Abstract
Background:
Per- and polyfluoroalkyl substances (PFAS) are chemical substances spread throughout the environment worldwide. Exposure during pregnancy represents a specific window of vulnerability for child health.
Objective:
Our objective was to assess the impact of prenatal exposure to multiple PFAS on emotional and behavioral functions in 12-y-old children.
Method:
In the PELAGIE mother–child cohort (France), prenatal exposure to nine PFAS was measured from concentrations in cord serum samples. Behavior was assessed at age 12 y using the parent-reported Strengths and Difficulties Questionnaire (SDQ) and the self-reported Dominic Interactive for Adolescents (DIA) for 444 children. Associations were estimated using negative binomial models for each PFAS. Bayesian kernel machine regression (BKMR) models were performed to assess the exposure mixture effect on children’s behavior.
Results:
In our study population, 73% of mothers had spent more than 12 y in education. Higher scores on SDQ externalizing subscale were observed with increasing cord-serum concentration of perfluorooctanoic acid (PFOA) and perfluorononanoic acid (PFNA) [adjusted mean ratio , 95% confidence interval (CI): 1.03, 1.34, and (95% CI: 1.00, 1.29) for every doubling of concentration, respectively]. Results for the hyperactivity score were similar [ (95% CI: 1.04, 1.40) and (95% CI: 1.02, 1.36), respectively]. With regard to major depressive disorder and internalizing subscales, perfluorodecanoic acid (PFDA) was associated with higher self-reported DIA scores [ (95% CI: 1.01, 1.27) and (95% CI: 1.02, 1.21), respectively]. In terms of the anxiety subscale, PFDA and PFNA were associated with higher scores [ (95% CI: 1.02, 1.21) and (95% CI: 1.01, 1.19), respectively]. Concurrent increases in the PFAS concentrations included in the BKMR models showed no change in the SDQ externalizing and DIA internalizing subscales scores.
Conclusion:
Prenatal exposure to PFNA and PFOA were associated with increasing scores for measures of externalizing behaviors, specifically hyperactivity. We also identified associations between PFNA and PFDA prenatal exposure levels and increasing scores related to internalizing behaviors (general anxiety and major depressive disorder), which adds to the as yet sparse literature examining the links between prenatal exposure to PFAS and internalizing disorders. https://doi.org/10.1289/EHP12540
Introduction
Per- and polyfluoroalkyl substances (PFAS) form a group of more than 9,000 synthetic chemicals that have been used worldwide for more than 70 y. Their surfactant properties have ensured their entry into the composition of everyday consumer products—including protectants for paper and cardboard packaging, carpets, leather, textiles, food packaging, nonstick cookware, and firefighting foam.1 As a result of their long half-lives (estimated between 2 and 30 y in humans), PFAS are both widespread and persistent in the environment.1 For the general population, the major exposure routes are via food and water ingestion, though exposure can also occur through inhalation and dust ingestion.1 The main PFAS dietary sources are fish, seafood and food items contaminated by coated food packaging.2 Many studies have highlighted the ubiquity of contamination among humans,3 including among pregnant women.4 PFAS have been also detected in breast milk and cord serum samples,5,6 and these finding highlight the ability of PFAS to cross the placental barrier,7 resulting in fetal exposure.8,9 Results from both animal and in vitro studies suggest that some PFAS are developmental neurotoxicants affecting several neurochemical targets in the developing brain.8,9 Mechanisms include changes in calcium homeostasis, protein kinase C, synaptic plasticity, cellular differentiation, and alterations to the thyroid hormones.10,11
Epidemiological studies have examined the links between prenatal exposure to PFAS and neurodevelopment end points, in particular with regard to behavioral disorders. A 2018 review by Liew et al.12 stated that these links were fairly inconclusive. For PFOA specifically, two other reviews drew the same conclusions.13,14 The majority of the 12 prospective cohort studies reviewed by Liew et al.12 had examined the associations between prenatal exposure to some PFAS and attention-deficit/hyperactivity disorder (ADHD) (3 studies), behavior disorders (4 studies) or autistic traits (4 studies) among children 5–12 y of age. Just one study noted that prenatal exposure to perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) was associated with hyperactivity in children age 5–9 y old.15 Recent studies have since reported the adverse impact of prenatal exposure to PFAS on child behavior, including increased ADHD,6,16 attention deficits,2 or externalizing behavioral difficulties17–23—including, moreover, conduct disorders and oppositional defiant disorder. However, other recent studies reported reverse24,25 or no associations,26–30 leaving conclusions still unclear. Several studies observed females showing more externalizing disorders following prenatal2,6,19 or postnatal exposure30 to several PFAS, in comparison with males. However, this literature remains both scarce and inconsistent.
Internalizing behavior disorders include emotional troubles, major depressive disorder, generalized anxiety disorder, and specific phobia.31 Children and adolescents showing internalizing disorders may also present with externalizing behavior problems, and some studies suggest that internalizing and externalizing disorders may involve common brain structures and circuits.32–34 Few human studies on the neurodevelopmental toxicity of PFAS have thus far considered internalizing behaviors,20,28,30 and even these have still provided only inconclusive results: Higher internalizing difficulties among children were reported in association with prenatal exposure to perfluorohexane sulfonic acid (PFHxS)20 as well as to perfluorodecanoic acid (PFDA), perfluorononanoic acid (PFNA) and perfluoroheptane sulfonic acid (PFHpS),19 whereas in two other studies, no association was observed.28,30 Liew et al.11 concluded that more studies are needed on internalizing difficulties such as depression and that (in the studies they reviewed) there was a lack of consideration of the PFAS mixture. Such effects of this mixture have been investigated in a few recent studies, with mixed results.16,18,19,30
The aim of the present study was to assess the association between prenatal exposure to several PFAS and externalizing and internalizing behavior among 12-y-olds. PFAS were studied both individually and as a mixture.
Methods
Study Population
The Perturbateurs endocriniens: Etude Longitudinale sur les Anomalies de la Grossesse, l’Infertilité et l’Enfance (PELAGIE) mother–child cohort included 3,421 pregnant women from Brittany, France and covered the period 2002–2006. Women were recruited at their first prenatal visit by their gynecologist, obstetrician, or ultrasonography practitioner. The inclusion criteria required that women completed the inclusion questionnaire before 19 wk of pregnancy and were still pregnant at that time. At the time of delivery, cord-blood samples were collected from 2,138 women (62%). By the time the children reached the age of 12 y, 887 (41.5%) families were lost to follow-up, and for 60 families (2.8%) no cord blood sample remained; 1,191 were thus eligible for the clinical exam. A total of 933 (78.4%) of these families were reached by telephone, and 559 (59.9%) children agreed to undergo a clinical examination in a hospital setting (see Figure S1).
At inclusion and when their children were 2, 6, and 12 y of age, mothers completed a self-administered questionnaire at home on their family, social and demographic characteristics, diet and lifestyle, as well as on the child’s health. At birth, hospitals provided information about the pregnancy, pregnancy outcome, and the child’s health. Cord blood samples were collected, and persistent organic pollutants (POPs) were measured.35
The adults participating in this study provided written informed consent and the 12-y-old children provided written assent. Written informed consent was obtained from parents/legal guardians for all participants under 18 y of age. The study was approved by the Advisory Committee on Information Processing in Health Research (CCTIRS; 2015; no. 15.326bis), the Committee for the Protection of Persons (CPP; 2015; no. 15/23-985), and the French National Commission for Information Technology and Civil Liberties (CNIL; 2002, 2015; no. 915420/2015-456).
Child’s Behavior Assessment
Parents assessed their children’s behavior the year the child turned 12 y old, using the French version of the Strengths and Difficulties Questionnaire (SDQ).36,37 A total of 420 of 444 questionnaires (94.6%) were completed by the mother alone, 14 (3.2%) by the father alone, and 10 (2.2%) by both parents. The SDQ asks questions about 25 attributes divided between 5 scales of 5 items each. Respondents use a 3-point Likert scale scored from 0 (not true), 1 (somewhat true), to 2 (certainly true) to indicate the extent to which each attribute applies to the child in question. Scores ranging from 0 to 10 (sum of item responses) are then generated for emotional symptoms, conduct problems, hyperactivity-inattention, peer problems, and prosocial behavior.37
Emotional symptoms and peer problems scores can be summed into an internalizing subscale, and hyperactivity/inattention and conduct problems scores can be summed into an externalizing subscale. As recommended by Goodman et al.,38 these broader internalizing and externalizing composite scales (ranging from 0 to 20) can be fruitfully deployed in analyses of low-risk samples from the general population. The parent-reported French SDQ versions have been shown to accurately measure psychopathological symptoms in young people (especially in cases of externalizing troubles).39
As children mature, the use of self-reported questionnaires should be considered, especially for measurement of internalizing troubles.31 The Dominic Interactive for Adolescents (DIA) was thus chosen for this study; it is a highly structured, multimedia self-report screen that uses visual and auditory stimuli to assess the current symptoms defining seven of the mental disorders most frequently found in adolescents 12–15 y of age. The DIA comprises 91 items (pictures and questions) and assesses four internalizing symptoms: major depressive disorder (MDD; 19 items), generalized anxiety disorder (GAD; 16 items), specific phobia (SPh; 8 items) and substance abuse disorder (SAD; 6 items), as well as three externalizing disorders: ADHD (18 items), oppositional defiant disorder (ODD; 9 items), and conduct disorder (CD; 15 items).40 The internalizing composite scale is the sum of major depressive disorder, generalized anxiety disorder, specific phobia, and substance abuse disorder subscales (48 items), and the externalizing composite scale is the sum of ADHD, ODD, and CD subscales (42 items). The health personnel conducting the clinical exam, who had no access to the results at the end of the test, offered the children the option of completing it alone in a room to minimize possible inhibition or control in children’s answers. The psychometric properties of the DIA were evaluated,40,41 and they showed good internal consistency; the range of coefficients remained fairly constant across age, sex, language, and type of subsample (school, clinical).41
In the main analysis, the parent-reported SDQ was used to assess children’s hyperactivity and externalizing disorders to allow comparisons, because evaluation of children’s behavioral disturbances in the literature is typically based on parents’ (or teachers’) reports. As mentioned above, the self-reported DIA test was preferred for the assessment of internalizing disorders, general anxiety, and major depressive disorder, because these are self-reported by children and meet the classification of the Diagnostic and Statistical Manual of Mental Disorders (DSM).40–42
Given that both informants (parent and child) might provide unique information about child’s behavior,43 complementary analyses were conducted on the externalizing and ADHD scales derived from the self-reported DIA and on internalizing behaviors derived from the parent-reported SDQ.
Where one or two items were missing from questionnaire data, the corresponding subscale score was extrapolated from the remaining available items (done for , including five participants with two missing values for hyperactivity scale and , including one participant with two missing values for the conduct troubles scale). Eleven children were excluded either because questionnaire data were missing () or because they had three (or more) missing SDQ items within a scale (), and some because they were receiving regular or prolonged treatment in the form of tranquilizers or antidepressant or psychostimulant neuroleptic drugs ().
Exposure Assessment
Prenatal exposure to nine PFAS [perfluorobutanoic acid (PFBA), perfluorohexanoic acid (PFHxA), PFOA, PFNA, PFDA, perfluoroundecanoic acid (PFUnDA), perfluorobutane sulfonic acid (PFBS), PFHxS, and PFOS] was assessed by measuring biomarkers in cord blood serum samples by the Centre de Toxicologie du Québec (CTQ) at the Institut National de Santé Publique du Québec (INSPQ).
The PFAS were extracted from the cord serum samples () using the solid phase extraction (SPE) technique with a weak anion exchange resin. The extracts were evaporated to dryness and taken up in a ammonium acetate in 40% methanol solution. Samples were analyzed using ultra performance liquid chromatography with tandem mass spectrometry (UPLC-MS/MS); the method has been described in more detail elsewhere.44 The limit of detection (LOD) varied from 0.05 to , depending on the analytes. The LOD was calculated using 3 times the standard deviation (SD) on 30 replicates, analyzed in different analytical runs, of a serum sample at a concentration between 5 and 7 times the estimated concentration, yielding a signal to noise of 3:1. Intraday precision varied between 2.6% and 8.1%, and interday precision varied between 5.4% and 7.4%, depending on the analytes.
Of the 548 children who had undergone the clinical examination at 12 y of age and did not meet the exclusion criteria (i.e., missing behavior score or treated on a regular or prolonged basis with tranquilizers or antidepressant or psychostimulant neuroleptic drugs, ), 444 had their cord blood samples analyzed for PFAS measurements. Concentrations were reported as wet weight (units of micrograms per liter). Missing data, because of analytical interferences in some samples for the analytes PFNA (), PFDA (), and PFUnDA (), confirmed by qualifying ion ratio that did not meet the expected criterion of , were then excluded.
Several POPs had already been measured, as reported previously,35 and these measurements were used in this study for sensitivity analysis on the influence of coexposures. Analyses were performed in cord-blood serum samples by the CTQ at the INSPQ: 14 polychlorinated biphenyls (PCBs), (PCBs 28, 52, 74, 99, 101, 118, 138, 153, 170, 180, 183, 187, 194, 203), 8 organochlorine pesticides [OCPs: (), (), hexachlorobenzene (HCB), dieldrin, dichlorodiphenyltrichloroethane (p,p′-DDT), dichlorodiphenyldichloroethylene (p,p′-DDE), heptachlor epoxide, ], and 3 polybrominated diphenyl ethers (PBDEs), 47, 99, 209. Total lipid level data (g/L) were also available.
Covariates and Potential Confounding
Potential covariates were identified a priori, based on existing literature on in utero risk factors for mental health and behavior [maternal stress and anxiety,45–47 smoking, drug or alcohol consumption during pregnancy,31,45–49 maternal prepregnancy body mass index (BMI),48 hypertensive disorders during pregnancy, preeclampsia and other pregnancy complications,46,48,49 exposure to environmental chemicals,31,45,47 and low birth weight and prematurity45–47,49]. Refined selection was made using a directed acyclic graph (DAG) approach (see Figure S2).50 Covariates were obtained from the PELAGIE questionnaires completed at inclusion (early in pregnancy), at birth, and up to the child’s 12th birthday. We considered child sex, birth weight, and small for gestational age (SGA; ); maternal age at inclusion, number of years in full-time education for mother, marital status at birth (single or not), parity, prepregnancy BMI, smoking status and alcohol intake during pregnancy, usual fish intake before pregnancy (because fish and seafood may accumulate persistent organic contaminants and heavy metals), and pregnancy complications (hypertension, infection in utero). The final adjustment set included: maternal age (in years), maternal prepregnancy BMI (in three categories: , , ), number of years in full-time education for mother (in three groups: y, 12 y, y), parity (nulliparous vs. multiparous), maternal prepregnancy fish intake ( once a month, at least once a month, at least twice a week), smoking status at inclusion (smoker vs. nonsmoker), and child sex. Selected covariates had very few missing data () and were then simply imputed with the mode.
Statistical Analyses
Comparisons between individuals included in this study and the original cohort were performed using chi-square or Fisher tests for categorical variables or Student’s -tests for mean comparison after having checked applicability criteria. For behavioral scores, internal consistency was checked using polychoric and tetrachoric Cronbach’s alpha calculation. Because some items in the DIA are used in multiple subscales, Cronbach’s alpha for composite internalizing and externalizing subscales was calculated using items only once. Score distributions between males and females were compared using Wilcoxon tests. Spearman rank correlations were used to estimate agreement between parent-reported SDQ and self-reported DIA internalizing and externalizing behavior scores.
Compounds detected in fewer than 5% of cord-serum samples were excluded from the analyses; this was the case for PFBA, PFHxA and PFBS. The other six PFAS cord-serum concentrations were detected in of the samples and were then after graphical check for distribution log-normality.
Because all machine concentration readings below the LOD were available for PFAS, these values (rather than data imputation) were used for the analyses. Spearman rank correlation coefficients were calculated between values of cord-serum PFAS concentrations.
Overall, 444 individuals with prenatal exposure to at least one PFAS compound measured in cord-serum sample and behavior scores were included in the analyses.
Single-pollutant approach.
For each PFAS, we used negative binomial regression models to explore associations between cord-serum concentrations and each of the scores (after checking for data overdispersion). Results are presented as adjusted mean ratio (aMR) of behavioral score with 95% confidence intervals (CIs), reflecting the relative change in the mean score with every doubling of the PFAS cord-serum concentration. All models were adjusted for the potential confounders listed above.
Linearity was investigated using generalized additive models (GAM) including restricted cubic splines. Nonlinearity was quantified using the effective degree of freedom (edf), which is a summary statistic of GAM. An edf equal to 1 reflects a linear relationship, corresponds to a weakly nonlinear relationship, and can be considered a nonlinear relationship.51,52 Models with exposure categorized in quartiles were retained when the edf was . To evaluate the possible sex-specific effect of PFAS on behavioral scores, we introduced to the models a cross-product term between child sex and PFAS exposure variable and retained statistical significance at . Stratified analyses on child sex were then performed to determine whether results were qualitatively similar between groups.
Multiple-pollutant approach: Bayesian kernel machine regression.
We used Bayesian kernel machine regression (BKMR), a nonparametric high-dimensional exposure–response function resulting from component-wise variable selection (50,000 iterations) to investigate nonlinear associations, interactions, and joint effects of the mixture of PFAS.53 Each compound within the model is assigned a posterior inclusion probability (PIP), indicating the relative importance of each exposure. We used a threshold for posterior inclusion probability of 0.5 (i.e., considered an important compound in a mixture).54
Only the six PFAS exposures accounting for more than 70% of detected data were included as continuous data and were centered on the median and interquartile range (IQR)scaled. Although our outcomes were score counts, we used Gaussian regression in the BKMR models. Potential confounders identified earlier were included in the models.
Sensitivity analysis.
Although breastfeeding has been associated with children’s mental health, it was not considered in our a priori set of potential confounders because prenatal PFAS concentrations have previously been associated with decreased breastfeeding duration.55,56 To control for possible confounding, single-pollutant models were additionally adjusted for breastfeeding duration (none, months, months) (excluding missing data ).
Both maternal and paternal mental health have been associated with children’s internalizing and externalizing behavior.57,58 Furthermore, parental mental health can influence the way they evaluate their own child’s behavior.59 At age 12 y, the respondent parent’s mental health was measured using the Short-Form 36 (SF36) questionnaire’s emotional well-being subscale.60 The parent completing the questionnaire (overwhelmingly the mother, as mentioned above) was answering both the SDQ for the child and the SF36 part for his or her own mental health. When both parents declared having answered the questionnaire (), we assumed that the SF36 questionnaire was completed by the mother. We then performed a second sensitivity analysis, adding this covariate into the single-pollutant models for each behavioral score.
Because prenatal exposure to other pollutants [including POPs such as PCBs and PBDEs or organochlorine (OC) pesticides] has already been identified as linked to child behavior,61,62 we performed a third sensitivity analysis to verify the potential influence of coexposures. PCBs, PBDEs, and OC pesticides were available for a reduced sample size, ranging from 401 to 415, depending on the compound considered. Values below the LOD were randomly imputed from a log-normal probability distribution, the parameters of which were estimated using a maximum-likelihood method.63 Concentrations were for compounds detected in of the population and categorized for compounds detected in . Compounds detected in fewer than 5% of samples were not included in the analyses. To identify potential coexposures linked to outcomes, we used separate negative binomial regression models adjusted for potential confounders (same set as for the main models). Given that PCBs, OC pesticides, and PBDEs are lipophilic compounds,64 total lipid levels were included in these models as covariates. Spearman rank correlation coefficients were also calculated between cord serum PFAS and POP concentrations. For each outcome and each PFAS, models performed in the single-pollutant approach were then run adding all coexposure variables linked at with the outcome. The multiple pollutant approach with BKMR models was also performed, adjusting models for potential confounders and coexposures.
Analyses were performed using R (version 4.0.2; R Development Core Team). Results were considered statistically significant when the -value was .
Results
Mother–child pairs’ characteristics are shown in Table 1. Mothers were a mean of 30.8 () years of age at inclusion, and 13% () were overweight or obese prior to pregnancy. Twenty-three percent of mothers reported that they were smokers at the beginning of pregnancy. Seventy-three percent had spent more than 12 y in education. At the time of the clinical exam, the children were 12.8 y of age on average.
Table 1.
Study population characteristics (), PELAGIE mother–child cohort, Brittany, France.
| Characteristics | (%) or |
|---|---|
| Mother | |
| Age at inclusion | |
| y | 30.80 () |
| Age group | |
| y | 0 |
| 20 to y | 23 (5.2) |
| 25 to y | 172 (38.7) |
| 30 to y | 178 (40.1) |
| 35 to y | 67 (15.1) |
| 40 y or more | 4 (0.9) |
| Parity | |
| No child | 169 (38.1) |
| At least one child | 275 (61.9) |
| Prepregnancy BMI | |
| kg/m² | 22.05 () |
| BMI group | |
| 38 (8.6) | |
| to | 345 (78.2) |
| to | 45 (10.2) |
| or more | 13 (2.9) |
| Mother’s education | |
| y | 48 (10.8) |
| 12 y | 71 (16) |
| y | 325 (73.2) |
| Tobacco statusa | |
| No or former smoker | 341 (77.3) |
| Smoker at the beginning of pregnancy | 100 (22.7) |
| Food intake–fish consumption before pregnancyb | |
| once/month | 52 (11.7) |
| At least once a month | 256 (57.8) |
| At least twice a week | 135 (30.5) |
| Birth | |
| Gestational age | |
| gestational weeks | 39.58 () |
| Birth weight | |
| g | 3,467.26 () |
| Small for gestational age () | |
| No | 431 (97.1) |
| Yes | 13 (2.9) |
| Breastfeedingc | |
| None | 106 (28.8) |
| months | 103 (28) |
| months months | 98 (26.6) |
| months | 61 (16.6) |
| Parental mental health (when child was age 12 y) | |
| Short-Form 36 – Emotional well-beingd,e | |
| 69.13 () | |
| Child sex at birth | |
| Males | 224 (50.5) |
| Females | 220 (49.5) |
| Child age at examination | |
| y | 12.81 () |
Note: BMI, body mass index; SD, standard deviation.
Missing data: .
Missing data: .
Missing data: .
Missing data: .
Responding parent: mother, .
In comparison to the original cohort (), women included in this study () were better educated [47% with y vs. 32% for women not included (), ], were older [mean age (SD) 30.80 y (3.88) vs. 29.99 y (4.35), ], were less likely to be primipara (), had lower prepregnancy BMI (), smoked less at the beginning of pregnancy (77% were nonsmoker vs. 71%, ), and ate fish more often before pregnancy (). Their babies were born with higher gestational age [: 39.58 wk (1.25) vs. 39.10 wk (2.48)] than those not included in the study () had higher birth weights [: () vs. (), ], and were less likely to be small for gestational age () than those not included. Details are displayed in Table S1.
Tables 2 and 3, respectively, present the results for parent-reported SDQ, as well as self-reported DIA scores for the entire sample population and for males and females separately. Detailed results of the SDQ and DIA subscales are available in Tables S2 and S3. Males had higher median scores than females on both the SDQ externalizing subscale [4 (range: 0–17) vs. 2 (range: 0–13), respectively; ] and the hyperactivity subscale [3 (range: 0–10) vs. 1 (range: 0–9) for males and females, respectively; ] as well as for externalizing [8 (range: 0–28) vs. 6 (range: 0–24); ] and ADHD scores [6 (range: 0–18) vs. 4 (range: 0–16); ] for males and females, respectively, on the DIA scale. Females had higher median scores than males on the DIA internalizing subscale [9 (range: 0–39) vs. 11 (range 0–31), respectively; ].
Table 2.
Parent-reported SDQ. Scores at age 12 y, PELAGIE mother–child cohort, Brittany, France (). Values reported are the median (minimum–maximum) scores.
| Sex | Externalizing | Hyperactivity | Internalizing |
|---|---|---|---|
| Total () | 3 (0–17) | 2 (0–10) | 3 (0–15) |
| Males () | 4 (0–17) | 3 (0–10) | 3 (0–14) |
| Females () | 2 (0–13) | 1 (0–9) | 4 (0–15) |
| -Valuea | 0.009 |
Note: SDQ, Strengths and Difficulties Questionnaire.
Wilcoxon test -value comparing males vs. females. See Table S2 for results for every subscale.
Table 3.
Self-reported DIA. Scores at age 12 y, PELAGIE mother–child cohort, Brittany, France (). Values reported are the median (minimum–maximum) scores.
| Sex | Externalizing | ADHD | Internalizing | Anxiety | Major depressive disorder |
|---|---|---|---|---|---|
| Total () | 7 (0–28) | 5 (0–18) | 10 (0–39) | 5 (0–15) | 3 (0–17) |
| Males () | 8 (0–28) | 6 (0–18) | 9 (0–39) | 5 (0–15) | 3 (0–17) |
| Females () | 6 (0–24) | 4 (0–16) | 11 (0–31) | 5 (0–15) | 3 (0–12) |
| -Valuea | 0.0001 | 0.002 | 0.002 | 0.4 |
Note: ADHD, attention-deficit/hyperactivity disorder; DIA Dominic Interactive for Adolscents.
Wilcoxon test -value comparing males vs. females. See Table S3 for results for every subscale.
Cronbach’s alpha (polychoric) was 0.84 and 0.86 for the SDQ hyperactivity and externalizing subscales, respectively, and 0.81 for the SDQ internalizing behavior subscale. For the DIA anxiety, major depressive, and internalizing subscales, tetrachoric Cronbach’s alpha was 0.80, 0.87, and 0.89, respectively, and 0.91 and 0.90 for ADHD and externalizing subscales (see Tables S4 and S5 for the coefficients for other subscales). Spearman rank correlations between SDQ and DIA were higher for externalizing behaviors () than for internalizing ().
Table 4 shows the distribution of cord serum concentrations of PFAS in our sample. Concentrations above the LOD were measured in more than 70% of samples for PFHxS (100%), PFOS (100%), PFOA (100%), PFNA (99.8%), PFDA (88.4%), and PFUnDA (73.2%). PFOS and PFOA had the highest medians [ and , respectively, and the strongest correlations were between PFNA and PFDA () and PFNA and PFOA (); see Figure S3].
Table 4.
Distribution of cord-blood serum concentrations of PFAS, including machine concentration readings below the LOD available, PELAGIE mother–child cohort, Brittany, France ().
| LOD () | ND (%) | Min | 5% | 10% | 25% | 50% | 75% | 90% | 95% | Max | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFOA | 0.07 | 444 | 0 (0) | 0.31 | 1.04 | 1.32 | 1.70 | 2.24 | 2.84 | 3.72 | 4.14 | 7.36 |
| PFNA | 0.09 | 433 | 1 (0.2) | 0.07 | 0.16 | 0.20 | 0.26 | 0.35 | 0.46 | 0.58 | 0.67 | 1.55 |
| PFDA | 0.06 | 406 | 47 (11.6) | 0.02 | 0.05 | 0.06 | 0.07 | 0.10 | 0.13 | 0.16 | 0.18 | 0.51 |
| PFUnDA | 0.05 | 422 | 113 (26.8) | 0.00 | 0.00 | 0.03 | 0.05 | 0.07 | 0.09 | 0.12 | 0.14 | 0.30 |
| PFHxS | 0.06 | 444 | 0 (0) | 0.06 | 0.26 | 0.31 | 0.42 | 0.56 | 0.70 | 0.95 | 1.09 | 3.17 |
| PFOS | 0.2 | 444 | 0 (0) | 0.54 | 2.24 | 2.78 | 3.52 | 4.61 | 5.89 | 7.63 | 8.50 | 27.17 |
Note: PFBA, PFHxA, and PFBS were detected in of samples (0.5%, 2.5% and 1.3% respectively). LOD, limit of detection; ND, nondetected; PFBA, perfluorobutanoic acid; PFBS, perfluorobutanesulfonic acid; PFDA, perfluorodecanoic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFUnDA, perfluoroundecanoic acid.
Associations between Cord-Blood PFAS Concentrations and Child Behavioral Scores
Single-pollutant approach: main analysis.
Table 5 presents the main results for the associations between PFAS prenatal exposure and SDQ externalizing and hyperactivity scores and DIA internalizing, general anxiety, and major depressive disorders scores. Linearity was not rejected for any PFAS (see Figure S4 for results of the splines regression models), and no heterogeneity was identified between males and females (results for interaction term significance and for stratified models are displayed in Figures S5 and S6 and Table S6). Higher scores on the SDQ externalizing subscale were observed with increasing cord serum concentration of PFOA and PFNA [ (95% CI: 1.03, 1.34) and (95% CI: 1.00, 1.29) for every doubling of PFAS concentration, respectively]. Hyperactivity score results were similar [ (95% CI: 1.04, 1.40) and (95% CI: 1.02, 1.36), respectively]. Concerning major depressive and internalizing subscales, higher PFDA concentrations were associated with higher scores [ (95% CI: 1.01, 1.27) and (95% CI: 1.02, 1.21), respectively, for every doubling of concentration]. In terms of the anxiety subscale, both PFDA and PFNA concentrations were associated with higher scores [ (95% CI: 1.02, 1.21) and (95% CI: 1.01, 1.19), respectively].
Table 5.
Associations between prenatal exposure measured in cord serum and behavior scores at age 12 y. Results from the single-pollutant approach, PELAGIE mother–child cohort, Brittany, France ().
| Outcome | Exposure | |||||
|---|---|---|---|---|---|---|
| PFOA (
) aMR (95% CI) |
PFNA (
) aMR (95% CI) |
PFDA (
) aMR (95% CI) |
PFUnDA (
) aMR (95% CI) |
PFHxS (
) aMR (95% CI) |
PFOS (
) aMR (95% CI) |
|
| Main analysis | ||||||
| SDQ externalizing | 1.18 (1.03, 1.34) | 1.14 (1.00, 1.29) | 1.07 (0.94, 1.21) | 1.00 (0.88, 1.13) | 1.02 (0.90, 1.16) | 1.06 (0.94, 1.21) |
| SDQ hyperactivity | 1.20 (1.04, 1.40) | 1.18 (1.02, 1.36) | 1.11 (0.96, 1.30) | 0.99 (0.85, 1.14) | 1.07 (0.93, 1.23) | 1.08 (0.94, 1.26) |
| DIA internalizing | 1.05 (0.96, 1.14) | 1.08 (1.00, 1.17) | 1.11 (1.02, 1.21) | 1.06 (0.97, 1.15) | 1.02 (0.94, 1.10) | 1.02 (0.94, 1.11) |
| DIA anxiety | 1.06 (0.98, 1.16) | 1.10 (1.01, 1.19) | 1.11 (1.02, 1.21) | 1.07 (0.98, 1.16) | 1.02 (0.94, 1.11) | 1.01 (0.93, 1.10) |
| DIA depression | 1.08 (0.97, 1.21) | 1.11 (1.00, 1.23) | 1.14 (1.01, 1.27) | 1.05 (0.95, 1.17) | 1.02 (0.92, 1.13) | 1.02 (0.92, 1.14) |
| Complementary analysis | ||||||
| DIA externalizing | 1.05 (0.94, 1.17) | 1.03 (0.93, 1.14) | 1.13 (1.01, 1.27) | 1.04 (0.94, 1.16) | 1.03 (0.93, 1.14) | 1.00 (0.90, 1.11) |
| DIA ADHD | 1.07 (0.96, 1.19) | 1.06 (0.96, 1.18) | 1.16 (1.04, 1.30) | 1.05 (0.95, 1.16) | 1.06 (0.96, 1.17) | 1.03 (0.92, 1.14) |
| SDQ internalizing | 1.06 (0.94, 1.20) | 0.94 (0.84, 1.05) | 0.97 (0.86, 1.09) | 0.95 (0.85, 1.06) | 1.04 (0.93, 1.17) | 1.00 (0.88, 1.13) |
Note: Negative binomial regression models adjusted for maternal age at the beginning of pregnancy (in years), maternal prepregnancy BMI (in three categories: , , ), maternal education (in three groups: y, 12 y, y), parity (nulliparous vs. multiparous), maternal prepregnancy fish intake ( once/month, at least once a month, at least twice a week) smoking status at inclusion (smoker vs. nonsmoker) and child sex. ADHD, attention-deficit/hyperactivity disorder; aMR, adjusted mean ratio; BMI, body mass index; CI, confidence interval; DIA, Dominic Interactive for Adolescents; PFDA, perfluorodecanoic acid; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFUnDA, perfluoroundecanoic acid; SDQ, Strengths and Difficulties Questionnaire.
Single-pollutant approach: complementary analysis.
When using externalizing and ADHD scores obtained from self-reported DIA, the results were similar on the whole: Associations were in the same direction (see Table 5), although they were diminished and no longer statistically significant for PFOA and PFNA and became statistically significant for PFDA. When using the parent-reported SDQ subscale for internalizing behavior, no association was identified with any PFAS.
BKMR models.
Concurrent increases in concentrations of PFAS included in the models (reference: 25th percentile) showed no, or only a very slight, increase in both SDQ externalizing and DIA internalizing scores (Figure 1), CI for mixture–outcome associations crossed the null value.
Figure 1.
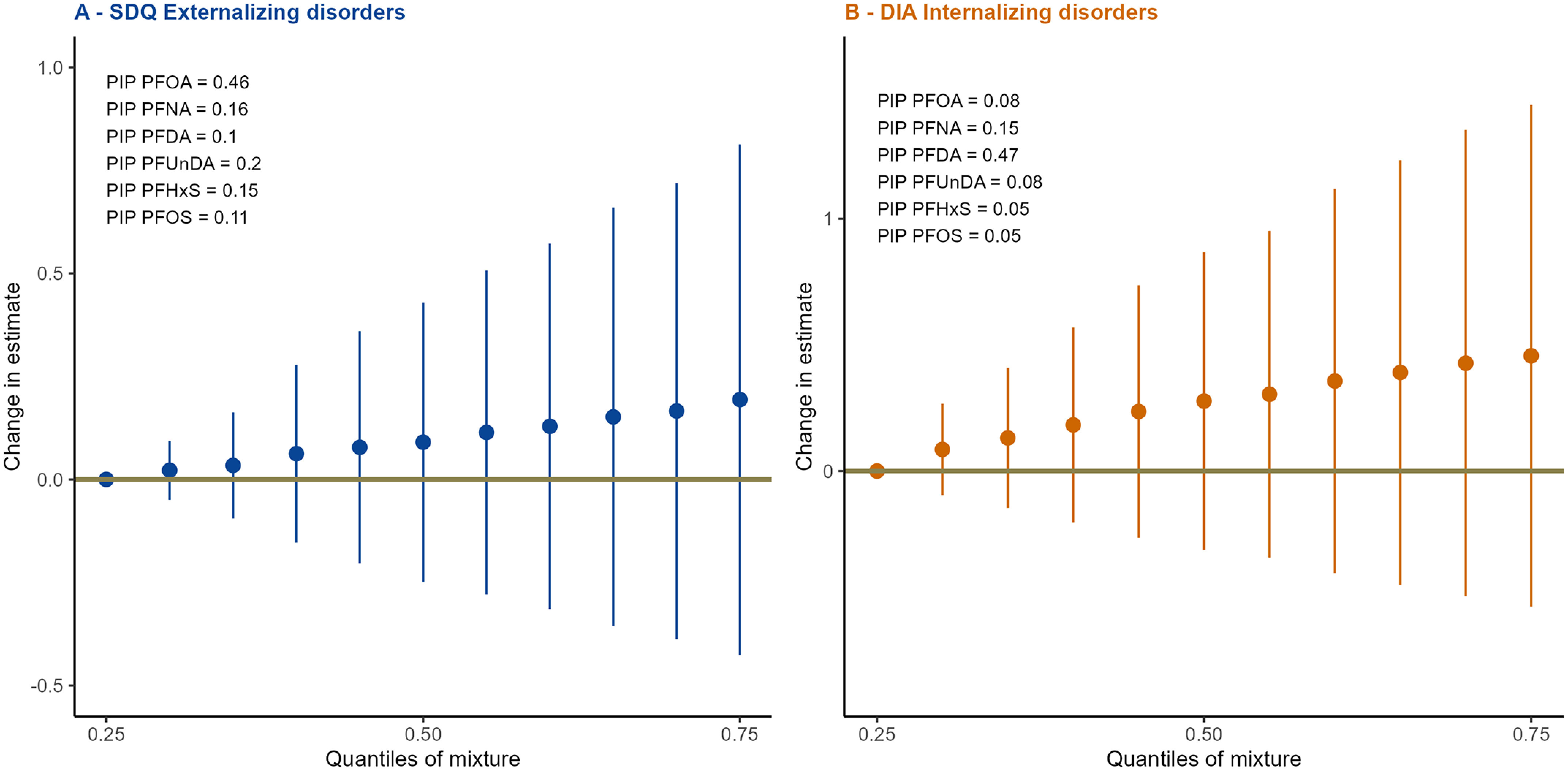
Overall PFAS mixture effect for SDQ Externalizing subscale (A) and DIA internalizing subscale (B) from BKMR models (), PELAGIE mother–child cohort, Brittany, France. Change in estimate are spotted as blue and orange dots and 95% CIs as blue and orange bars (for externalizing and internalizing subscales, respectively) for concurrent increase of exposures in comparison with the 25th percentile. PIP are provided on the figure. Mixture was composed of PFOA, PFNA, PFDA, PFUnDA, PFHxS, and PFOS. Models were adjusted for maternal age at the beginning of pregnancy (in years), maternal prepregnancy BMI (in three categories: , , ), maternal education (in three groups: y, 12 y, y), parity (nulliparous vs. multiparous), maternal prepregnancy fish intake ( once/month, at least once a month, at least twice a week), smoking status at inclusion (smoker vs. nonsmoker) and child sex. Numeric data can be found in Table S13. Note: BKMR, Bayesian kernel machine regression; BMI, body mass index; CI, confidence interval; DIA, Dominic Interactive for Adolescents; PFAS, per- and polyfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFUnDA, perfluoroundecanoic acid; PIP, posterior inclusion probabilities; SDQ, Strengths and Difficulties Questionnaire.
For the externalizing score, PFOA was identified as the predominant component of the joint effect (; see Figure 1) followed by PFUnDA (). The single-exposure effect of PFOA suggested a weak association with a higher score on the SDQ externalizing subscale (see Table S14 and Figure S7A).
For the internalizing subscale, PFDA was the predominant component of the joint effect (; see Figure 1), followed by PFNA (). The single-exposure effect of PFDA suggested a weak association with higher score on the DIA internalizing subscale (see Table S14 and Figure S7B).
No evidence of either interaction or nonlinear association was identified, other than for PFDA prenatal exposure and DIA internalizing subscale, where the response curve seemed to reach a plateau as concentration increased (see Figures S8 and S9 and Table S15 for exposure levels).
Sensitivity Analyses
Sensitivity analyses performed for the single-pollutant approach on multivariable negative binomial regression models revealed results similar, on the whole, to those of the main analyses rerun on the same sample sizes (see Tables S7 and S8). The association between prenatal exposure to PFDA and the SDQ hyperactivity score became statistically significant (; 95% CI: 1.00, 1.38, ) after controlling for breastfeeding (Table S7). When models were rerun on the sample using data for parental emotional well-being measured by SF36 (with and without adjusting for SF36-EWB, see Table S8), effect sizes for anxiety and internalizing behavior scores were slightly amplified, and the association between PFOA and anxiety score appeared statistically significant (; 95% CI: 1.00, 1.20).
Potential persistent organic pollutant coexposures considered in sensitivity analyses included PCB 118, 138, 153, 170, 180, 183, 187, 194, 203, 74, and 99: PBDE 209, 47, and 99; dieldrin; heptachlor epoxide; HCB; p,p′-DDE; ; and . The proportion of nondetected values and the distributions for each compound is displayed in Table S9. Correlations between coexposure (OC, PCB, and PBDE) and PFAS are displayed in Figure S3. Higher correlations were seen between PFUnDA and PCBs (0.27 for PCB118 to 0.34 for PCB153). Detailed results for models studying associations between prenatal exposure to POP and behavior scores are displayed in Table S10. Furthermore, , detected vs. nondetected, was associated () with higher scores on the SDQ hyperactivity scale (; 95% CI: 1.0, 2.0) and with DIA internalizing, major depressive, and anxiety subscales (; 95% CI: 1.0, 1.6). Dieldrin (values greater than median in reference to nondetected values) was also negatively associated with major depressive subscale [ (95% CI: 0.9, 1.3) and (95% CI: 0.7, 1.0)]. When models were further adjusted for these coexposures, the conclusions were no different from the main analysis. The associations between PFUnDA and anxiety and internalizing subscales became statistically significant [ (95% CI: 1.01, 1.20) and (95% CI: 1.00, 1.18), respectively; see Table S11]. In the BKMR models, results were also similar to those of the main analysis (see Table S12).
Discussion
Our objective was to assess the link between prenatal exposure to several PFAS and behavior among 12-y-olds. The results of the single-pollutant approach showed increased scores on externalizing and hyperactivity subscales associated with increasing cord-blood serum levels of PFOA and PFNA. The study also highlighted the fact that prenatal exposures to increasing levels of PFNA and PFDA were associated with increasing scores on depressive, anxiety, and internalizing subscales. Our results also suggested a weak association between prenatal PFUnDA and scores on anxiety and internalizing subscales. For both internalizing and externalizing subscales, the overall mixture effect from BKMR modeling suggested no, or very slight, deleterious effects.
In light of our findings, the discussion below focuses on studies also conducted in European areas and on the associations between prenatal exposure to PFOA and PFNA and externalizing behaviors, on the one hand, and on PFDA and PFNA and any other PFAS and internalizing behaviors on the other.
The cord serum samples used in our study were collected between 2002 and 2006. The median cord serum concentration of PFAS was generally within the range reported by other contemporaneous studies using cord blood measurements. For PFOA, we observed a median of , whereas studies in European countries observed median values of in Germany,65 from the Danish Birth Cohort study,66 in a Norwegian study67 (), in Belgium68 (), and [geometric mean (GM)] in a pooled analyses of four cohorts from Belgium and Slovakia.69 The median cord concentration of PFNA of in our study was slightly higher than in the pooled analysis from Belgium and Slovakia (GM of ) or the Norwegian study (median ). The median level of cord-blood PFDA in our study () was higher than in the Norwegian study (median ) and similar to that found in the Faroese Islands70,71 (GM of and ). For PFOS cord serum concentration, median concentration was in our sample and was lower than that found in other studies (medians of in Germany65 and in Denmark66).
In the INUENDO cohort (Greenland, Ukraine, and Poland), prenatal exposure to PFOA was associated with a small-to-moderate effect on behavior (especially hyperactivity) in 5- to 9-y-olds, as assessed using the parent-reported SDQ (0.5 point higher hyperactivity score for high exposure in comparison with low exposure).15 In the Faroese Islands birth cohort, prenatal PFOA exposure was reported to be associated with higher externalizing behaviors, as assessed using the SDQ at age 7 y.21 In contrast, in the Danish Birth Cohort study, increased prenatal exposure to PFOA (second and third quartiles) was statistically significantly associated with lower odds ratios (OR) of children scoring higher in terms of total difficulties, emotional symptoms, and hyperactivity assessed using the SDQ, in comparison with the lowest quartile of exposure.72
Studies focusing on ADHD diagnosis or symptoms were also identified. In a nested case–control study in the Norwegian Mother, Father and Child Cohort study (MoBa), prenatal exposure to PFOA was associated with a higher risk of ADHD.16 In the Human Milk Study (HUMIS), exposure to PFOA measured in breast milk was positively associated with ADHD in single-pollutant analysis—though this association disappeared in the multipollutant approach.6 In the Danish Birth Cohort study, even though no association was found between prenatal exposure to PFAS and risk of an ADHD diagnosis, when categorized in quartiles, the PFOA concentration was positively associated with ADHD [relative risk (95% CI: 1.14, 1.88) and (95% CI: 1.49, 2.75)],73 where PFAS were entered into models simultaneously.
In the present study, we detected no difference between females and males in the association between prenatal PFOA exposure and scores on externalizing and hyperactivity subscales. Effect modification by sex has been reported previously,2,17–20,24,29,74,75 but few studies have found any such effect for the association between prenatal PFOA exposure and externalizing behaviors. In the Dutch mother–child cohort Linking Maternal Nutrition to Child Health (LINC), males having the highest prenatal exposure to PFOA showed lower scores on the Externalizing Problem Scale of the Child Behavior Checklist.74 In Denmark, though no associations between maternal PFOA concentrations in serum and symptoms of ADHD at the age of 2.5 or 5 y were seen overall, a sex difference was suggested, with a higher risk among females (though the association was not statistically significant).75 In a meta-analysis performed on nine European birth cohorts, Forns et al. identified no overall association between prenatal exposure to PFOA and ADHD, but they did suggest a sex-specific association between early-life exposure (including prenatal) to PFOA and ADHD, with females being more at risk than males, as seen in the two studies cited previously.14
Ten other European studies have also reported no association between prenatal exposure to PFOA and externalizing behaviors, making it difficult to draw any firm conclusions from these studies. For sex-specific effects, it is even more complicated, although the studies identified do tend to suggest that females are at a higher risk.2,19,25,26,28,30,72,73,76,77
In our study, prenatal PFNA exposure (cord-serum median concentration ) was associated with higher externalizing and hyperactivity subscales scores measured by SDQ. This finding is in accordance with the literature. A study from Greenland, Ukraine, and Poland22 reported prenatal PFNA exposure (median maternal plasma concentration ) to be associated with higher SDQ total difficulties and hyperactivity scores among children 5 to 9 y of age. In the Danish National Birth Cohort,19 an increasing prenatal PFNA exposure was associated with externalizing difficulties at 7 and 11 y of age, reported (via SDQ) by parents and children; no sex-specific differences were identified, although associations measured at age 7 y appeared stronger in females.
In Europe, in the other nine studies identified, no associations were seen in term of links between prenatal exposure to PFNA and externalizing behaviors.2,16,21,25,26,30,73–75
Beyond Europe, three studies have suggested associations between prenatal exposure to PFNA and externalizing behavior: lower scores for inattention and oppositional defiant subscales in 7-y-olds,78 increased hyperactivity and hyperactive-type ADHD symptoms and diagnostic criteria in children 5 and 8 y of age,20 higher ADHD and hyperactivity-impulsivity scores among males.24
Our study reported that prenatal exposure to PFNA was associated with higher scores on the anxiety subscale (as well as on internalizing subscale, though to a lesser extent) measured by the self-reported DIA. A similar association was also suggested with SDQ self-reported internalizing difficulties at 11 y of age in the Danish National Birth Cohort.19 Beyond Europe, a study has suggested an association between prenatal exposure to PFNA and less somatic complaints problems in 2- and 4-y-old males in China.18
Using the DIA, we also observed that prenatal PFDA exposure was associated with higher scores for internalizing, depressive, and anxiety subscales. Although there is a scarcity of literature regarding the effect of prenatal PFDA exposure on behavior, our result is in line with that of the study in the Danish National Birth Cohort, in which a suggestive association was also observed between prenatal PFDA exposure (median maternal plasma concentration ) and higher SDQ self-reported internalizing difficulties scores at 11 y of age.19
In our study, a further association was suggested between PFUnDA and scores on anxiety and internalizing subscales. Only one study (performed in China) identified prenatal exposure to both PFUnDA and perfluorotridecanoic acid (PFTrDA) as associated with internalizing problems, measured using the Child Behavior Checklist (CBCL) in 2- and 4-y-old males.18 Few other studies have explored associations between prenatal exposure to PFAS and internalizing disorders. In Europe, a study of the association between prenatal exposure to PFOA and PFOS and depression diagnosis identified through the prescription of antidepressants reported no association,28 whereas an inverse association was identified between PFOS and SDQ parent-reported internalizing subscale score in children 3 to 7 y of age.25 In the United States, among 8-y-olds, prenatal PFHxS was associated with more severe internalizing problems, especially depression and somatization, measured using the Behavior Assessment System for Children, 2nd edition (BASC-2).20
Because we are all exposed to a mixture of many intercorrelated PFAS, including before we are born, there is a growing interest in addressing this issue, including through the use of more complex statistical approaches.79–81 We therefore used a BKMR model, retaining six compounds in the mixture: PFOA, PFNA, PFDA, PFUnDA, PFHxS, and PFOS. We did not find any statistically significant association of the PFAS mixture observed in our French population with externalizing or internalizing behavior scores of children. The fact that we contrasted all PFAS compounds in the mixture model may have somewhat diluted or concealed stronger compound-specific effect. However, in the BKMR models, when we introduced only the PFAS that were associated with outcomes in the single-pollutant approach, the mixture effect still did not reach statistical significance. This mixture approach did not raise any concern about interactions between PFAS, and suggested that it may be PFOA and PFDA that predominantly affect behavioral scores (externalizing and internalizing, respectively), although both PIP were , which is the threshold identified in the literature at which a compound is considered to be of importance.54 In contrast with findings using the single-pollutant approach, PFNA was not identified as contributing much to the mixture effect on behavior scores. The associations seen with PFNA and externalizing behavior and internalizing behavior may be because of strong correlations between PFOA and PFNA and PFDA and PFNA (see Figure S2).
Using Weighted Quantile Sum (WQS) regression, Luo et al.19 found that the mixture of six PFAS (PFOA, PFNA, PFDA, PFOS, PFHxS, PFHpS) was associated with higher total SDQ difficulties in Danish children at age 7 y, though the effect sizes of the associations decreased by the age of 11 y. They also identified higher (though not statistically significant) externalizing troubles scores in parental and child reports at 7 and 11 y, associated with the prenatal PFAS mixture, with PFNA contributing most to the mixture effect. Using structural equation modeling with mutual adjustment for pre- and postnatal exposures, Oulhote et al. did not identify any effect of prenatal exposure to a PFAS mixture (PFOA, PFNA, PFDA, PFOS, PFHxS) on the SDQ in 7-y-olds from the Faroe Islands, though they did find that postnatal exposure to PFAS measured at 5 and 7 y of age was associated with the latent function of several SDQ subscales, i.e., total difficulties, externalizing subscale, conduct problems, and autism spectrum disorder scores.30 Again, no association between PFAS mixture (PFOA, PFNA, PFDA, PFUnDA, PFOS, PFHxS, PFHpS) and risk of ADHD was observed using quantile G-computation, though there was a lower risk of autism spectrum disorder among prenatally exposed Norwegian children (PFNA, PFOA, and PFOS had the highest positive influence in the mixture, whereas PFDA, PFUnDA, PFHpS, and PFHxS had the highest negative influence).16 A few studies have included several families of POPs in a mixture model. Oulhote et al.21 identified an overall adverse effect of a mixture of mercury, PCBs, and PFAS measured prenatally on behavioral scores measured via the SDQ and using the G-formula combined with SuperLearner.
In animal studies (mostly focused on PFOA and PFOS), the most consistent neurobehavioral deficit associated with developmental exposure has been impaired motor activity82 (increased or decreased, both of which were associated with disruption of the cholinergic system as reported earlier for PCBs and PBDEs83). Starnes et al. argued in their review that the interpretation of tests relying on exploratory and motor behavior could be impaired due to concurrent physical and emotional (anxiety-like) reactions in the face of new environments.82 Impaired learning and memory were also identified, though less frequently, in the literature. A few studies have focused on other PFAS (mostly long-chained),84 and data on PFNA and PFDA are sparse; Kawabata et al. did not identify PFDA exposure as being associated with alteration in cognitive function or anxiety in adult rat models,85 and Johansson et al. identified no effect in rats exposed neonatally to PFDA.83
The major mechanisms identified to explain PFAS neurotoxicity are modification in calcium homeostasis, alterations in neurotransmitters [glutamate, acid (GABA), dopamine, acetylcholine, and serotonin levels] and neuroendocrine disruption (including sex, stress, and thyroid hormones).82 PFAS neurotoxicity might increase with carbon chain length and fluorination level.82 It has also been suggested that the biological mechanisms of developmental neurotoxicity could include inhibition of DNA synthesis, deficits in cell numbers and growth, oxidative stress, and reduced cell viability, differing from one PFAS to another.86 PFAS toxicity to the brain and behavior might also express itself through indirect pathways, such as alteration of immune functions, or dysregulation of peroxisome proliferator–activated receptors (PPARs), which intervene in regulating cellular differentiation, proliferation, and metabolic routes.82
One strength of our study is that we used different tests to evaluate both externalizing and internalizing behaviors based on the parental reports and the child self-reports, respectively. The SDQ has been shown to accurately capture externalizing disorders in low-risk community samples38 and has been very widely used in studies on associations between chemical prenatal exposure and behavior in childhood. This approach allowed us to compare our results to other published data. When considering externalizing behavior assessed with the DIA, associations with prenatal exposures to PFAS were on the whole similar, highlighting the consistency of these results. Given that, thus far, internalizing disorders have rarely been studied at ages at which children were capable of self-reporting, the DIA has not been used in such studies. Nevertheless it remains a very promising tool because it is easy to implement and allows the assessment of disorders meeting the DSM classification.40–42 The fact that our complementary analysis using the parent-reported SDQ internalizing composite scale did not show any association highlights the importance of using self-reported questionnaire to accurately assess internalizing behaviors in adolescents.
Prenatal exposure to PFAS was assessed in cord-blood samples collected at delivery in our study. We believe that this is likely to represent direct fetal exposure to PFAS; indeed, it has been shown that transplacental transfer of PFAS from maternal blood to fetal blood differs, depending on type of PFAS (sulfonate compounds such as PFOS and PFHxS show lower transplacental transfer rates than carboxylates) and carbon chain length.87,88 The relationship between transplacental transfer rate and carbon chain length seemed U-shaped: Compounds including 8 carbons (such as PFOA) showed a higher level of transplacental transfer rate than compounds with 9, 10, or 11 carbons (such as PFNA, PFDA and PFUnDA, respectively), and the rate seemed to increase then with chain length87,88; this observation might be explained by differences in binding affinity to serum proteins according to chain length. It has also been reported that the PFAS concentration in maternal blood decreases throughout the pregnancy.89 Cord-blood samples might reflect exposure at the end of pregnancy and then underestimate it by assuming the PFAS transplacental transfer rate to be constant throughout pregnancy. Had they been available, maternal blood samples collected in early pregnancy would certainly have contributed other valuable information and potentially allowed the indirect effects of PFAS exposure on behavior in later life to be included via mediation related to alterations in maternal thyroid function90 and, to a lesser extent, in newborns and infants91 as a result of exposure to PFAS. These alterations in maternal thyroid function may impair a child’s neuropsychological development, including in terms of externalizing behaviors such as ADHD and internalizing behaviors like anxiety and depression.92
As we stated earlier, postnatal exposure to PFAS has been shown to be associated with behavioral outcomes among children. In our study population, we did not measure postnatal exposure to PFAS during childhood. Breastfeeding is known to be a source of exposure to PFAS during infancy, and because of their long half-lives, we can assume that postnatal exposure in infancy is driven by breastfeeding. For our study, we performed a sensitivity analysis adjusting our models for breastfeeding (as a way of somehow controlling for early postnatal exposure), and there was no effect on results, which supports our findings regarding associations between prenatal exposure to some PFAS and behavior at age 12 y.
Although maternal education level and age in our study sample were higher than those in the original cohort, all regression models were adjusted for these two covariates, which minimized the effect of a potential selection bias on the results. In this prospective longitudinal cohort, we were able to take account of many confounding factors, including prenatal coexposure to other persistent organic pollutants, such as PCBs,61 PBDEs,62 and OCPs. We cannot, however, exclude residual confounding from other unmeasured or unknown confounders. Because the socioeconomic status of our study population is both high and fairly homogenous, confounding linked to socioeconomic status is unlikely to occur. We measured parent emotional well-being using the SF36 questionnaire; it allowed us to partially take parent mental health into consideration in our study. However, it is worth noting that parent mental health assessment occurred at the same time as the child behavior assessment. Previous measure was not available in our study.
In recent years, exposure levels to PFOA and PFOS have decreased for the general population in Europe and the United States, as a result of the inclusion of PFOA and PFOS in the Stockholm convention list of banned or restricted POPs.93,94 Yet this trend is mixed; exposure levels to PFNA and PFDA and other PFAS (such as PFBA, PFHxA or PFBS that were detected in in our study) are increasing.93–95 Both these compounds and the new generation of PFAS (such as GenX and C604) are now spreading worldwide and demand fresh investigation.
Conclusion
This study has highlighted prenatal PFOA and PFNA exposures as being associated with higher scores on externalizing and hyperactivity subscales among 12-y-olds, which is in accordance with the literature regarding younger children. We also found prenatal exposure to PFNA and PFDA to be associated with scores on internalizing subscale; this result adds some information to the as yet sparse literature examining the links between prenatal exposure to PFAS and internalizing disorders. The toxicological plausibility of the association between prenatal exposure to PFAS and internalizing behavior has yet to be studied, but the links and relationships between externalizing and internalizing behaviors (especially among adolescents) are known, and this field warrants further studies. Indeed, internalizing disorders such as depression and anxiety can impact future mental health.
Supplementary Material
Acknowledgments
The authors are grateful to the physicians and nurses and all the families who participated in the study (and continue to do so). The authors especially thank the UIC health personnel, S. Métayé and C. Réminiac, for their rigorous work in examining the children, and N. Costet, V. Villalon, and I. Coiffec, who contributed to the PELAGIE cohort in general. The authors also thank V. Kovess-Masfety, Department of Psychiatry at McGill University (Montréal, Canada) and at the Laboratory of Psychopathology and Health Process the University of Paris; G. Muckle, School of Psychology, Faculty of Social Sciences, Laval University (Québec, Canada); and V. Siroux, Institute for Advanced Biosciences (IAB) Centre de recherche UGA – Inserm U 1209 – CNRS UMR5309, for their help and advice. The authors particularly thank J. Roffe for the English editing.
The PELAGIE cohort has been funded (since its inception) by the Institut national de la santé et de la recherche médicale (Inserm), the French Ministries of Health (2003–2004), Labor (2002–2003), and Research (ATC 2003–2004), the French National Institute for Public Health Surveillance (InVS, 2002–2006), the National Agency for Research (ANR, 2005–2008, 2010–2012, 2015–2019), the French Agency for Environmental Health Safety (Afsset/ANSES, 2007–2009, 2009–2012, 2019–2023), the French Agency for Drug Safety (2013–2017), the Fondation de France (2014–2017, 2015–2018, 2017–2020, 2019–2021, 2021–2024), the French Ministry of Ecology (PNRPE 2014–2016), the Research Institute of Public Health (IResP 2011–2014), and the following European programs: Hi-WATE (2007–2009), ENRIECO (2008–2010), and OBERON (2019–2023), REMEDIA (2020–2024). This research is part of a PhD project funded by the French network of doctoral programs and coordinated by École des hautes études en santé publique (EHESP), the French School of Public Health.
The PELAGIE cohort data comply with the European regulation on the protection of personal data (25 May 2018). This regulation is based on a logic of compliance and increased responsibility of the actors who access the data. In addition, the cohort study complies with the French “informatique et liberté” law (law no. 78-17, January 1978, 2018). Access to data is thus possible following the agreement of the cohort principal investigators (C.C., C.W.) and on condition that the actors demonstrate respect for these European and French principles of personal data protection to strengthen the rights of individuals. Further enquiries can be directed to the corresponding author.
References
- 1.Agency for Toxic Substances and Disease Registry. 2021. Toxicological Profile for Perfluoroalkyls. https://stacks.cdc.gov/view/cdc/59198 [accessed 7 July 2022]. [PubMed]
- 2.Bach CC, Liew Z, Matthiesen NB, Henriksen TB, Bech BH, Nøhr EA, et al. 2022. In utero exposure to perfluoroalkyl and polyfluoroalkyl substances and attention and executive function in the offspring: a study in the Danish National Birth Cohort. Environ Res 212(pt B):113262, PMID: , 10.1016/j.envres.2022.113262. [DOI] [PubMed] [Google Scholar]
- 3.Fromme H, Tittlemier SA, Völkel W, Wilhelm M, Twardella D. 2009. Perfluorinated compounds – exposure assessment for the general population in Western countries. Int J Hyg Environ Health 212(3):239–270, PMID: , 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Bjerregaard-Olesen C, Bossi R, Liew Z, Long M, Bech BH, Olsen J, et al. 2017. Maternal serum concentrations of perfluoroalkyl acids in five international birth cohorts. Int J Hyg Environ Health 220(2 pt A):86–93, PMID: , 10.1016/j.ijheh.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Cariou R, Veyrand B, Yamada A, Berrebi A, Zalko D, Durand S, et al. 2015. Perfluoroalkyl acid (PFAA) levels and profiles in breast milk, maternal and cord serum of French women and their newborns. Environ Int 84:71–81, PMID: , 10.1016/j.envint.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Lenters V, Iszatt N, Forns J, Čechová E, Kočan A, Legler J, et al. 2019. Early-life exposure to persistent organic pollutants (OCPs, PBDEs, PCBs, PFASs) and attention-deficit/hyperactivity disorder: a multi-pollutant analysis of a Norwegian birth cohort. Environ Int 125:33–42, PMID: , 10.1016/j.envint.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Ma D, Lu Y, Liang Y, Ruan T, Li J, Zhao C, et al. 2022. A critical review on transplacental transfer of per- and polyfluoroalkyl substances: prenatal exposure levels, characteristics, and mechanisms. Environ Sci Technol 56(10):6014–6026, PMID: , 10.1021/acs.est.1c01057. [DOI] [PubMed] [Google Scholar]
- 8.van de Bor M. 2019. Fetal toxicology. In: Handbook of Clinical Neurology, vol. 162. Amsterdam, The Netherlands: Elsevier, 31–55. [DOI] [PubMed] [Google Scholar]
- 9.Grandjean P, Landrigan PJ. 2014. Neurobehavioural effects of developmental toxicity. Lancet Neurol 13(3):330–338, PMID: , 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau C, Butenhoff JL, Rogers JM. 2004. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol Appl Pharmacol 198(2):231–241, PMID: , 10.1016/j.taap.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 11.Liew Z, Goudarzi H, Oulhote Y. 2018. Developmental exposures to perfluoroalkyl substances (PFASs): an update of associated health outcomes. Curr Environ Health Rep 5(1):1–19, PMID: , 10.1007/s40572-018-0173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liew Z, Ritz B, Bach CC, Asarnow RF, Bech BH, Nohr EA, et al. 2018. Prenatal exposure to perfluoroalkyl substances and IQ scores at age 5; a study in the Danish National Birth Cohort. Environ Health Perspect 126(6):067004, PMID: , 10.1289/EHP2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steenland K, Fletcher T, Stein CR, Bartell SM, Darrow L, Lopez-Espinosa M-J, et al. 2020. Review: evolution of evidence on PFOA and health following the assessments of the C8 Science Panel. Environ Int 145:106125, PMID: , 10.1016/j.envint.2020.106125. [DOI] [PubMed] [Google Scholar]
- 14.Forns J, Verner M-A, Iszatt N, Nowack N, Bach CC, Vrijheid M, et al. 2020. Early life exposure to perfluoroalkyl substances (PFAS) and ADHD: a meta-analysis of nine European population-based studies. Environ Health Perspect 128(5):57002, PMID: , 10.1289/EHP5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Høyer BB, Ramlau-Hansen CH, Obel C, Pedersen HS, Hernik A, Ogniev V, et al. 2015. Pregnancy serum concentrations of perfluorinated alkyl substances and offspring behaviour and motor development at age 5–9 years – a prospective study. Environ Health 14:2, PMID: , 10.1186/1476-069X-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skogheim TS, Weyde KVF, Aase H, Engel SM, Surén P, Øie MG, et al. 2021. Prenatal exposure to per- and polyfluoroalkyl substances (PFAS) and associations with attention-deficit/hyperactivity disorder and autism spectrum disorder in children. Environ Res 202:111692, PMID: , 10.1016/j.envres.2021.111692. [DOI] [PubMed] [Google Scholar]
- 17.Ghassabian A, Bell EM, Ma W-L, Sundaram R, Kannan K, Buck Louis GM, et al. 2018. Concentrations of perfluoroalkyl substances and bisphenol A in newborn dried blood spots and the association with child behavior. Environ Pollut 243(pt B):1629–1636, PMID: , 10.1016/j.envpol.2018.09.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Z, Tan J, Fang G, Ji H, Miao M, Tian Y, et al. 2022. Associations between prenatal exposure to perfluoroalkyl substances and neurobehavioral development in early childhood: a prospective cohort study. Ecotoxicol Environ Saf 241:113818, PMID: , 10.1016/j.ecoenv.2022.113818. [DOI] [PubMed] [Google Scholar]
- 19.Luo J, Xiao J, Gao Y, Ramlau-Hansen CH, Toft G, Li J, et al. 2020. Prenatal exposure to perfluoroalkyl substances and behavioral difficulties in childhood at 7 and 11 years. Environ Res 191:110111, PMID: , 10.1016/j.envres.2020.110111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vuong AM, Webster GM, Yolton K, Calafat AM, Muckle G, Lanphear BP, et al. 2021. Prenatal exposure to per- and polyfluoroalkyl substances (PFAS) and neurobehavior in US children through 8 years of age: the HOME study. Environ Res 195:110825, PMID: , 10.1016/j.envres.2021.110825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oulhote Y, Coull B, Bind M-A, Debes F, Nielsen F, Tamayo I, et al. 2019. Joint and independent neurotoxic effects of early life exposures to a chemical mixture. Environ Epidemiol 3(5):e063, PMID: , 10.1097/ee9.0000000000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Høyer BB, Bonde JP, Tøttenborg SS, Ramlau-Hansen CH, Lindh C, Pedersen HS, et al. 2018. Exposure to perfluoroalkyl substances during pregnancy and child behaviour at 5 to 9 years of age. Horm Behav 101:105–112, PMID: , 10.1016/j.yhbeh.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Liu J. 2004. Childhood externalizing behavior: theory and implications. J Child Adolesc Psychiatr Nurs 17(3):93–103, PMID: , 10.1111/j.1744-6171.2004.tb00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itoh S, Yamazaki K, Suyama S, Ikeda-Araki A, Miyashita C, Ait Bamai Y, et al. 2022. The association between prenatal perfluoroalkyl substance exposure and symptoms of attention-deficit/hyperactivity disorder in 8-year-old children and the mediating role of thyroid hormones in the Hokkaido study. Environ Int 159:107026, PMID: , 10.1016/j.envint.2021.107026. [DOI] [PubMed] [Google Scholar]
- 25.Jedynak P, Maitre L, Guxens M, Gützkow KB, Julvez J, López-Vicente M, et al. 2021. Prenatal exposure to a wide range of environmental chemicals and child behaviour between 3 and 7 years of age – an exposome-based approach in 5 European cohorts. Sci Total Environ 763:144115, PMID: , 10.1016/j.scitotenv.2020.144115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skogheim TS, Villanger GD, Weyde KVF, Engel SM, Surén P, Øie MG, et al. 2020. Prenatal exposure to perfluoroalkyl substances and associations with symptoms of attention-deficit/hyperactivity disorder and cognitive functions in preschool children. Int J Hyg Environ Health 223(1):80–92, PMID: , 10.1016/j.ijheh.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vuong AM, Braun JM, Yolton K, Wang Z, Xie C, Webster GM, et al. 2018. Prenatal and childhood exposure to perfluoroalkyl substances (PFAS) and measures of attention, impulse control, and visual spatial abilities. Environ Int 119:413–420, PMID: , 10.1016/j.envint.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strøm M, Hansen S, Olsen SF, Haug LS, Rantakokko P, Kiviranta H, et al. 2014. Persistent organic pollutants measured in maternal serum and offspring neurodevelopmental outcomes–a prospective study with long-term follow-up. Environ Int 68:41–48, PMID: , 10.1016/j.envint.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Harris MH, Oken E, Rifas-Shiman SL, Calafat AM, Bellinger DC, Webster TF, et al. 2021. Prenatal and childhood exposure to per- and polyfluoroalkyl substances (PFAS) and child executive function and behavioral problems. Environ Res 202:111621, PMID: , 10.1016/j.envres.2021.111621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oulhote Y, Steuerwald U, Debes F, Weihe P, Grandjean P. 2016. Behavioral difficulties in 7-year old children in relation to developmental exposure to perfluorinated alkyl substances. Environ Int 97:237–245, PMID: , 10.1016/j.envint.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Chen X, Lewis G. 2011. Childhood internalizing behaviour: analysis and implications. J Psychiatr Ment Health Nurs 18(10):884–894, PMID: , 10.1111/j.1365-2850.2011.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beauchaine TP, Hinshaw SP, Levy F, Hawes DJ, Johns A. 2015. Externalizing and internalizing comorbidity. In: The Oxford Handbook of Externalizing Spectrum Disorders. Beauchaine TP, Hinshaw SP, eds. Oxford, UK: Oxford University Press, 10.1093/oxfordhb/9780199324675.013.007. [DOI] [Google Scholar]
- 33.Angold A, Costello EJ, Erkanli A. 1999. Comorbidity. J Child Psychol Psychiatry 40(1):57–87, PMID: , 10.1111/1469-7610.00424. [DOI] [PubMed] [Google Scholar]
- 34.Andre QR, Geeraert BL, Lebel C. 2020. Brain structure and internalizing and externalizing behavior in typically developing children and adolescents. Brain Struct Funct 225(4):1369–1378, PMID: , 10.1007/s00429-019-01973-y. [DOI] [PubMed] [Google Scholar]
- 35.Chevrier C, Warembourg C, Gaudreau E, Monfort C, Le Blanc A, Guldner L, et al. 2013. Organochlorine pesticides, polychlorinated biphenyls, seafood consumption, and time-to-pregnancy. Epidemiology 24(2):251–260, PMID: , 10.1097/EDE.0b013e31827f53ec. [DOI] [PubMed] [Google Scholar]
- 36.Goodman R. 1997. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry 38(5):581–586, PMID: , 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 37.Goodman R. 2001. Psychometric properties of the strengths and difficulties questionnaire. J Am Acad Child Adolesc Psychiatry 40(11):1337–1345, PMID: , 10.1097/00004583-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Goodman A, Lamping DL, Ploubidis GB. 2010. When to use broader internalising and externalising subscales instead of the hypothesised five subscales on the Strengths and Difficulties Questionnaire (SDQ): data from British parents, teachers and children. J Abnorm Child Psychol 38(8):1179–1191, PMID: , 10.1007/s10802-010-9434-x. [DOI] [PubMed] [Google Scholar]
- 39.Capron C, Thérond C, Duyme M. 2007. Psychometric properties of the French version of the self-report and teacher Strengths and Difficulties Questionnaire (SDQ). Eur J Psychol Assess 23(2):79–88, 10.1027/1015-5759.23.2.79. [DOI] [Google Scholar]
- 40.Bergeron L, Smolla N, Valla J-P, St-Georges M, Berthiaume C, Piché G, et al. 2010. Psychometric properties of a pictorial instrument for assessing psychopathology in youth aged 12 to 15 years: the Dominic Interactive for Adolescents. Can J Psychiatry 55(4):211–221, PMID: , 10.1177/070674371005500404. [DOI] [PubMed] [Google Scholar]
- 41.Bergeron L, Smolla N, Berthiaume C, Renaud J, Breton J-J, St-Georges M, et al. 2017. Reliability, validity, and clinical utility of the Dominic Interactive for Adolescents–revised: a DSM-5–based self-report screen for mental disorders, borderline personality traits, and suicidality. Can J Psychiatry 62(3):211–222, PMID: , 10.1177/0706743716670129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.American Psychiatric Association, DSM-5 Task Force. 2013. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™. 5th ed. Washington, DC: American Psychiatric Publishing, Inc. [Google Scholar]
- 43.De Los Reyes A, Augenstein TM, Wang M, Thomas SA, Drabick DAG, Burgers DE, et al. 2015. The validity of the multi-informant approach to assessing child and adolescent mental health. Psychol Bull 141(4):858–900, PMID: , 10.1037/a0038498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Barrios J, Drysdale M, Ratelle M, Gaudreau É, LeBlanc A, Gamberg M, et al. 2021. Biomarkers of poly- and perfluoroalkyl substances (PFAS) in sub-Arctic and Arctic communities in Canada. Int J Hyg Environ Health 235:113754, PMID: , 10.1016/j.ijheh.2021.113754. [DOI] [PubMed] [Google Scholar]
- 45.Banaschewski T, Becker K, Döpfner M, Holtmann M, Rösler M, Romanos M, et al. 2017. Attention-deficit/hyperactivity disorder. Dtsch Arztebl Int 114(9):149–159, PMID: , 10.3238/arztebl.2017.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Latimer K, Wilson P, Kemp J, Thompson L, Sim F, Gillberg C, et al. 2012. Disruptive behaviour disorders: a systematic review of environmental antenatal and early years risk factors: risk factors for disruptive behaviour disorders. Child Care Health Dev 38(5):611–628, PMID: , 10.1111/j.1365-2214.2012.01366.x. [DOI] [PubMed] [Google Scholar]
- 47.Thapar A, Cooper M. 2016. Attention deficit hyperactivity disorder. Lancet Lond Lancet 387(10024):1240–1250, PMID: , 10.1016/S0140-6736(15)00238-X. [DOI] [PubMed] [Google Scholar]
- 48.Kim JH, Kim JY, Lee J, Jeong GH, Lee E, Lee S, et al. 2020. Environmental risk factors, protective factors, and peripheral biomarkers for ADHD: an umbrella review. Lancet Psychiatry 7(11):955–970, PMID: , 10.1016/S2215-0366(20)30312-6. [DOI] [PubMed] [Google Scholar]
- 49.Millichap JG. 2008. Etiologic classification of attention-deficit/hyperactivity disorder. Pediatrics 121(2):e358–e365, PMID: , 10.1542/peds.2007-1332. [DOI] [PubMed] [Google Scholar]
- 50.Greenland S, Pearl J, Robins JM. 1999. Causal diagrams for epidemiologic research. Epidemiology 10(1):37–48, PMID: . [PubMed] [Google Scholar]
- 51.Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed Effects Models and Extensions in Ecology with R. New York, NY: Springer. 10.1007/978-0-387-87458-6. [DOI] [Google Scholar]
- 52.Hunsicker ME, Kappel CV, Selkoe KA, Halpern BS, Scarborough C, Mease L, et al. 2016. Characterizing driver–response relationships in marine pelagic ecosystems for improved ocean management. Ecol Appl 26(3):651–663, PMID: , 10.1890/14-2200. [DOI] [PubMed] [Google Scholar]
- 53.Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16(3):493–508, PMID: , 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barbieri MM, Berger JO. 2004. Optimal predictive model selection. Ann Stat 32(3):870–897. 10.1214/009053604000000238. [DOI] [Google Scholar]
- 55.Romano ME, Xu Y, Calafat AM, Yolton K, Chen A, Webster GM, et al. 2016. Maternal serum perfluoroalkyl substances during pregnancy and duration of breastfeeding. Environ Res 149:239–246, PMID: , 10.1016/j.envres.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nielsen C, Li Y, Lewandowski M, Fletcher T, Jakobsson K. 2022. Breastfeeding initiation and duration after high exposure to perfluoroalkyl substances through contaminated drinking water: a cohort study from Ronneby, Sweden. Environ Res 207:112206, PMID: , 10.1016/j.envres.2021.112206. [DOI] [PubMed] [Google Scholar]
- 57.Pietikäinen JT, Kiviruusu O, Kylliäinen A, Pölkki P, Saarenpää-Heikkilä O, Paunio T, et al. 2020. Maternal and paternal depressive symptoms and children’s emotional problems at the age of 2 and 5 years: a longitudinal study. J Child Psychol Psychiatry 61(2):195–204, PMID: , 10.1111/jcpp.13126. [DOI] [PubMed] [Google Scholar]
- 58.Amrock SM, Weitzman M. 2014. Parental psychological distress and children’s mental health: results of a national survey. Acad Pediatr 14(4):375–381, PMID: , 10.1016/j.acap.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 59.De Los Reyes A, Kazdin AE. 2005. Informant discrepancies in the assessment of childhood psychopathology: a critical review, theoretical framework, and recommendations for further study. Psychol Bull 131(4):483–509, PMID: , 10.1037/0033-2909.131.4.483. [DOI] [PubMed] [Google Scholar]
- 60.Cuijpers P, Smits N, Donker T, ten Have M, de Graaf R. 2009. Screening for mood and anxiety disorders with the five-item, the three-item, and the two-item Mental Health Inventory. Psychiatry Res 168(3):250–255, PMID: , 10.1016/j.psychres.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 61.Pessah IN, Lein PJ, Seegal RF, Sagiv SK. 2019. Neurotoxicity of polychlorinated biphenyls and related organohalogens. Acta Neuropathol 138(3):363–387, PMID: , 10.1007/s00401-019-01978-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gibson EA, Siegel EL, Eniola F, Herbstman JB, Factor-Litvak P. 2018. Effects of polybrominated diphenyl ethers on child cognitive, behavioral, and motor development. Int J Environ Res Public Health 15(8), PMID: , 10.3390/ijerph15081636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jin Y, Hein MJ, Deddens JA, Hines CJ. 2011. Analysis of lognormally distributed exposure data with repeated measures and values below the limit of detection using SAS. Ann Occup Hyg 55(1):97–112, PMID: , 10.1093/annhyg/meq061. [DOI] [PubMed] [Google Scholar]
- 64.Schisterman EF, Whitcomb BW, Buck Louis GM, Louis TA. 2005. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect 113(7):853–857, PMID: , 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Midasch O, Drexler H, Hart N, Beckmann MW, Angerer J. 2007. Transplacental exposure of neonates to perfluorooctanesulfonate and perfluorooctanoate: a pilot study. Int Arch Occup Environ Health 80(7):643–648, PMID: , 10.1007/s00420-006-0165-9. [DOI] [PubMed] [Google Scholar]
- 66.Fei C, McLaughlin JK, Tarone RE, Olsen J. 2007. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect 115(11):1677–1682, PMID: , 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gützkow KB, Haug LS, Thomsen C, Sabaredzovic A, Becher G, Brunborg G, et al. 2012. Placental transfer of perfluorinated compounds is selective – a Norwegian Mother and Child sub-cohort study. Int J Hyg Environ Health 215(2):216–219, PMID: , 10.1016/j.ijheh.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 68.Cai A, Portengen L, Govarts E, Martin LR, Schoeters G, Legler J, et al. 2023. Prenatal exposure to persistent organic pollutants and changes in infant growth and childhood growth trajectories. Chemosphere 314:137695, PMID: , 10.1016/j.chemosphere.2022.137695. [DOI] [PubMed] [Google Scholar]
- 69.Fábelová L, Beneito A, Casas M, Colles A, Dalsager L, Den Hond E, et al. 2023. PFAS levels and exposure determinants in sensitive population groups. Chemosphere 313:137530, PMID: , 10.1016/j.chemosphere.2022.137530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blomberg AJ, Shih Y-H, Messerlian C, Jørgensen LH, Weihe P, Grandjean P, et al. 2021. Early-life associations between per- and polyfluoroalkyl substances and serum lipids in a longitudinal birth cohort. Environ Res 200:111400, PMID: , 10.1016/j.envres.2021.111400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eryasa B, Grandjean P, Nielsen F, Valvi D, Zmirou-Navier D, Sunderland E, et al. 2019. Physico-chemical properties and gestational diabetes predict transplacental transfer and partitioning of perfluoroalkyl substances. Environ Int 130:104874, PMID: , 10.1016/j.envint.2019.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fei C, Olsen J. 2011. Prenatal exposure to perfluorinated chemicals and behavioral or coordination problems at age 7 years. Environ Health Perspect 119(4):573–578, PMID: , 10.1289/ehp.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liew Z, Ritz B, von Ehrenstein OS, Bech BH, Nohr EA, Fei C, et al. 2015. Attention deficit/hyperactivity disorder and childhood autism in association with prenatal exposure to perfluoroalkyl substances: a nested case-control study in the Danish National Birth Cohort. Environ Health Perspect 123(4):367–373, PMID: , 10.1289/ehp.1408412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quaak I, de Cock M, de Boer M, Lamoree M, Leonards P, van de Bor M, et al. 2016. Prenatal exposure to perfluoroalkyl substances and behavioral development in children. Int J Environ Res Public Health 13(5):511, 10.3390/ijerph13050511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dalsager L, Jensen TK, Nielsen F, Grandjean P, Bilenberg N, Andersen HR, et al. 2021. No association between maternal and child PFAS concentrations and repeated measures of ADHD symptoms at age 2½ and 5 years in children from the Odense Child Cohort. Neurotoxicol Teratol 88:107031, PMID: , 10.1016/j.ntt.2021.107031. [DOI] [PubMed] [Google Scholar]
- 76.Ode A, Källén K, Gustafsson P, Rylander L, Jönsson BAG, Olofsson P, et al. 2014. Fetal exposure to perfluorinated compounds and attention deficit hyperactivity disorder in childhood. PLoS One 9(4):e95891, PMID: , 10.1371/journal.pone.0095891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Forns J, Lertxundi N, Aranbarri A, Murcia M, Gascon M, Martinez D, et al. 2012. Prenatal exposure to organochlorine compounds and neuropsychological development up to two years of life. Environ Int 45:72–77, PMID: , 10.1016/j.envint.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 78.Lien G-W, Huang C-C, Shiu J-S, Chen M-H, Hsieh W-S, Guo Y-L, et al. 2016. Perfluoroalkyl substances in cord blood and attention deficit/hyperactivity disorder symptoms in seven-year-old children. Chemosphere 156:118–127, PMID: , 10.1016/j.chemosphere.2016.04.102. [DOI] [PubMed] [Google Scholar]
- 79.Gibson EA, Nunez Y, Abuawad A, Zota AR, Renzetti S, Devick KL, et al. 2019. An overview of methods to address distinct research questions on environmental mixtures: an application to persistent organic pollutants and leukocyte telomere length. Environ Health 18(1):76, PMID: , 10.1186/s12940-019-0515-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Braun JM, Gennings C, Hauser R, Webster TF. 2016. What can epidemiological studies tell Us about the impact of chemical mixtures on human health? Environ Health Perspect 124(1):A6–A9, PMID: , 10.1289/ehp.1510569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lazarevic N, Barnett AG, Sly PD, Knibbs LD. 2019. Statistical methodology in studies of prenatal exposure to mixtures of endocrine-disrupting chemicals: a review of existing approaches and new alternatives. Environ Health Perspect 127(2):26001, PMID: , 10.1289/EHP2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Starnes HM, Rock KD, Jackson TW, Belcher SM. 2022. A critical review and Meta-Analysis of impacts of per- and polyfluorinated substances on the brain and behavior. Front Toxicol 4:881584, PMID: , 10.3389/ftox.2022.881584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johansson N, Fredriksson A, Eriksson P. 2008. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology 29(1):160–169, PMID: , 10.1016/j.neuro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 84.Piekarski D, Diaz K, McNerney M. 2020. Perfluoroalkyl chemicals in neurological health and disease: human concerns and animal models. Neurotoxicology 77:155–168, PMID: , 10.1016/j.neuro.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 85.Kawabata K, Matsuzaki H, Nukui S, Okazaki M, Sakai A, Kawashima Y, et al. 2017. Perfluorododecanoic acid induces cognitive deficit in adult rats. Toxicol Sci 157(2):421–428, PMID: , 10.1093/toxsci/kfx058. [DOI] [PubMed] [Google Scholar]
- 86.Slotkin TA, MacKillop EA, Melnick RL, Thayer KA, Seidler FJ. 2008. Developmental neurotoxicity of perfluorinated chemicals modeled in vitro. Environ Health Perspect 116(6):716–722, PMID: , 10.1289/ehp.11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng P, Liu Y, An Q, Yang X, Yin S, Ma LQ, et al. 2022. Prenatal and postnatal exposure to emerging and legacy per-/polyfluoroalkyl substances: levels and transfer in maternal serum, cord serum, and breast milk. Sci Total Environ 812:152446, PMID: , 10.1016/j.scitotenv.2021.152446. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y, Han W, Wang C, Zhou Y, Shi R, Bonefeld-Jørgensen EC, et al. 2019. Efficiency of maternal-fetal transfer of perfluoroalkyl and polyfluoroalkyl substances. Environ Sci Pollut Res Int 26(3):2691–2698, PMID: , 10.1007/s11356-018-3686-3. [DOI] [PubMed] [Google Scholar]
- 89.Kato K, Wong L-Y, Chen A, Dunbar C, Webster GM, Lanphear BP, et al. 2014. Changes in serum concentrations of maternal poly- and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in a multiethnic cohort of Cincinnati, Ohio pregnant women during 2003–2006. Environ Sci Technol 48(16):9600–9608, PMID: , 10.1021/es501811k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boesen SAH, Long M, Wielsøe M, Mustieles V, Fernandez MF, Bonefeld-Jørgensen EC, et al. 2020. Exposure to perflouroalkyl acids and foetal and maternal thyroid status: a review. Environ Health 19(1):107, PMID: , 10.1186/s12940-020-00647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun M, Cao X, Wu Y, Shen L, Wei G. 2022. Prenatal exposure to endocrine-disrupting chemicals and thyroid function in neonates: a systematic review and meta-analysis. Ecotoxicol Environ Saf 231:113215, PMID: , 10.1016/j.ecoenv.2022.113215. [DOI] [PubMed] [Google Scholar]
- 92.Salazar P, Villaseca P, Cisternas P, Inestrosa NC. 2021. Neurodevelopmental impact of the offspring by thyroid hormone system-disrupting environmental chemicals during pregnancy. Environ Res 200:111345, PMID: , 10.1016/j.envres.2021.111345. [DOI] [PubMed] [Google Scholar]
- 93.Kim K, Bennett DH, Calafat AM, Hertz-Picciotto I, Shin H-M. 2020. Temporal trends and determinants of serum concentrations of per- and polyfluoroalkyl substances among Northern California mothers with a young child, 2009–2016. Environ Res 186:109491, PMID: , 10.1016/j.envres.2020.109491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Göckener B, Weber T, Rüdel H, Bücking M, Kolossa-Gehring M. 2020. Human biomonitoring of per- and polyfluoroalkyl substances in German blood plasma samples from 1982 to 2019. Environ Int 145:106123, PMID: , 10.1016/j.envint.2020.106123. [DOI] [PubMed] [Google Scholar]
- 95.Zheng G, Schreder E, Dempsey JC, Uding N, Chu V, Andres G, et al. 2021. Per- and polyfluoroalkyl substances (PFAS) in breast milk: concerning trends for current-use PFAS. Environ Sci Technol 55(11):7510–7520, PMID: , 10.1021/acs.est.0c06978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


