ABSTRACT
To search for subunit vaccine candidates, immunogenic chlamydial antigens identified in humans were evaluated for protection against both infection and pathology in a mouse genital tract infection model under three different immunization regimens. The intramuscular immunization regimen was first used to evaluate 106 chlamydial antigens, which revealed that two antigens significantly reduced while 11 increased genital chlamydial burden. The two infection-reducing antigens failed to prevent pathology and 23 additional antigens even exacerbated pathology. Thus, intranasal mucosal immunization was tested next since intranasal inoculation with live Chlamydia muridarum prevented both genital infection and pathology. Two of the 29 chlamydial antigens evaluated were found to prevent genital infection but not pathology and three exacerbate pathology. To further improve protection efficacy, a combinational regimen (intranasal priming + intramuscular boosting + a third intraperitoneal/subcutaneous boost) was tested. This regimen identified four infection-reducing antigens, but only one of them prevented pathology. Unfortunately, this protective antigen was not advanced further due to its amino acid sequence homology with several human molecules. Two pathology-exacerbating antigens were also found. Nevertheless, intranasal mucosal priming with viable C. muridarum in control groups consistently prevented both genital infection and pathology regardless of the subsequent boosters. Thus, screening 140 different chlamydial antigens with 21 repeated multiple times in 17 experiments failed to identify a subunit vaccine candidate but demonstrated the superiority of viable chlamydial organisms in inducing immunity against both genital infection and pathology, laying the foundation for developing a live-attenuated Chlamydia vaccine.
KEYWORDS: immunogenic C. trachomatis antigens, C. muridarum mouse model, protection efficacy, mucosal priming with live C. muridarum , protective antigens
INTRODUCTION
Chlamydia trachomatis is a leading cause of sexually transmitted infection in the human genital tract, which, if not treated in time, may lead to pathological sequelae in the upper genital tract such as hydrosalpinx, resulting in loss of reproductive function (1 – 4). Although the precise mechanism remains unclear, invading the genital tract epithelial cells is considered a key determinant in chlamydial pathogenicity (5 – 7). Chlamydia mainly targets epithelial cells for infection, starting with the entry by an infectious elementary body (EB). The internalized EB differentiates into a non-infectious but metabolically active reticulate body (RB) that replicates, during which, new molecules are synthesized for secretion outside of the chlamydial organisms (8 – 13) and metabolites (14) are generated that may also be released into host cells. After completing the replication, the progeny RBs differentiate back into EBs for spreading to neighboring host cells. It is worth noting that chlamydial secretion molecules and/or metabolites may not become part of EBs but can interact with host cells, impacting both chlamydial pathogenicity (15 – 18) and host responses (19, 20). It will be interesting to determine whether the chlamydial infection-dependent induction of host responses is necessary for preventing subsequent chlamydial infection and pathogenicity.
Although effective antibiotics are available for treating chlamydial infection, the challenge is that C. trachomatis infection is often asymptomatic or lacks specific symptoms, thus significantly hindering the treatment efficacy. Obviously, vaccination offers a long-term solution to this challenge. However, despite the extensive efforts in more than half-century (21 – 39), there is still no licensed Chlamydia vaccine for humans. The Chlamydia muridarum intravaginal infection mouse model has been extensively used to study C. trachomatis pathogenesis and immunology (19, 40 – 46). Although C. muridarum causes no known diseases in humans, it can infect the female mouse genital tract productively (41) and following a single intravaginal inoculation, C. muridarum induces many different strains of mice to develop upper genital tract pathologies such as hydrosalpinx that closely resembles that in the human genital tracts induced by C. trachomatis (42, 47). Thus, this mouse genital tract infection model has been extensively used to search for chlamydial vaccine candidates. Since our whole genome-scale soluble protein-based proteome array analyses of sera from C. trachomatis-infected women had systemically identified chlamydial immunogenic antigens in humans (3, 48, 49), it was logical to use the murine model to evaluate the protection efficacy of the chlamydial antigens recognized by humans.
The current manuscript reports a portion of the results from our past vaccine research efforts. The goal of these experiments was to search for subunit vaccine antigens using the C. muridarum infection of mouse genital tract model. To maximize the protection efficacy induced by chlamydial antigens, the C. muridarum homologs of the C. trachomatis antigens were used as the immunogens in most cases. Mice were immunized three times with chlamydial antigens and 1 month after the last immunization, mice were intravaginally challenged with C. muridarum and monitored for genital tract chlamydial burden and pathology. Three different vaccination regimens were compared. Chlamydial antigens adjuvanted with CpG and incomplete Freund’s adjuvant (IFA) were used for intramuscular (IM), intraperitoneal (IP), or subcutaneous (SC) immunization while those adjuvanted with CpG alone for intranasal (IN) mucosal inoculation. The exception was that low doses of live C. muridarum were intranasally inoculated without any adjuvant. An intramuscular immunization regimen was first used to screen a total of 106 chlamydial antigens, which revealed two antigens that significantly reduced genital chlamydial burden while surprisingly 11 increased the burden compared to the negative immunization controls. The two infection-reducing antigens are recombinant protein TC0420 and native major outer membrane protein (MOMP) extracted from C. muridarum and their protection efficacies were as potent as that induced by adjuvanted UV-inactivated C. muridarum but significantly less potent than that induced by adjuvanted live C. muridarum. Unfortunately, the intramuscular immunization-induced immunity failed to reproducibly prevent genital pathology regardless of the immunogens used. Further, under the intramuscular immunization regimen, 23 antigens were found to significantly exacerbate genital pathology, raising a concern on the safety of this regimen. Thus, an intranasal mucosal immunization regimen was tested next since intranasal inoculation with live C. muridarum consistently protected against both genital tract infection and pathology although UV-inactivated C. muridarum failed to do so (50). Two of the 29 chlamydial antigens evaluated were found to be protective against genital infection but not pathology. Further, three antigens were found to exacerbate pathology. To further boost the immunogenicity of chlamydial antigens, a combinational regimen (intranasal priming + intramuscular boosting + a final intraperitoneal or subcutaneous booster) was tested, which identified four antigens that significantly prevented genital infection with only one of them also preventing pathology. However, the antigen that induced protection against both genital infection and pathology was not recommended to move forward further due to its amino acid sequence homology with human molecules. In addition, two other antigens were also found to exacerbate pathology under this regimen. Nevertheless, in the control groups, as long as mice were intranasally primed with live C. muridarum, protection against both genital chlamydial infection and pathology was always induced regardless of the subsequent boosters, which has again demonstrated the dependence of inducing immunity against both genital infection and pathology on viable chlamydial organisms. Thus, although screening 140 different chlamydial antigens with 21 of them repeated multiple times in 17 independent experiments failed to identify a subunit chlamydial vaccine antigen for humans, the efforts have revealed multiple detrimental antigens and demonstrated thsuperiority of viable chlamy organisms in priming protective responses against both genital infection and pathology. Our findings suggest that a live-auated Chlamydia vaccine strategy should be considered given both the past success in using similar strategies for developing human vaccines (51 – 53) and the progress in producing attenuated chlamydial mutants (54 – 59).
MATERIALS AND METHODS
Chlamydial vaccine antigen expression and purification
The chlamydial vaccine antigens were identified by using a whole genome-scale soluble GST (glutathione-s-transferase) fusion protein array consisting of 908 distinct C. trachomatis antigens to screen sera from 99 women infected with C. trachomatis (48). A total of 27 antigens were found to react with 50% or more patient sera, which were designated as immunodominant antigens, and 719 antigens were recognized by at least one patient serum, which were designated as immunogenic antigens. The most immunogenic chlamydial antigen identified in humans was the C. trachomatis serovar D plasmid-encoded glycoprotein 3 (Pgp3 encoded by pCT03, its homolog in C. muridarum is encoded by pCM03) that was recognized by 96% patients followed by the chlamydial protease/proteosome-like activity factor (CPAF, encoded by ORF CT858, its homolog in C. muridarum is encoded by TC0248) recognized by 94% and the outer membrane complex protein B (OmcB by ORF CT443, its homolog in C. muridarum is encoded by TC0727) by 89%. The major outer membrane protein (MOMP encoded by ORF CT681, its homolog in C. muridarum is encoded by TC0052) was ranked the 22nd since it was recognized by only 58% patient sera. A total of 140 different chlamydial antigens (not counting repeating or antigens in different versions or with different fusion partners) fop of the immunogenic antigen list were selected for protection efficacy evaluation in a C. muridarum infection of mouse genital tract model (see Table S1 for a list of all antigens tested). To maximize the induction of protection efficacy in the C. muridarum-mouse model, the corresponding C. muridarum homologs were used as immunogens. The open reading frames (ORFs) coding for each of the 140 C. muridarum antigens were cloned from the C. muridarum genome and plasmid [NCBI accession# NC_002620.2; reference (60)] into pGEX vector (Amersham Pharmacia Biotech, Inc., Piscataway, NJ, USA) and expressed as fusion proteins with glutathione-s-transferase (GST) fused to the N-terminus of the chlamydial proteins. The resultant fusion proteins were purified using glutathione-conjugated agarose beads as described previously (61 – 63). In most cases, the full-length chlamydial antigens were purified and used as immunogens. When it was difficulty in expressing an antigen in full length, only a fragment of that antigen was expressed instead. The C-terminal region was indicated with a “c,” N-terminus with “n,” middle region with “m,” while “s” was used to indicate the short form of Pgp3 as described previously (64, 65). For some experiments, two antigens were fused together as a bi-valent vaccine antigen or for manipulating the conformation of a target antigen/fragment, including the fusion of the full-length C. muridarum MOMP with the C-terminus of pCM03 (TC0052-pCM03c) and the MOMP C- and N-terminal regions with Pgp3c (TC0052c-pCM03c and TC0052n-pCM03c), respectively. The N- and C-terminal regions of OmcB were also fused to CPAFc and CM Pgp3c (TC0727n-TC0248c and TC0727c-pCM03c), respectively. In most cases, chlamydial antigens were purified by cleaving from the beads and the soluble proteins were concentrated via centrifugal concentrators. When cleavage was difficult, the GST-chlamydial fusion proteins were eluted off the beads and used as immunogens. Free GST was eluted as negative immunization control antigen. In some cases, the elution of fusion protein was difficult, and the bead complexes after extensive washing were then used as immunogens for intramuscular or intraperitoneal injection.
For positive immunization controls, live C. muridarum (Nigg3 strain) EBs were used. The organisms were grown in HeLa cells (ATCC, Manassas, VA, USA, 20108), purified, and titrated as described previously (66, 67). To prepare UV-inactivated C. muridarum EBs (UV-EB or UV-CM), EBs were exposed to UV light from a G36T5L/C UV lamp (Universal light source, San Francisco, CA, USA) at 5 cm for 45 min at room temperature. To ensure that the UV-CM organisms were completely inactivated, each UV-EB preparation was tested on HeLa cells and no inclusion-forming unit (IFU) was recovered after incubation for 24 h (data not shown). Aliquots of both live CM and UV-CM organisms were stored at −80°C till use.
Mouse immunization
Female Balb/c mice were purchased at the age of 4 weeks old from Charles River Laboratories, Inc. (Wilmington, MA, USA). For intramuscular, intraperitoneal, or subcutaneous immunization, 30 µg of each chlamydial antigen or GST protein or 1 × 105 IFUs of UV-CM or live CM plus 10 µg CpG in a total volume of 50 µL phosphate-buffered saline (PBS) emulsified in equal volume of IFA was used for each injection. The CpG with a sequence of 5′-TCC.ATG.ACG.TTC.CTG.ACG.TT-3′ (all nucleotides are phosphorothioate-modified at the 3′ inter-nucleotide linkage) was purchased from Integrated DNA Technologies (IDT, Coralville, IA, USA) and the IFA from Sigma-Aldrich (St. Louis, MO, USA). We used the CpG-IFA as adjuvants for the intramuscular injection because they have been shown to induce Th1 dominant immune responses (68, 69). For intranasal mucosal immunization, 30 µg of chlamydial antigen or GST protein or 1 × 106 IFUs of UV-CM plus 10 µg CpG or 50 or 500 IFUs of live CM alone in a total volume of 20 µL PBS was used for each inoculation. In some experiments, DDA [dimetyldiocta-decylammonium bromide (product no. 890810P)] and TDB [D-(+)-trehalose 6,6′-dibehenate (product no. 890808P), both from Avanti Polar Lipids (Alabaster, AL, USA)] were used since DDA/TDB were reported to promote immunogenicity of chlamydial antigens (70). The procedure for formulating DDA/TDB at a final inoculation dose of 250 µg DDA and 50 µg TDB in a total immunization volume of 200 µL per injection per mouse was described previously (70).
Three different immunization regimens were compared in the current study: Intramuscular, intranasal, and combinational regimens. For all regimens, mice were immunized three times, on day 0, day 20, and day 30, respectively. The exception is the intranasal immunization with live CM, which was given only once at the beginning of the immunization schedule. The combinational regimen consists of an intranasal priming and an intramuscular boosting with or without a final intraperitoneal or subcutaneous booster.
Mouse challenge infection
Thirty days after the final immunization, each mouse was inoculated intravaginally with 2 × 104 IFUs of live C. muridarum organisms in 20 µL of SPG (sucrose-phosphate-glutamate buffer consisting of 218 mM sucrose, 3.76 mM KH2PO4, 7.1 mM K2HPO4, 4.9 mM glutamate, pH 7.2). Five days prior to the inoculation, each mouse was injected with 2.5 mg Depo-provera (Pharmacia Upjohn, Kalamazoo, MI, USA) subcutaneously to synchronize the menstrual cycle and increase mouse susceptibility to C. muridarum infection.
Monitoring vaginal C. muridarum burdens
To monitor vaginal live organism shedding, vaginal swabs were taken on different days after the intravaginal infection. As indicated in different data sets, slightly different schedules for collecting vaginal swabs were followed in different experiments. However, the overall schedule was once every week until two consecutive negative detection results were obtained from the same mouse. Most mice cleared infection by day 28 regardless of the immunization, which was why the chlamydial burdens from the 1st month were used for comparison between groups. For determining the chlamydial burden in swabs, each swab was soaked in 0.5 mL of SPG and vortexed with glass beads and the chlamydial organisms released into the supernatants were titrated on HeLa cell monolayers in duplicates as described previously (71). Briefly, the serially diluted swab samples were inoculated onto HeLa cell monolayers grown on coverslips in 24-well plates. After incubation for 24 h in the presence of 2 µg/mL cycloheximide, the cultures were processed for immunofluorescence assay (see below) and the inclusions were counted under a fluorescence microscope. Five random fields were counted per coverslip. For coverslips with less than one IFU per field, the entire coverslips were counted. Samples showing obvious cytotoxicity of HeLa cells were excluded. The number of IFUs per swab was calculated based on the number of IFUs per field, number of fields per coverslip, dilution factors and inoculation and total sample volumes. An average was taken from the serially diluted and duplicate samples for any given swab. The IFUs/swabs were converted into log10 for calculating both chlamydial burden group means and standard deviation at each time point and area-under-curve (AUC) from each mouse and group comparison (see statistics below).
Evaluating mouse genital tract pathology hydrosalpinx
Mice were sacrificed 50–60 days after infection for evaluating urogenital tract tissue pathology. Before removing the genital tract tissues from mice, an in situ gross examination was performed for evidence of hydrosalpinx formation and any other gross abnormalities. The severity of oviduct hydrosalpinx was semi-quantitatively scored as the following (71): oviduct with no hydrosalpinx was scored with 0; hydrosalpinx can only be seen after amplification under a stereoscope was scored with 1; Hydrosalpinx clearly visible with naked eyes but with a size smaller than that of the ovary on the same side was scored with 2; Hydrosalpinx with a size similar to that of ovary was scored with 3 while larger than the ovary scored with 4. The scores from both oviducts from the same mouse were added as the hydrosalpinx score of the mouse. The genital tissue gross pathology was documented with a digital camera.
Immunofluorescence assay
HeLa cells grown on glass coverslips in 24-well plates were pretreated with DMEM containing 30 mg/mL of DEAE-Dextran (Sigma, St. Louis, MO, USA) for 10 min. After the DEAE-Dextran solution was removed, the serially diluted swab samples were added to the monolayers and allowed to attach to the cell monolayers for 2 h at 37°C. The infected cells were cultured in DMEM with 10% FCS and 2 µg/mL of cycloheximide (Sigma) for 24 h. The infected cultures were processed by fixing with 2% paraformaldehyde dissolved in PBS for 30 min at room temperature, followed by permeabilization with 2% saponin (Sigma) for 1 h. After washing and blocking, the cell samples were labeled with Hoechst (blue, Sigma) for visualizing DNA and a rabbit anti-chlamydial organisms (unpublished data) plus a goat anti-rabbit IgG conjugated with Cy2 (green; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for visualizing chlamydial inclusions. The immuno-labeled cell samples were quantitated as described above and used for image acquisition with an Olympus AX-70 fluorescence microscope equipped with multiple filter sets (Olympus, Melville, NY, USA) as described previously (66, 72).
Statistical analyses
For IFU data, the time course IFUs from each mouse were calculated into the AUC by adding up all measured IFUs and then dividing by the number of time points measured within the first 4 weeks after the challenge infection. As a result, each mouse was rendered with one AUC value for group comparisons. For hydrosalpinx scores (HS) from each mouse, the scores from both oviducts were added up as the score for the mouse. When ANOVA test (http://www.physics.csbsju.edu/stats/anova.html) was performed to analyze the IFUs (AUCs) and HS among multiple groups from a given immunization regimen, significant differences were found for both IFUs and HS. Then, Wilcoxon signed-rank test (two-tailed) was used to compare each antigen group with the corresponding negative and/or positive immunization control groups (http://faculty.vassar.edu/lowry/wilcoxon.html). The Fisher’s exact test (https://www.danielsoper.com/statcalc/calculator.aspx?id=29) was used to compare the incidence of positivity for infection or hydrosalpinx. Since the analyses were carried out after the completion of all 17 independent experiments, we were able to pool the controls from different experiments under the same regimen together for comparing with different antigen groups. Similarly, data for the same antigen repeated in different experiments under the same immunization regimen was also pooled for statistical analyses.
RESULTS
Intramuscular immunization with adjuvanted C. muridarum induced significant protection against subsequent chlamydial infection in the female mouse genital tract
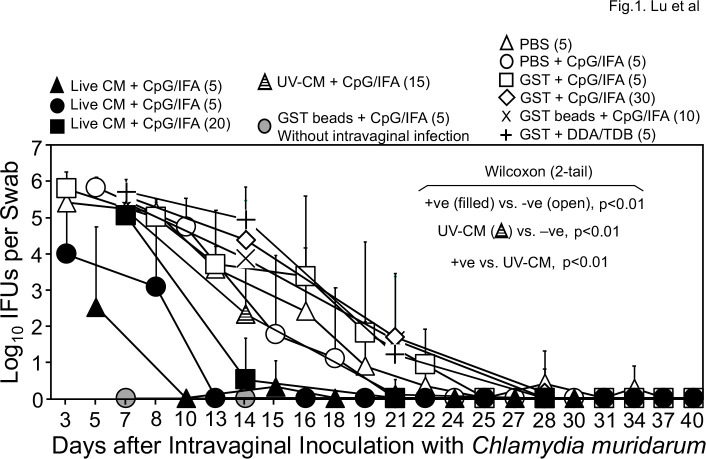
Since intramuscular injection is a well-accepted route for vaccinating humans, per our industrial partner’s recommendation, we first evaluated whether the whole C. muridarum (CM) organisms without (live CM) or with UV-light inactivation (UV-CM) can induce protective immunity against subsequent challenge infection with C. muridarum in the genital tract. Because our goal is to establish a mouse model for evaluating protection efficacy of chlamydial antigens identified in humans (48), which requires adjuvants to enhance immunity, we used CpG and IFA to emulsify live CM or UV-CM. The adjuvanted CM preps were intramuscularly injected into mice three times and 1 month after the last immunization, mice were intravaginally challenged with live CM for evaluating the protection efficacy. As shown in Fig. 1, all 30 mice that received intramuscular injection with adjuvanted live CM developed significantly reduced and shortened shedding courses of live chlamydial organisms from the genital tract (thus, designated as positive immunization controls), while 60 mice that received PBS with or without adjuvants or the adjuvanted control antigen glutathione-s-transferase (GST) displayed significantly high levels of live chlamydial organism shedding (collectively designated as mock or negative immunization controls). Interestingly, 15 mice that received adjuvanted UV-CM still developed moderate levels of genital shedding, which were significantly lower than those of the negative control groups but still higher than those of the positive immunization controls. The results summarized from eight independent experiments have demonstrated that the intramuscular immunization with CpG and IFA as adjuvants can be used to evaluate the protection efficacy of chlamydial antigens.
Fig 1.
Chlamydia muridarum shedding courses from the genital tracts of mice intramuscularly immunized with adjuvanted C. muridarum or adjuvant alone. Groups of female mice were intramuscularly immunized three times on days 0, 20, and 30, respectively, with UV light-inactivated C. muridarum (UV-CM, hatched triangle, n = 15) or live CM (filled or solid symbols) adjuvanted with CpG and incomplete Freund’s adjuvant (IFA) or PBS buffer or GST (glutathione-s-transferase) with or without CpG/IFA (open symbols) or DDA/TDB adjuvants (+). For some groups, GST-bound to agarose beads were used as immunogens (X & gray circle). One month after the last immunization, all mice (except for the no infection control group, gray circle, n = 5) were challenged intravaginally with 2 × 104 IFUs (inclusion forming units) of C. muridarum organisms and vaginal swabs were taken once or twice weekly as indicated along the x-axis for monitoring genital chlamydial burdens. The vaginal swab collection from different groups started on different days, including days 3 (filled circle, n = 5; open triangle, n = 5; open square, n = 5), 5 (filled triangle, n = 5; open circle, n = 5), or 7 (gray circle, n = 5; filled square, n = 20; open diamond, n = 30; cross, n = 10; plus, n = 5) after intravaginal infection. The vaginal swabs were titrated for chlamydial burdens in terms of IFUs which were converted into Log10 for calculating group mean and standard deviation at a given time point as displayed along the y-axis. The IFU data were from eight independent experiments. The live CM-immunized mice were regarded as positive immunization groups (+ve, filled symbols, n = 30) while mice immunized with buffer or GST with or without adjuvants were considered negative immunization control groups (open symbols and cross/plus symbols, n = 60). The area-under-curve (AUC) was compared between the +ve and −ve groups using Wilcoxon, P < 0.01. The UV-EB group was different from both the +ve and −ve controls (P < 0.01).
The intramuscular immunization regimen reveals more chlamydial antigens that induce detrimental responses and fewer antigens that induce protection
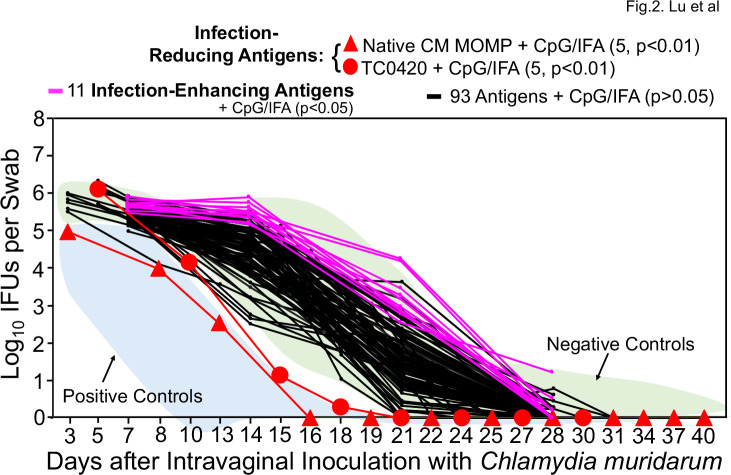
Using the established intramuscular immunization and adjuvant regimen, we evaluated 106 chlamydial antigens for protecting against the subsequent chlamydial infection in the female genital tract (Fig. 2). The native MOMP was purified from C. muridarum (73), while the remaining 105 antigens were purified from a bacterial expression system (48). When the vaginal chlamydial burdens of the antigen-immunized groups were compared with those of the negative immunization controls, two antigens were found to induce significant protection against genital chlamydial infection (thus designated as infection-reducing antigens), while surprisingly 11 were found to significantly exacerbate genital chlamydial infection (infection-enhancing antigens). The remaining 93 antigens did not significantly alter the live chlamydial shedding courses. The two infection-reducing antigens are native MOMP and TC0420. Native MOMP has been shown to induce significant protection (73 – 75), while TC0420 is a hypothetical protein that contains T cell epitopes (76) and has been shown to induce protective immunity against chlamydial infection (77). Thus, our screening has validated previous observations and also identified 11 infection-enhancing antigens.
Fig 2.
Chlamydia muridarum shedding courses from the genital tracts of mice intramuscularly immunized with different chlamydial antigens. Groups of female mice (n = 4–10) were intramuscularly immunized with ~30 µg of each of 106 chlamydial antigens adjuvanted with CpG + IFA or DDA/TDB for three times for evaluating the intramuscular immunization-induced protection against genital tract infection as described in Fig. 1 legend. The areas under curves of positive (+ve, light blue) and negative (−ve, light green) control groups from Fig. 1 were shaded as references (indicated by arrows). A two-tailed Wilcoxon analysis revealed that two antigens (native MOMP, filled red square; TC0420, filled red circle) significantly reduced genital chlamydial burdens (designated as infection-reducing antigens, red), while 11 antigens significantly enhanced chlamydial burdens (designated as infection-enhancing antigens, pink) when compared with the negative controlsve). Immunization with any of the remaining 93 antigens failed to significantly alter the chlamydial shedding courses (black curves). The IFU data were from the same eight independent experiments as described in Fig. 1 legend. To maintain clarity, the standard deviations were omitted from the plot.
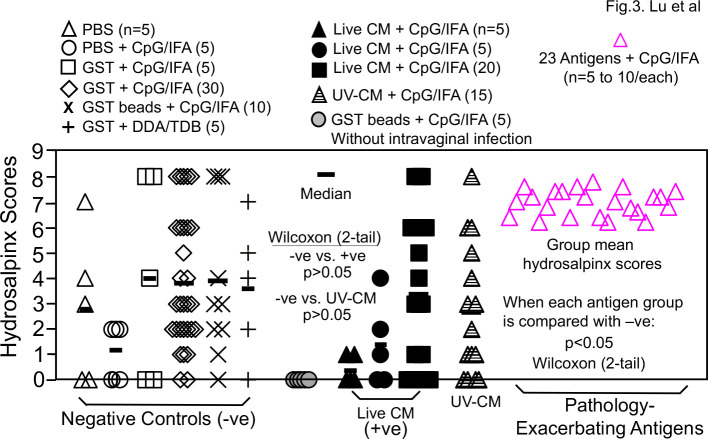
When the upper genital tract pathology was compared between the negative immunization control groups and the chlamydial antigen-immunized groups (Fig. 3), no antigen groups, including the two infection-reducing antigen groups, significantly reduced hydrosalpinx scores. Surprisingly, 23 antigens significantly increased hydrosalpinx scores (designated as pathology-enhancing/exacerbating antigens; also see Fig. S1). Further, only 1 of the 23 pathology-exacerbating antigens significantly enhanced genital infection and the remaining 22 antigens did not, suggesting that the 22 antigens might have induced host responses for selectively promoting chlamydial pathogenicity without enhancing chlamydial infection. The identification of large number of pathology-enhancing antigens raised a concern on the safety of the intramuscular immunization regimen. Further, the 30 positive immunization control mice (that were intramuscularly immunized with live CM, marked as +ve in Fig. 1) prevented genital chlamydial infection but not pathology.
Fig 3.
Hydrosalpinx scores of mice intramuscularly immunized with adjuvanted C. muridarum organisms or antigens or buffer/control antigen/adjuvant alone. Groups of female mice (n = 4–10) were intramuscularly immunized and intravaginally challenged as described in Fig. 1. legend. On day 56 after intravaginal challenge, all mice were sacrificed for semi-quantitatively scoring upper genital tract pathology hydrosalpinx as described in the Materials and Methods section and the hydrosalpinx scores were displayed along the y-axis. A two-tailed Wilcoxon analysis revealed no statistically significant difference in hydrosalpinx scores between the negative immunization controls (n = 60, −ve) and positive controls (n = 30, +ve) or UV-CM (n = 15). Mice intramuscularly immunized with adjuvanted GST beads but without intravaginal challenge infection (gray circle) did not develop any significant pathology. When the hydrosalpinx scores from groups of mice (n = 4–10) immunized with each of the 106 chlamydial antigens as described in Fig. 2 legend were compared with that of the negative controls (n = 60), 23 antigens were found to significantly increase hydrosalpinx (each pink open triangle represents the mean score from each of the 23 groups; for scores from individual mice in these 23 groups, please see Fig.S1). There was no significant difference in hydrosalpinx score between any of the remaining 83 antigen groups and the negative immunization control mice (data not shown). The hydrosalpinx score data were from the same eight independent experiments as described in Fig. 1 and 2 legends.
Intranasal mucosal immunization with live chlamydial organisms reproducibly prevents both chlamydial infection and pathogenicity in the genital tract
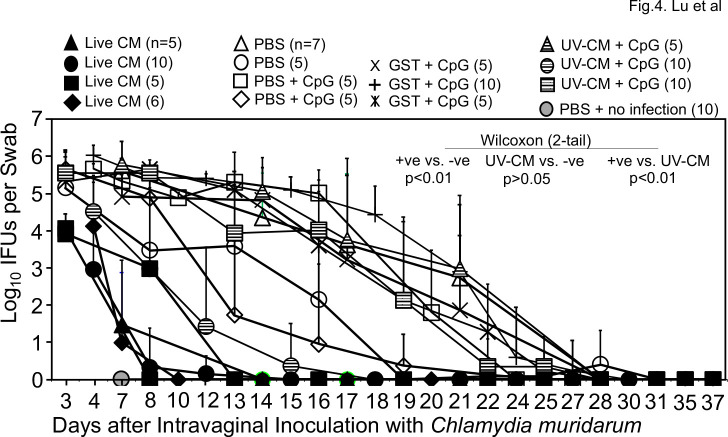
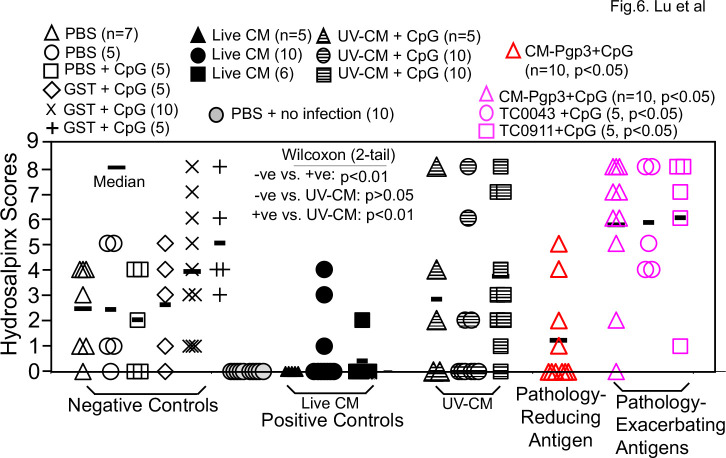
To identify a more sensitive/appropriate immunization regimen for evaluating the protection efficacy of chlamydial vaccine antigen candidates, we next tested the intranasal mucosal immunization regimen. As shown in Fig. 4, all 26 mice each intranasally inoculated with a single dose of 50 or 500 IFUs of live CM developed immunity that significantly reduced genital chlamydial burden when compared with that of the 42 mice that were intranasally inoculated with PBS with or without GST and/or CpG adjuvant. Thus, the live CM-immunized mice were designated as positive immunization controls or +ve while mice receiving buffer or GST with or without CpG as negative immunization controls or -ve. It is worth noting that the 25 mice that were intranasally inoculated with CpG-adjuvanted UV-CM also significantly reduced vaginal chlamydial burden but their protection efficacy was significantly less potent than that induced by live CM. When the pathology was compared (Fig. 6), live CM-immunization significantly prevented pathology while UV-CM-immunization failed to do so.
Fig 4.
Chlamydia muridarum shedding courses from the genital tracts of mice intranasally immunized with C. muridarum or buffer/control antigen/adjuvant alone. Groups of mice were intranasally immunized once with live C. muridarum in PBS buffer (live CM, filled symbols) or immunized three times on days 0, 20 & 30 respectively with UV light-inactivated C. muridarum adjuvanted with CpG (UV-CM +CpG, hatched symbols) or PBS buffer or GST (glutathione-s-transferase) with or without CpG (open symbols). One month after the last immunization (2 months after live CM), all mice (except for the no infection control group, gray circle, n = 5) were challenged intravaginally with 2 × 104 IFUs of C. muridarum and vaginal swabs were taken once or twice weekly as indicated along the x-axis for measuring the number of live chlamydial organisms. The vaginal swab collection from different groups started on different days, including days 3 (filled triangle & square, n = 5/each; open circle, n = 5; hatched square, n = 10), 5 (filled circle, n = 10; filled diamond, n = 6; open square, n = 5; plus, n = 10; hatched circle, n = 10) or 7 (gray circle, n = 10; cross, n = 5; hatched triangle, n = 5) after intravaginal infection. The vaginal swabs were titrated for chlamydial burdens in terms of IFUs which were converted into Log10 for calculating group mean and standard deviation at a given time point as displayed along the y-axis. The IFU data were from five independent experiments. The live CM-immunized mice were used as positive immunization controls (+ve, filled symbols, n = 26) while mice immunized with buffer or GST with or without adjuvants as negative immunization controls (-ve, open symbols and cross/plus symbols, n = 42). The area-under-curve (AUC) was used to compare between the +ve and -ve groups using Wilcoxon (P < 0.01). The UV-CM groups (n = 25) were significantly different from the +ve (P < 0.01) but not the -ve (P > 0.05) controls, indicating that intranasal inoculation with live CM but not killed CM significantly prevented genital chlamydial infection.
The intranasal immunization with chlamydial antigens fails to induce transmucosal immunity for preventing both genital chlamydial infection and pathogenicity
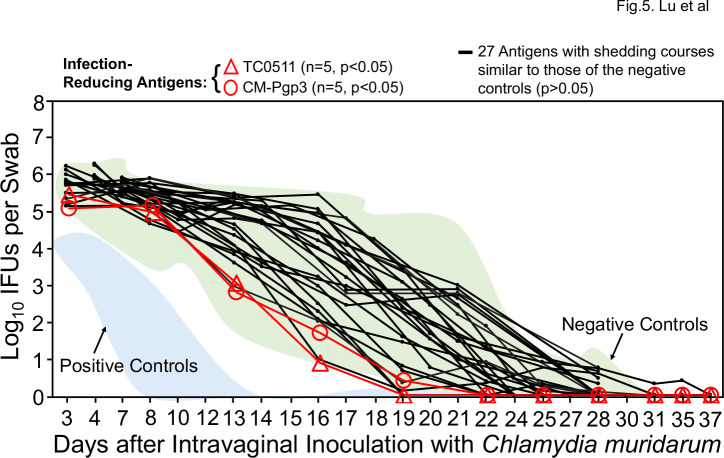
We next used the intranasal immunization regimen for evaluating the protection efficacy of 29 chlamydial antigens (including antigens that were repeated multiple times such as various versions of Pgp3 and CPAF). Only two antigens were found to significantly reduce vaginal chlamydial burden (Fig. 5) and they were TC0511 and pCM03 or Pgp3. TC0511 is the recombination protein RecR with 203 amino acids (Genbank #AAF39353). Although TC0511 is a highly conserved chlamydial protein with significant homology with its bacterial homologs, its homolog (CT240) in C. trachomatis does not have any significant homology with any human proteins based on blastp search. Thus, CT240 could be considered a human vaccine candidate if it were also able to prevent pathology. The second infection-reducing antigen is the most immunodominant C. muridarum plasmid-encoded glycoprotein Pgp3 (78). A total of 35 mice in five different groups with 5–10 mice per group were intranasally immunized with Pgp3 in four independent experiments (Table S1). It is worth emphasizing that Although intranasal immunization with Pgp3 was found to significantly reduce genital chlamydial burden in the initial experiment with 5 mice per group, when Pgp3 was repeated in three additional experiments with 10 mice per group, no significant protection was found anymore. When all Pgp3-immunized 35 mice were compared with the 42 negative control mice, no significant difference in genital chlamydial burden was found. These results have demonstrated that pCM03 may not be considered a protective antigen. Moreover, when the pathology was compared (Fig. 6), only one group of 10 mice immunized with Pgp3 significantly reduced the hydrosalpinx score but without impacting the genital chlamydial burden. Paradoxically, another group of 10 mice intranasally immunized with Pgp3 was found to significantly increase the hydrosalpinx score but without enhancing genital chlamydial infection. When the hydrosalpinx scores of all 35 mice intranasally immunized with Pgp3 were compared with those of the corresponding negative control mice, no more significant difference was found. Thus, Pgp3 might not be a reliable vaccine candidate. This conclusion was confirmed by the results from experiments in which Pgp3 was tested using other immunization regimens (see Table S1). Further, the intranasal immunization also revealed two additional pathology-exacerbating antigens (Fig. 6): TC0043 and TC0911. TC0043 is the chlamydial type III secretion translocase SctQ with 373 amino acids (Genbank #AAF73521), while TC0911 is a conserved hypothetical protein with 831 amino acids [Genbank #AAF39704; reference (60)]. Intranasal inoculation with TC0043 or TC0911 significantly exacerbated oviduct hydrosalpinx but without significantly altering the genital infection, suggesting that these two antigens-induced immune responses might directly promote pathogenicity in the upper genital tract. It will be interesting to further characterize the pathology-exacerbating responses.
Fig 5.
Chlamydia muridarum shedding courses from the genital tracts of mice intranasally immunized with different chlamydial antigens. Groups of female mice (n = 5–10) were intranasally immunized with ~30 µg of each of 29 chlamydial antigens adjuvanted with CpG for three times for evaluating the intranasal immunization-induced protection against genital tract infection as described in Fig. 4 legend. The areas under curves of positive (+ve, light blue) and negative (−ve, light green) control groups from Fig. 4 were shaded as references (indicated by arrows). A two-tailed Wilcoxon revealed that mice immunized with TC0511 (red open triangle) or CM-Pgp3 (red open circle) significantly reduced genital chlamydial burdens (designated as infection-reducing antigens) when compared with the negative controls (−ve). Immunization with any of the remaining 27 antigens failed to significantly alter the chlamydial shedding courses (black). The IFU data were from the same five independent experiments as described in Fig. 4 legend. To maintain the presentation clarity, the error bars were not omitted.
Fig 6.
Hydrosalpinx scores of mice intranasally immunized with C. muridarum organisms or antigens or buffer/control antigen/adjuvant alone. Groups of mice were intranasally immunized and intravaginally challenged as described in Fig. 4 and 5 legends. On day 56 after intravaginal challenge, all mice were sacrificed for semi-quantitatively scoring the upper genital tract pathology hydrosalpinx as described in the Materials and Methods section and the hydrosalpinx scores were displayed along the Y-axis. A 2-tailed wilcoxon analysis revealed statistically significant difference in hydrosalpinx scores between the negative immunization controls (-ve, open, n = 37) and positive controls (+ve, filled, n = 21, P < 0.01) but not the UV-CM (hatched symbols, n = 25, P > 0.05). Mice intranasally immunized with PBS but without intravaginal challenge infection (gray circle) did not develop any significant pathology. The hydrosalpinx score data were from the same five independent experiments as described in Fig. 4 and 5 legends except for groups from VacExp B, the pathology data were missing (see Table S1). When each of the 29 chlamydial antigen groups (n = 4 to 10) was compared with the negative controls (n = 37), the plasmid-encoded Pgp3 significantly reduced the hydrosalpinx score (red open triangle) while the same Pgp3 in a different experiment (pink open triangle) and antigens TC0043 (pink open circle) & TC0911 (pink open square) significantly increased the hydrosalpinx scores. Note that intranasal immunization with live CM but not UV-CM significantly prevented genital pathology while CpG-adjuvanted chlamydial antigens either reduced or exacerbated pathology in different experiments. Particularly, Pgp3 was repeated five times with a total of 35 mice under the same intranasal immunization regimen (see Table S1) and the hydrosalpinx scores of the 35 mice were not significantly different from those of the negative control mice.
A combinational immunization regimen identifies a chlamydial antigen that prevents both chlamydial infection and pathology in the female genital tract
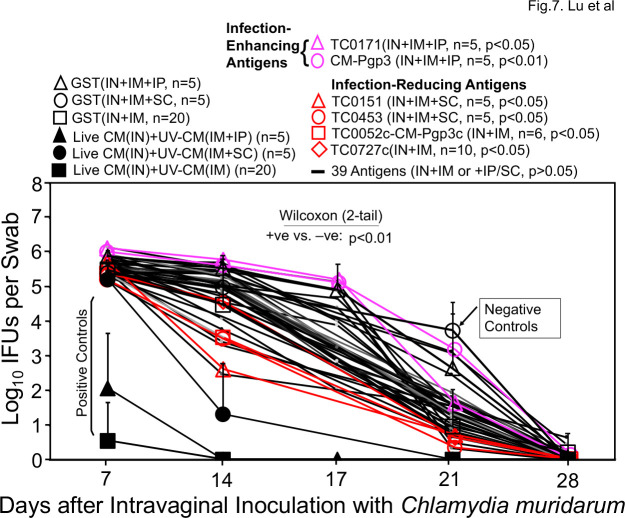
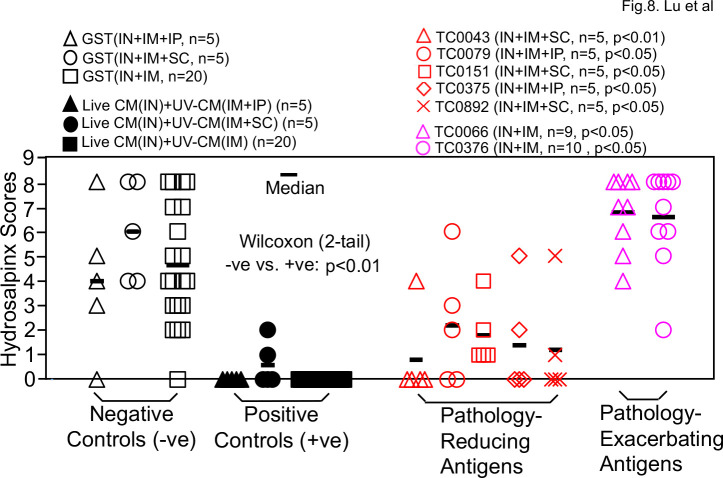
Since neither the intramuscular nor the intranasal immunization regimen has identified chlamydial antigens that can prevent both chlamydial infection and pathology, we next tested a combinational immunization regimen consisting of intranasal priming, intramuscular boosting with or without a third boost intraperitoneally or subcutaneously. As shown in Fig. 7, all 30 mice receiving intranasal priming with live CM and intramuscular boosting with adjuvanted UV-CM with or without the third booster significantly reduced the shedding of live chlamydial organisms comparing to the negative control mice. When groups of mice immunized with individual chlamydial antigens were compared with the negative control mice, four antigens were found to significantly reduce genital chlamydial infection: TC0151, TC0453, TC0052c-Pgp3c fusion protein, and TC0727c. TC0151 is beta-ketoacyl-[acyl-carrier-protein] synthase II (fabF, WP_010229532.1), while TC0453 is a hypothetical protein (WP_010230489.1). TC0052 is the MOMP, while TC0727 is the outer membrane complex protein B (OmcB). The rationale of testing the TC0052c-Pgp3c fusion protein was to take advantage of the Pgp3 C-terminal trimerization domain to trimerize the C-terminal half of MOMP that contains variable domains (VD) III and IV (79). The rationale of testing the C-terminus of OmcB was that the immunogenicity of OmcB in humans and chlamydia-infected animals was largely mapped to its C-terminus (80, 81). We also found that TC0171 (a DUF4339 domain-containing protein, WP_010229701.1) and Pgp3 significantly enhanced chlamydial infection under the same combinational immunization regimen, which once again suggested that Pgp3 is not a safe vaccine antigen. When the pathology was compared (Fig. 8), all live CM-primed mice were prevented from any significant pathology while significant pathology developed in the negative control groups (P < 0.01). Mice immunized with chlamydial antigens TC0043 (SctQ, WP_010229202.1), TC0079 (ATP-dependent Clp protease proteolytic subunit, WP_010229313.1), TC0151 (beta-ketoacyl-ACP synthase II, fabF, WP_010229532.1), TC0375 (thioredoxin-disulfide reductase, trxB, WP_010230279.1), or TC0892 (peroxiredoxin, WP_010231862.1) all significantly reduced pathology. Among the five pathology-reducing antigens, only TC0151 significantly prevented chlamydial infection in the genital tract as shown in Fig. 7. Thus, we have designated TC0151 as a protective chlamydial antigen. Finally, Fig. 8 also showed that mice immunized with TC0066 or TC0376 significantly enhanced pathology although these two antigens failed to significantly promote chlamydial infection. It is worth noting that although TC0043 reduced pathology under the combinational immunization regimen, the same antigen exacerbated pathology under the intranasal immunization regimen although TC0043 did not affect chlamydial challenge infection courses in the genital tract under either regimen. These observations suggest that the immunization routes or adjuvants could affect the immune phenotypes that can either reduce or exacerbate pathology. Despite the variable results from the individual antigen-immunized mice, all live CM-primed mice (regardless of the subsequent boosts) consistently prevented both genital chlamydial infection and pathology.
Fig 7.
Chlamydia muridarum shedding courses from the genital tracts of mice immunized via a combinational regimen. Groups of mice were immunized three times with intranasal (IN) priming on day 0, intramuscular (IM) boosting on day 20 and a final intraperitoneal (IP) or subcutaneous (SC) booster on day 30, respectively, with C. muridarum (filled symbols as positive immunization controls, +ve), GST (open symbols as negative immunization controls, −ve) or different chlamydial antigens (red and pink symbols and black lines). One month after the last immunization, all mice were challenged intravaginally with 2 × 104 IFUs of C. muridarum and vaginal swabs were taken once weekly as indicated along the x-axis for monitoring chlamydial burdens in terms of log10 IFUs as displayed along the y-axis. The area-under-curves (AUCs) were compared between the +ve and −ve groups using a two-tailed Wilcoxon (P < 0.01). Comparing to the -ve controls, chlamydial antigens TC0151, TC0453, TC0052c-Pgp3c, and TC0727c were found to significantly reduce genital chlamydial burdens (designated as infection-reducing antigens, red) while antigens TC0171 and Pgp3 significantly enhanced chlamydial burdens (designated as infection-enhancing antigens, pink). The IFU data were from the four independent experiments.
Fig 8.
Hydrosalpinx scores of mice immunized via a combinational regimen. Groups of female mice (n = 4–10) were immunized via a combinational regimen and intravaginally challenged as described in Fig. 7 legend. On day 56 after intravaginal challenge, all mice were sacrificed for semi-quantitatively scoring the upper genital tract pathology hydrosalpinx as described in the Materials and Methods section and the hydrosalpinx scores were displayed along the y-axis. A two-tailed Wilcoxon analysis revealed statistically significant difference in hydrosalpinx scores between the negative immunization controls (−ve, open, n = 30) and positive controls (+ve, filled, n = 30, P < 0.01). Comparing to the −ve controls, chlamydial antigens TC0043, TC0079, TC0151, TC0375, and TC0892 were found to significantly reduce hydrosalpinx (designated as pathology-reducing antigens, red) while antigens TC0066 and TC0376 significantly enhanced hydrosalpinx (designated as pathology-enhancing antigens, pink). The hydrosalpinx score data were from the same four independent experiments as shown in Fig. 7. Note that only TC0151 prevented both genital chlamydial infection and pathology.
DISCUSSION
The successful isolation of C. trachomatis from human ocular tissues by Tang et al. (82, 83) more than 60 years ago had laid the foundation for developing a Chlamydia vaccine. Since then, extensive efforts have been made for achieving the goal (21 – 38). However, no licensed C. trachomatis vaccine is available today. The current study was designed to search for chlamydial subunit vaccine antigens by screening 140 C. trachomatis antigens identified to be immunogenic in women infected with C. trachomatis (48) using a C. muridarum infection of female mouse genital tract model (41). The C. muridarum homologs (60) were used as immunogens in most cases to match the genital challenging infection organisms. The advantage of this mouse model is that it can evaluate vaccine efficacy by simultaneously monitoring both genital infection and pathology (42, 47, 84).
We started with the intramuscular priming plus two boosts regimen with CpG and IFA as adjuvants since intramuscular injection is a well-accepted route for vaccinating humans and CpG and IFA can promote both antibody and Th1 responses. After screening 106 chlamydial antigens, we did not find any chlamydial antigen that can prevent both chlamydial infection and pathology in the female mouse genital tract. Instead, we found that only two antigens reduced but 11 promoted chlamydial infection and no antigen prevented genital pathology but 23 exacerbated pathology. Although the failed human trachoma vaccine trials based on intramuscular immunization with formalin-fixed chlamydial organisms (21 – 24) had already suggested that chlamydial organisms might contain antigens that can induce detrimental immune responses for exacerbating chlamydial infection/pathogenicity, it was still surprising to find so many chlamydial antigens that enhanced chlamydial infection/pathogenicity in the mouse model. This phenomenon was rarely reported in previous mouse model-based chlamydial vaccine studies, which was probably because most studies were only focused on one or a few chlamydial antigens. More importantly, although the positive immunization control mice (mice immunized with adjuvanted live CM) reduced genital infection in multiple independent experiments, these mice were not always protected from pathology. None of the UV-CM-immunized mice was protected from pathology under the intramuscular immunization regimen. Thus, we tested the intranasal mucosal immunization regimen since intranasal inoculation with C. muridarum is known to prevent both genital tract infection and pathology (50). After evaluating 29 chlamydial antigens, we identified two infection-reducing antigens but no infection-enhancing antigens, which is an improvement over the intramuscular immunization regimen. However, neither of the infection-reducing antigens significantly prevented the genital tract pathology, suggesting that the prevention of pathology might require stronger immunity. Thus, we further tested a combinational immunization regimen with intranasal mucosal priming followed by intramuscular boosting with or without additional intraperitoneal or subcutaneous boosters. Among the 44 antigens evaluated, we found 4 infection-reducing antigens, 2 infection-enhancing antigens, 5 pathology-reducing antigens, and 2 pathology-enhancing antigens. Clearly, the combinational regimen identified more protective antigens than detrimental antigens at both the anti-infection and anti-pathology levels. More importantly, TC0151 was found to prevent both genital tract infection and pathology. It is worth noting that all mice intranasally primed with live CM were protected from both genital chlamydial infection and pathogenicity. The subsequent boosting with intramuscular injection of adjuvanted UV-CM did not alter the protective phenotype of the immune responses, suggesting that mucosal priming with live CM is important for maintaining the protective phenotype of the induced immune responses.
Combinational immunization with TC0151 prevented both genital tract infection and pathology. TC0151 is a highly conserved beta-ketoacyl-[acyl-carrier-protein] synthase II (fabF, WP_010229532.1) with 418 amino acids that shares 98% amino acid identity with the C. trachomatis fabF (WP_009872915.1). FabF is involved in the condensation step of fatty acid biosynthesis in which the malonyl donor group is decarboxylated and the resulting carbanion used to attack and extend the acyl group attached to the acyl carrier protein. C. trachomatis fabF is immunogenic in C. trachomatis-infected women (48), suggesting that fabF-based vaccine-primed immune responses can be recalled during C. trachomatis infection in women. However, C. trachomatis fabF was found to have significant levels of amino acid identity/similarity with the various isoforms of human fatty acid synthase (e.g., 24.5% identity against AAH63242.1) and human mitochondrial beta-ketoacyl ACP synthase (47.78% against XP_006713280.1). Careful comparison has revealed identical or similar amino acids (without gaps) in ~10 different regions (each with 5–10 amino acids in length) between C. trachomatis fabF and its human homologs, raising a serious safety concern (on potentially inducing auto-reactive immune responses in humans when a C. trachomatis fabF-based vaccine is applied). Thus, although chlamydial fabF protected against both chlamydial infection and pathology in the female mouse genital tract, it was recommended not to move forward to the next step.
Some chlamydial antigens were found to significantly reduce genital tract infection but without reducing pathology, while others significantly prevented pathology but without preventing genital infection. These antigens were not recommended to move forward for vaccine development. This is because an effective vaccine should prevent both infection and pathology. The discrepancy displayed by these antigens might be caused by either the inability of an antigen to induce adequate protective response or the inappropriate immunization regimens and adjuvants. The C. muridarum genital infection mouse model is considered sensitive and specific for measuring the protection efficacy against both genital infection and pathology. This conclusion is supported by the identification of chlamydial fabF as a protective antigen for preventing both genital chlamydial infection and pathology reported in the current study. The finding that mice intranasally primed with live CM were always protected against both genital chlamydial infection and pathology has further demonstrated the reproducibility of the mouse model. Thus, if a chlamydial antigen fails to protect against both genital infection and pathology in the mouse model, it is unlikely that this antigen will be successful in large animal models or humans. Obviously, an effective subunit vaccine may combine multiple infection-reducing antigens with pathology-reducing antigens to form multi-valent vaccines. The antigen combination idea was tested in the current study by genetically fusing Pgp3 fragments with MOMPc or fragments of OmcB with fragments of CPAF in various versions. Only the fusion protein TC0052c-pCM03c was found to significantly reduce genital chlamydial infection but without preventing pathology. Although we still have plans to re-evaluate the protection efficacy of different antigen combinations, we are also aware of the safety concern that antigen combinations might also increase the risk of promoting infection and/or exacerbating pathology.
Many chlamydial antigens were found to induce detrimental responses for promoting genital infection and/or exacerbating pathology. The failed human trachoma vaccine trials had suggested that some chlamydial antigens might induce detrimental responses for exacerbating mucosal pathology upon chlamydial infection (21 – 23). Although the chlamydial antigens responsible for detrimental responses in humans and mice are not necessary the same, it will be interesting to investigate the mechanisms by which some of the antigens promote genital chlamydial infection and/or pathogenicity. It is worth pointing out that the chlamydial antigen-induced detrimental immune responses have not been widely reported using mouse models, but on the other hand, to the best of our knowledge, no large-scale antigen screen has been performed for any chlamydia. Most detrimental chlamydial antigens were identified under the intramuscular immunization regimen, which is consistent with the observation made in the failed human trachoma vaccine trials since the trachoma vaccines were also based on intramuscular immunization with formalin-fixed whole chlamydial organisms. The intranasal immunization regimen enabled more chlamydial antigens to induce protective responses in the female mouse genital tract. Consistently, all mice intranasally immunized with live CM prevented both genital chlamydial infection and pathology. It will be interesting to investigate the mechanisms by which intranasal immunization with chlamydial antigens tend to induce protective responses.
A consistent finding in the current study is that immunization with live CM is always more effective than UV-CM in inducing protection against genital chlamydial infection and pathology. Even under the intramuscular immunization regimen, adjuvanted live CM induced significantly stronger protection against genital chlamydial infection than adjuvanted UV-CM did. Although emulsification with adjuvants is expected to inactivate CM and muscle cells are considered non-permissible to chlamydial infection, it is possible that some viable CM organisms may remain after emulsification. The remaining viable CM may still transiently infect mouse muscle cells and produce infection-dependent antigens for directly activating protective responses. Nevertheless, although intramuscular injection with adjuvanted live CM prevented genital tract chlamydial infection, it failed to consistently prevent pathology. Only after intranasal inoculation, was live CM able to fully prevent both genital chlamydial infection and pathology. This may be because the intranasally inoculated live CM can undergo productive infection in the airway, triggering strong protective immune responses. Consistently, live chlamydial infection-dependent protective immunity has been frequently observed (73, 85 – 88). The protective immunity induced by live CM was correlated with a Th1-dominant but Th17-low T cell response coupled with immune responses to chlamydial secretion proteins (50). Immune responses with similar phenotypes are thought to be important for clearing chlamydial infection (43). The challenge is how to induce the infection-dependent protective immune responses using non-infectious chlamydial antigen platforms. Some have tested surrogate live vectors such as adenovirus (89), poliovirus (90), or influenza viruses (91), while others have evaluated non-viable vectors such as Vibrio cholerae ghosts [VCGs; reference (26, 27)] and the immunogenic bacteriophage MS2 virus-like particle [VLP; reference (92)] for enhancing chlamydial antigen immunogenicity. These vector systems may function as both adjuvants and presentation platforms. For example, VCG was found to exert immunomodulatory effect on dendritic cells for enhanced antigen presentation and induction of protective immunity (93). A recent study has evaluated the protection efficacy of UV-inactivated EBs after modification with nanoparticles and pattern-recognition receptor ligands (34). However, none of these has been advanced to the stage of clinical evaluation. The most advanced chlamydia vaccine is CTH522 adjuvanted with CAS01 liposomes or aluminum hydroxide, for which the phase I trial was completed in 2019 (35).
Both the difficulty in developing subunit Chlamydia vaccines (29, 33) and the acquisition of genital tract pathogenicity-attenuated chlamydial mutants (54, 56, 58) have reignited the interest in developing whole chlamydial organism-based vaccines. In particular, the discovery that chlamydial colonization in the gastrointestinal tract is non-pathological and can induce transmucosal protection against subsequent chlamydial infection in the female genital tract has laid the foundation for developing a live-attenuated oral chlamydial vaccine (57). Consistently, the current study has demonstrated that mice with intranasal mucosal priming with live CM significantly prevented both genital chlamydial infection and pathogenicity but similar priming with UV-CM failed to do so. Oral immunization with various genital pathogenicity-attenuated CM mutants has been shown to induce protective immunity against both chlamydial infection and pathology in the female genital tract (54, 56, 58). Efforts are underway to move some of the attenuated mutants to clinical evaluation. The efforts are motivated by the fact that live-attenuated C. psittaci (94) and C. abortus (95) vaccines are approved for protecting animals. It is worth emphasizing that even if sharing our vaccine screening experience as described in the current manuscript stimulates the development of a subunit Chlamydia vaccine, there may still be a need to develop other versions of Chlamydia vaccines given the diverse human populations affected by Chlamydia. It is well known that both IPV (inactivated polio vaccine) and OPV (oral live polio vaccine) have contributed to the worldwide eradication of the polio pandemic and their combinational usage is continuing to maintain human herd immunity (96). Further, although mRNA vaccines have been successful in ending the COVID-19 pandemic in many countries, a live-attenuated mucosal vaccine may still be needed for blocking the spreading of SARS-CoV-2 and preventing the emergence of new variants (55). Thus, developing a live-attenuated Chlamydia may be both timely and necessary. The question is whether oral CM-induced immunity can cross-protect humans against C. trachomatis infection and pathology in the genital tract? First, there are successful examples using animal-adapted microbes as vaccines for protecting humans against human pathogens, including cowpox virus as a live vaccine to protect humans against smallpox (53), BCG of M. bovis against M. tuberculosis (51, 97) and meningococcal vaccine against gonorrhea (98). Second, prior exposure to CM has been reported to induce protective immunity against genital infection by multiple serovars of the human pathogen C. trachomatis (99). Thus, the next steps are to test whether oral delivery of an attenuated CM can protect against C. trachomatis in the genital tracts of large animal models or humans. Obviously, the safety of any live-attenuated vaccines is a primary concern. For example, the live-attenuated C. abortus vaccine was found to still induce abortion (100). This is probably because the C. abortus vaccine lacks genomic evidence of attenuation (101), which is consistent with its phenotype of infecting placenta and shedding live organisms. However, the proposed CM-based oral vaccine intrOv is genetically defined to carry loss of function mutations (71, 102) and can no longer induce genital tract pathology even following a direct genital tract inoculation (71). More importantly, although oral intrOv is able to induce robust transmucosal protective immunity in the genital tract (58), the orally inoculated intrOv itself is restricted to the GI tract (103, 104) and cleared from the gut by IFNγ-producing group 3-like innate lymphoid cells (105 – 107).
ACKNOWLEDGMENTS
This work was supported in part by grants (to G.Z.) from Merck and NIAID.
Contributor Information
Guangming Zhong, Email: Zhongg@uthscsa.edu.
Sunny Shin, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA .
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/iai.00349-23.
Fig.1S shows the individual antigen-exacerbated hydrosalpinx scores from Fig.3 while Table 1S lists both chlamydial burdens and hydrosalpinx scores for all antigens tested.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. CDC . 2020. Sexually transmitted diseases. Services USDoHaH. Available from: https://www.cdc.gov/std/statistics/2020/
- 2. Brunham RC, Maclean IW, Binns B, Peeling RW. 1985. Chlamydia trachomatis: its role in tubal infertility. J Infect Dis 152:1275–1282. doi: 10.1093/infdis/152.6.1275 [DOI] [PubMed] [Google Scholar]
- 3. Rodgers AK, Budrys NM, Gong S, Wang J, Holden A, Schenken RS, Zhong G. 2011. Genome-wide identification of Chlamydia trachomatis antigens associated with tubal factor infertility. Fertil Steril 96:715–721. doi: 10.1016/j.fertnstert.2011.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Budrys NM, Gong S, Rodgers AK, Wang J, Louden C, Shain R, Schenken RS, Zhong G. 2012. Chlamydia trachomatis antigens recognized in women with tubal factor infertility, normal fertility, and acute infection. Obstet Gynecol 119:1009–1016. doi: 10.1097/AOG.0b013e3182519326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stephens RS. 2003. The cellular paradigm of chlamydial pathogenesis. Trends Microbiol 11:44–51. doi: 10.1016/s0966-842x(02)00011-2 [DOI] [PubMed] [Google Scholar]
- 6. Igietseme JU, Omosun Y, Nagy T, Stuchlik O, Reed MS, He Q, Partin J, Joseph K, Ellerson D, George Z, Goldstein J, Eko FO, Bandea C, Pohl J, Black CM. 2018. Molecular pathogenesis of Chlamydia disease complications: epithelial-mesenchymal transition and fibrosis. Infect Immun 86:e00585-17. doi: 10.1128/IAI.00585-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhong G. 2009. Killing me softly: chlamydial use of proteolysis for evading host defenses. Trends Microbiol 17:467–474. doi: 10.1016/j.tim.2009.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Z, Chen D, Zhong Y, Wang S, Zhong G. 2008. The chlamydial plasmid-encoded protein pgp3 is secreted into the cytosol of Chlamydia-infected cells. Infect Immun 76:3415–3428. doi: 10.1128/IAI.01377-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhong G. 2011. Chlamydia trachomatis secretion of proteases for manipulating host signaling pathways. Front Microbiol 2:14. doi: 10.3389/fmicb.2011.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qi M, Lei L, Gong S, Liu Q, DeLisa MP, Zhong G. 2011. Chlamydia trachomatis secretion of an immunodominant hypothetical protein (CT795) into host cell cytoplasm. J Bacteriol 193:2498–2509. doi: 10.1128/JB.01301-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gong S, Lei L, Chang X, Belland R, Zhong G. 2011. Chlamydia trachomatis secretion of hypothetical protein CT622 into host cell cytoplasm via a secretion pathway that can be inhibited by the type III secretion system inhibitor compound 1. Microbiology (Reading) 157:1134–1144. doi: 10.1099/mic.0.047746-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferrell JC, Fields KA. 2016. A working model for the type III secretion mechanism in Chlamydia. Microbes Infect 18:84–92. doi: 10.1016/j.micinf.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Valdivia RH. 2008. Chlamydia effector proteins and new insights into chlamydial cellular microbiology. Curr Opin Microbiol 11:53–59. doi: 10.1016/j.mib.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 14. Barker JR, Koestler BJ, Carpenter VK, Burdette DL, Waters CM, Vance RE, Valdivia RH, Taylor R. 2013. STING-dependent recognition of cyclic di-AMP mediates type I interferon responses during Chlamydia trachomatis infection. mBio 4:e00018-13. doi: 10.1128/mBio.00018-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang Z, Tang L, Shao L, Zhang Y, Zhang T, Schenken R, Valdivia R, Zhong G. 2016. The Chlamydia-secreted protease CPAF promotes chlamydial survival in the mouse lower genital tract. Infect Immun 84:2697–2702. doi: 10.1128/IAI.00280-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Y, Huang Y, Yang Z, Sun Y, Gong S, Hou S, Chen C, Li Z, Liu Q, Wu Y, Baseman J, Zhong G. 2014. Plasmid-encoded Pgp3 is a major virulence factor for Chlamydia muridarum to induce hydrosalpinx in mice. Infect Immun 82:5327–5335. doi: 10.1128/IAI.02576-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shao L, Zhang T, Melero J, Huang Y, Liu Y, Liu Q, He C, Nelson DE, Zhong G, Roy CR. 2018. The genital tract virulence factor pGP3 is essential for Chlamydia muridarum colonization in the gastrointestinal tract. Infect Immun 86:e00429-17. doi: 10.1128/IAI.00429-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang T, Huo Z, Ma J, He C, Zhong G. 2019. The plasmid-encoded pGP3 promotes Chlamydia evasion of acidic barriers in both stomach and vagina. Infect Immun 87:e00844-18. doi: 10.1128/IAI.00844-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murthy AK, Li W, Guentzel MN, Zhong G, Arulanandam BP. 2011. Vaccination with the defined chlamydial secreted protein CPAF induces robust protection against female infertility following repeated genital chlamydial challenge. Vaccine 29:2519–2522. doi: 10.1016/j.vaccine.2011.01.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Su X, Xu H, French M, Zhao Y, Tang L, Li X-D, Chen J, Zhong G. 2022. Evidence for cGAS-STING signaling in the female genital tract resistance to Chlamydia trachomatis infection. Infect Immun 90:e0067021. doi: 10.1128/iai.00670-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dhir SP, Agarwal LP, Gupta SB, Detels R, Wang SP, Grayston JT. 1968. Trachoma vaccine trial in india: results of two-year follow-up. Indian J Med Res 56:1289–1294. [PubMed] [Google Scholar]
- 22. Woolridge RL, Grayston JT, Chang IH, Cheng KH, Yang CY, Neave C. 1967. Field trial of a monovalent and of a bivalent mineral oil adjuvant trachoma vaccine in Taiwan school children. Am J Ophthalmol 63:Suppl. doi: 10.1016/0002-9394(67)94158-x [DOI] [PubMed] [Google Scholar]
- 23. Woolridge RL, Grayston JT, Chang IH, Yang CY, Cheng KH. 1967. Long-term follow-up of the initial (1959-1960) trachoma vaccine field trial on Taiwan. Am J Ophthalmol 63:Suppl. doi: 10.1016/0002-9394(67)94159-1 [DOI] [PubMed] [Google Scholar]
- 24. Grayston JT, Wang SP, Woolridge RL, Alexander ER. 1964. Prevention of trachoma with vaccine. Arch Environ Health 8:518–526. doi: 10.1080/00039896.1964.10663711 [DOI] [PubMed] [Google Scholar]
- 25. Pais R, Omosun Y, He Q, Blas-Machado U, Black C, Igietseme JU, Fujihashi K, Eko FO. 2017. Rectal administration of a chlamydial subunit vaccine protects against genital infection and upper reproductive tract pathology in mice. PLoS One 12:e0178537. doi: 10.1371/journal.pone.0178537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ekong EE, Okenu DN, Mania-Pramanik J, He Q, Igietseme JU, Ananaba GA, Lyn D, Black C, Eko FO. 2009. A Vibrio cholerae ghost-based subunit vaccine induces cross-protective chlamydial immunity that is enhanced by CTA2B, the nontoxic derivative of cholera toxin. FEMS Immunol Med Microbiol 55:280–291. doi: 10.1111/j.1574-695X.2008.00493.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ifere GO, He Q, Igietseme JU, Ananaba GA, Lyn D, Lubitz W, Kellar KL, Black CM, Eko FO. 2007. Immunogenicity and protection against genital Chlamydia infection and its complications by a multisubunit candidate vaccine. J Microbiol Immunol Infect 40:188–200. [PubMed] [Google Scholar]
- 28. Morrison SG, Su H, Caldwell HD, Morrison RP. 2000. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infect Immun 68:6979–6987. doi: 10.1128/IAI.68.12.6979-6987.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de la Maza LM, Darville TL, Pal S. 2021. Chlamydia trachomatis vaccines for genital infections: where are we and how far is there to go? Expert Rev Vaccines 20:421–435. doi: 10.1080/14760584.2021.1899817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhong G, Brunham RC, de la Maza LM, Darville T, Deal C. 2019. National institute of allergy and infectious diseases workshop report: "Chlamydia vaccines: the way forward" Vaccine 37:7346–7354. doi: 10.1016/j.vaccine.2017.10.075 [DOI] [PubMed] [Google Scholar]
- 31. Pal S, Tifrea DF, Zhong G, de la Maza LM. 2018. Transcervical inoculation with Chlamydia trachomatis induces infertility in HLA-DR4 transgenic and wild-type mice. Infect Immun 86:e00722-17. doi: 10.1128/IAI.00722-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rockey DD, Wang J, Lei L, Zhong G. 2009. Chlamydia vaccine candidates and tools for chlamydial antigen discovery. Expert Rev Vaccines 8:1365–1377. doi: 10.1586/erv.09.98 [DOI] [PubMed] [Google Scholar]
- 33. Borges ÁH, Follmann F, Dietrich J. 2022. Chlamydia trachomatis vaccine development - a view on the current challenges and how to move forward. Expert Rev Vaccines 21:1555–1567. doi: 10.1080/14760584.2022.2117694 [DOI] [PubMed] [Google Scholar]
- 34. Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, Basto PA, Perro M, Vrbanac VD, Tager AM, Shi J, Yethon JA, Farokhzad OC, Langer R, Starnbach MN, von Andrian UH. 2015. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 348:aaa8205. doi: 10.1126/science.aaa8205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abraham S, Juel HB, Bang P, Cheeseman HM, Dohn RB, Cole T, Kristiansen MP, Korsholm KS, Lewis D, Olsen AW, McFarlane LR, Day S, Knudsen S, Moen K, Ruhwald M, Kromann I, Andersen P, Shattock RJ, Follmann F. 2019. Safety and immunogenicity of the Chlamydia vaccine candidate CTH522 adjuvanted with CAF01 liposomes or aluminium hydroxide: a first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect Dis 19:1091–1100. doi: 10.1016/S1473-3099(19)30279-8 [DOI] [PubMed] [Google Scholar]
- 36. Kari L, Whitmire WM, Olivares-Zavaleta N, Goheen MM, Taylor LD, Carlson JH, Sturdevant GL, Lu C, Bakios LE, Randall LB, Parnell MJ, Zhong G, Caldwell HD. 2011. A live-attenuated chlamydial vaccine protects against trachoma in nonhuman primates. J Exp Med 208:2217–2223. doi: 10.1084/jem.20111266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kari L, Whitmire WM, Crane DD, Reveneau N, Carlson JH, Goheen MM, Peterson EM, Pal S, de la Maza LM, Caldwell HD. 2009. Chlamydia trachomatis native major outer membrane protein induces partial protection in nonhuman primates: Implication for a trachoma transmission-blocking vaccine. J Immunol 182:8063–8070. doi: 10.4049/jimmunol.0804375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bryan ER, Trim LK, Sadowski P, Paramasivan S, Kim JJ, Gough K, Worley S, Maidment TI, Carey AJ, Mihalas B, McLaughlin EA, Beagley KW. 2023. Prophylactic and therapeutic vaccination protects sperm health from Chlamydia muridarum-induced abnormalities. Biol Reprod 108:758–777. doi: 10.1093/biolre/ioad021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eko FO, Okenu DN, Singh UP, He Q, Black C, Igietseme JU. 2011. Evaluation of a broadly protective Chlamydia-cholera combination vaccine candidate. Vaccine 29:3802–3810. doi: 10.1016/j.vaccine.2011.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhong G, Brunham R, Chernesky M, Clarke I, Darville T, JC de Vires H, Gaydos C, Greub G, W. Hook III E, Mabey D, Moncada J, Morre S, Paavonen J, Stary A, Valdivia R, Van Der Pol B, West S. Proceedings of the Julius Schachter Fifteenth International Symposium on Human Chlamydial Infections UT Print; University of Texas Health Science Center at San Antonio; [Google Scholar]
- 41. Zhong G, Quayle A, Aiyar A, Zhang T. 2020. Animal models of Chlamydia Trachomatis infection, p 459–482. In Hegemann H, Sütterlin C (ed), Chlamydia biology: From genome to disease. Caister Academic Press, U.K. [Google Scholar]
- 42. Chen J, Zhang H, Zhou Z, Yang Z, Ding Y, Zhou Z, Zhong E, Arulanandam B, Baseman J, Zhong G, Kwaik YA. 2014. Chlamydial induction of hydrosalpinx in 11 strains of mice reveals multiple host mechanisms for preventing upper genital tract pathology. PLoS ONE 9:e95076. doi: 10.1371/journal.pone.0095076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morrison RP, Caldwell HD. 2002. Immunity to murine chlamydial genital infection. Infect Immun 70:2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Beatty WL, Byrne GI, Morrison RP. 1994. Repeated and persistent infection with Chlamydia and the development of chronic inflammation and disease. Trends Microbiol 2:94–98. doi: 10.1016/0966-842x(94)90542-8 [DOI] [PubMed] [Google Scholar]
- 45. Li LX, McSorley SJ. 2015. A re-evaluation of the role of B cells in protective immunity to Chlamydia infection. Immunol Lett 164:88–93. doi: 10.1016/j.imlet.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao L, Lundy SR, Eko FO, Igietseme JU, Omosun YO. 2023. Genital tract microbiome dynamics are associated with time of Chlamydia infection in mice. Sci Rep 13:9006. doi: 10.1038/s41598-023-36130-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shah AA, Schripsema JH, Imtiaz MT, Sigar IM, Kasimos J, Matos PG, Inouye S, Ramsey KH. 2005. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis 32:49–56. doi: 10.1097/01.olq.0000148299.14513.11 [DOI] [PubMed] [Google Scholar]
- 48. Wang J, Zhang Y, Lu C, Lei L, Yu P, Zhong G. 2010. A genome-wide profiling of the humoral immune response to Chlamydia trachomatis infection reveals vaccine candidate antigens expressed in humans . J Immunol 185:1670–1680. doi: 10.4049/jimmunol.1001240 [DOI] [PubMed] [Google Scholar]
- 49. Lu C, Holland MJ, Gong S, Peng B, Bailey RL, Mabey DW, Wu Y, Zhong G. 2012. Genome-wide identification of Chlamydia trachomatis antigens associated with trachomatous trichiasis. Invest Ophthalmol Vis Sci 53:2551–2559. doi: 10.1167/iovs.11-9212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lu C, Zeng H, Li Z, Lei L, Yeh IT, Wu Y, Zhong G. 2012. Protective immunity against mouse upper genital tract pathology correlates with high IFNgamma but low IL-17 T cell and anti-secretion protein antibody responses induced by replicating chlamydial organisms in the airway. Vaccine 30:475–485. doi: 10.1016/j.vaccine.2011.10.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Angelidou A, Conti M-G, Diray-Arce J, Benn CS, Shann F, Netea MG, Liu M, Potluri LP, Sanchez-Schmitz G, Husson R, Ozonoff A, Kampmann B, van Haren SD, Levy O. 2020. Licensed bacille calmette-guerin (BCG) formulations differ markedly in bacterial viability, RNA content and innate immune activation. Vaccine 38:2229–2240. doi: 10.1016/j.vaccine.2019.11.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hawgood BJ. 1999. Doctor albert calmette 1863-1933: founder of antivenomous serotherapy and of antituberculous BCG vaccination. Toxicon 37:1241–1258. doi: 10.1016/s0041-0101(99)00086-0 [DOI] [PubMed] [Google Scholar]
- 53. Riedel S. 2005. Edward Jenner and the history of smallpox and vaccination. Proc (Bayl Univ Med Cent) 18:21–25. doi: 10.1080/08998280.2005.11928028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhou Z, Tian Q, Wang L, Zhong G, Roy CR. 2022. Chlamydia deficient in plasmid-encoded glycoprotein 3 (pGP3) as an attenuated live oral vaccine. Infect Immun 90. doi: 10.1128/iai.00472-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nouailles G, Adler JM, Pennitz P, Peidli S, Teixeira Alves LG, Baumgardt M, Bushe J, Voss A, Langenhagen A, Langner C, et al. 2023. Live-attenuated vaccine sCPD9 elicits superior mucosal and systemic immunity to SARS-CoV-2 variants in hamsters. Nat Microbiol 8:860–874. doi: 10.1038/s41564-023-01352-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Morrison SG, Giebel AM, Toh E, Banerjee A, Nelson DE, Morrison RP. 2020. A genital infection-attenuated Chlamydia muridarum mutant infects the gastrointestinal tract and protects against genital tract challenge. mBio 11:e02770-20. doi: 10.1128/mBio.02770-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang L, Zhu C, Zhang T, Tian Q, Zhang N, Morrison S, Morrison R, Xue M, Zhong G, Roy CR. 2018. Nonpathogenic colonization with Chlamydia in the gastrointestinal tract as oral vaccination for inducing transmucosal protection. Infect Immun 86:e00630-17. doi: 10.1128/IAI.00630-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang Y, He R, Winner H, Gauduin MC, Zhang N, He C, Zhong G. 2023. Induction of transmucosal protection by oral vaccination with an attenuated Chlamydia. Infect Immun 91:e0004323. doi: 10.1128/iai.00043-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhu C, Lin H, Tang L, Chen J, Wu Y, Zhong G. 2018. Oral Chlamydia vaccination induces transmucosal protection in the airway. Vaccine 36:2061–2068. doi: 10.1016/j.vaccine.2018.03.015 [DOI] [PubMed] [Google Scholar]
- 60. Read TD, Brunham RC, Shen C, Gill SR, Heidelberg JF, White O, Hickey EK, Peterson J, Utterback T, Berry K, Bass S, Linher K, Weidman J, Khouri H, Craven B, Bowman C, Dodson R, Gwinn M, Nelson W, DeBoy R, Kolonay J, McClarty G, Salzberg SL, Eisen J, Fraser CM. 2000. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res 28:1397–1406. doi: 10.1093/nar/28.6.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sharma J, Bosnic AM, Piper JM, Zhong G. 2004. Human antibody responses to a Chlamydia-secreted protease factor. Infect Immun 72:7164–7171. doi: 10.1128/IAI.72.12.7164-7171.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sharma J, Dong F, Pirbhai M, Zhong G. 2005. Inhibition of proteolytic activity of a chlamydial proteasome/protease-like activity factor by antibodies from humans infected with Chlamydia trachomatis. Infect Immun 73:4414–4419. doi: 10.1128/IAI.73.7.4414-4419.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sharma J, Zhong Y, Dong F, Piper JM, Wang G, Zhong G. 2006. Profiling of human antibody responses to Chlamydia trachomatis urogenital tract infection using microplates arrayed with 156 chlamydial fusion proteins. Infect Immun 74:1490–1499. doi: 10.1128/IAI.74.3.1490-1499.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen D, Lei L, Lu C, Galaleldeen A, Hart PJ, Zhong G. 2010. Characterization of Pgp3, a Chlamydia trachomatis plasmid-encoded immunodominant antigen. J Bacteriol 192:6017–6024. doi: 10.1128/JB.00847-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Galaleldeen A, Taylor AB, Chen D, Schuermann JP, Holloway SP, Hou S, Gong S, Zhong G, Hart PJ. 2013. Structure of the Chlamydia trachomatis immunodominant antigen Pgp3. J Biol Chem 288:22068–22079. doi: 10.1074/jbc.M113.475012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Greene W, Xiao Y, Huang Y, McClarty G, Zhong G. 2004. Chlamydia-infected cells continue to undergo mitosis and resist induction of apoptosis. Infect Immun 72:451–460. doi: 10.1128/IAI.72.1.451-460.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cheng W, Shivshankar P, Zhong Y, Chen D, Li Z, Zhong G. 2008. Intracellular interleukin-1alpha mediates interleukin-8 production induced by Chlamydia trachomatis infection via a mechanism independent of type I interleukin-1 receptor. Infect Immun 76:942–951. doi: 10.1128/IAI.01313-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pollack KE, Meneveau MO, Melssen MM, Lynch KT, Koeppel AF, Young SJ, Turner S, Kumar P, Sol-Church K, Mauldin IS, Slingluff CL. 2020. Incomplete freund’s adjuvant reduces arginase and enhances Th1 dominance, TLR signaling and CD40 ligand expression in the vaccine site microenvironment. J Immunother Cancer 8:e000544. doi: 10.1136/jitc-2020-000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Facciolà A, Visalli G, Laganà A, Di Pietro A. 2022. An overview of vaccine adjuvants: current evidence and future perspectives. Vaccines (Basel) 10:819. doi: 10.3390/vaccines10050819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yu H, Jiang X, Shen C, Karunakaran KP, Jiang J, Rosin NL, Brunham RC. 2010. Chlamydia muridarum T-cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection that correlates with a high frequency of gamma interferon (IFN-gamma)/tumor necrosis factor alpha and IFN-gamma/Interleukin-17 double-positive CD4+ T cells. Infect Immun 78:2272–2282. doi: 10.1128/IAI.01374-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Conrad TA, Gong S, Yang Z, Matulich P, Keck J, Beltrami N, Chen C, Zhou Z, Dai J, Zhong G. 2016. The chromosome-encoded hypothetical protein TC0668 is an upper genital tract pathogenicity factor of Chlamydia muridarum. Infect Immun 84:467–479. doi: 10.1128/IAI.01171-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fan T, Lu H, Hu H, Shi L, McClarty GA, Nance DM, Greenberg AH, Zhong G. 1998. Inhibition of apoptosis in Chlamydia-infected cells: blockade of mitochondrial cytochrome C release and caspase activation. J Exp Med 187:487–496. doi: 10.1084/jem.187.4.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pal S, Peterson EM, de la Maza LM. 2005. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect Immun 73:8153–8160. doi: 10.1128/IAI.73.12.8153-8160.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sun G, Pal S, Sarcon AK, Kim S, Sugawara E, Nikaido H, Cocco MJ, Peterson EM, de la Maza LM. 2007. Structural and functional analyses of the major outer membrane protein of Chlamydia trachomatis. J Bacteriol 189:6222–6235. doi: 10.1128/JB.00552-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pal S, Peterson EM, Rappuoli R, Ratti G, de la Maza LM. 2006. Immunization with the Chlamydia trachomatis major outer membrane protein, using adjuvants developed for human vaccines, can induce partial protection in a mouse model against a genital challenge. Vaccine 24:766–775. doi: 10.1016/j.vaccine.2005.08.074 [DOI] [PubMed] [Google Scholar]
- 76. Yu H, Jiang X, Shen C, Karunakaran KP, Brunham RC. 2009. Novel Chlamydia muridarum T cell antigens induce protective immunity against lung and genital tract infection in murine models. J Immunol 182:1602–1608. doi: 10.4049/jimmunol.182.3.1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yu H, Karunakaran KP, Jiang X, Shen C, Andersen P, Brunham RC. 2012. Chlamydia muridarum T cell antigens and adjuvants that induce protective immunity in mice. Infect Immun 80:1510–1518. doi: 10.1128/IAI.06338-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhong G. 2017. Chlamydial plasmid-dependent pathogenicity. Trends Microbiol 25:141–152. doi: 10.1016/j.tim.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Stephens RS, Wagar EA, Schoolnik GK. 1988. High-resolution mapping of serovar-specific and common antigenic determinants of the major outer membrane protein of Chlamydia trachomatis. J Exp Med 167:817–831. doi: 10.1084/jem.167.3.817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Qi M, Gong S, Lei L, Liu Q, Zhong G. 2011. A Chlamydia trachomatis OmcB C-terminal fragment is released into the host cell cytoplasm and is immunogenic in humans. Infect Immun 79:2193–2203. doi: 10.1128/IAI.00003-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hou S, Lei L, Yang Z, Qi M, Liu Q, Zhong G. 2013. Chlamydia trachomatis outer membrane complex protein B (OmcB) is processed by the protease CPAF. J Bacteriol 195:951–957. doi: 10.1128/JB.02087-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tang FF, Chang HL, Huang YT, Wang KC. 1957. Studies on the etiology of trachoma with special reference to isolation of the virus in chick embryo. Chin Med J 75:429–447. [PubMed] [Google Scholar]
- 83. Wang Y. 1999. Etiology of trachoma: a great success in isolating and cultivating Chlamydia trachomatis. Chin Med J (Engl) 112:938–941. [PubMed] [Google Scholar]
- 84. Sun X, Yang Z, Zhang H, Dai J, Chen J, Tang L, Rippentrop S, Xue M, Zhong G, Wu G. 2015. Chlamydia muridarum induction of glandular duct dilation in mice. Infect Immun 83:2327–2337. doi: 10.1128/IAI.00154-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chen L, Lei L, Chang X, Li Z, Lu C, Zhang X, Wu Y, Yeh IT, Zhong G. 2010. Mice deficient in MyD88 develop a Th2-dominant response and severe pathology in the upper genital tract following Chlamydia muridarum infection . J Immunol 184:2602–2610. doi: 10.4049/jimmunol.0901593 [DOI] [PubMed] [Google Scholar]
- 86. Cheng W, Shivshankar P, Li Z, Chen L, Yeh IT, Zhong G. 2008. Caspase-1 contributes to Chlamydia trachomatis-induced upper urogenital tract inflammatory pathologies without affecting the course of infection. Infect Immun 76:515–522. doi: 10.1128/IAI.01064-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Olsen AW, Theisen M, Christensen D, Follmann F, Andersen P. 2010. Protection against Chlamydia promoted by a subunit vaccine (CTH1) compared with a primary intranasal infection in a mouse genital challenge model. PLoS One 5:e10768. doi: 10.1371/journal.pone.0010768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yu H, Karunakaran KP, Kelly I, Shen C, Jiang X, Foster LJ, Brunham RC. 2011. Immunization with live and dead Chlamydia muridarum induces different levels of protective immunity in a murine genital tract model: correlation with MHC class II peptide presentation and multifunctional Th1 cells. J Immunol 186:3615–3621. doi: 10.4049/jimmunol.1002952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Brown THT, David J, Acosta-Ramirez E, Moore JM, Lee S, Zhong G, Hancock REW, Xing Z, Halperin SA, Wang J. 2012. Comparison of immune responses and protective efficacy of intranasal prime-boost immunization regimens using adenovirus-based and CpG/HH2 adjuvanted-subunit vaccines against genital Chlamydia muridarum infection. Vaccine 30:350–360. doi: 10.1016/j.vaccine.2011.10.086 [DOI] [PubMed] [Google Scholar]
- 90. Murdin AD, Su H, Manning DS, Klein MH, Parnell MJ, Caldwell HD. 1993. A poliovirus hybrid expressing a neutralization epitope from the major outer membrane protein of Chlamydia trachomatis is highly immunogenic. Infect Immun 61:4406–4414. doi: 10.1128/iai.61.10.4406-4414.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. He Q, Martinez-Sobrido L, Eko FO, Palese P, Garcia-Sastre A, Lyn D, Okenu D, Bandea C, Ananaba GA, Black CM, Igietseme JU. 2007. Live-attenuated influenza viruses as delivery vectors for Chlamydia vaccines. Immunology 122:28–37. doi: 10.1111/j.1365-2567.2007.02608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Collar AL, Linville AC, Core SB, Frietze KM. 2022. Epitope-based vaccines against the Chlamydia trachomatis major outer membrane protein variable domain 4 elicit protection in mice. Vaccines (Basel) 10:875. doi: 10.3390/vaccines10060875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Eko FO, Mania-Pramanik J, Pais R, Pan Q, Okenu DMN, Johnson A, Ibegbu C, He C, He Q, Russell R, Black CM, Igietseme JU. 2014. Vibrio cholerae ghosts (VCG) exert immunomodulatory effect on dendritic cells for enhanced antigen presentation and induction of protective immunity. BMC Immunol 15:584. doi: 10.1186/s12865-014-0056-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wills JM, Gruffydd-Jones TJ, Richmond SJ, Gaskell RM, Bourne FJ. 1987. Effect of vaccination on feline Chlamydia psittaci infection. Infect Immun 55:2653–2657. doi: 10.1128/iai.55.11.2653-2657.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Burall LS, Rodolakis A, Rekiki A, Myers GSA, Bavoil PM. 2009. Genomic analysis of an attenuated Chlamydia abortus live vaccine strain reveals defects in central metabolism and surface proteins. Infect Immun 77:4161–4167. doi: 10.1128/IAI.00189-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Estivariz CF, Kovacs SD, Mach O. 2023. Review of use of inactivated poliovirus vaccine in campaigns to control type 2 circulating vaccine derived poliovirus (cVDPV) outbreaks. Vaccine 41 Suppl 1:A113–A121. doi: 10.1016/j.vaccine.2022.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li J, Zhan L, Qin C. 2021. The double-sided effects of mycobacterium bovis bacillus calmette-guérin vaccine. NPJ Vaccines 6:14. doi: 10.1038/s41541-020-00278-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Abara WE, Bernstein KT, Lewis FMT, Schillinger JA, Feemster K, Pathela P, Hariri S, Islam A, Eberhart M, Cheng I, Ternier A, Slutsker JS, Mbaeyi S, Madera R, Kirkcaldy RD. 2022. Effectiveness of a serogroup B outer membrane vesicle meningococcal vaccine against gonorrhoea: a retrospective observational study. Lancet Infect Dis 22:1021–1029. doi: 10.1016/S1473-3099(21)00812-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ramsey KH, Cotter TW, Salyer RD, Miranpuri GS, Yanez MA, Poulsen CE, DeWolfe JL, Byrne GI. 1999. Prior genital tract infection with a murine or human biovar of Chlamydia trachomatis protects mice against heterotypic challenge infection. Infect Immun 67:3019–3025. doi: 10.1128/IAI.67.6.3019-3025.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Caspe SG, Livingstone M, Frew D, Aitchison K, Wattegedera SR, Entrican G, Palarea-Albaladejo J, McNeilly TN, Milne E, Sargison ND, Chianini F, Longbottom D, Dean D. 2020. The 1B vaccine strain of Chlamydia abortus produces placental pathology indistinguishable from a wild type infection. PLoS ONE 15:e0242526. doi: 10.1371/journal.pone.0242526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Longbottom D, Sait M, Livingstone M, Laroucau K, Sachse K, Harris SR, Thomson NR, Seth-Smith HMB. 2018. Genomic evidence that the live Chlamydia abortus vaccine strain 1B is not attenuated and has the potential to cause disease. Vaccine 36:3593–3598. doi: 10.1016/j.vaccine.2018.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Chen C, Zhou Z, Conrad T, Yang Z, Dai J, Li Z, Wu Y, Zhong G. 2015. In vitro passage selects for Chlamydia muridarum with enhanced infectivity in cultured cells but attenuated pathogenicity in mouse upper genital tract. Infect Immun 83:1881–1892. doi: 10.1128/IAI.03158-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Shao L, Zhang T, Liu Q, Wang J, Zhong G, Roy CR. 2017. Chlamydia muridarum with mutations in chromosomal genes tc0237 and/or tc0668 is deficient in colonizing the mouse gastrointestinal tract. Infect Immun 85:e00321-17. doi: 10.1128/IAI.00321-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Koprivsek JJ, Zhang T, Tian Q, He Y, Xu H, Xu Z, Zhong G. 2019. Distinct roles of chromosome- versus plasmid-encoded genital tract virulence factors in promoting Chlamydia muridarum colonization in the gastrointestinal tract. Infect Immun 87:e00265-19. doi: 10.1128/IAI.00265-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Koprivsek JJ, He Y, Song C, Zhang N, Tumanov A, Zhong G. 2020. Evasion of innate lymphoid cell-regulated gamma interferon responses by Chlamydia muridarum to achieve long-lasting colonization in mouse colon. Infect Immun 88:e00798-19. doi: 10.1128/IAI.00798-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. He Y, Xu H, Song C, Koprivsek JJ, Arulanandam B, Yang H, Tao L, Zhong G. 2021. Adoptive transfer of group 3-like innate lymphoid cells restores mouse colon resistance to colonization of a gamma interferon-susceptible Chlamydia muridarum mutant. Infect Immun 89:e00533-20. doi: 10.1128/IAI.00533-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. He Y, Wang Y, He R, Abdelsalam AM, Zhong G. 2023. IL-23 receptor signaling licenses group 3-like innate lymphoid cells to restrict a live-attenuated oral Chlamydia vaccine in the gut. bioRxiv. doi: 10.1101/2023.09.11.557246 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig.1S shows the individual antigen-exacerbated hydrosalpinx scores from Fig.3 while Table 1S lists both chlamydial burdens and hydrosalpinx scores for all antigens tested.