Summary
Background
This study investigated the early safety and mid-term outcomes of stepwise implementation of transcatheter aortic valve implantation (TAVI) in Vietnamese patients with severe aortic stenosis (AS) at a single center, following the process of technical transfer.
Methods
From 2017 to 2022, 90 patients with symptomatic severe AS underwent TAVI at a tertiary hospital in Vietnam. The first 30 cases received support for technology transfer from international proctors. One-year outcomes were evaluated using the Valve Academic Research Consortium-2 (VARC-2) criteria.
Findings
Forty patients (45.5%) had bicuspid aortic valve (BAV). The Medtronic Evolut R/Pro self-expanding valve system was used in 98.9% of all cases, with a 29-mm valve being the most common. Device success was achieved in 95.6% of cases, whereas one procedural death occurred. At one year, four deaths (5.3%) occurred and all were in the BAV group. Other complications included stroke (2.8%), hospital readmission due to the valve or worsening heart failure (2.8%), permanent pacemaker implantation (9.9%), and moderate paravalvular leak (3.0%). The left ventricular ejection fraction and mean transvalvular gradient significantly improved after TAVI. There were no significant differences in procedural success and mortality when the proctor support period and the subsequent solo operator period were compared.
Interpretation
TAVI procedure is safe for treating severe AS in Vietnamese patients, despite the high prevalence of BAV. The procedural complication rate was low, with promising outcomes at one year. These results also highlight the effectiveness of the TAVI technical transfer model in Vietnam.
Funding
No funding was provided for this study.
Keywords: TAVI, Early safety, 1-Year outcomes, Vietnam
Research in context.
Evidence before this study
Since the first TAVI procedure on humans in 2002, up until mid-2022, there have been over 1.5 million TAVI cases worldwide. Several studies have indicated that Asian patients exhibit anatomical characteristics of the aortic valve region that differ from those of Western populations, such as a smaller aortic annulus and a higher prevalence of BAV. However, comprehensive studies conducted in Asia, predominantly in developed countries, have reported optimistic outcomes for TAVI in this continental population, comparable with those observed in Western populations. Nevertheless, there is a lack of data on TAVI outcomes in the Vietnamese population, which has a relatively high incidence of BAV compared to other populations, and where the adoption of TAVI technology has been delayed compared to many developed countries. We conducted a search on PubMed regarding TAVI in Vietnam, the prevalence of BAV in the TAVI population, and the technology transfer models for TAVI in developing countries, including Vietnam, using the terms TAVI OR TAVR AND Vietnam, BAV AND TAVI OR TAVR, technology transfer of TAVI OR TAVR, without language restrictions.
There have been no reports on TAVI outcomes in the Vietnamese population with severe aortic valve stenosis. The prevalence of BAV among TAVI patients ranges from 5 to 10%, except in China, where it is reported to be 48.5%. When using newer valve designs, the outcomes of TAVI for BAV are comparable to those for TAV, with no significant differences in short- and mid-term clinical outcomes. Additionally, there is scarce documentation regarding the implementation and technology transfer models in countries where TAVI adoption has been delayed, such as Vietnam.
Added value of this study
The first study on 90 Vietnamese patients with symptomatic severe aortic valve stenosis undergoing TAVI revealed a high prevalence of BAV (45.5%) and a relatively “younger” mean age (70.7 ± 8.8 years) compared to other studies. The procedure demonstrated a high success rate (95.6%) and low mortality at 30 days (2.3%) and 1 year (5.6%), which can be compared favorably to findings in other populations. According to the VARC-2 criteria, there were no significant differences in outcomes between BAV and TAV at the time of the procedure, 30 days, and 1 year, except for a higher mortality rate at 1 year in the BAV group (13.8% vs. 0.0%, p = 0.027).
Furthermore, these TAVI results highlight the effectiveness of the successful technology transfer model applied in Vietnam. The Heart Team underwent training at a professional TAVI center, followed by a phase of TAVI performance with the support of proctors, and finally the transition to independent procedure execution as certified solo operators.
Implications of all the available evidence
This study provides valuable insights into TAVI outcomes in the Vietnamese population, characterized by a relatively high prevalence of BAV. Despite this, the results of TAVI in this patient group are promising and overall show no significant differences in short- and mid-term outcomes between BAV and TAV. Additionally, the successful technology transfer model of TAVI demonstrated in this study can potentially be applicable to other countries with similar economic and healthcare conditions as Vietnam.
Introduction
AS due to degeneration increases with age and is commonly observed in older adults. Patients with severe AS have poor prognosis once symptoms develop and if untreated. For over 50 years, surgical aortic valve replacement (SAVR) has been the gold standard treatment, improving symptoms and survival in these patients.1 However, at least 30% of severe AS patients are deemed inoperable due to advanced age or comorbidities.2 Since the first TAVI procedure in 2002 for an inoperable patient with severe AS, more than 1.5 million TAVI procedures have been performed worldwide, and randomized controlled trials and registries across various populations have demonstrated TAVI’s safety and efficacy regardless of surgical risk.3,4 The first TAVI procedure in Vietnam for severe AS was performed in 2011, and over 200 TAVI procedures have been performed at 10 centers across the country. TAVI is one of the most complex cardiac interventions that requires substantial investment in both equipment and skills to achieve optimal outcomes. In countries with delayed access to TAVI procedures, such as Vietnam, the technology transfer model for the Heart Team from an academic TAVI center, followed by the support phase of proctors, and ultimately independent performance of the procedure, has initially yielded results that need to be re-evaluated. Furthermore, some reports within the country indicate a relatively high prevalence of BAV stenosis in the Vietnamese population–a characteristic that is often considered unfavorable for this intervention. To date, TAVI outcomes in the Vietnamese population remain unknown despite published registries in Asia.5, 6, 7 Therefore, this study aimed to evaluate the early safety and clinical efficacy, and feasibility of technology transfer of TAVI in the treatment of severe AS in older patients in Vietnam at 1-year follow-up.
Methods
Patients
From March 2017 to December 2022, we recruited all patients aged ≥60 years (defined as elderly in Vietnam) with severe AS who underwent TAVI at Vinmec Central Park International Hospital in Ho Chi Minh City, Vietnam. Severe AS was defined as an aortic valve area of ≤1.0 cm2 and a mean transvalvular gradient of ≥40 mmHg. All patients had symptoms of New York Heart Association (NYHA) functional class ≥II. The surgical risk for TAVI was calculated using the Society of Thoracic Surgeons (STS) score, and the decision to perform TAVI was made by a Heart Team, with the final decision left to the patient/family.
We excluded patients with recent myocardial infarction within 30 days, stroke within 6 months, severe heart failure with left ventricular ejection fraction (LVEF) < 20%, active infection or bleeding, concomitant coronary artery disease or other valve diseases requiring surgical intervention, life expectancy <1 year, aortic annulus diameter <18 mm or >30 mm, or those who required SAVR during the follow-up period (the study did not continue to evaluate outcomes after valve replacement).
Currently, most TAVI devices in Vietnam are self-expanding valve systems from Medtronic, USA (Evolut R and Evolut Pro). In recent years, the balloon-expandable valve system from Edwards, USA (Sapien 3), has also been introduced. Therefore, almost all patients in this study underwent TAVI with the Evolut R/Pro system, with 2 patients receiving the Portico self-expanding valves (Abbott, USA) and one receiving the Sapien 3 valve.
To prepare for the implementation of the TAVI technique, in addition to the appropriate investment in equipment, our Heart Team (consisting of general cardiologists, interventional cardiologists, cardiac surgeons, imaging specialists, intensivists, and anesthesiologists) underwent extensive training on TAVI at the Georges-Pompidou European Hospital (Paris, France). Subsequently, we performed the first thirty TAVI cases with the support of international proctors, and starting from the thirty-first case, we independently performed the procedures as certified solo operators.
We performed TAVI in a hybrid catheterization laboratory with a single team of three interventional cardiologists. All patients underwent coronary angiography, echocardiography, and computerized tomographic imaging to evaluate the coronary artery system, the aortic valve, and vascular access. At our center, we prioritized femoral artery access, followed by trans-subclavian and trans-carotid access, depending on the individual’s anatomy and suitability for TAVI. Initially, we induced general anesthesia and used transesophageal echocardiography to assist valve deployment and create rapid ventricular pacing with a standard electrode wire in the right ventricle. However, later cases were predominantly performed under local anesthesia without transesophageal echocardiography, and some used a left-ventricular-driven guidewire for rapid pacing at a frequency of 120–180 beats/min. In most cases where the femoral artery was used for valve delivery, we closed the artery with two Proglide devices (“pre-closing” technique) and used a sheath measuring 14–18 F, depending on the case. A Terumo hydrophilic guidewire was used to cross the aortic valve with support from an Amplatz catheter. In most cases, the transvalvular gradient was measured using two pigtail catheters, one placed at the aortic root and the other in the left ventricle, before pre-dilation and/or valve implantation. We used the Confida guidewire for most valve deployments using coplanar or cusp-overlap techniques. Most patients with BAV underwent pre-dilation. In cases with an aortic angulation ≥70°, we used snare catheter technique to assist in passing the TAVI valve through the aortic root. After valve implantation, we re-measured the transvalvular gradient and acquired images of the aortic root (in addition to calculating the aortic regurgitation index) to assess the transvalvular gradient and the degree of paravalvular leakage. The patients underwent transthoracic echocardiography before the completion of the procedure. Temporary electrode was kept for at least 24–48 h in cases of transient atrioventricular or complete bundle branch block during the procedure. The patients were re-examined and evaluated at 1-week, 30-day, 6-month, and 1-year intervals after discharge. In cases where patients could not return for follow-up visits because of death, we contacted the guardians/relatives via telephone to obtain information.
The clinical outcomes used to evaluate the results of this study were defined according to the Valve Academic Research Consortium (VARC)-2 criteria.8 Device success was defined as no mortality, correct positioning of the valve according to the anatomical location with a single valve, and no bioprosthetic valve mismatch (mean transvalvular gradient ≤20 mmHg and no regurgitation more than mild). Early safety within the first 30 days after TAVI was evaluated based on the rate of events, including all-cause mortality, stroke, life-threatening bleeding, stage 2 or 3 acute kidney injury, coronary artery obstruction requiring intervention, major vascular complications, and repeat procedures (including TAVI, SAVR, or balloon aortic valvuloplasty) for TAVI dysfunction. Clinical effectiveness at 1-year follow-up was evaluated based on the rates of mortality, stroke, hospital readmission due to valve-related symptoms or heart failure exacerbation, valve-related dysfunction (mean transvalvular gradient >20 mmHg and/or moderate-to-severe aortic regurgitation), and NYHA functional class III–IV status. Thrombosis and endocarditis of the TAVI valve were documented to evaluate the safety of the valve over time.
Descriptive statistics are presented as mean ± standard deviation for continuous variables and as numbers (%) for categorical variables. Descriptive statistics were performed for the entire sample and the TAV and BAV groups. The two groups were compared using the Wilcoxon rank-sum test and Fisher’s exact test based on continuous and categorical variables. The Kaplan–Meier test was used to estimate the survival rate. A univariate Cox regression model was used to identify the factors associated with 1-year mortality. The results were reported as hazard ratios (HR), 95% confidence intervals, and p-values. Changes in clinical parameters (LVEF, mean transvalvular gradient, and NYHA classification) were represented by plots at baseline, discharge, 30 days, and 12 months. Changes in clinical parameters were compared using the Wilcoxon signed rank test. All tests were two-tailed, and a p-value of < 0.05 was considered statistically significant. Statistical analysis was performed using R statistical software version 4.1.0.
The study was approved by the Ethics Committee of the University of Medicine and Pharmacy in Ho Chi Minh City, and all patients provided written consent for the research.
Role of the finding source
No funding was provided for this study.
Results
Baseline characteristics
From March 2017 to December 2022, 90 symptomatic patients with severe AS and NYHA functional class ≥II underwent TAVI at Vinmec Central Park International Hospital. The number of patients meeting the study criteria and undergoing follow-ups for assessment of in-hospital, 30-day, and 1-year outcomes was 90, 88, and 71, respectively. The mean aortic valve area was 0.62 ± 0.18 cm2, and the mean transvalvular gradient was 64.0 ± 20.4 mmHg. The mean age of the patients was 70.7 ± 8.8 years (range: 60–90 years), and 53.3% were male. Most patients had severe heart failure with NYHA functional class III–IV (86.6%), and the mean STS score was 5.8 ± 1.0%. Forty patients (45.5%) had BAV, and 2 patients had degenerated bioprosthetic valves and coexisting biological mitral valve prostheses. Compared with TAV patients, BAV patients had a smaller mean aortic valve area, a higher mean transvalvular gradient, and a larger aortic angulation (all p < 0.05) (Table 1).
Table 1.
Baseline characteristics of the patients.
| All (N = 90) | TAV (N = 48) | BAV (N = 40) | p-value | |
|---|---|---|---|---|
| Age, years | 70.7 ± 8.8 | 71.7 ± 9.4 | 69.6 ± 8.2 | 0.264 |
| 60–79 | 69 (76.7) | 33 (68.8) | 34 (85.0) | 0.085 |
| ≥80 | 21 (23.3) | 15 (31.2) | 6 (15.0) | |
| Sex male | 48 (53.3) | 27 (56.2) | 21 (52.5) | 0.830 |
| BMI, kg/m2 | 22.6 ± 3.0 | 22.5 ± 2.8 | 22.9 ± 3.2 | 0.602 |
| NYHA functional classification | 0.781 | |||
| II | 12 (13.3) | 8 (16.7) | 4 (10.0) | |
| III | 66 (73.3) | 34 (70.8) | 30 (75.0) | |
| IV | 12 (13.3) | 6 (12.5) | 6 (15.0) | |
| STS score, % | 5.8 ± 1.0 | 5.7 ± 0.9 | 5.9 ± 1.1 | 0.323 |
| Hypertension | 75 (83.3) | 37 (77.1) | 36 (90.0) | 0.156 |
| Hyperlipidemia | 66 (73.3) | 31 (64.6) | 33 (82.5) | 0.092 |
| Chronic heart failure | 27 (30.0) | 10 (20.8) | 15 (37.5) | 0.100 |
| Diabetes mellitus | 25 (27.8) | 11 (22.9) | 14 (35.0) | 0.242 |
| Prior PCI | 17 (18.9) | 11 (22.9) | 6 (15.0) | 0.422 |
| Chronic pulmonary disease | 16 (17.) | 9 (18.8) | 7 (17.5) | 1 |
| Peripheral arterial disease | 13 (14.4) | 7 (14.6) | 6 (15.0) | 1 |
| Chronic atrial fibrillation | 9 (10.0) | 4 (8.3) | 3 (7.5) | 1 |
| Cerebral vascular disease | 6 (6.7) | 2 (4.2) | 4 (10.0) | 0.405 |
| Chronic kidney disease | 14 (15.6) | 4 (8.3) | 8 (20.0) | 0.131 |
| Chronic kidney dialysis | 1 (1.1) | 1 (2.1) | 0 (0.0) | 1 |
| eGFR, ml/min/1.73 m2 | 67.4 ± 18.7 | 67.5 ± 18.6 | 69.9 ± 19.0 | 0.561 |
| Prior permanent pacemaker | 1 (1.1) | 1 (2.1) | 0 (0.0) | 1 |
| Prior biological aortic valve prosthesis | 2 (2.2) | |||
| Prior biological mitral valve prosthesis | 2 (2.2) | |||
| Echocardiographic findings | ||||
| LVEF, % | 60.8 ± 14.5 | 60.5 ± 14.5 | 61.2 ± 14.9 | 0.819 |
| Aortic valve area, cm2 | 0.62 ± 0.18 | 0.67 ± 0.18 | 0.57 ± 0.15 | 0.008 |
| Mean pressure gradient, mmHg | 64.0 ± 20.4 | 58.2 ± 18.2 | 71.7 ± 20.7 | 0.002 |
| Moderate/severe aortic regurgitation | 6 (6.7) | 3 (6.2) | 1 (2.5) | 0.623 |
| Moderate/severe aortic calcification | 76 (84.4) | 35 (72.9) | 40 (100.0) | <0.001 |
| MSCT findings | ||||
| Bicuspid aortic valve | 40 (45.5) | |||
| Annulus diameter, mm | 23.8 ± 2.8 | 23.9 ± 2.5 | 24.1 ± 2.6 | 0.630 |
| Aortic angulation, degree | 49.1 ± 10.0 | 46.2 ± 9.1 | 52.6 ± 10.3 | 0.003 |
Summary statistics are presented as mean ± standard deviation or n (%).
Apart from 48 patients with TAV and 40 patients with BAV, 2 patients with failed aortic bioprosthetic surgery underwent valve-in-valve TAVI.
BAV, bicuspid aortic valve; BMI, body mass index; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; MSCT, multi-slice computed tomography; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons; TAV, transcatheter aortic valve.
The baseline characteristics and main outcomes of TAVI are shown in Table 2. All 90 eligible patients underwent TAVI at the hybrid catheterization laboratory of Vinmec Central Park International Hospital. Two patients underwent valve-in-valve TAVI with a degenerated surgical aortic valve. General anesthesia was used in the early phase but was later switched to local anesthesia (73.3%). Almost all patients underwent TAVI via the femoral artery (87 cases, 96.7%); two underwent TAVI via the subclavian artery, and one via the trans-carotid artery due to the unsuitable anatomy of the femoral artery. The self-expanding valve system from Medtronic (USA) was the most commonly used (89/92 valves, 98.9%); two patients used the Portico self-expanding valve system from Abbott (USA), and only one patient used the balloon-expandable Sapien 3 valve system from Edwards (USA). The 29-mm valve was the most commonly used (44 cases, 48.9%), and the 25-mm or 27-mm valve was used only once for each size (1.1%). These procedural characteristics do not show statistically significant differences between the two patient groups, TAV and BAV (all p > 0.05). One procedural death occurred in a patient with pelvic and aortic root dissection when the operator used a snare to push the 26-mm Evolut R valve through the horizontal aorta (at an angle of 66°). One patient had a stroke due to embolization of the Evolut R 29-mm valve, leading to aortic root dissection and emergency surgery to replace the valve. Procedural mortality and stroke events occurred exclusively in BAV patients; however, the difference was not statistically significant compared to TAV patients (2.5% vs. 0.0%, p = 0.405). Another patient underwent embolization of an Evolut R 29-mm valve that was subsequently snared and replaced with a second Evolut R 26-mm valve, leading to aortic root dissection. The three cases of aortic root dissection and two cases of life-threatening bleeding in our study were the BAV patients. One patient had coronary occlusion after deployment of an Evolut R 29-mm valve at a high position (left coronary ostium height of 11.9 mm) and required emergency valve replacement surgery. Three patients required emergency surgery, including two valve replacements and one procedural mortality, with no statistically significant difference between the BAV and TAV groups (5.0% vs. 2.1%, p = 0.589). Six patients (6.7%) had complete atrioventricular block, and one patient (1.1%) experienced paravalvular leak ≥ moderate, with higher rates but not statistically significant in the BAV group compared to the TAV group (10.0% vs. 4.2%, p = 0.405 and 2.6% vs. 0.0%, p = 0.420, respectively). The mean transvalvular pressure gradient, measured by transthoracic echocardiography immediately after transcatheter aortic valve deployment, was 9.5 ± 4.2 mmHg, and this gradient did not differ significantly between the BAV and TAV groups (10.2 ± 4.2 mmHg vs. 8.9 ± 4.3 mmHg, p = 0.151). According to the VARC-2 criteria, device success was achieved in 86 of the 90 patients (95.6%), and this rate was higher in TAV patients compared to BAV patients (97.9% vs. 92.5%, p = 0.326), but the difference was not statistically significant. Furthermore, among the first 30 TAVI cases with international proctor support, the overall procedural success rate as well as within each TAV and BAV group did not significantly differ from the subsequent 60 cases when we performed TAVI as solo operators (93.3% vs. 96.7%, p = 0.598; 100.0% vs. 96.8%, p > 0.999 and 84.6% vs. 96.3%, p = 0.242, respectively) (Table 3). The mean length of the hospital stay was 9 days.
Table 2.
Procedural characteristics and outcomes.
| All (N = 90) | TAV (N = 48) | BAV (N = 40) | p-value | |
|---|---|---|---|---|
| Procedural data | ||||
| Valve-in-valve implantation | 2 (2.2) | |||
| Local anesthesia | 66 (73.3) | 34 (70.8) | 30 (75.0) | 0.811 |
| Rapid pacing via left ventricular guidewire | 13 (14.4) | 4 (8.3) | 8 (20.0) | 0.131 |
| Access route | 0.498 | |||
| Transfemoral | 87 (96.7) | 45 (93.8) | 40 (100.0) | |
| Trans-subclavian | 2 (2.2) | 2 (4.2) | 0 (0.0) | |
| Transcarotid | 1 (1.1) | 1 (2.1) | 0 (0.0) | |
| Types of valves | 0.729 | |||
| Evolut R | 80 (88.9) | 43 (89.6) | 34 (85.0) | |
| Evolut Pro | 9 (10.0) | 3 (6.2) | 5 (12.5) | |
| Portico | 2 (2.2) | 1 (2.1) | 1 (2.5) | |
| Sapien 3 | 1 (1.1) | 1 (2.1) | 0 (0.0) | |
| Valve size (mm) | 0.444 | |||
| 29 mm | 44 (48.9) | 27 (56.2) | 16 (40.0) | |
| 26 mm | 21 (23.3) | 9 (18.8) | 11 (27.5) | |
| 34 mm | 16 (17.8) | 7 (14.6) | 9 (22.5) | |
| 23 mm | 9 (10.0) | 4 (8.3) | 3 (7.5) | |
| 25 mm | 1 (1.1) | 1 (2.1) | 0 (0.0) | |
| 27 mm | 1 (1.1) | 0 (0.0) | 1 (2.5) | |
| Procedural outcomes | ||||
| Mortality | 1 (1.1) | 0 (0.0) | 1 (2.5) | 0.455 |
| Disabling stroke | 1 (1.1) | 0 (0.0) | 1 (2.5) | 0.455 |
| Life-threatening bleeding | 2 (2.2) | 0 (0.0) | 2 (5.0) | 0.204 |
| Major vascular complication | 3 (3.3) | 0 (0.0) | 3 (7.5) | 0.090 |
| Coronary artery obstruction requiring intervention | 1 (1.1) | 1 (2.1) | 0 (0.0) | 1 |
| Annulus rupture or ventricular septal perforation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 |
| Valve malpositioning | 2 (2.2) | 0 (0.0) | 2 (5.0) | 0.204 |
| Conversion to open heart surgery | 3 (3.3) | 1 (2.1) | 2 (5.0) | 0.589 |
| Conversion to SAVR | 2 (2.2) | 1 (2.1) | 1 (2.5) | 1 |
| Complete atrioventricular block | 6 (6.7) | 2 (4.2) | 4 (10.0) | 0.405 |
| Paravalvular leak ≥ moderate | 1 (1.1) | 0 (0.0) | 1 (2.6) | 0.420 |
| Implantation of 2 valves | 2 (2.2) | 0 (0.0) | 2 (5.0) | 0.204 |
| Device success | 86 (95.6) | 47 (97.9) | 37 (92.5) | 0.326 |
Summary statistics are presented as mean ± standard deviation or n (%).
Two patients with failed aortic bioprosthetic surgery underwent valve-in-valve TAVI.
BAV, bicuspid aortic valve; SAVR, surgical aortic valve replacement; TAV, transcatheter aortic valve.
Table 3.
Baseline characteristics of the patients and outcomes between proctor support and solo operator phases.
| All |
TAV |
BAV |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1–30 (N = 30) | 31–90 (N = 60) | p-value | 1–30 (N = 17) | 31–90 (N = 31) | p-value | 1–30 (N = 13) | 31–90 (N = 27) | p-value | |
| Age, years | 70.5 ± 9.5 | 70.8 ± 8.5 | 0.872 | 72.4 ± 10.5 | 71.3 ± 8.9 | 0.726 | 68.1 ± 7.7 | 70.3 ± 8.5 | 0.426 |
| NYHA III–IV | 26 (86.7%) | 52 (86.7%) | >0.999 | 15 (88.2%) | 25 (80.6%) | 0.694 | 11 (84.6%) | 25 (92.6%) | 0.584 |
| STS score, % | 6.2 ± 0.9 | 5.7 ± 1.0 | 0.025 | 6.0 ± 0.8 | 5.5 ± 0.9 | 0.048 | 6.3 ± 1.0 | 5.7 ± 1.2 | 0.125 |
| Chronic heart failure | 5 (16.7%) | 22 (36.7%) | 0.056 | 4 (23.5%) | 6 (19.4%) | 0.727 | 1 (7.7%) | 14 (51.9%) | 0.013 |
| Cerebral vascular disease | 1 (3.3%) | 5 (8.3%) | 0.659 | 1 (5.9%) | 1 (3.2%) | >0.999 | 0 (0.0%) | 4 (14.8%) | 0.284 |
| Mean transaortic pressure gradient, mmHg | 65.1 ± 20.7 | 63.4 ± 20.4 | 0.711 | 54.1 ± 10.7 | 60.5 ± 21.1 | 0.176 | 79.4 ± 22.2 | 68.0 ± 19.3 | 0.127 |
| Aortic valve area, cm2 | 0.66 ± 0.18 | 0.60 ± 0.17 | 0.119 | 0.74 ± 0.17 | 0.63 ± 0.18 | 0.042 | 0.56 ± 0.15 | 0.57 ± 0.16 | 0.827 |
| Bicuspid aortic valve (MSCT) | 13 (43.3%) | 27 (46.6%) | 0.824 | - | - | - | 13 (100.0%) | 27 (100.0%) | >0.999 |
| Procedural time, min | 208.7 ± 76.8 | 182.3 ± 46.8 | 0.092 | 207.4 ± 64.8 | 184.2 ± 41.3 | 0.196 | 210.4 ± 93.0 | 180.9 ± 54.5 | 0.306 |
| Fluoroscopy time, min | 33.4 ± 11.0 | 28.9 ± 12.2 | 0.081 | 32.2 ± 10.4 | 27.5 ± 11.1 | 0.154 | 35.1 ± 11.9 | 31.0 ± 13.5 | 0.340 |
| Device success | 28 (93.3%) | 58 (96.7%) | 0.598 | 17 (100.0%) | 30 (96.8%) | >0.999 | 11 (84.6%) | 26 (96.3%) | 0.242 |
| 30-day all-cause mortality | 1 (3.4%) | 1 (1.7%) | >0.999 | 0 (0.0%) | 0 (0.0%) | – | 1 (8.3%) | 1 (3.7%) | 0.526 |
| 1-year all-cause mortality | 2 (6.9%) | 2 (4.8%) | >0.999 | 0 (0.0%) | 0 (0.0%) | – | 2 (16.7%) | 2 (11.8%) | >0.999 |
Summary statistics are presented as n (%).
TAV, transcatheter aortic valve; BAV, bicuspid aortic valve.
The main clinical outcomes according to VARC-2 at 30 days and 1 year after the procedure are presented in Table 4. Additional one cardiac-related death occurred on day 30 after discharge, resulting in a cumulative mortality rate of 2.3% on day 30 after TAVI. Similarly, the incidence of stroke, moderate or severe aortic regurgitation, and permanent pacemaker implantation increased by 1 patient each, resulting in cumulative rates of 2.3%, 2.3%, and 8.0%, respectively. Major life-threatening bleeding did not increase compared to in-hospital bleeding (two patients, 2.3%). The rates of all-cause mortality (5.1% vs. 0.0%, p = 0.202), cardiac mortality (5.1% vs. 0.0%, p = 0.202), stroke (5.1% vs. 0.0%, p = 0.202), life-threatening bleeding (5.1% vs. 0.0%, p = 0.202), and permanent pacemaker implantation (10.3% vs. 6.4%, p = 0.694) in the BAV group tend to be higher than those in the TAV group.
Table 4.
Outcomes at 30 days and 1 year.
| Outcomes | All | TAV | BAV | p-value |
|---|---|---|---|---|
| 30-day outcomes | N = 88 | N = 47 | N = 39 | |
| All-cause mortality | 2 (2.3) | 0 (0.0) | 2 (5.1) | 0.202 |
| Cardiovascular mortality | 2 (2.3) | 0 (0.0) | 2 (5.1) | 0.202 |
| Disabling stroke | 2 (2.3) | 0 (0.0) | 2 (5.1) | 0.202 |
| Life-threatening bleeding | 2 (2.3) | 0 (0.0) | 2 (5.1) | 0.202 |
| Stage 2 or 3 acute kidney injury | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 |
| Coronary artery obstruction requiring intervention | 1 (1.1) | 1 (2.1) | 0 (0.0) | 1 |
| Major vascular complication | 3 (3.4) | 0 (0.0) | 3 (7.7) | 0.089 |
| Valve-related dysfunction requiring repeat procedure (BAV, TAVI, or SAVR) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 |
| New pacemaker implantation | 7 (8.0) | 3 (6.4) | 4 (10.3) | 0.694 |
| Moderate/severe paravalvular leaka | 2 (2.3) | 1 (2.1) | 1 (2.6) | 1 |
| 1-year outcomes | N = 71 | N = 40 | N = 29 | |
| All-cause mortality | 4 (5.6) | 0 (0.0) | 4 (13.8) | 0.027 |
| Disabling stroke | 2 (2.8) | 0 (0.0) | 2 (6.9) | 0.173 |
| Requiring hospitalizations for valve-related symptoms or worsening heart failure | 2 (2.8) | 0 (0.0) | 2 (6.9) | 0.173 |
| NYHA III or IV | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 |
| Valve-related disfunction | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 |
| New pacemaker implantation | 7 (9.9) | 3 (7.5) | 4 (13.8) | 0.442 |
| Moderate/severe paravalvular leakb | 2 (3.0) | 1 (2.5) | 1 (3.4) | 1 |
| Prosthetic valve endocarditis or thrombosis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 |
Summary statistics are presented as mean ± standard deviation or n (%).
BAV, bicuspid aortic valve; NYHA, New York Heart Association; SAVR, surgical aortic valve replacement; TAV, transcatheter aortic valve; TAVI, transcatheter aortic valve implantation.
The number of calculated patients is 86.
The number of calculated patients is 67.
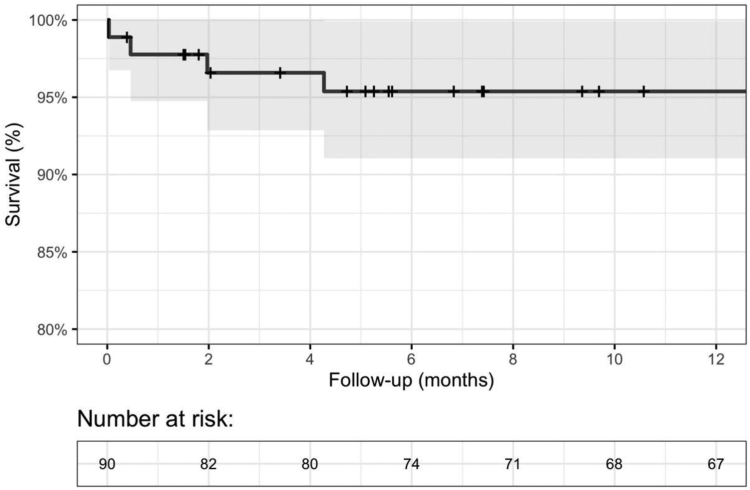
From day 30 to 1 year after TAVI, two additional patients died (one with acute heart failure and one with sudden death at home), resulting in a cumulative mortality rate of four patients (5.6%) due to any cause at 1 year. All four deaths occurred in the BAV group, and the difference was statistically significant compared to that in the TAV group (13.8% vs. 0.0%, p = 0.027). Fig. 1 shows the survival rate over time at 1 year. The incidence of stroke (two patients, 2.8%), permanent pacemaker implantation (seven patients, 9.9%), and moderate or severe aortic regurgitation (two patients, 2.8%) remained stable for 1 year after TAVI. Two patients (2.8%) were readmitted because of worsening heart failure, and one patient died. None of the patients had NYHA functional classes III–IV.
Fig. 1.
Kaplan–Meier survival curve for 1-year all-cause mortality after TAVI. The backline represents the Kaplan–Meier estimate, and the gray region represents the 95% confidence interval. TAVI, transcatheter aortic valve implantation.
During the solo operator phase, there was one procedural-related mortality. However, there was no significant difference in all-cause mortality at 30 days (3.4% vs. 1.7%) and 1 year (6.9% vs. 4.8%) between the TAVI cases performed with proctor support and those performed by solo operators. Furthermore, there was no significant difference in procedure time and fluoroscopy time between the two phases, although these times showed a decreasing trend in the later phase. Some baseline patient characteristics and outcomes between the two stages of TAVI performed with proctor assistance and solo operator are presented in Table 3.
Using univariate analysis, our study showed statistically significant factors associated with mortality 1 year after TAVI, including chronic heart failure, cerebrovascular disease, STS score, mean transvalvular gradient, and failure of device implantation (Table 5). However, in the multivariable model including these five factors, no significant association was found due to the limited number of one-year mortality following TAVI (Supplementary Table 4B).
Table 5.
Predictors of 1-year mortality.
| HR (95% CI) | p-value | |
|---|---|---|
| Chronic heart failure | 6.54 (1.17–36.7) | 0.033 |
| Cerebral vascular disease | 16.7 (3.33–83.4) | <0.001 |
| STS score | 21.2 (4.38–102) | <0.001 |
| Mean transaortic pressure gradient | 1.04 (1.01–1.08) | 0.014 |
| Failure of device implantation | 49.8 (6.54–380) | <0.001 |
CI, confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction.
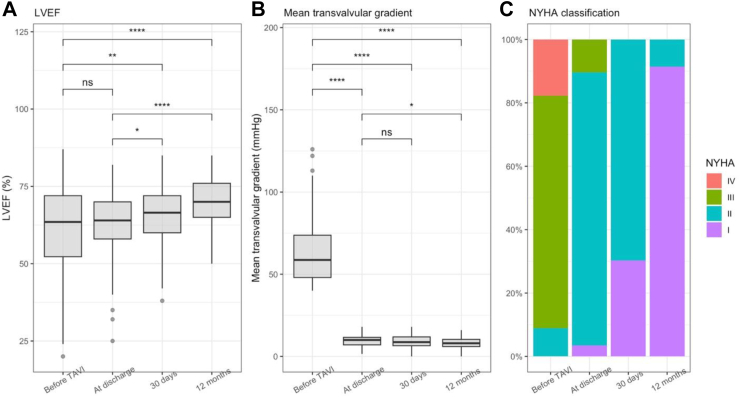
Compared to baseline LVEF (60.8 ± 14.5%), patients’ LVEF improved immediately at discharge (62.5 ± 10.6%), after 30 days (65.9 ± 9.5%), and after 1 year (69.3 ± 8.5%). The difference was significant when comparing the LVEF at 30 days and 1 year to baseline (Fig. 2A).
Fig. 2.
Clinical parameters before and after the intervention. ns; not significant (p > 0.05); ∗, p ≤ 0.05; ∗∗, p ≤ 0.01; ∗∗∗, p ≤ 0.001; ∗∗∗∗, p ≤ 0.0001 LVEF; NYHA, New York Heart Association, left ventricular ejection fraction; TAVI, transcatheter aortic valve implantation.
The mean pressure gradient across the valve decreased sharply after valve implantation and continued to decrease at discharge, 30 days, and 1 year, with significant differences compared to before TAVI. The mean gradient at 1 year decreased significantly compared to that at discharge (p = 0.002) (Fig. 2B).
At baseline, most patients had NYHA III–IV (86.6%), and none had NYHA I. At discharge, most patients had NYHA II (86.2%), and NYHA III–IV only accounted for 6.9% of all patients. From day 30 onwards, there were no patients with NYHA III–IV (0.0%) (Fig. 2C). Moderate or greater aortic regurgitation before TAVI was present in 6 patients and remained the same at 30 days and 1 year after TAVI (two patients).
Discussion
TAVI has been one of the most challenging techniques in structural heart interventions in our country, particularly during the initial phases of its implementation. At that time, only a few TAVI cases were performed nationwide, and all were conducted by foreign proctors. Our primary objective was to perform TAVI safely and effectively by ourselves on Vietnamese patients with symptomatic severe aortic valve stenosis, especially those who were considered inoperable, to reduce mortality rates and improve their quality of life. To achieve this goal, our Heart Team received thorough training and step-by-step technical transfer for TAVI with the strong support by Professor Antoine Lafont (Georges-Pompidou European Hospital, France). After this phase, we continued to receive support from international proctors for the first 30 TAVI cases at our center. These initial steps played a crucial role in the subsequent development of our center and have yielded promising outcomes.
This is the first study of 90 patients with severe AS undergoing TAVI at a single center in Vietnam. Overall, the study results showed a low rate of procedural complications for TAVI in this population, according to the VARC-2 criteria, and was effective at both 30 days and 1 year, which is comparable to TAVI studies in other populations. One of the highlights of our study was the relatively young age of the patients and the high rate of BAV compared to other studies.
Several randomized controlled trials and registry studies with data collected before 2017 showed that the average age for TAVI was 75–80 years, and patients were considered “young” if they were ≤75 years old.9, 10, 11 However, the average age tended to be younger since the CRT studies demonstrated that TAVI was safe and equally effective as SAVR on severe AS patients with moderate (in 2017) and low (in 2019) surgical risk.12, 13, 14 ACC/AHA 202015 and ESC/EACTS 202116 guidelines subsequently set age thresholds of 75 and 65 years for considering TAVI or SAVR in AS patients. In the STS-ACC TVT registry study (n = 276,316)17 of TAVI patients from 2011 to 2019, the average age also decreased gradually, from 84 years (range: 78–88) before 2013 to 80 years (range: 73–85) in 2019. The average age in our study was 70.7 ± 8.8 years with moderate surgical risk (STS 5.8 ± 1.0), relatively “young” compared to TAVI studies before 2017, with most patients having a high surgical risk. This difference was not significant when compared with TAVI studies on patients after 2017, especially after 2019. In registry studies of Gulf (n = 795)18 and China (n = 1204),19 the average age was 74.56 and 73.8 ± 6.5 years, respectively. An analysis of a registry study of 7097 TAVI patients from February 2011 to June 2018 in Switzerland showed that the patients in the 70–79 years age group accounted for nearly 30% of all patients, and older age influenced short- and long-term mortality, stroke, and permanent pacemaker implantation.20 Therefore, the age of the patients included in our study may also contribute to the low rate of these events. The BAV patient group in our study had a younger average age than the TAV group (69.6 ± 8.2), which is also a demographic characteristic in BAV patients undergoing TAVI in many other studies.21,22
The main randomized controlled trials of TAVI in the treatment of AS excluded patients with BAV, as BAV has anatomical features that are not favorable for TAVI, such as severe valve calcification and raphe, an elliptical valve annulus, a large annular size, a horizontal aorta, and accompanying arterial pathology.21,23 However, in the real world, approximately 5–10% of the population with BAV undergoes TAVI.22 The evidence of TAVI in this population mainly comes from observational studies and registries.21 In the early stages, patients who underwent TAVI for BAV had worse clinical outcomes than TAV, with higher rates of all-cause mortality, permanent pacemaker implantation, and moderate or severe paravalvular leak.24 In later studies, with better evaluation of the aortic valve region using multi-slice computed tomography, newer generations of transcatheter aortic valves, and improved techniques, TAVI for BAV showed no significant difference compared to TAV. In a study by Yoon et al. on 546 pairs of BAV and TAV patients with the same baseline characteristics who underwent TAVI, there was no difference in procedural complications or 30-day, 1-year, or 2-year mortality rates when using newer generations of valves (Evolut R, Sapien 3, and Lotus).25 Similar trends were also observed in two registry studies by Forrest et al.26 (n = 929 propensity-score matched pairs of BAV and TAV, using Evolut R and Evolut Pro valves) and Makkar et al.27 (n = 2691 propensity-score matched pairs of BAV and TAV, using the Sapien 3 valve) based on data from the STS-ACC TVT from 2015 to 2018. In our study, a significant proportion of patients had BAV (40 of 88 native aortic valves, accounting for 45.5%). This proportion is very different from that in many other studies, except for a registry study of 1024 TAVI patients in China, in which 48.5% of patients had BAV.19,22 Vietnam and China are two neighboring countries with many similar ethnic characteristics, which may explain the similarity in the proportion of patients with BAV in the TAVI population mentioned above. In addition, the relatively “young” age of the study population may explain the high proportion of patients with BAV. The results of TAVI for BAV compared to TAV in our study were also similar to those of other studies, with no significant differences in device success, procedural complications, or 30-day mortality. However, at the 1-year mark, patients with BAV had a significantly higher mortality rate.
The device success rate according to the VARC-2 criteria was 95.6%, and this success rate did not significantly differ between the TAV and BAV patient groups. In the first 30 TAVI cases, we received support from international proctors, but starting from the 31st case, as certified solo operators, we performed TAVI independently. Thanks to comprehensive training, skills, and experience gained from the initial 30 TAVI cases, the device success rate in the subsequent 60 cases did not significantly differ from the initial phase with expert support. In other Asian studies, with the self-expanding CoreValve/Evolut R system being dominant, the success rate ranged from 90.6 to 95%.28 The Asian TAVR registry29 (n = 848, CoreValve 35.3%, and Sapien 64.7%) reported a device success rate of 85.5%. The incidence of common TAVI complications in our study was similar to that of other studies, except for acute kidney injury stage 2 or 3 (using AKIN criteria), which was 0.0% in our study.6,17,19
Within the first 30 days after TAVI, two cases died (2.3%) due to cardiovascular causes. This result is similar to other TAVI studies in Asia, including the Korean TAVI registry (2.6%),30 China TAVR registry (2.3%),19 Asian TAVR (2.5%),29 Asia Pacific TAVI registry (2.5%),6 STS-ACC TVT registry of TAVR (3.32%),17 and SURTAVI study (2.8%)12 with patients at intermediate surgical risk. At 1 year, there were four deaths (5.6%), which was higher than that in the Chinese registry (4.5%)19 but lower than that in many other studies (Table 6). Our study sample was small and had a young average age, which may explain the low mortality rate at 1-year follow-up after TAVI. Univariate regression analysis showed that chronic heart failure, cerebrovascular disease, STS score, mean transaortic pressure gradient, and failure of device implantation were significant predictors of 1-year mortality. These predictors have also been observed in other studies, except failure of device implantation. Transfemoral TAVI was also shown to significantly increase 1-year mortality, but the rate was very low in our study (3.3%); therefore, the difference in mortality between the transfemoral and non-transfemoral approaches was not significant. Overall, the main results of our study, according to VARC-2, within the first 30 days and 1 year after TAVI were favorable compared to those of other studies, with mortality rates compared in Table 6. These results demonstrate the feasibility of TAVI in real-world settings for the older population in Vietnam and the success of the TAVI technology transfer based on the appropriate step-by-step approaches. The comparisons made here are only for relative reference, as there are many differences between our study and those being compared.
Table 6.
Comparisons of 30-day and 1-year outcomes between our study and others.
| Our study | CARRY29 | Asia Pacific TAVI6 | FRANCE TAVI10 | STS-ACC TVTa,17 | SURTAVI12 | |
|---|---|---|---|---|---|---|
| Time to collect TAVI data, year | 2017–2022 | 2012–2020 | 2009–2017 | 2013–2015 | 2011–2019 | 2012–2016 |
| Sample size | 90 | 1204 | 1125 | 12,804 | 276,316 | 864 |
| Mean age, year | 70.7 ± 8.8 | 73.8 ± 6.5 | 79.9 ± 8.1 | 83.4 ± 7.2 | 81 | 79.9 ± 6.2 |
| STS or Log EuroSCORE | 5.8 ± 1.0 | 6.0 | 7.1 ± 6.2 | 17.9 ± 12.3 | 5.22 | 4.4 ± 1.5 |
| Med-Eds valves, %b | 98.9–1.1 | – | 32.7–38.9 | 34.9–64.3 | – | 100.0–0.0 |
| 30-day all-cause mortality, % | 2.3 | 2.3 | 2.5 | 5.4 | 3.32 | 2.8 |
| 1-year all-cause mortality, % | 5.6 | 4.5 | 8.8 | – | 15.62 | 8.1 |
STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
Nonmissing data.
Med-Eds valves: CoreValve, Evolut R, Evolut Pro–Sapien, Sapien XT, Sapien 3 valves.
One of the main limitations of our study compared to other registry studies on TAVI outcomes in other populations is the relatively small sample size, which may have led to different results. The proportion of TAVI procedures via the non-transfemoral approach or in patients with low or high surgical risk in our study was also very low; therefore, we could not analyze the impact of these factors on the clinical outcomes of patients. In addition, the presence of pre-existing frailty, which is a prognostic factor for adverse outcomes in patients undergoing valve interventions (including TAVI and SAVR), was not recorded or analyzed in our study.
The results of the first study of 90 older patients (≥60 years old) with severe AS treated with TAVI at a single center in Vietnam from March 2017 to December 2022 showed that TAVI in this population, with high rate of BAV, is safe with a low complication rate and is effective at the 1-year follow-up, compared to other clinical studies. An appropriate strategy for technology transfer, careful patient selection, the use of new-generation transcatheter aortic valves, and advanced techniques will help achieve favorable outcomes for TAVI in the Vietnamese population when indicated for intervention in patients with AS.
Contributors
Vo Thanh Nhan and Nguyen Quoc Khoa contributed equally to this study, including the study design, data collection, analysis, and manuscript writing. Nguyen Duc Cong and Le Quoc Su were involved in the study design and manuscript writing. La Thi Thuy and Nguyen Van Duong participated in the data collection and manuscript writing. Nguyen Van Tan and Than Ha Ngoc The contributed to the data analysis and manuscript writing. Nguyen Lam Vuong contributed to analyzing statistical data. Arik Finkelstein and Antoine Lafont contributed to the review and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Data sharing statement
The data presented in this study are available by email to the corresponding author upon request.
Declaration of interests
The authors declare no conflicts of interest.
Acknowledgements
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100956.
Contributor Information
Vo Thanh Nhan, Email: drnhanvo@gmail.com.
Nguyen Quoc Khoa, Email: khoanguyenql@gmail.com.
La Thi Thuy, Email: thuyla04@gmail.com.
Nguyen Van Duong, Email: dr.duongnguyen1989@gmail.com.
Nguyen Van Tan, Email: nguyenvtan10@ump.edu.vn.
Than Ha Ngoc The, Email: the2509@ump.edu.vn.
Nguyen Lam Vuong, Email: nguyenlamvuong@ump.edu.vn.
Nguyen Duc Cong, Email: cong1608@gmail.com.
Le Quoc Su, Email: lequocsu2015@gmail.com.
Ariel Finkelstein, Email: afinkel@tasmc.health.gov.il.
Antoine Lafont, Email: antoinelafont75@gmail.com.
Appendix A. Supplementary data
References
- 1.Geicu L., Busuttil O., D'Ostrevy N., et al. Updates on the latest surgical approach of the aortic stenosis. J Clin Med. 2021;10:5140. doi: 10.3390/jcm10215140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leon M.B., Smith C.R., Mack M., et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 3.Cribier A. Invention and uptake of TAVI over the first 20 years. Nat Rev Cardiol. 2022;19:427–428. doi: 10.1038/s41569-022-00721-w. [DOI] [PubMed] [Google Scholar]
- 4.Mesnier J., Panagides V., Nuche J., Rodés-Cabau J. Evolving indications of transcatheter aortic valve replacement-where are we now, and where are we going. J Clin Med. 2022;11:3090. doi: 10.3390/jcm11113090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee C.H., Inohara T., Hayashida K., Park D.W. Transcatheter aortic valve replacement in Asia: present status and future perspectives. JACC Asia. 2021;1:279–293. doi: 10.1016/j.jacasi.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tay E., Khaing T., Yin W.H., et al. Asia Pacific TAVI registry (an APSIC initiative): initial report of early outcomes: Asia Pacific TAVI registry. Asiaintervention. 2021;7:54–59. doi: 10.4244/aij-d-18-00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vo N.T., Nguyen D.V., La T.T., Tran N.H., Nguyen K.Q. Transcatheter aortic valve replacement through the carotid artery in A 60-year-old-man with aortic stenosis and chronic dialysis: a Case report. Med. Pharmres. 2022;6:50–54. doi: 10.32895/UMP.MPR.6.3.S10. [DOI] [Google Scholar]
- 8.Kappetein A.P., Head S.J., Généreux P., et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33:2403–2418. doi: 10.1093/eurheartj/ehs255. [DOI] [PubMed] [Google Scholar]
- 9.Adams D.H., Popma J.J., Reardon M.J., et al. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 10.Auffret V., Lefevre T., Van Belle E., et al. Temporal trends in transcatheter aortic valve replacement in France: FRANCE 2 to France TAVI. J Am Coll Cardiol. 2017;70:42–55. doi: 10.1016/j.jacc.2017.04.053. [DOI] [PubMed] [Google Scholar]
- 11.Navarese E.P., Andreotti F., Kołodziejczak M., et al. Age-related 2-year mortality after transcatheter aortic valve replacement: the YOUNG TAVR registry. Mayo Clin Proc. 2019;94:1457–1466. doi: 10.1016/j.mayocp.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Reardon M.J., Van Mieghem N.M., Popma J.J., et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321–1331. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 13.Mack M.J., Leon M.B., Thourani V.H., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 14.Popma J.J., Deeb G.M., Yakubov S.J., et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–1715. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 15.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;143:e35–e71. doi: 10.1161/cir.0000000000000932. [DOI] [PubMed] [Google Scholar]
- 16.Vahanian A., Beyersdorf F., Praz F., et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 17.Carroll J.D., Mack M.J., Vemulapalli S., et al. STS-ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol. 2020;76:2492–2516. doi: 10.1016/j.jacc.2020.09.595. [DOI] [PubMed] [Google Scholar]
- 18.Alasnag M., AlMerri K., Almoghairi A., et al. One-year outcomes for patients undergoing transcatheter aortic valve replacement: the Gulf TAVR registry. Cardiovasc Revasc Med. 2022;41:19–26. doi: 10.1016/j.carrev.2021.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Li Y.M., Xiong T.Y., Xu K., et al. Characteristics and outcomes following transcatheter aortic valve replacement in China: a report from China aortic valve transcatheter replacement registry (CARRY) Chin Med J (Engl) 2021;134:2678–2684. doi: 10.1097/cm9.0000000000001882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Attinger-Toller A., Ferrari E., Tueller D., et al. Age-related outcomes after transcatheter aortic valve replacement: insights from the SwissTAVI registry. JACC Cardiovasc Interv. 2021;14:952–960. doi: 10.1016/j.jcin.2021.01.042. [DOI] [PubMed] [Google Scholar]
- 21.Vincent F., Ternacle J., Denimal T., et al. Transcatheter aortic valve replacement in bicuspid aortic valve stenosis. Circulation. 2021;143:1043–1061. doi: 10.1161/circulationaha.120.048048. [DOI] [PubMed] [Google Scholar]
- 22.Xiong T.Y., Ali W.B., Feng Y., et al. Transcatheter aortic valve implantation in patients with bicuspid valve morphology: a roadmap towards standardization. Nat Rev Cardiol. 2023;20:52–67. doi: 10.1038/s41569-022-00734-5. [DOI] [PubMed] [Google Scholar]
- 23.Yoon S.H., Kim W.K., Dhoble A., et al. Bicuspid aortic valve morphology and outcomes after transcatheter aortic valve replacement. J Am Coll Cardiol. 2020;76:1018–1030. doi: 10.1016/j.jacc.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Wijesinghe N., Ye J., Rodés-Cabau J., et al. Transcatheter aortic valve implantation in patients with bicuspid aortic valve stenosis. JACC Cardiovasc Interv. 2010;3:1122–1125. doi: 10.1016/j.jcin.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Yoon S.H., Bleiziffer S., De Backer O., et al. Outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol. 2017;69:2579–2589. doi: 10.1016/j.jacc.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Forrest J.K., Kaple R.K., Ramlawi B., et al. Transcatheter aortic valve replacement in bicuspid versus tricuspid aortic valves from the STS/ACC TVT registry. JACC Cardiovasc Interv. 2020;13:1749–1759. doi: 10.1016/j.jcin.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Makkar R.R., Yoon S.H., Leon M.B., et al. Association between transcatheter aortic valve replacement for bicuspid vs tricuspid aortic stenosis and mortality or stroke. JAMA. 2019;321:2193–2202. doi: 10.1001/jama.2019.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y.H., Chang H.H., Chen P.L., et al. Procedural characteristics and outcomes of transcatheter aortic valve implantation: a single-center experience of the first 100 inoperable or high surgical risk patients with severe aortic stenosis. Acta Cardiol Sin. 2017;33:339–349. doi: 10.6515/acs20170620a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon S.H., Ahn J.M., Hayashida K., et al. Clinical outcomes following transcatheter aortic valve replacement in Asian population. JACC Cardiovasc Interv. 2016;9:926–933. doi: 10.1016/j.jcin.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 30.Yu C.W., Kim W.J., Ahn J.M., et al. Trends and outcomes of transcatheter aortic valve implantation (TAVI) in Korea: the results of the first cohort of Korean TAVI registry. Korean Circ J. 2018;48:382–394. doi: 10.4070/kcj.2018.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.