats were euthanized by exsanguination from the abdominal aorta while under 3% isoflurane anesthesia through inhalation as recommended68. Blood and liver were collected for isolation of serum and proteomics analysis.
Measurement of total nitrogen and TiO2 content in diets and feces
Diets and feces were freeze-dried and ground. Total nitrogen (TN) content was determined by Combustion Analysis using the Association of Official Agricultural Chemists Official Method 990.03, 2006. Crude protein was calculated using a conversion factor of 6.25 from TN. TiO2 content was measured using the procedure as described by Myers et al.67.
Fecal true protein digestibility
The fecal true protein digestibility (TPD) was calculated using the TN-to-TiO2 ratio in the diets and feces according to the following equation69:
The TN-to-TiO2 ratio in the feces of the rats on a protein-free diet or endogenous fecal nitrogen excretion (Fk) from a separate study with the same design (Supplementary Table 1) was used in the calculations.
Determination of amino acids in diet and serum samples
Serum samples were deproteinized using sulfosalicylic acid solution, and the free amino acids and some of their metabolites were analysed using automated cation-exchange column liquid chromatography with post column ninhydrin reaction and dual wavelength detection in a Beckman high standard amino acid analyser. Amino acids in the diets were determined using the Association of Official Agricultural Chemists Official Method 982. 30 E (a,b) chp. 45.3.05, 2006. Serum carnitine was measured using the carnitine assay kit (Abcam Inc., Toronto, Canada). Serum ammonia levels were determined using the ammonia assay kit (Sigma-Aldrich, Oakville, Canada).
EAA scores and protein quality in diets
The EAA scores in diets were the lowest ratios of the EAA content (mg/g protein) to their requirements30 in rats. The protein quality of the diets was determined using Protein Digestibility-Corrected Amino Acid Score (PDCAAS). PDCAAS = the limiting EAA score × TPD.
Statistical analysis
Data are presented as mean ± SEM. All data were evaluated for equality of variance prior to statistical analysis. Variables with skewed distribution were logarithmically transformed. The effects of different levels of dietary protein and supplemental Lys as well as their interaction were analyzed by two-way Analysis of Variance. Differences between individual means were determined by Tukey’s honestly significant difference post-hoc test. A probability of p < 0.05 was considered to be significant. The correlation between the ratios of supplemental Lys to protein content and PDCAAS was assessed using a linear regression model. The correlation between the ratios of supplemental Lys to protein content and BWG was analysed using segmented linear regression modelling from the ‘chngpt’ R-package (4.03). Its breakpoint and 95% confidence intervals were estimated using a grid search method and bootstrap approach, respectively70–72. All other data were analyzed using STATISTICA version 13 (StatSoft, Inc., Tulsa, OK, USA).
Ethics approval
The animal experimental protocol (ACC#2014-018) was approved by the Health Canada-Ottawa Animal Care Committee, and all animal handling and care followed the guidelines of the Canadian Council for Animal care.
Supplementary Information
Acknowledgements
This research was funded by Health Canada A-base fund. We thank the technicians in the Scientific Service Division (SSD), Food Directorate, Health Canada for their outstanding assistance during the animal experimentation phase.
Abbreviations
- α-AAA
α-Aminoadipic acid
- AA
Amino acid
- Arg
l-arginine
- BB
γ-Butyrobetaine
- BCAA
Branched chain amino acids
- BWG
Body weight gain
- EAA
Essential amino acid
- Glns
Glutamine synthetase
- Lys
l-lysine
- PDCAAS
Protein digestibility-corrected amino acid score
- TML
Trimethyllysine
- TN
Total nitrogen
- TPD
True protein digestibility
Author contributions
C.W.X. and J.B. designed the experiments; C.W.X., A.H., L.K. and J.B. conducted the experiments and sample analyses; C.W.X. analysed the data and wrote the first draft and had the primary responsibility for the final content. All the authors revised the manuscript, read and approved the final manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-47321-3.
References
- 1.Le DT, Chu HD, Le NQ. Improving nutritional quality of plant proteins through genetic engineering. Curr. Genom. 2016;17:220–229. doi: 10.2174/1389202917666160202215934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hussain T, Abbas S, Khan MA, Scrimshaw NS. Lysine fortification of wheat flour improves selected indices of the nutritional status of predominantly cereal-eating families in Pakistan. Food Nutr. Bull. 2004;25:114–122. doi: 10.1177/156482650402500202. [DOI] [PubMed] [Google Scholar]
- 3.Joint WHO/FAO/UNU Expert Consultation Protein and amino acid requirements in human nutrition. World Health Organ Tech. Rep. Ser. 2007;935:1–265. [PubMed] [Google Scholar]
- 4.Tomé D, Bos C. Lysine requirement through the human life cycle. J. Nutr. 2007;137:1642s–1645s. doi: 10.1093/jn/137.6.1642S. [DOI] [PubMed] [Google Scholar]
- 5.Trumbo P, Schlicker S, Yates AA, Poos M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet Assoc. 2002;102:1621–1630. doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- 6.Longo N, Frigeni M, Pasquali M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta. 1863;2422–2435:2016. doi: 10.1016/j.bbamcr.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato T, Ito Y, Nagasawa T. Regulation of skeletal muscle protein degradation and synthesis by oral administration of lysine in rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2013;59:412–419. doi: 10.3177/jnsv.59.412. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe RR. Protein supplements and exercise. Am. J. Clin. Nutr. 2000;72:551s–557s. doi: 10.1093/ajcn/72.2.551S. [DOI] [PubMed] [Google Scholar]
- 9.Sato T, Ito Y, Nagasawa T. Dietary l-lysine suppresses autophagic proteolysis and stimulates Akt/mTOR signaling in the skeletal muscle of rats fed a low-protein diet. J. Agric. Food Chem. 2015;63:8192–8198. doi: 10.1021/acs.jafc.5b03811. [DOI] [PubMed] [Google Scholar]
- 10.Knopf RF, et al. Plasma growth hormone repsonse to intravenous administration of amino acids. J. Clin. Endocrinol. Metab. 1965;25:1140–1144. doi: 10.1210/jcem-25-8-1140. [DOI] [PubMed] [Google Scholar]
- 11.Suminski RR, et al. Acute effect of amino acid ingestion and resistance exercise on plasma growth hormone concentration in young men. Int. J. Sport Nutr. 1997;7:48–60. doi: 10.1123/ijsn.7.1.48. [DOI] [PubMed] [Google Scholar]
- 12.Chromiak JA, Antonio J. Use of amino acids as growth hormone-releasing agents by athletes. Nutrition. 2002;18:657–661. doi: 10.1016/s0899-9007(02)00807-9. [DOI] [PubMed] [Google Scholar]
- 13.Unni US, et al. The effect of a controlled 8-week metabolic ward based lysine supplementation on muscle function, insulin sensitivity and leucine kinetics in young men. Clin. Nutr. 2012;31:903–910. doi: 10.1016/j.clnu.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Griffith RS, Norins AL, Kagan C. A multicentered study of lysine therapy in Herpes simplex infection. Dermatologica. 1978;156:257–267. doi: 10.1159/000250926. [DOI] [PubMed] [Google Scholar]
- 15.Griffith RS, et al. Success of l-lysine therapy in frequently recurrent herpes simplex infection. Treatment and prophylaxis. Dermatologica. 1987;175:183–190. doi: 10.1159/000248823. [DOI] [PubMed] [Google Scholar]
- 16.Mahmood ZA. Microbial Biotechnology: Progress and Trends. Taylor & Francis Group; 2015. [Google Scholar]
- 17.Gaby AR. Natural remedies for Herpes simplex. Altern. Med. Rev. 2006;11:93–101. [PubMed] [Google Scholar]
- 18.Zyzak DV, et al. Acrylamide formation mechanism in heated foods. J. Agric. Food Chem. 2003;51:4782–4787. doi: 10.1021/jf034180i. [DOI] [PubMed] [Google Scholar]
- 19.Robert F, et al. Acrylamide formation from asparagine under low-moisture Maillard reaction conditions. 1. Physical and chemical aspects in crystalline model systems. J. Agric. Food Chem. 2004;52:6837–6842. doi: 10.1021/jf0492464. [DOI] [PubMed] [Google Scholar]
- 20.Yu M, et al. Effect of ten amino acids on elimination of acrylamide in a model reaction system. Afr. J. Food Sci. 2013;7:329–333. doi: 10.5897/AJFS2013.1031. [DOI] [Google Scholar]
- 21.Kobayashi A, et al. Elimination of acrylamide by moderate heat treatment below 120°C with lysine and cysteine. Food Sci. Technol. Res. 2014;20:979–985. doi: 10.3136/fstr.20.979. [DOI] [Google Scholar]
- 22.Kim CT, Hwang E-S, Lee HJ. Reducing acrylamide in fried snack products by adding amino acids. J. Food Sci. 2005;70:C354–358. doi: 10.1111/j.1365-2621.2005.tb09966.x. [DOI] [Google Scholar]
- 23.Friedman M. Chemistry, biochemistry, and safety of acrylamide. A review. J. Agric. Food Chem. 2003;51:4504–4526. doi: 10.1021/jf030204+. [DOI] [PubMed] [Google Scholar]
- 24.FAO/WHO. Protein quality evaluation. Report of the joint FAO/WHO expert consultation. FAO Food Nutr. Pap.51, 1–66 (1991).
- 25.Harper AE. Balance and imbalance of amino acids. Ann. N. Y. Acad. Sci. 1958;69:1025–1038. doi: 10.1111/j.1749-6632.1958.tb49733.x. [DOI] [PubMed] [Google Scholar]
- 26.Kurpad AV. 90th anniversary commentary: Amino acid imbalances: Still in the balance. J. Nutr. 2018;148:1647–1649. doi: 10.1093/jn/nxy195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toohey JI. Sulfur amino acids in diet-induced fatty liver: A new perspective based on recent findings. Molecules. 2014;19:8334–8349. doi: 10.3390/molecules19068334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao CW, Wood C, Bertinato J. Dietary supplementation with l-lysine affects body weight and blood hematological and biochemical parameters in rats. Mol. Biol. Rep. 2019;46:433–442. doi: 10.1007/s11033-018-4492-1. [DOI] [PubMed] [Google Scholar]
- 29.Xiao CW, Faddoul C, Wood C. Dietary l-lysine supplementation altered the content of pancreatic polypeptide, enzymes involved in glutamine metabolism, and β-actin in rats. Amino Acids. 2018;50:1729–1737. doi: 10.1007/s00726-018-2648-x. [DOI] [PubMed] [Google Scholar]
- 30.National Research Council Subcommittee on Laboratory Animal Nutrition . Nutrient Requirements of Laboratory Animals. 4. National Academies Press; 1995. [Google Scholar]
- 31.Morales A, et al. Low-protein amino acid-supplemented diets for growing pigs: Effect on expression of amino acid transporters, serum concentration, performance, and carcass composition. J. Anim. Sci. 2015;93:2154–2164. doi: 10.2527/jas.2014-8834. [DOI] [PubMed] [Google Scholar]
- 32.Yen JT, Kerr BJ, Easter RA, Parkhurst AM. Difference in rates of net portal absorption between crystalline and protein-bound lysine and threonine in growing pigs fed once daily. J. Anim. Sci. 2004;82:1079–1090. doi: 10.2527/2004.8241079x. [DOI] [PubMed] [Google Scholar]
- 33.Boirie Y, et al. Acute postprandial changes in leucine metabolism as assessed with an intrinsically labeled milk protein. Am. J. Physiol. 1996;271:E1083–1091. doi: 10.1152/ajpendo.1996.271.6.E1083. [DOI] [PubMed] [Google Scholar]
- 34.Weijzen MEG, et al. Ingestion of free amino acids compared with an equivalent amount of intact protein results in more rapid amino acid absorption and greater postprandial plasma amino acid availability without affecting muscle protein synthesis rates in young adults in a double-blind randomized trial. J. Nutr. 2022;152:59–67. doi: 10.1093/jn/nxab305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ronnestad I, Conceição LE, Aragão C, Dinis MT. Free amino acids are absorbed faster and assimilated more efficiently than protein in postlarval Senegal sole (Solea senegalensis) J. Nutr. 2000;130:2809–2812. doi: 10.1093/jn/130.11.2809. [DOI] [PubMed] [Google Scholar]
- 36.Metges CC, et al. Kinetics of l-[1-(13)C]leucine when ingested with free amino acids, unlabeled or intrinsically labeled casein. Am. J. Physiol. Endocrinol. Metab. 2000;278:E1000–1009. doi: 10.1152/ajpendo.2000.278.6.E1000. [DOI] [PubMed] [Google Scholar]
- 37.Pencharz PB, Elango R, Ball RO. An approach to defining the upper safe limits of amino acid intake. J. Nutr. 2008;138:1996s–2002s. doi: 10.1093/jn/138.10.1996S. [DOI] [PubMed] [Google Scholar]
- 38.Gropper SS, Gropper DM, Acosta PB. Plasma amino acid response to ingestion of l-amino acids and whole protein. J. Pediatr. Gastroenterol. Nutr. 1993;16:143–150. doi: 10.1097/00005176-199302000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Fuentes JM, Campo ML, Soler G. Kinetics and inhibition by some aminoacids of lactating rat mammary gland arginase. Arch. Int. Physiol. Biochim. Biophys. 1994;102:255–258. doi: 10.3109/13813459409003940. [DOI] [PubMed] [Google Scholar]
- 40.Batista ED, et al. Efficiency of lysine utilization by growing steers. J. Anim. Sci. 2016;94:648–655. doi: 10.2527/jas.2015-9716. [DOI] [PubMed] [Google Scholar]
- 41.Teixeira PD, et al. Effect of ruminally protected arginine and lysine supplementation on serum amino acids, performance, and carcass traits of feedlot steers1. J. Anim. Sci. 2019;97:3511–3522. doi: 10.1093/jas/skz191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Si H, et al. Effects of arginine supplementation on serum metabolites and the rumen bacterial community of sika deer (Cervus nippon) Front. Vet. Sci. 2021;8:630686. doi: 10.3389/fvets.2021.630686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheeseman CI. Characteristics of lysine transport across the serosal pole of the anuran small intestine. J. Physiol. 1983;338:87–97. doi: 10.1113/jphysiol.1983.sp014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brosnan ME, Brosnan JT. Renal arginine metabolism. J. Nutr. 2004;134:2791S–2795S. doi: 10.1093/jn/134.10.2791S. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz IF, et al. Augmented arginine uptake, through modulation of cationic amino acid transporter-1, increases GFR in diabetic rats. Kidney Int. 2004;65:1311–1319. doi: 10.1111/j.1523-1755.2004.00508.x. [DOI] [PubMed] [Google Scholar]
- 46.Kato T, Sano M, Mizutani N. Inhibitory effect of intravenous lysine infusion on urea cycle metabolism. Eur. J. Pediatr. 1987;146:56–58. doi: 10.1007/bf00647285. [DOI] [PubMed] [Google Scholar]
- 47.Fico ME, Hassan AS, Milner JA. The influence of excess lysine on urea cycle operation and pyrimidine biosynthesis. J. Nutr. 1982;112:1854–1861. doi: 10.1093/jn/112.10.1854. [DOI] [PubMed] [Google Scholar]
- 48.Edmonds MS, Baker DH. Failure of excess dietary lysine to antagonize arginine in young pigs. J. Nutr. 1987;117:1396–1401. doi: 10.1093/jn/117.8.1396. [DOI] [PubMed] [Google Scholar]
- 49.Roy N, Lapierre H, Bernier JF. Whole-body protein metabolism and plasma profiles of amino acids and hormones in growing barrows fed diets adequate or deficient in lysine. Can. J. Anim. Sci. 2000;80:585–595. doi: 10.4141/a98-057. [DOI] [Google Scholar]
- 50.Henry Y, Colléaux Y, Sève B. Effects of dietary level of lysine and of level and source of protein on feed intake, growth performance, and plasma amino acid pattern in the finishing pig. J. Anim. Sci. 1992;70:188–195. doi: 10.2527/1992.701188x. [DOI] [PubMed] [Google Scholar]
- 51.Wang XQ, et al. Effects of dietary lysine levels on apparent nutrient digestibility and cationic amino acid transporter mRNA abundance in the small intestine of finishing pigs, Sus scrofa. Anim. Sci. J. 2012;83:148–155. doi: 10.1111/j.1740-0929.2011.00941.x. [DOI] [PubMed] [Google Scholar]
- 52.Canfield C-A, Bradshaw PC. Amino acids in the regulation of aging and aging-related diseases. Transl. Med. Aging. 2019 doi: 10.1016/j.tma.2019.09.001. [DOI] [Google Scholar]
- 53.Falcón P, Escandón M, Brito Á, Matus S. Nutrient sensing and redox balance: GCN2 as a new integrator in aging. Oxid. Med. Cell Longev. 2019;2019:5730532. doi: 10.1155/2019/5730532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lushchak O, et al. Implications of amino acid sensing and dietary protein to the aging process. Exp. Gerontol. 2019;115:69–78. doi: 10.1016/j.exger.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 55.Cummings NE, Lamming DW. Regulation of metabolic health and aging by nutrient-sensitive signaling pathways. Mol. Cell Endocrinol. 2017;455:13–22. doi: 10.1016/j.mce.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aris JP, et al. Amino acid homeostasis and chronological longevity in Saccharomyces cerevisiae. In: Breitenbach M, Michal Jazwinski S, Laun P, et al., editors. Aging Research in Yeast. Springer; 2012. pp. 161–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McBean GJ. Inhibition of the glutamate transporter and glial enzymes in rat striatum by the gliotoxin, alpha aminoadipate. Br. J. Pharmacol. 1994;113:536–540. doi: 10.1111/j.1476-5381.1994.tb17022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Razquin C, et al. Lysine pathway metabolites and the risk of type 2 diabetes and cardiovascular disease in the PREDIMED study: Results from two case-cohort studies. Cardiovasc. Diabetol. 2019;18:151. doi: 10.1186/s12933-019-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang TJ, et al. 2-Aminoadipic acid is a biomarker for diabetes risk. J. Clin. Invest. 2013;123:4309–4317. doi: 10.1172/jci64801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee HJ, et al. 2-Aminoadipic acid (2-AAA) as a potential biomarker for insulin resistance in childhood obesity. Sci. Rep. 2019;9:13610. doi: 10.1038/s41598-019-49578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi M, et al. Genetic architecture of plasma alpha-aminoadipic acid reveals a relationship with high-density lipoprotein cholesterol. J. Am. Heart Assoc. 2022;11:e024388. doi: 10.1161/jaha.121.024388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arora P. Metabolomics yield a novel biomarker for predicting diabetes mellitus risk in humans. Circ. Cardiovasc. Genet. 2014;7:95–96. doi: 10.1161/circgenetics.114.000528. [DOI] [PubMed] [Google Scholar]
- 64.Davis AT, Kruggel EM, Randall S. Excess dietary lysine increases skeletal muscle and plasma trimethyllysine in rats. J. Nutr. 1993;123:1109–1116. doi: 10.1093/jn/123.6.1109. [DOI] [PubMed] [Google Scholar]
- 65.Fischer M, Hirche F, Kluge H, Eder K. A moderate excess of dietary lysine lowers plasma and tissue carnitine concentrations in pigs. Br. J. Nutr. 2009;101:190–196. doi: 10.1017/s0007114508994770. [DOI] [PubMed] [Google Scholar]
- 66.Kilkenny C, et al. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Myers WD, Ludden PA, Nayigihugu V, Hess BW. Technical note: A procedure for the preparation and quantitative analysis of samples for titanium dioxide. J. Anim. Sci. 2004;82:179–183. doi: 10.2527/2004.821179x. [DOI] [PubMed] [Google Scholar]
- 68.Leary, S. et al. AVMA guidelines for the euthanasia of animals: 2020 Edition. (2020).
- 69.Ravindran V, Hew LI, Ravindran G, Bryden WL. A comparison of ileal digesta and excreta analysis for the determination of amino acid digestibility in food ingredients for poultry. Br. Poult. Sci. 1999;40:266–274. doi: 10.1080/00071669987692. [DOI] [PubMed] [Google Scholar]
- 70.Fong Y, Di C, Huang Y, Gilbert PB. Model-robust inference for continuous threshold regression models. Biometrics. 2017;73:452–462. doi: 10.1111/biom.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elder A, Fong Y. Estimation and inference for upper hinge regression models. Environ. Ecol. Stat. 2019;26:287–302. doi: 10.1007/s10651-019-00428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fong Y. Fast bootstrap confidence intervals for continuous threshold linear regression. J. Comput. Graph. Stat. 2019;28:466–470. doi: 10.1080/10618600.2018.1537927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.