Abstract
Microscopic colitis (MC) is a chronic inflammatory disease that affects the older population. Its clinical presentation includes a variety of gastrointestinal manifestations. The main symptom is chronic watery, nonbloody diarrhea. The disease has a female predominance. The diagnosis might be challenging since the symptoms are similar to other differential diagnoses, such as celiac disease, irritable bowel syndrome, Crohn’s disease, bacterial overgrowth, and infectious colitis. The golden diagnostic tool for diagnosis is performing colonoscopy to obtain the colonic biopsy, which demonstrates the characteristic histological evidence needed for diagnosis. The treatment starts with an accurate diagnosis and trial of any possible offending medications. Alternatively, there are many medications, such as bismuth or budesonide, which are very effective in treating this disease. The primary objective of this detailed review is to enhance knowledge and understanding of this condition among healthcare providers to guide them with detailed information regarding epidemiology, clinical presentation, diagnosis, and appropriate management. In the assessment of individuals presenting with persistent chronic diarrhea, it is essential for healthcare providers to consider MC as a probable differential diagnosis.
Keywords: inflammatory bowel disease, lymphocytic colitis, collagenous colitis, diarrhea, microscopic colitis
Introduction and background
Microscopic colitis (MC) is considered a common cause of persistent watery diarrhea in elderly people [1]. It is a chronic inflammatory disease of the large intestine, primarily affecting individuals in the middle-aged demographic, with a higher prevalence among females [2]. The large intestine exhibits a normal mucosal morphology on colonoscopy in people with MC. The accurate diagnosis is confirmed by performing a colonoscopy with random colonic biopsies showing the characteristic features that establish the diagnosis. It was discovered globally in 1980, and it has two different types, namely, lymphocytic colitis (LC) and collagenous colitis (CC) [2,3]. Each of the two subtypes has distinct histologic characteristics, such as intraepithelial lymphocytosis, a significant inflammatory infiltration in the lamina propria, and subepithelial collagen band [4].
The clinical feature of MC is nonspecific and includes mainly a history of chronic watery, nonbloody diarrhea along with other gastrointestinal symptoms [1,4-6]. The disease course and severity range from mild intermittent symptoms to more progressive and chronic symptoms with dehydration and electrolyte derangement requiring hospitalization and proper management. Furthermore, while some individuals achieve spontaneous remission, others may require long-term maintenance treatment [7]. Increased awareness and knowledge of this disease in the past few decades has resulted in early diagnosis and proper management [8]. Despite being deemed a benign condition with no increase in mortality, as is the scenario with other inflammatory bowel diseases (IBDs), MC can lead to a significant negative impact on an individual’s quality of life.
Review
Epidemiology
In the late 20th century, multiple studies from the America and European continents revealed a substantial increase in the prevalence of MC [9], whereas more recent statistics indicate that the incidence rates have plateaued [10,11]. Based on previous studies, the incidence of the collagenous type is estimated to be between 2 and 10.8 per 100,000 per year, and the incidence of the lymphocytic type is between 2.3 and 16 per 100,000 per year, with a greater incidence in northern Europe and North America [7,12-14]. The average age upon diagnosis is 65 years old [1]. Moreover, the diagnosis of MC occurs in around one-quarter of individuals before the age of 45. On the other hand, it has seldom been noticed in children [15-17]. MC has a female gender predominance [7,13,18]. Female predominance seems to be more evident in collagenous colitis than in lymphocytic colitis (female-to-male incidence rate ratios, 3.0 and 1.9, respectively) [10]. At present, Denmark has the greatest incidence, with around 16 collagenous cases per 100,000 people-years and around 11 lymphocytic cases per 100,000 people-years [19].
Risk factors
Several risk factors have been determined to have a significant impact on the development of MC, some of which are modifiable and others are not. The likelihood of getting the disease is higher in females, particularly in collagenous colitis as opposed to lymphocytic colitis, when comparing the two types [2].
Another major risk factor for MC includes active smoking. There exists evidence indicating a confirmed association between smoking and the pathogenesis of MC and adverse clinical outcomes [7,20,21]. When Yen et al. looked at 340 people with MC, they found that smoking cigarettes, whether in the past or at present, made the likelihood of getting the disease much higher [21]. In addition, smokers might develop the disease more than 10 years earlier than non-smokers [22].
Moreover, the presence of any personal medical background of autoimmune disorders, such as rheumatoid arthritis, diabetes mellitus, thyroid disorders, or celiac disease, is regarded as a significant risk factor [7,23-25]. Furthermore, the use of certain medications is considered one of the most common etiologies for MC [26,27]. For instance, the utilization of nonsteroidal anti-inflammatory drugs (NSAIDs), such as aspirin; proton pump inhibitors (PPIs), such as lansoprazole; and selective serotonin reuptake inhibitors are the major precipitant medications to cause the disease. In addition, various medications with different mechanisms of action including beta-blockers, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers or statins have been reported in previous studies [10,26,27]. Duloxetine, an antidepressant that inhibits the reuptake of serotonin and norepinephrine, has been associated with MC [28].
Pathophysiology
The etiology of MC remains uncertain; however, it is probably a complex process influenced by various factors, including mucosal immunological reactions in response to luminal factors in people with a hereditary predisposition [29]. The association between the development of MC and genetic predisposition remains uncertain. Nevertheless, there have been documented instances within families [4,7,30]. It is noteworthy that individuals within the same family exhibited the development of either lymphocytic or collagenous colitis, providing evidence for a shared underlying pathophysiological mechanism. Previous studies have demonstrated a correlation between MC and HLA-DQ2 or DQ1,3. In addition, HLA-DR3DQ2 haplotype and tumor necrosis factor 2 allele carriers were observed more frequently in MC patients compared to normal populations [31,32].
Meanwhile, the dense collagen band in collagenous colitis may be caused by a defective collagen metabolism. The dominant subepithelial matrix deposition has a major role in enhancing the expression of the principal fibrogenic genes, metalloproteinase inhibitor, and procollagen I by myofibroblastic cells along with poor fibrinolysis [30,33]. Moreover, an increased expression of transforming growth factor (TGF) beta-1 has been linked with collagen storage in tissues of individuals diagnosed with collagenous colitis [34].
One potential hypothesis is that an impairment in the function of the epithelial barrier and the presence of luminal substances could result in an elevated permeability of antigens and bacteria across the mucosal layer, thus leading to immune dysregulation and the manifestation of intestinal inflammation observed in cases of MC [35]. The proposed hypothesis that supported the involvement of certain bacteria in the pathogenesis of MC was obtained from the improvement of symptoms and histology following a treatment with a bismuth course in patients diagnosed with lymphocytic colitis and after a successful treatment of Helicobacter pylori in patients diagnosed with collagenous colitis [1,24,36]. The species that were observed in the literature along with H. pylori were Campylobacter species, Clostridium difficile, and Yesinia species [24]. Nevertheless, this theory lacks reliability since the administration of budesonide, a local immunosuppressive therapy, has proven to be quite effective in treating MC [8,24,37].
Autoimmunity has been identified as a potential factor in the pathogenesis and development of MC. This correlation had been identified on several studies. One of the largest studies looked at this correlation was a case control study conducted in Denmark by Wildt et al. in 2021 [38]. It involved 155,910 controls and 15,597 patients diagnosed with MC and documented a notable association between autoimmune disease and MC, particularly celiac disease, Crohn's disease, and ulcerative colitis. The study also revealed a higher occurrence of autoimmune illness in individuals with collagenous colitis as opposed to those with lymphocytic colitis. An additional study of 103 patients diagnosed with MC revealed that 39% (n=40) of the patients had a coexisting autoimmune disease. Particularly, there was no significant variation observed in the prevalence of autoimmune diseases between patients diagnosed with collagenous colitis and those diagnosed with lymphocytic colitis. Among the population of interest, Hashimoto thyroiditis emerged as the most observed autoimmune illness, affecting 14 individuals, accounting for 35% of the cases. This was followed by rheumatoid arthritis, which was present in seven patients, constituting 17.5% of the sample. Similarly, Sjogren's syndrome was also identified in seven patients, representing another 17.5% of the total cases [39].
The understanding of the relationship between bile acid malabsorption and MC is currently limited, mostly due to the complexity of the physiology and metabolism of bile acids inside the intestinal tract [40-42]. Furthermore, the intestinal tract has several receptors that have the ability to bind bile acids. These receptors play a significant role in physiological processes and contribute to the complexity of analyzing bile acid physiology in the colon. The manifestation of diarrhea linked to bile acid malabsorption can be attributed to either an augmentation in the production of bile acids or a reduction in their absorption in the terminal ileum. Furthermore, the conversion of primary bile acids to secondary bile acids is based upon the presence of specific bacteria within the colon. Any modifications in the intestinal bacteria could potentially impact the composition of bile acids within the colon. Bile acids can potentially induce MC in certain persons. Conversely, these acids may potentially exert a secondary influence by exacerbating diarrhea symptoms in individuals diagnosed with pre-existing MC [43]. The etiology of bile acid malabsorption in individuals diagnosed with MC is believed to involve multiple pathways. Certain patients with MC have evidence of villous atrophy and inflammation in the ileum, which may result in bile acid malabsorption and heightened levels of bile acids in the colon [44,45]. The presence of the ileum in these individuals may indicate the potential dissemination of the disease from the colon to the ileum. Moreover, it is plausible that a subset of these individuals may possess undetected celiac disease, a condition that affects the ileum [25]. These individuals may experience reduced absorption of bile in the terminal ileum, leading to elevated levels of bile acids in the colon. This, in turn, could potentially impact the onset and duration of diarrhea [46].
Clinical presentation
The typical presentation of the disease is chronic watery, nonbloody diarrhea. It is often present during the night and early morning and is frequently associated with fecal urgency, incontinence, and abdominal pain [1,47]. In the majority of cases, the symptoms develop in a gradual and progressive course, but around 40% of patients might develop sudden onset symptoms [47]. The mean frequency of diarrhea episodes typically ranges from three to eight loose stools per day, and in rare severe situations, it could reach up to 15 episodes per day [1,48]. Furthermore, the diarrhea might be accompanied by abnormal weight loss or other extraintestinal symptoms, such as uveitis, arthralgia, or arthritis. Collagenous colitis appears to be a more severe form of bowel inflammation than lymphocytic colitis, which tends to manifest earlier in life [49]. There exists a third entity characterized by patients exhibiting typical clinical symptoms but lacking the precise histological markers associated with either the collagenous or lymphocytic subtype. This entity is called incomplete MC or MC not otherwise specified (NOS) [50,51]. It is crucial for primary healthcare providers to offer an early referral to gastroenterologist if they have any patient with unexplained chronic diarrhea for proper evaluation and diagnosis, which will have a great positive impact on the patient's quality of life and disease prognosis.
Diagnosis
The presence of chronic diarrhea and related associated symptoms should raise suspicions for the potential diagnosis of MC, especially in middle-aged and older persons. The establishment of a diagnosis is challenging and requires ruling out another differential diagnosis of chronic diarrhea. The laboratory workup should include a routine stool culture or polymerase chain reaction (PCR) testing for different possible causative bacterial organisms, such as Campylobacter, Shigella, Salmonella, Yersinia, Clostridioides difficile, and Escherichia coli O157:H7 [39]. In addition, parasitic infections need to be ruled out by performing stool for ova and parasites along with the Giardia stool antigen [39]. Celiac serologies are also recommended to exclude the possibility of celiac disease [4,25].
The primary diagnosis method for this condition involves obtaining histologic samples from various sites (specifically, two to four biopsies of colon segments) during a colonoscopy procedure. This approach is essential since mucosal inflammation may not always be apparent during colonoscopy. However, it is important to highlight that there are certain nonspecific macroscopic signs of colitis, which might be indicative of the presence of underlying MC [5]. These signs include a slightly swollen mucosa, friability, exudative lesions, and hyperemic bowel wall [5,52].
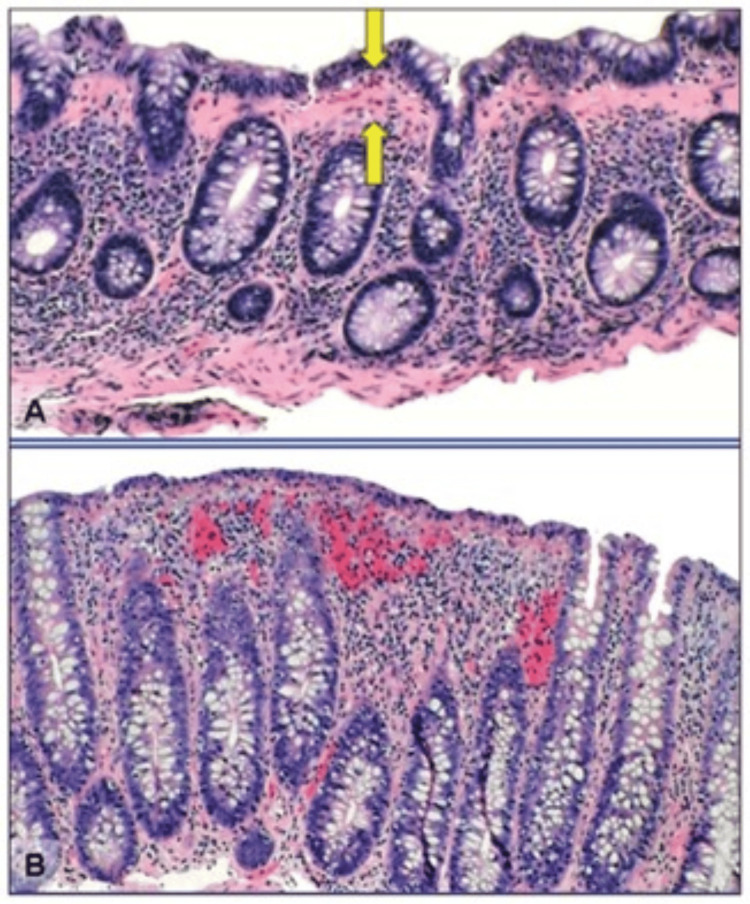
Colonoscopy with subsequent biopsies from the right- and left-sided colon is generally considered to be a safe procedure for patients who have been diagnosed with MC. The incidence of perforations has been documented in individuals with substantial collagen accumulations, sometimes referred to as fractured colon. However, such occurrences are infrequent [53]. The justification for conducting a colonoscopy instead of a narrower assessment of the left colon using flexible sigmoidoscopy lies in the fact that MC has the potential to exhibit patchy characteristics. Furthermore, there is a gradual decrease in the degree of histologic alterations as one moves from the proximal to the distal colon [6,19]. The inflammatory cellular activity in collagenous and lymphocytic colitis exhibits similarities, characterized predominantly by mononuclear infiltrates in the lamina propria with eosinophils. Nevertheless, specific histologic characteristics are taken into consideration to differentiate between collagenous and lymphocytic colitis [3,17,18,48]. The colonic mucosa in the collagenous type showed evidence of a subepithelial collagen band of at least 10 micrometers in diameter between crypts [54]. Meanwhile, lymphocytic colitis is characterized by at least 20 intraepithelial lymphocytes per 100 surface epithelial cells with no evidence of crypt architecture distortion [55-57]. The histological feature of MC is shown in Figure 1 [58].
Figure 1. Histological features of microscopic colitis.
(A) Collagenous colitis is associated with an increased lymphocytic infiltrate of the lamina propria and intraepithelial lymphocytosis. There is a markedly thickened (>10 μm) collagen band underlying the colonic epithelial cells (arrows). (B) Lymphocytic colitis is associated with an increased lymphocytic infiltrate of the lamina propria and intraepithelial lymphocytosis. The collagen band underlying the colonic epithelial cells is normal in width (<10 μm) [58].
Management
The main objective of managing individuals diagnosed with MC is to accomplish clinical remission (characterized by a reduction in daily stool frequency to one to two bowel motions and the absence of watery stool over the course of seven days) to enhance the overall quality of life experienced by the patients. The currently available management options include a range of interventions, starting with avoidance of triggering factors followed by a variety of pharmaceutical treatments targeting conservative treatment of diarrheal episodes. In cases where initial conservative measures fail to control symptoms, more potent lines of management, such as oral corticosteroids, immunomodulators, or biologic agents, can be introduced. Finally, in refractory and severe cases, surgical interventions might have a role as the last management option.
Lifestyle Modifications
The initial management strategy involves the identification of modifiable factors, such as smoking and drug-induced cases. Patients must be counseled regarding the significant role of smoking in disease pathogenesis and progression [21]. It is crucial for healthcare providers to actively promote tobacco cessation among patients and advocate for their participation in national smoking cessation programs.
In addition to smoking cessation, it is necessary to review the patient’s medication list as an essential part of the initial management given its potential for reversibility and avoid possible offending medications, such as nonsteroidal anti-inflammatory drugs, proton pump inhibitors, antidepressant medications, beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or statins. These medications need to be screened, and it is recommended to discontinue or provide a safer alternative if possible [59].
Antidiarrheal Medications
The primary objective of antidiarrheal medications is to reduce the frequency of nighttime attacks. Loperamide is considered the preferred therapeutic agent [37,39,60]. Loperamide is a synthetic phenylpiperidine opioid with a high degree of lipophilicity. Loperamide functions as an agonist of the mu-opioid receptor. At recommended dosages, loperamide exerts its effects by interacting directly with the mu-opioid receptors located in the muscles of the colon. This interaction leads to a reduction in the duration of time for substances to pass through the colon, an inhibition of peristalsis, a reduction in electrolyte loss, and an optimization of rectal activities [39,60,61].
Bismuth
Bismuth subsalicylate and bismuth subcitrate enemas showed some benefits in treating MC in a few limited studies. In a prospective study including 12 patients with MC who were treated with bismuth subsalicylate 262 mg daily for eight weeks, a significant clinical remission was observed in 11 patients, with a histological response in nine patients [62]. The average time to response was two weeks, and almost three quarters of the patient population remained in remission at 28 months' follow-up [62].
Budesonide
For patients who exhibit active diseases, characterized by a minimum of three stools daily or at least one watery stool daily, or those who experience persistent diarrhea despite the administration of antidiarrheals, it is advisable to incorporate oral budesonide at a dosage of 9 mg per day for a duration of six to eight weeks [4,60,63]. Budesonide is a potent and effective glucocorticoid that has a broad spectrum of anti-inflammatory properties. The administration of budesonide orally typically results in primarily local effects, with minimal systemic exposure as a result of its pharmacokinetic action and extensive first-pass effect [39,47,60,61]. According to the current guidelines, it is recommended that budesonide be utilized initially for treatment induction and to maintain remission in MC [4,8,39,47,60,61]. The treatment's success rate for budesonide was 88% in collagenous colitis, whereas only one-third of patients treated with a placebo had a significant clinical response based on Kafil et al.'s study [64]. Meanwhile, the remission rate for the budesonide-treated group in lymphocytic colitis was 84% compared with less than half of the total patients who were treated with a placebo [65]. There was also a strong evidence of histological response based on Chande et al.'s study [65].
Cholestyramine
Cholestyramine is a pharmaceutical agent classified as a bile-acid binder, which has demonstrated efficacy in the management of diarrhea in individuals presenting with bile acid malabsorption [66]. It can be used in patients with mild, persistent diarrhea despite budesonide, as an adjunctive medication with loperamide, and the recommended dosage of cholestyramine is 4 grams every six hours.
Aminosalicylates
Aminosalicylates, such as mesalamine, appear ineffective in treating collagenous colitis and lymphocytic colitis [1,67]. In an eight-week randomized clinical trial, a total of 92 patients diagnosed with active collagenous colitis were administered either oral budesonide (9 mg daily), mesalamine (3 grams daily), or a placebo. The study findings revealed that the remission rates observed with mesalamine were similar to those shown with the placebo [68]. Maintenance therapy has not been investigated. In the same study, 57 patients with active lymphocytic colitis were randomized to receive therapy with budesonide, mesalamine (at a dosage of 3 grams per day), or a placebo for a duration of eight weeks. The results indicated that there was no statistically significant difference in the rates of clinical and histologic remission between the mesalamine group and the placebo group [68].
Probiotics
Taking into consideration the potential role of gut microflora in the pathogenesis of MC, one study evaluated the effectiveness of probiotics as a possible treatment option. Wildt et al. studied the association of Lactobacillus acidophilus LA-5 and Bifidobacterium species in treating MC and found no superiority in comparison to a placebo [69]. Further studies are needed to assess the effectiveness of probiotics in patients with MC.
Immunomodulators and Biological Agents
Immunomodulators and biological agents are administered to selected individuals who are glucocorticoid-resistant or intolerant. Limited evidence from small case series and retrospective studies suggests that immunomodulators and biological agents can induce remission in refractory cases of MC. Azathioprine and 6-mercaptopurine are immunomodulators of choice, and they can achieve clinical remission up to 28% and 46%, respectively, based on Munch et al.'s study [70]. Biological therapies, such as anti-tumor necrosis factor drugs (infliximab and adalimumab) and integrin receptor antagonists (vedolizumab), are the agents to be considered in such clinical scenarios [71-74].
Surgical interventions
When medical treatments fail to induce remission, surgery may be considered for refractory and severe cases. The preferred procedure is diverting ileostomy, but a total colectomy may also be considered [60]. The evidence for surgical intervention in refractory came from few case reports and case series. For example, a middle-aged man who was diagnosed with collagenous colitis for five years and failed multiple lines of medical therapy eventually underwent total protocolectomy followed by ileal pouch anal anastomosis, and on the two-year follow-up after the surgery, he had a reasonable control of disease symptoms [75]. Once again, it is observed that most patients tend to exhibit positive responses to the medical management of MC. Consequently, surgical interventions should only be offered for individuals who have failed all alternative therapies, eliminated all other potential precipitating factors, and are experiencing severe and incapacitating diarrhea.
Disease monitoring and long-term assessment
Disease monitoring and long-term assessment are considered essential components to ensure good compliance to the management plan and to assess the need for an alternative plan in the case of disease relapse. Regular follow-up is usually given to the patient upon starting the treatment until clinical remission is achieved. After that, the aim of the primary physician will be to maintain the remission over time with an annual assessment and easy clinic booking in the case of flaring-up of symptoms. Moreover, a close monitoring of any possible treatment side effect is crucial. For example, the patient must be provided with calcium and vitamin D supplements in the case of long-term corticosteroid therapy.
Disease prognosis
MC might improve within week or few months with interventions. In some cases, spontaneous recovery has been noticed. Clinical relapse after a successful treatment were observed to range from 30% to 60% based on few studies; therefore, the long-term follow-up is very crucial. In the literature review, there were a few studies that looked at the natural history and disease prognosis after proper management. Fernández et al. examined 37 patients with collagenous colitis and 44 patients with lymphocytic colitis in a three-year prospective study, which involved the administration of a different line of management, and found that two-third of the targeted group maintained long-term remission while the remaining third had a relapse on the long-term follow-up [76]. There is currently no evidence suggesting a greater risk of developing colorectal cancer in individuals with MC. Furthermore, the disease transition from collagenous colitis to lymphocytic colitis is infrequent.
Future prospectives for research and awareness on MC
Although the prevalence of MC as a common cause of chronic diarrhea, particularly among the older population, is growing, there is a need to enhance awareness among healthcare professionals in countries where the reported incidence is relatively low. The understanding of the function of the gut microbiota or specific gene profiles has the potential to create opportunities for the development of novel evidence-based therapeutic approaches aimed at minimizing disease progression and flares and potentially achieving a curative outcome. The current therapeutic interventions for MC exhibit some constraints, hence necessitating the exploration of novel therapy modalities. To date, the primary objective in the care of MC has been to address symptoms and enhance the quality of life for patients in the immediate term. There is potential for additional medications, beyond those commonly used in the treatment of traditional types of IBD, to have a positive impact on reducing relapse risk and sustaining remission over an extended period of time. Furthermore, there remains uncertainties regarding the appropriate categorization of collagenous colitis and lymphocytic colitis as distinct entities. An area that requires attention is the identification and verification of noninvasive biomarkers, such as fecal calprotectin or mucosal and fecal neutrophil gelatinase-associated lipocalin, with the aim of serving as potential indicators for MC. These biomarkers have the potential to evaluate and anticipate the clinical activity of the disease.
Conclusions
MC is a gastrointestinal condition distinguished by gastroenterologists in their practice and characterized mainly by chronic persistent watery diarrhea. The prevalence and subsequent recognition of this disease have been on the rise. Maintaining a heightened level of suspicion is of utmost importance when evaluating individuals from specific demographics who exhibit symptoms of persistent diarrhea. This is particularly relevant for middle-aged women and the elderly. In such cases, it is imperative to make appropriate referrals for lower gastrointestinal endoscopy with biopsy when deemed necessary. There are two histological distinctive types, collagenous colitis and lymphocytic colitis. Although lifestyle modifications, such as smoking cessation, and a comprehensive medication history review to eliminate potential drug-induced etiology, have proven advantageous, budesonide has been proven by various international societies as the primary treatment for MC. Although budesonide has demonstrated high rates of success, there exists a potential for recurrence. The administration of low-dose maintenance budesonide is an effective approach in treating cases of relapsing MC cases; however, additional data are required to further support these findings. The literature discusses several alternative pharmaceutical options; although specific options have minimal supporting evidence, others lack any empirical data.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Khalid I. AlHussaini
Drafting of the manuscript: Khalid I. AlHussaini
Critical review of the manuscript for important intellectual content: Khalid I. AlHussaini
References
- 1.Collagenous colitis: a retrospective study of clinical presentation and treatment in 163 patients. Bohr J, Tysk C, Eriksson S, Abrahamsson H, Järnerot G. Gut. 1996;39:846–851. doi: 10.1136/gut.39.6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chronic diarrhea of unknown origin. Read NW, Krejs GJ, Read MG, Santa Ana CA, Morawski SG, Fordtran JS. https://pubmed.ncbi.nlm.nih.gov/7350049/#:~:text=Chronic%20diarrhea%20of%20unknown%20origin%20Gastroenterology.%201980%20Feb%3B78,other%20institutions%20had%20failed%20to%20reveal%20a%20diagnosis. Gastroenterology. 1980;78:264–271. [PubMed] [Google Scholar]
- 3.Lymphocytic ("microscopic") colitis: a comparative histopathologic study with particular reference to collagenous colitis. Lazenby AJ, Yardley JH, Giardiello FM, Jessurun J, Bayless TM. Hum Pathol. 1989;20:18–28. doi: 10.1016/0046-8177(89)90198-6. [DOI] [PubMed] [Google Scholar]
- 4.European guidelines on microscopic colitis: United European Gastroenterology and European Microscopic Colitis Group statements and recommendations. Miehlke S, Guagnozzi D, Zabana Y, et al. United European Gastroenterol J. 2021;9:13–37. doi: 10.1177/2050640620951905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Microscopic colitis: considerations for gastroenterologists, endoscopists, and pathologists. Kotze LM, Kotze PG, Kotze LR, Nisihara R. Arq Gastroenterol. 2023;60:188–193. doi: 10.1590/S0004-2803.20230222-143. [DOI] [PubMed] [Google Scholar]
- 6.Microscopic colitis in Northern Ireland: an updated clinicopathological audit and assessment of compliance with European guidelines. Moore M, Coleman HG, Allen PB, Loughrey MB. Colorectal Dis. 2022;24:1584–1590. doi: 10.1111/codi.16254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Microscopic colitis-defining incidence rates and risk factors: a population-based study. Williams JJ, Kaplan GG, Makhija S, Urbanski SJ, Dupre M, Panaccione R, Beck PL. Clin Gastroenterol Hepatol. 2008;6:35–40. doi: 10.1016/j.cgh.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 8.Treatment of microscopic colitis: the role of budesonide and new alternatives for refractory patients. Rojo E, Casanova MJ, Gisbert JP. Rev Esp Enferm Dig. 2020;112:53–58. doi: 10.17235/reed.2019.6655/2019. [DOI] [PubMed] [Google Scholar]
- 9.The epidemiology of microscopic colitis: a population based study in Olmsted County, Minnesota. Pardi DS, Loftus EV Jr, Smyrk TC, et al. Gut. 2007;56:504–508. doi: 10.1136/gut.2006.105890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Incidence, prevalence, and temporal trends of microscopic colitis: a systematic review and meta-analysis. Tong J, Zheng Q, Zhang C, Lo R, Shen J, Ran Z. Am J Gastroenterol. 2015;110:265–276. doi: 10.1038/ajg.2014.431. [DOI] [PubMed] [Google Scholar]
- 11.The epidemiology of microscopic colitis in Olmsted County from 2002 to 2010: a population-based study. Gentile NM, Khanna S, Loftus EV Jr, et al. Clin Gastroenterol Hepatol. 2014;12:838–842. doi: 10.1016/j.cgh.2013.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The epidemiology of microscopic colitis: a 10-year pathology-based nationwide Danish cohort study. Bonderup OK, Wigh T, Nielsen GL, Pedersen L, Fenger-Grøn M. Scand J Gastroenterol. 2015;50:393–398. doi: 10.3109/00365521.2014.940378. [DOI] [PubMed] [Google Scholar]
- 13.Incidence of collagenous and lymphocytic colitis: a 5-year population-based study. Fernandez-Banares F, Salas A, Forne M, Esteve M, Espinos J, Viver JM. Am J Gastroenterol. 1999;94:418–423. doi: 10.1111/j.1572-0241.1999.00870.x. [DOI] [PubMed] [Google Scholar]
- 14.The epidemiology of microscopic colitis in Olmsted County, Minnesota: population-based study from 2011 to 2019. Tome J, Sehgal K, Kamboj AK, et al. Clin Gastroenterol Hepatol. 2022;20:1085–1094. doi: 10.1016/j.cgh.2021.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collagenous colitis in children. Yardley JH, Lazenby AJ, Kornacki S. Gastroenterology. 1993;105:647–648. doi: 10.1016/0016-5085(93)90762-2. [DOI] [PubMed] [Google Scholar]
- 16.Collagenous colitis in children. Gremse DA, Boudreaux CW, Manci EA. Gastroenterology. 1993;104:906–909. doi: 10.1016/0016-5085(93)91030-l. [DOI] [PubMed] [Google Scholar]
- 17.Lymphocytic and collagenous colitis in children and adolescents: Comprehensive clinicopathologic analysis with long-term follow-up. Windon AL, Almazan E, Oliva-Hemker M, et al. Hum Pathol. 2020;106:13–22. doi: 10.1016/j.humpath.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Are collagenous colitis and lymphocytic colitis distinct syndromes? Jackson BK. Dig Dis. 1995;13:301–311. doi: 10.1159/000171510. [DOI] [PubMed] [Google Scholar]
- 19.Microscopic colitis in Denmark and Sweden: incidence, putative risk factors, histological assessment and endoscopic activity. Davidson S, Sjöberg K, Engel PJ, Lo Rinc E, Fiehn AK, Vigren L, Munck LK. Scand J Gastroenterol. 2018;53:818–824. doi: 10.1080/00365521.2018.1476583. [DOI] [PubMed] [Google Scholar]
- 20.Smoking status influences clinical outcome in collagenous colitis. Münch A, Tysk C, Bohr J, et al. J Crohns Colitis. 2016;10:449–454. doi: 10.1093/ecco-jcc/jjv235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Current and past cigarette smoking significantly increase risk for microscopic colitis. Yen EF, Pokhrel B, Du H, Nwe S, Bianchi L, Witt B, Hall C. Inflamm Bowel Dis. 2012;18:1835–1841. doi: 10.1002/ibd.22838. [DOI] [PubMed] [Google Scholar]
- 22.Is smoking a risk factor for collagenous colitis? Vigren L, Sjöberg K, Benoni C, et al. Scand J Gastroenterol. 2011;46:1334–1339. doi: 10.3109/00365521.2011.610005. [DOI] [PubMed] [Google Scholar]
- 23.Microscopic colitis: a large retrospective analysis from a health maintenance organization experience. Kao KT, Pedraza BA, McClune AC, et al. World J Gastroenterol. 2009;15:3122–3127. doi: 10.3748/wjg.15.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Microscopic colitis: epidemiology, pathophysiology, diagnosis and current management-an update 2013. Storr MA. ISRN Gastroenterol. 2013;2013:352718. doi: 10.1155/2013/352718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Celiac disease and risk of microscopic colitis: a nationwide population-based matched cohort study. Bergman D, Khalili H, Lebwohl B, Roelstraete B, Green PH, Ludvigsson JF. United European Gastroenterol J. 2023;11:189–201. doi: 10.1002/ueg2.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drug exposure and risk of microscopic colitis: a nationwide Danish case-control study with 5751 cases. Bonderup OK, Fenger-Grøn M, Wigh T, Pedersen L, Nielsen GL. Inflamm Bowel Dis. 2014;20:1702–1707. doi: 10.1097/MIB.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 27.Increased risk of microscopic colitis with use of proton pump inhibitors and non-steroidal anti-inflammatory drugs. Masclee GM, Coloma PM, Kuipers EJ, Sturkenboom MC. Am J Gastroenterol. 2015;110:749–759. doi: 10.1038/ajg.2015.119. [DOI] [PubMed] [Google Scholar]
- 28.Microscopic lymphocytic colitis due to duloxetine: case report and review of the literature. Millán-Nohales C, Ordieres-Ortega L, García-Martínez R. Gastroenterol Hepatol. 2021;44:222–223. doi: 10.1016/j.gastrohep.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Chronic colitis with thickening of the subepithelial collagen layer (collagenous colitis): histopathologic findings in 15 patients. Jessurun J, Yardley JH, Giardiello FM, Hamilton SR, Bayless TM. Hum Pathol. 1987;18:839–848. doi: 10.1016/s0046-8177(87)80059-x. [DOI] [PubMed] [Google Scholar]
- 30.Microscopic colitis: what do we know about pathogenesis? Pisani LF, Tontini GE, Vecchi M, Pastorelli L. Inflamm Bowel Dis. 2016;22:450–458. doi: 10.1097/MIB.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 31.Predisposing HLA-DQ2 and HLA-DQ8 haplotypes of coeliac disease and associated enteropathy in microscopic colitis. Fernández-Bañares F, Esteve M, Farré C, et al. Eur J Gastroenterol Hepatol. 2005;17:1333–1338. doi: 10.1097/00042737-200512000-00011. [DOI] [PubMed] [Google Scholar]
- 32.High prevalence of celiac sprue-like HLA-DQ genes and enteropathy in patients with the microscopic colitis syndrome. Fine KD, Do K, Schulte K, Ogunji F, Guerra R, Osowski L, McCormack J. Am J Gastroenterol. 2000;95:1974–1982. doi: 10.1111/j.1572-0241.2000.02255.x. [DOI] [PubMed] [Google Scholar]
- 33.Synthesis of collagen I in collagenous sprue. Daum S, Foss HD, Schuppan D, Riecken EO, Zeitz M, Ullrich R. Clin Gastroenterol Hepatol. 2006;4:1232–1236. doi: 10.1016/j.cgh.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Increased presence of eosinophilic granulocytes expressing transforming growth factor-beta1 in collagenous colitis. Ståhle-Bäckdahl M, Maim J, Veress B, Benoni C, Bruce K, Egesten A. Scand J Gastroenterol. 2000;35:742–746. doi: 10.1080/003655200750023426. [DOI] [PubMed] [Google Scholar]
- 35.Collagenous colitis: are bacterial cytotoxins responsible? Andersen T, Andersen JR, Tvede M, Franzmann MB. https://pubmed.ncbi.nlm.nih.gov/8438843/ Am J Gastroenterol. 1993;88:375–377. [PubMed] [Google Scholar]
- 36.Resolution of collagenous colitis after treatment for Helicobacter pylori. Narayani RI, Burton MP, Young GS. Am J Gastroenterol. 2002;97:498–499. doi: 10.1111/j.1572-0241.2002.05513.x. [DOI] [PubMed] [Google Scholar]
- 37.Microscopic colitis: pathophysiology and clinical management. Miehlke S, Verhaegh B, Tontini GE, Madisch A, Langner C, Munch A. Lancet Gastroenterol Hepatol. 2019;4:305–314. doi: 10.1016/S2468-1253(19)30048-2. [DOI] [PubMed] [Google Scholar]
- 38.Autoimmunity as an Etiological Factor of Cancer: The Transformative Potential of Chronic Type 2 Inflammation. Li CM, Chen Z. Front Cell Dev Biol. 2021;9:664305. doi: 10.3389/fcell.2021.664305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Microscopic colitis in older adults: impact, diagnosis, and management. Fedor I, Zold E, Barta Z. Ther Adv Chronic Dis. 2022;13:20406223221102821. doi: 10.1177/20406223221102821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bile acid receptors and gastrointestinal functions. Ticho AL, Malhotra P, Dudeja PK, Gill RK, Alrefai WA. https://doi.org/10.1016/j.livres.2019.01.001. Liver Res. 2019;3:31–39. doi: 10.1016/j.livres.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bile acid-related regulation of mucosal inflammation and intestinal motility: from pathogenesis to therapeutic application in IBD and microscopic colitis. Di Vincenzo F, Puca P, Lopetuso LR, et al. Nutrients. 2022;14 doi: 10.3390/nu14132664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bile acid sequestrants in microscopic colitis: clinical outcomes and utility of bile acid testing. Tome J, Sehgal K, Kamboj AK, Harmsen WS, Khanna S, Pardi DS. Clin Gastroenterol Hepatol. 2023 doi: 10.1016/j.cgh.2023.04.031. [DOI] [PubMed] [Google Scholar]
- 43.Systematic review: bile acids and intestinal inflammation-luminal aggressors or regulators of mucosal defence? Pavlidis P, Powell N, Vincent RP, Ehrlich D, Bjarnason I, Hayee B. https://doi.org/10.1111/apt.13333. Aliment Pharmacol Ther. 2015;42:802–817. doi: 10.1111/apt.13333. [DOI] [PubMed] [Google Scholar]
- 44.Primary ileal villous atrophy associated with microscopic colitis. Germain E, Roblin X, Colle PE, Barnoud R, Faucheron JL, Bonaz B. https://doi.org/10.1111/j.1440-1746.2005.03990.x. J Gastroenterol Hepatol. 2005;20:1800–1801. doi: 10.1111/j.1440-1746.2005.03990.x. [DOI] [PubMed] [Google Scholar]
- 45.The terminal ileum is affected in patients with lymphocytic or collagenous colitis. Sapp H, Ithamukkala S, Brien TP, et al. Am J Surg Pathol. 2002;26:1484–1492. doi: 10.1097/00000478-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Primary ileal villous atrophy is often associated with microscopic colitis. Marteau P, Lavergne-Slove A, Lemann M, et al. Gut. 1997;41:561–564. doi: 10.1136/gut.41.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Symptomatic overlap between microscopic colitis and irritable bowel syndrome: a prospective study. Abboud R, Pardi DS, Tremaine WJ, Kammer PP, Sandborn WJ, Loftus EV Jr. Inflamm Bowel Dis. 2013;19:550–553. doi: 10.1097/MIB.0b013e31827febfd. [DOI] [PubMed] [Google Scholar]
- 48.Microscopic colitis: clinical and pathologic perspectives. Münch A, Langner C. Clin Gastroenterol Hepatol. 2015;13:228–236. doi: 10.1016/j.cgh.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 49.Microscopic colitis: a descriptive clinical cohort study of 795 patients with collagenous and lymphocytic colitis. Mellander MR, Ekbom A, Hultcrantz R, Löfberg R, Öst Å, Björk J. Scand J Gastroenterol. 2016;51:556–562. doi: 10.3109/00365521.2015.1124283. [DOI] [PubMed] [Google Scholar]
- 50.Atypical forms of microscopic colitis: morphological features and review of the literature. Chang F, Deere H, Vu C. Adv Anat Pathol. 2005;12:203–211. doi: 10.1097/01.pap.0000175115.63165.6b. [DOI] [PubMed] [Google Scholar]
- 51.Lymphocytic and collagenous colitis: epidemiologic differences and similarities. Sonnenberg A, Genta RM. Dig Dis Sci. 2013;58:2970–2975. doi: 10.1007/s10620-013-2718-6. [DOI] [PubMed] [Google Scholar]
- 52.Does lymphocytic colitis always present with normal endoscopic findings? Park HS, Han DS, Ro YO, Eun CS, Yoo KS. Gut Liver. 2015;9:197–201. doi: 10.5009/gnl13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colonic perforation in collagenous colitis: a systematic review of a rare complication and guidance on management. Hussain Z, Kelly S, Clarke A, Adams S, Miller G. Surg Endosc. 2010;24:2930–2934. doi: 10.1007/s00464-010-1086-y. [DOI] [PubMed] [Google Scholar]
- 54.Subepithelial collagen table thickness in colon specimens from patients with microscopic colitis and collagenous colitis. Lee E, Schiller LR, Vendrell D, Santa Ana CA, Fordtran JS. Gastroenterology. 1992;103:1790–1796. doi: 10.1016/0016-5085(92)91436-8. [DOI] [PubMed] [Google Scholar]
- 55.Microscopic colitis syndrome. Veress B, Löfberg R, Bergman L. Gut. 1995;36:880–886. doi: 10.1136/gut.36.6.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Immunohistochemical characterization of lymphocytes in microscopic colitis. Göranzon C, Kumawat AK, Hultgren-Hörnqvist E, Tysk C, Eriksson S, Bohr J, Nyhlin N. J Crohns Colitis. 2013;7:0–42. doi: 10.1016/j.crohns.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 57.Intraepithelial and lamina propria lymphocytes do not correlate with symptoms or exposures in microscopic colitis. Sandler RS, Hansen JJ, Peery AF, Woosley JT, Galanko JA, Keku TO. Clin Transl Gastroenterol. 2022;13:0. doi: 10.14309/ctg.0000000000000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Microscopic colitis: a review for the surgical endoscopist. Datta I, Brar SS, Andrews CN, Dupre M, Ball CG, Buie WD, Beck PL. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2769103/ Can J Surg. 2009;52:0–72. [PMC free article] [PubMed] [Google Scholar]
- 59.Microscopic colitis: etiopathology, diagnosis, and rational management. Nielsen OH, Fernandez-Banares F, Sato T, Pardi DS. Elife. 2022;11 doi: 10.7554/eLife.79397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Management of microscopic colitis: challenges and solutions. Shor J, Churrango G, Hosseini N, Marshall C. Clin Exp Gastroenterol. 2019;12:111–120. doi: 10.2147/CEG.S165047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Microscopic colitis: an update. Fărcaş RA, Grad S, Dumitraşcu DL. Med Pharm Rep. 2022;95:370–376. doi: 10.15386/mpr-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Efficacy of open-label bismuth subsalicylate for the treatment of microscopic colitis. Fine KD, Lee EL. Gastroenterology. 1998;114:29–36. doi: 10.1016/s0016-5085(98)70629-8. [DOI] [PubMed] [Google Scholar]
- 63.Budesonide treatment for microscopic colitis: systematic review and meta-analysis. Sebastian S, Wilhelm A, Jessica L, Myers S, Veysey M. Eur J Gastroenterol Hepatol. 2019;31:919–927. doi: 10.1097/MEG.0000000000001456. [DOI] [PubMed] [Google Scholar]
- 64.Interventions for treating collagenous colitis. Kafil TS, Nguyen TM, Patton PH, MacDonald JK, Chande N, McDonald JW. Cochrane Database Syst Rev. 2017;11:0. doi: 10.1002/14651858.CD003575.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Interventions for treating lymphocytic colitis. Chande N, Al Yatama N, Bhanji T, Nguyen TM, McDonald JW, MacDonald JK. Cochrane Database Syst Rev. 2017;7:0. doi: 10.1002/14651858.CD006096.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Role of bile acids and bile acid binding agents in patients with collagenous colitis. Ung KA, Gillberg R, Kilander A, Abrahamsson H. Gut. 2000;46:170–175. doi: 10.1136/gut.46.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.P752 Oral once daily budesonide granules rapidly induce clinical remission in Lymphocytic Colitis: A double-blind, double-dummy, multi-centre, randomised trial. Miehlke S, Aust D, Mihaly E, et al. J Crohns Colitis. 2018;12:491. [Google Scholar]
- 68.Budesonide is more effective than mesalamine or placebo in short-term treatment of collagenous colitis. Miehlke S, Madisch A, Kupcinskas L, et al. Gastroenterology. 2014;146:1222–1230. doi: 10.1053/j.gastro.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 69.Probiotic treatment of collagenous colitis: a randomized, double-blind, placebo-controlled trial with Lactobacillus acidophilus and Bifidobacterium animalis subsp. Lactis. Wildt S, Munck LK, Vinter-Jensen L, et al. Inflamm Bowel Dis. 2006;12:395–401. doi: 10.1097/01.MIB.0000218763.99334.49. [DOI] [PubMed] [Google Scholar]
- 70.Azathioprine and mercaptopurine in the management of patients with chronic, active microscopic colitis. Münch A, Fernandez-Banares F, Munck LK. Aliment Pharmacol Ther. 2013;37:795–798. doi: 10.1111/apt.12261. [DOI] [PubMed] [Google Scholar]
- 71.Efficacy of anti-TNF therapies in refractory severe microscopic colitis. Esteve M, Mahadevan U, Sainz E, Rodriguez E, Salas A, Fernández-Bañares F. J Crohns Colitis. 2011;5:612–618. doi: 10.1016/j.crohns.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 72.Vedolizumab-induced remission in 3 patients with refractory microscopic colitis: a tertiary care center case series. Jennings JJ, Charabaty A. Inflamm Bowel Dis. 2019;25:0. doi: 10.1093/ibd/izz042. [DOI] [PubMed] [Google Scholar]
- 73.Successful use of infliximab in the treatment of corticosteroid dependent collagenous colitis. Pola S, Fahmy M, Evans E, Tipps A, Sandborn WJ. Am J Gastroenterol. 2013;108:857–858. doi: 10.1038/ajg.2013.43. [DOI] [PubMed] [Google Scholar]
- 74.Vedolizumab for the induction of remission in treatment-refractory microscopic colitis in a pediatric patient. Wenzel AA, Strople J, Melin-Aldana H, Brown JB. J Pediatr Gastroenterol Nutr. 2020;71:0–8. doi: 10.1097/MPG.0000000000002739. [DOI] [PubMed] [Google Scholar]
- 75.Total proctocolectomy and ileal pouch anal anastomosis to successfully treat a patient with collagenous colitis. Williams RA, Gelfand DV. Am J Gastroenterol. 2000;95:2147. doi: 10.1111/j.1572-0241.2000.02225.x. [DOI] [PubMed] [Google Scholar]
- 76.Collagenous and lymphocytic colitis. Evaluation of clinical and histological features, response to treatment, and long-term follow-up. Fernández-Bañares F, Salas A, Esteve M, Espinós J, Forné M, Viver JM. Am J Gastroenterol. 2003;98:340–347. doi: 10.1111/j.1572-0241.2003.07225.x. [DOI] [PubMed] [Google Scholar]