Abstract
Background and Aims:
Refractory pruritus and other complications of cholestasis are indications for liver transplantation (LT) in patients with Alagille syndrome (ALGS). We evaluated predictors of event-free survival and transplant-free survival in patients with ALGS treated with maralixibat (MRX), an ileal bile acid transporter inhibitor.
Approach and Results:
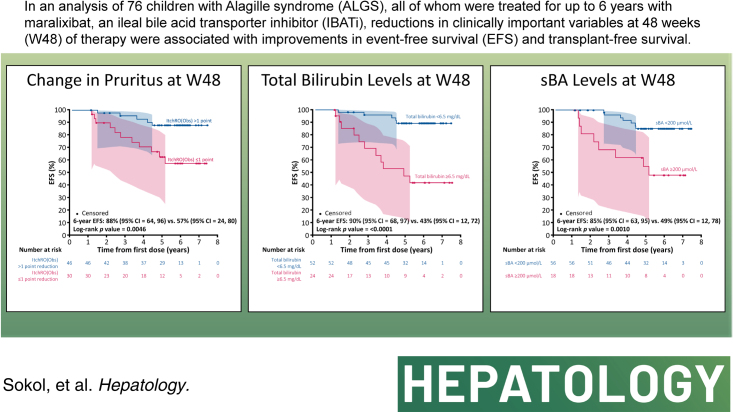
We assessed patients with ALGS from 3 clinical trials of MRX with up to 6 years of follow-up. Event-free survival was defined as the absence of LT, surgical biliary diversion, hepatic decompensation, or death; transplant-free survival was the absence of LT or death. Forty-three potential predictors were evaluated, including age, pruritus (ItchRO[Obs] 0–4 scale), biochemistries, platelets, and serum bile acids. Harrell’s concordance statistic assessed goodness-of-fit, and then, Cox proportional hazard models confirmed the statistical significance of the predictors identified. A further analysis was performed to identify cutoffs using a grid search. Seventy-six individuals met the criteria of receiving MRX for ≥48 weeks with laboratory values available at week 48 (W48). The median duration of MRX was 4.7 years (IQR: 1.6–5.8); 16 had events (10 LT, 3 decompensation, 2 death, and 1 surgical biliary diversion). The 6-year event-free survival improved with a clinically meaningful >1-point ItchRO(Obs) reduction from baseline to W48 (88% vs. 57%; p = 0.005), W48 bilirubin < 6.5 mg/dL (90% vs. 43%; p < 0.0001), and W48 serum bile acid < 200 µmol/L (85% vs. 49%; p = 0.001). These parameters were also predictive of 6-year transplant-free survival.
Conclusions:
Improvement in pruritus by 48 weeks, and lower W48 bilirubin and serum bile acid levels were associated with fewer events. These data may help identify potential markers of disease progression for ALGS patients treated with MRX.
INTRODUCTION
Alagille syndrome (ALGS) is a genetic, multisystem disease characterized by cholestasis with the paucity of interlobular bile ducts, cardiac anomalies, skeletal abnormalities, angiodysplasias of the renal and cerebral arteries, hypercholesterolemia, xanthomas, and ocular changes.1,2 Liver histopathology is notable, with bile duct paucity associated with an early cholestatic phase and progresses in a substantial proportion of patients to cirrhosis, end-stage liver disease, and the need for liver transplantation.3,4 Pruritus in ALGS may be constant and intractable, leading to a severely impaired quality of life. Intractable pruritus was an indication for liver transplant in 69% of patients with ALGS, as reported in the largest natural history cohort from the Global ALagille Alliance study.3,4 Native liver survival in this cohort was reported to be 40.3% by age 18.4 Currently, treatment for ALGS-related pruritus involves off-label use of drugs with variable benefits, including ursodeoxycholic acid, antihistamines, rifampin, opioid antagonists, and bile acid sequestrants.5,6 Impaired bile flow causes a significant elevation of total serum bile acids (sBA), which likely contributes to the pruritus that is seen in most patients, although the precise mechanism linking sBA to pruritus is incompletely understood.5,7 The role of sBA in causing pruritus in ALGS is supported by the antipruritic benefit seen in some patients with ALGS undergoing a surgical biliary diversion (SBD), which interrupts the enterohepatic circulation and reabsorption of conjugated bile acids in the terminal ileum, although this procedure has limitations (eg, surgical complications and stigma from cutaneous stoma) and has variable efficacy in ALGS.8–10
Maralixibat (MRX), an ileal bile acid transporter inhibitor (IBATi), is the first FDA-approved treatment of cholestatic pruritus in patients with ALGS who are 3 months of age and older.11 The primary mechanism of action of an IBATi is to reduce the reuptake of bile acids in the ileum, thereby increasing their fecal excretion and lowering portal venous and sBA levels.5 Approval of MRX was based on the pivotal ICONIC study,12 a placebo-controlled, randomized-withdrawal period phase 2b study with an open-label extension in children aged 1–18 years with ALGS who demonstrated significant and durable reductions in pruritus and sBA. In addition, improvements were observed in cholesterol, xanthomas, pediatric quality of life measures, and height z-scores.12 The MRX clinical trial program, which consisted of ICONIC and 2 additional clinical trials and extension studies, gave participants the option to continue receiving MRX, many of whom have now been followed up for >6 years. These studies provide an opportunity to identify potentially important predictors for long-term outcomes among patients with ALGS treated with MRX. In this analysis, we examined clinical outcomes for up to 6 years of treatment in participants with ALGS enrolled in MRX clinical trials. Our primary aim was to identify predictors of event-free survival (EFS; absence of SBD, hepatic decompensation, liver transplant, or death) and transplant-free survival (TFS; absence of liver transplant or death) in children with ALGS treated with MRX. Once predictors were identified, a further analysis was performed to determine cutoffs for a response. These data will help clarify how clinical and laboratory characteristics, while on MRX therapy, could predict long-term outcomes for patients with ALGS. This, in turn, may be used as a guide to clinical decision-making for physicians and patients.
METHODS
Study population
This analysis integrated data from 3 clinical trials of IBATi in children with ALGS and cholestatic pruritus (Figure 1; Supplemental Tables S1–3, http://links.lww.com/HEP/H881). All trials used Itch-Reported Outcome (Observer) (ItchRO[Obs]) as a primary endpoint as this instrument has previously been validated for the measurement of pruritus in children with ALGS and which defines a >1 point reduction as clinically meaningful.13 Inclusion and exclusion criteria, dosing, and endpoints were similar for all 3 trials. ICONIC (NCT02160782) was a double-blind, randomized-withdrawal study of MRX in children with ALGS, described elsewhere.12 Briefly, ICONIC was a placebo-controlled, phase 2b study with an open-label extension in children with ALGS (1–18 y of age) with more than 3 times the upper limit of normal sBA levels and intractable pruritus. After 18 weeks of MRX, participants were randomized 1:1 to continue MRX or receive a placebo for 4 weeks, after which all participants received open-label MRX up to week 48. Participants could then enroll in a long-term extension study. ITCH (NCT02057692) was a double-blind, randomized controlled trial conducted in North America of MRX for 13 weeks to evaluate safety and efficacy in the reduction of pruritus and sBA by 3 doses of MRX or placebo, with results that have been published.14 Participants enrolled in ITCH were then eligible to enroll in a long-term, open-label extension study (IMAGINE-II) in which all participants received MRX (NCT02117713) for up to 220 weeks. Finally, IMAGO (NCT01903460) had a similar study design as ITCH but was conducted in the United Kingdom, and participants were eligible to enroll in a long-term extension study (IMAGINE; NCT02047318) for up to 288 weeks.30,31 Although 84 participants were enrolled across all 3 clinical trials and their long-term extension studies, this integrated analysis is derived from the 76 participants for whom we had complete data, including laboratory values at baseline and week 48 (with a window of −12 to +6 wk); 8 participants were not included in this analysis because of missing week 48 data (n = 7), events before week 48 (n = 3), or both (n = 2). Written informed consent for the 3 initial clinical trials and extension studies was obtained from caregivers, and assent was obtained when appropriate from the child according to local IRB rules. The study protocols conformed to the ethical guidelines of the 1975 Declaration of Helsinki, the 2018 edition of the Declaration of Istanbul, and were approved by institutional review boards or ethic committees from each study site. Written informed consent was obtained from each participant, parent or legal guardian, as appropriate.
FIGURE 1.
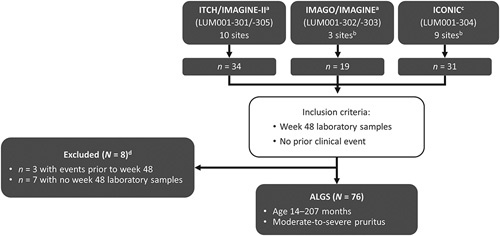
Patient selection for the combined ALGS study population. ITCH: NCT02057692; IMAGINE-II: NCT02117713; IMAGO: NCT01903460; IMAGINE: NCT02047318; and ICONIC: NCT02160782. aMaralixibat dose: up to 266 µg/kg/d. bThere was 1 common site for the IMAGO/IMAGINE and ICONIC trials; the total number of different sites for the overall study population (N = 76) was 21. cMaralixibat dose: 380 µg/kg/d. dTwo participants had events before week 48 and also did not have week 48 laboratory samples. Abbreviation: ALGS, Alagille syndrome.
Outcomes
EFS was defined as the absence of SBD, hepatic decompensation (including the onset of ascites or variceal bleeding), liver transplant, or death. TFS was defined as the absence of liver transplant or death. These outcomes were selected given that they are of paramount importance to patients, families, and clinicians. EFS and TFS are commonly used in adult trials and natural history studies examining long-term outcomes of patients with diseases associated with portal hypertension and chronic liver failure. In addition, while the benefit of IBAT inhibitors on the reduction of pruritus in specific cholestatic disorders is understood, there is a paucity of data regarding these long-term outcomes from clinical trials of IBATis in any liver disease. Participants were followed from the start of MRX treatment for a minimum of 53 weeks and a maximum of 380 weeks. For participants who were still actively receiving MRX, on-study adverse events were collected that identified and described events, including outcome data. For participants who discontinued enrollment in an MRX study, follow-up data on outcome events were collected through an appropriate IRB/ethics committee approval and consent process, and then, data were obtained by the physician through record review or telephone encounters with the participant’s family.
Potential predictors of outcomes and thresholds
The following prespecified variables were considered potential predictors of long-term EFS and TFS: age at enrollment, weight z-score, and height z-score (as assessed using the World Health Organization definitions for children <2 years and the Centers for Disease Control and Prevention guidelines for children ≥2 y),15,16 change in pruritus as assessed by ItchRO(Obs), Peds Quality of Life fatigue score, alanine aminotransferase, aspartate aminotransferase, platelet count, aspartate aminotransferase to platelet ratio index, alkaline phosphatase, total bilirubin, sBA, and clinical trial. Predictors were assessed at multiple timepoints including baseline and week 48, as maximum and minimum values, and as percentage change from baseline. In total, 43 predictors based on specific parameters, timepoints, and values were considered. Harrell’s concordance statistic (C-statistic) was computed for each predictor, with the C-statistic defined as the proportion of observations that the variable can order correctly in terms of survival times indicating the goodness of fit. In general, a value ≤0.5 indicates a very poor model, values ≥0.7 indicate a good model, and values ≥0.8 indicate a strong model.17 C-statistics across all predictors were then ranked, and only predictors with C-statistic ≥ 0.7 were considered for additional analyses although a percent reduction in sBA (ie, 50%) at any time in the first 48 weeks was also evaluated, given that this was the definition for a clinical response in the ICONIC trial.12 Only 1 timepoint was selected per variable of interest. When variables assessed at multiple timepoints (eg, total serum bilirubin at baseline and week 48) showed strong concordance, the variable assessed at week 48 was chosen given its closer proximity to the outcome and the expected likelihood of predicting the outcome. Once variables and timepoints with the highest C-statistics were identified, a survival analysis using a Cox regression model for each result was carried out to confirm that each predictor (treated as a continuous variable) was a statistically significant predictor of the outcomes of interest assessed at the α = 0.05 confidence level for 2-sided comparisons. Finally, a third analysis was performed to identify the optimal threshold for each variable that discriminated between outcomes of interest. In this analysis, the threshold was identified using a grid to determine the highest sustained C-statistic for a given range of the variable; in the context of the grid search, the optimal threshold was no longer expected to perform similarly (eg, ≥0.7 = “good”) since this analysis is performed under repeated testing. Rather, the grid search provided information on the optimal threshold through relative ranking rather than an absolute threshold. For this analysis, the optimal threshold was defined as the value corresponding to a maximal and sustained value.
Additional analyses using unadjusted and adjusted Cox proportional hazard models also explored whether age was independently associated with outcomes (ie, TFS and EFS) or predictive as an independent variable given its relationship to other variables of interest; models were fitted using maximum partial likelihood estimation, and additional fitting with Firth’s correction was explored as a sensitivity analysis. The Cox proportional hazard models were unadjusted, as well as adjusted for age, to determine the independent effect of each risk factor adjusted for age.
Performance over time
The ability of a specific variable and timepoint to predict EFS and TFS over time was assessed from week 50 to 260 using a third analysis of concordance statistics. In this analysis, the plot of the time-dependent AUC was generated using the inverse probability of censoring weighting method to compute the receiver operating characteristic curve.18 Briefly, this technique uses the nearest neighbor to compute the curves. Utilizing this approach, time is a variable in the model, and therefore, the C-statistic values change over the follow-up time; while C-statistics provide overall measures of predictive accuracy, time-dependent receiver operating characteristic curves and AUC functions summarize the predictive accuracy at specific times. In addition, Gwet’s AC1 was calculated to determine whether the concordance between predictors identified at a specific timepoint (eg, week 48) was consistent with predictors identified at other timepoints (ie, week 12 and 24).
Statistical analysis
The baseline was defined as the start of MRX treatment, and follow-up was defined as the time from the start of MRX treatment. Cox regression models with Firth’s correction were used to assess the statistical significance of the predictors identified. Kaplan-Meier curves were used to evaluate the ability of each variable-threshold combination to predict outcomes of interest. Log-rank tests were performed to evaluate the difference between EFS and TFS. To account for potential bias, where individuals exposed to something cannot be expected to have an event before the exposure, or events shortly after exposure are not likely to be causally related, Kaplan-Meier curves were created with events pruned for the first 48 weeks since laboratory values were assessed at that time; EFS, TFS, and log-rank tests were determined as a conditional survival analysis requiring that patients survived up to 48 weeks. Given insufficient evidence to indicate variables that were not normally distributed, Pearson correlation coefficients were used to assess the relationship between all identified predictors. The impact of predictors adjusted for age was also assessed using a Cox proportional hazard model with 95% CIs. Statistical significance was assessed at the α = 0.05 confidence level for 2-sided comparisons. Statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Study population
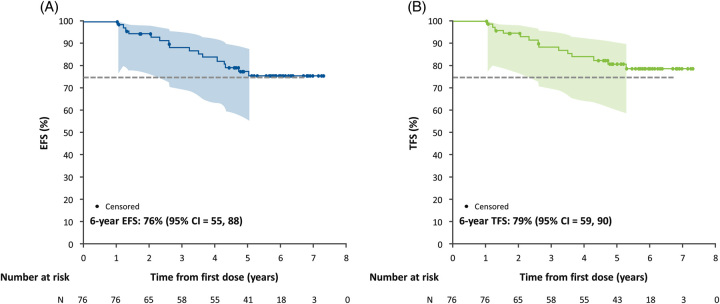
This analysis was derived from 76 pediatric participants with baseline (at time of enrollment into the clinical trial) median (Q1, Q3) age of 70 (33–126) months, weight z-score of −1.41 (−2.15, −0.67), and height z-score of −1.50 (−2.29, −0.95) (Table 1). Participants had a baseline median ItchRO(Obs) score of 2.7 (2.1, 3.1), total bilirubin of 2.3 (0.9, 8.4) mg/dL, direct bilirubin of 1.7 (0.6, 7.0) mg/dL, alanine aminotransferase of 134 (95, 193) U/L, and sBA levels of 184 (78, 361) µmol/L. Among all participants, events occurred in 16 (22%) individuals, including 10 (13%) liver transplants, 3 (4%) hepatic decompensations, 2 (3%) deaths, and 1 (1%) SBD. Overall, EFS and TFS for the cohort at 6 years of follow-up (ie, from the start of MRX) were 76% and 79%, respectively (Figure 2).
TABLE 1.
Clinical and laboratory characteristics
| Clinical and laboratory characteristics | Baseline (N = 76) | Week 48 (N = 76) |
|---|---|---|
| ItchRO(Obs) scorea | 2.7 (2.1, 3.1) | 1.14 (0.24, 1.86) |
| Weight z-score | −1.41 (−2.15, −0.67) | −1.40 (−2.05, −0.63) |
| Height z-score | −1.50 (−2.29, −0.95) | −1.45 (−2.35, −0.81 |
| Total bilirubin (mg/dL) | 2.3 (0.9, 8.4) | 2.0 (0.9, 8.1) |
| Direct bilirubin (mg/dL) | 1.7 (0.6, 7.0) | 1.7 (0.6, 6.8) |
| sBA (μmol/L) | 184 (78, 361) | 100 (43, 194) |
| Albumin (g/dL) | 4.6 (4.3, 4.7) | 4.6 (4.3, 4.7) |
| ALT (U/L) | 134 (95, 193) | 181 (125, 237) |
| AST (U/L) | 130 (96, 185) | 155 (110, 209) |
| GGT (U/L) | 392 (188, 751) | 418 (285, 663) |
| International normalized ratio | 1.00 (1.00, 1.10) | 1.00 (1.00, 1.10) |
| Platelets (×109/L) | 293 (223, 383) | 252 (195, 346) |
Note: All data are median (Q1, Q3).
ItchRO(Obs) score recorded as weekly morning average.
Abbreviations: GGT, gamma-glutamyl transferase; ItchRO(Obs), Itch-Reported Outcome (Observer); sBA, serum bile acids.
FIGURE 2.
Kaplan-Meier estimates of overall 6-year outcomes in children with Alagille syndrome treated with maralixibat. (A) EFS. (B) TFS. The dashed line indicates a 75% survival threshold. The shaded area indicates 95% confidence limits. Abbreviations: EFS, event-free survival; TFS, transplant-free survival.
Identification of predictors
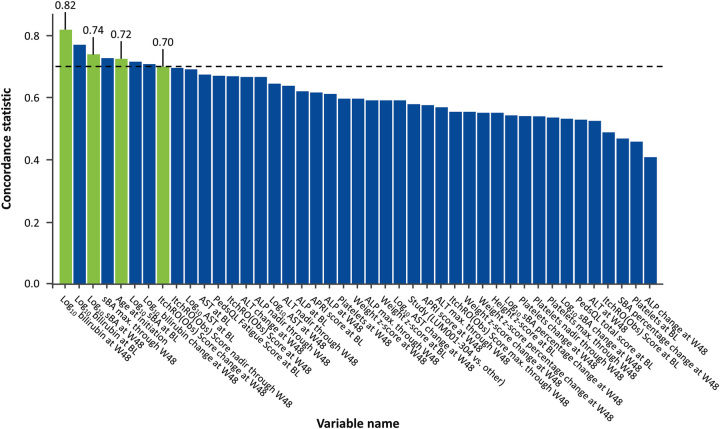
C-statistics for the prediction of EFS were determined for prespecified variables at different timepoints in the study follow-up and organized by descending value (Figure 3). Week 48 log10 bilirubin was the strongest predictor of EFS (0.82), followed by week 48 log10 sBA (0.74). The next highest unique C-statistic was the age at initiation of MRX (0.72). Although the C-statistic for the nadir of ItchRO(Obs) >48 weeks was slightly higher than the value at 48 weeks (0.71 vs. 0.70), the latter timepoint was selected for future modeling given its closer proximity to outcomes of interest. All other variables had C-statistics < 0.7 and were not included in additional analyses. Similar C-statistics were seen for the prediction of TFS (Supplemental Figure S1, http://links.lww.com/HEP/H881). The predictive ability of these 4 variables was reassessed using the Cox proportional hazard model and continued to demonstrate a strong association between these variables and EFS (Supplemental Table S4, http://links.lww.com/HEP/H881).
FIGURE 3.
Concordance statistic for prediction of event-free survival in children with Alagille syndrome treated with maralixibat, according to demographics, and clinical and laboratory characteristics. Variables selected for inclusion in the model (ie, those with a concordance statistic ≥0.7) were total bilirubin levels at W48, change in pruritus (as assessed by ItchRO[Obs]) from baseline to W48, sBA (total serum bile acids) levels at W48, and age at enrollment. Abbreviations: ALP, alkaline phosphatase; APRI, AST to platelet ratio index; BL, baseline; ItchRO(Obs), Itch-Reported Outcome (Observer); PedsQL, Pediatric Quality of Life InventoryTM 4.0 Generic Core Scales; sBA, serum bile acids; W48, week 48.
Identification of threshold for pruritus, bilirubin, and sBA
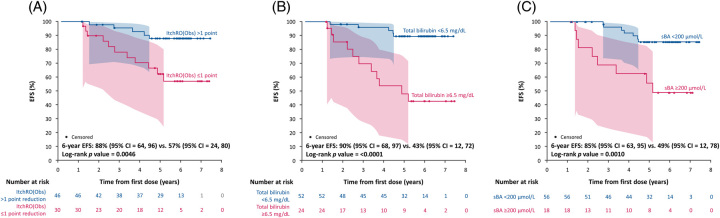
With predictors identified and their statistical significance confirmed, a separate analysis to determine cutoffs was performed. Using a grid search, the optimal threshold for ItchRO(Obs) to discriminate between the presence and absence of an event was a >1-point reduction with a C-statistic of 0.67 (Supplemental Figure S2A, http://links.lww.com/HEP/H881). At 6 years of follow-up, children who had >1 point reduction in ItchRO(Obs), compared with those with ≤1 point reduction, had increased EFS (88% vs. 57%; p = 0.0046; Figure 4A; Table 2) and TFS (93% vs. 57%; p = 0.0007). For bilirubin, the grid search identified the optimal threshold to discriminate between the presence and absence of an event as week 48 log10 bilirubin of 2.045 µmol/L (corresponding to 6.5 mg/dL) with a C-statistic of 0.73 (Supplemental Figure S2B, http://links.lww.com/HEP/H881). At 6 years of follow-up, children with week 48 bilirubin <6.5 mg/dL, compared with those with higher values, had increased EFS (90% vs. 43%; p < 0.0001; Figure 4B) and TFS (94% vs. 42%; p < 0.0001). Finally, the grid search identified for sBA the optimal threshold to discriminate between the presence and absence of an event as week 48 log10 sBA 2.3 µmol/L (corresponding to 200 µmol/L) with a C-statistic of 0.68 (Supplementary Figure 2C, http://links.lww.com/HEP/H881). At 6 years of follow-up, children with week 48 sBA < 200 µmol/L, compared with those with higher values, had increased EFS (85% vs. 49%; p = 0.0010; Figure 4C) and TFS (90% vs. 49%; p = 0.0001). Similarly, at 6 years of follow-up, children with a 50% reduction in sBA, compared with those that did not achieve this degree of reduction, had increased EFS (86% vs. 39%; p < 0.0001; Supplemental Figure S3A, http://links.lww.com/HEP/H881) and TFS (90% vs. 39%; p < 0.0001; Supplemental Figure S3B, http://links.lww.com/HEP/H881).
FIGURE 4.
Kaplan-Meier estimates of 6-year EFS in children with Alagille syndrome treated with maralixibat for each predictive variable. (A) Change in pruritus (as measured by ItchRO[Obs]) from baseline to week 48 (>1 point reduction vs. ≤1 point reduction). (B) Total bilirubin levels at week 48 (<6.5 mg/dL vs. ≥6.5 mg/dL). (C) sBA levels at week 48 (<200 µmol/L vs. ≥200 µmol/L). The shaded area indicates 95% confidence limits. Abbreviations: EFS, event-free survival; ItchRO(Obs), Itch-Reported Outcome (Observer); sBA, serum bile acids.
TABLE 2.
Probability of EFS and TFS in children with Alagille syndrome treated with maralixibat according to the threshold of predictor
| Variable | ||||||
|---|---|---|---|---|---|---|
| Change in pruritusa from baseline to week 48 | Total bilirubin levels at week 48 | sBA levels at week 48 | ||||
| Improved: > 1 point reduction n = 46 | Worse: ≤ 1 point reduction n = 30 | Improved: < 6.5 mg/dL n = 52 | Worse: ≥ 6.5 mg/dL n = 24 | Improved: < 200 µmol/L n = 56 | Worse: ≥ 200 µmol/L n = 18 | |
| 6-y EFS (%) | 88 | 57 | 90 | 43 | 85 | 49 |
| C-statistic | 0.70 | 0.82 | 0.74 | |||
| p b | 0.0046 | <0.0001 | 0.0010 | |||
| 6-y TFS (%) | 93 | 57 | 94 | 42 | 90 | 49 |
| C-statistic | 0.77 | 0.85 | 0.79 | |||
| p b | 0.0007 | < 0.0001 | 0.0001 | |||
Note: “improved” or “worse” refers to the predicted outcomes for either EFS or TFS.
As assessed by ItchRO(Obs).
p-value from the log-rank test.
Abbreviations: EFS, event-free survival; ItchRO(Obs), Itch-Reported Outcome (Observer); sBA, serum bile acids; TFS, transplant-free survival.
Impact of age
Age was identified as a predictor and was seen to be statistically significant in a Cox regression model. The grid search identified the optimal age threshold to discriminate between the presence and absence of an event as 36 months with a C-statistic of 0.72. Age was associated with bilirubin and pruritus such that patients <36 months and those ≥36 months had higher median total bilirubin (9.60 vs. 1.80 mg/dL; p = 0.006), direct bilirubin (8.10 vs. 1.10 mg/dL; p = 0.004), and sBA (298 vs. 144 µmol/L; p = 0.023), indicating worse cholestasis in the younger cohort and the importance of age as a potentially confounding variable in the relationship between other predictors with EFS and TFS (Supplemental Table S5, http://links.lww.com/HEP/H881). Because age was strongly correlated with ItchRO(Obs), bilirubin, and sBA, Cox proportional hazard models with maximum partial likelihood estimation were developed to assess the independent effect of age on EFS. Age below 36 months was associated with increased risk of an event in an unadjusted Cox proportional hazard model (HR: 3.636; 95% CI, 1.360, 9.721; Supplemental Table S6, http://links.lww.com/HEP/H881). With respect to pruritus, children who had a <1 point reduction in ItchRO(Obs) had a significantly increased risk of an event in an unadjusted model (HR: 4.108; 95% CI, 1.425, 11.839). In a model that assessed the independent effect of ItchRO(Obs) and age on EFS, a <1 point reduction in ItchRO(Obs) continued to be associated with increased risk of an event (adjusted HR [aHR]: 3.120; 95% CI, 1.023, 9.519), whereas age was no longer associated with an event (aHR: 2.506; 95% CI, 0.887, 7.081), indicating that the association between age and EFS can largely be attributed to the association between age and ItchRO(Obs). Similarly, children who had week 48 bilirubin > 6.5 mg/dL had a significantly increased risk of an event in an unadjusted model (HR: 7.427; 95% CI, 2.570, 21.465). In a model that assessed the independent effect of bilirubin and age, elevated week 48 bilirubin continued to be associated with reduced EFS (aHR: 6.019; 95% CI, 1.992, 18.186), but age was no longer associated with reduced EFS (aHR: 2.183; 95% CI, 0.780, 6.109). Finally, children with sBA > 200 µmol/L had an increased risk of an event in an unadjusted model (HR: 4.688; 95% CI, 1.699, 12.937). In a model that assessed the independent effect of sBA and age, elevated week 48 sBA continued to be associated with reduced EFS (aHR: 4.261; 95% CI, 1.533, 11.849), whereas age was not independently associated with reduced EFS (aHR 2.781; 95% CI, 0.995, 7.770). Sensitivity analyses were also run using the Cox proportional hazard model fitted with Firth’s correction yielding similar results (Supplemental Table S7, http://links.lww.com/HEP/H881).
Prediction over time
C-statistics in the change in ItchRO(Obs), bilirubin, and sBA from week 48 to >250 weeks were consistently >0.7 (Supplemental Figure S4, http://links.lww.com/HEP/H881), suggesting that the predictive ability of these variables was stable over several years. Additional sensitivity analyses evaluated whether individuals consistently remained above or below a response threshold at week 12, week 24, and week 48, and these analyses showed that concordance across all 3 timepoints was very good for bilirubin (92.2%; 95% CI, 84.5, 99.8; p < 0.0001), good for pruritus (62.1%; 95% CI, 47.3, 76.9; p < 0.0001), and moderate for sBA (46.5%; 95% CI, 31.0, 62.1; p < 0.0001).
Relationship between predictors
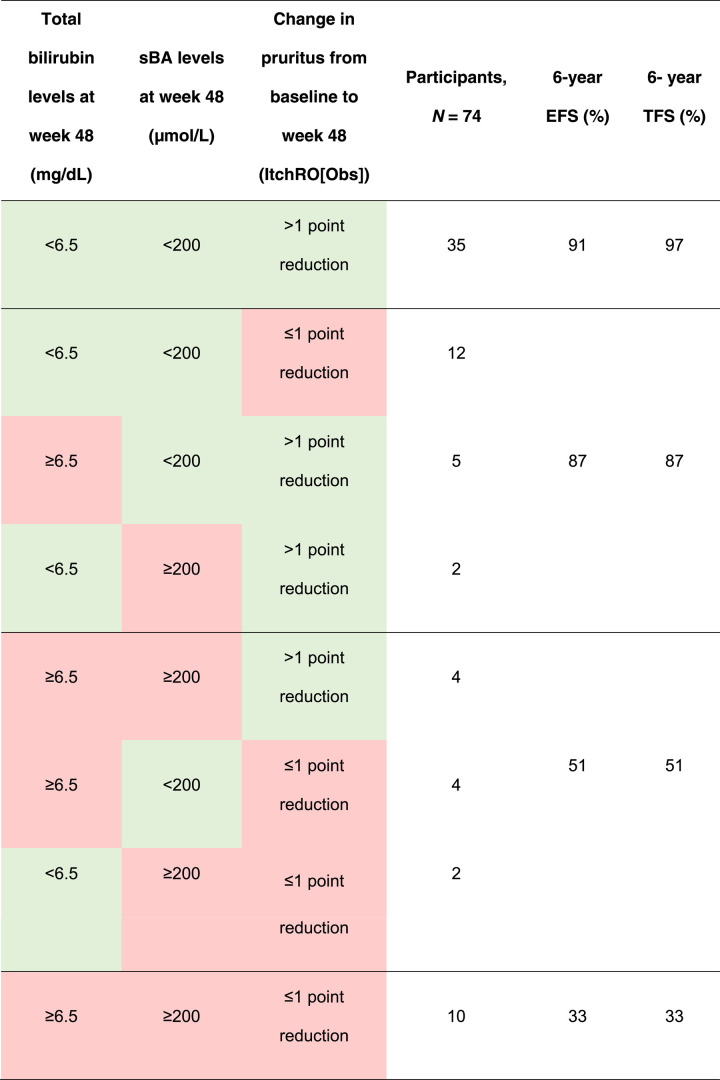
Week 48 log10 bilirubin, change in ItchRO(Obs), and week 48 sBA from baseline were all correlated, with the strongest correlation observed between week 48 log10 bilirubin and week 48 sBA (r = 0.72; p < 0.0001; Table 3). Complete data for the 3 variables were available for 74 children. Aggregating variables further served to help predict EFS (Table 4). Individuals with protective factors (ie, within the range of the threshold that predicted better outcomes) for all 3 variables (n = 35) or 2 variables (n = 19) had 6-year EFS of 91% and 87%, respectively. Individuals who had protective factors for 1 variable (n = 10) or no variables (n = 10) had 6-year EFS of 51% and 33%, respectively. Similarly, the impact of a 1-point ItchRO(Obs) reduction was also noted even in the presence of high bilirubin (ie, >6.5 mg/dL) with 6-year EFS of 69% for those that achieved a week 48 1-point reduction compared with 31% for those that did not achieve this reduction (Supplemental Figure S5, http://links.lww.com/HEP/H881). Similar relationships were identified for TFS (Table 4). In a sensitivity analysis in patients only with elevated bilirubin, reductions in pruritus led to improvements in EFS (56% vs. 17%) and improvements in TFS (55% vs. 17%; Supplemental Figure S5, http://links.lww.com/HEP/H881).
TABLE 3.
Relationship between predictors of event-free survival in children with Alagille syndrome treated with maralixibat
| Variable 1 | Variable 2 | Pearson correlation coefficient | p |
|---|---|---|---|
| Total bilirubin levels at week 48 | sBA levels at week 48 | 0.72 | <0.0001 |
| Total bilirubin levels at week 48 | Change in pruritusa from baseline to week 48 | 0.35 | 0.0019 |
| sBA levels at week 48 | Change in pruritusa from baseline to week 48 | 0.39 | 0.0005 |
As assessed by ItchRO(Obs).
Abbreviations: ItchRO(Obs), Itch-Reported Outcome (Observer); sBA, serum bile acids.
TABLE 4.
Relative effect of multiple predictive factors on EFS in children with Alagille syndrome treated with maralixibat
Note: green denotes a threshold predictive of improved EFS, and red denotes a threshold predictive of worse EFS.
Abbreviations: EFS, event-free survival; ItchRO(Obs), Itch-Reported Outcome (Observer); sBA, serum bile acids; TFS, transplant-free survival.
DISCUSSION
In this analysis of 76 children with ALGS, all of whom were treated with MRX for up to 6 years, reductions in clinically important variables were associated with improvements in EFS and TFS. Specifically, treatment response to MRX (ie, a clinically significant 1-point reduction in ItchRO[Obs] at week 48) was associated with substantially improved EFS, from 57% in those without this reduction in ItchRO(Obs) to 88% in those with a ≥1-point reduction, and improved TFS, from 57% to 93%, respectively. Similar improvements in EFS and TFS were observed in MRX-treated individuals who had lower bilirubin and sBA at week 48 of therapy, further supporting the importance of these biomarkers in the progression of ALGS. Individuals who had multiple predictors of poor outcomes while receiving MRX had much lower (<50%) EFS and TFS. The presence of a single predictor in isolation (eg, bilirubin >6.5 mg/dL) still provides a strong positive predictive EFS and TFS of 87% if other risk factors demonstrate improvement. Furthermore, reduction in pruritus as measured by ItchRO(Obs), the primary therapeutic endpoint for MRX in clinical trials, continued to be associated with improved EFS even among those individuals with high bilirubin.
Although the benefit of MRX to treat pruritus in patients with ALGS has been demonstrated,12 this analysis provides important new findings on the impact on longer clinical outcomes in patients with ALGS receiving an IBATi and elucidates important predictors of EFS and TFS. These findings suggest that patients treated with long-term MRX with clinically meaningful responses may have improved survival and clinical outcomes, including liver transplant and SBD.12 There is also a paucity of data on the role of sBA to predict outcomes in ALGS, and thus, sBA levels are rarely obtained in the clinical management of ALGS.4 In this analysis, the better outcomes observed in those with lower sBA may also represent the continued effectiveness of MRX as an IBATi. These data identify potential prognostic markers that may better inform patient-provider discussions on the possible benefits of MRX treatment. It should also be noted that, while the best predictor for any given biomarker was selected, baseline bilirubin was the second strongest predictor and only slightly surpassed by week 48 bilirubin, yet the former may have important implications in informing patient expectations and in managing the care of the patients (ie, proceed to pretransplant evaluation to avoid delay) at the onset of therapy. At the same time, as thresholds were identified using a grid search to identify optimal thresholds that were both maximal and sustained, variability exists around these thresholds and, therefore, should not be seen as defining markedly different clinical states or implying that patients slightly above or below a threshold would have substantially different outcomes, as demonstrated by improvement in EFS and TFS in patients with high bilirubin who have improved pruritus while on MRX. Ultimately, the values around the predictors should be seen as providing some clinical guidance of how changes in disease characteristics (pruritus response) and laboratory characteristics (bilirubin and sBA) may inform long-term outcomes, but these values should not be considered definitive or concrete, and indeed outcomes will undoubtedly vary for individual patients.
The identification of younger age as a predictor for EFS and TFS in patients treated with MRX may seem unusual, as it may be taken to imply that physicians should wait to start therapy. The likely explanation is that the probability of transplant for children with ALGS is not constant throughout childhood, and there is a survivor bias such that older children are inherently healthier or they would have already undergone transplantation. We attempted to account for this bias by adjusting for bilirubin and sBA. In these analyses, age was no longer statistically associated with EFS or TFS though some relationship between younger age with these outcomes persisted. Likely, there is still residual confounding of disease severity that is not fully accounted for after adjustment of these biomarkers, and these findings should not be interpreted to suggest that a delay in therapy is beneficial to the patient.
It is not surprising that MRX, a drug with a primary mechanism of action to reduce the bile salt pool size, and consequently sBA and pruritus, would ultimately lead to a delay in the need for a liver transplant for children with ALGS or a reduction in the number of transplants over a fixed time period. The majority of children with ALGS and cholestasis as a child will require a transplant, with 2 large prospective cohorts estimating that 50%–75% of patients with ALGS will require a liver transplant by adulthood.3,4 For these patients, pruritus was an important indication for transplant. In a recent analysis from the Global ALagille Alliance cohort, which included natural history data from >1400 patients, 358 patients required a liver transplant, with 69% being transplanted for intractable pruritus.4 In addition to liver transplant, SBD has been used for intractable pruritus in patients with ALGS with approximately 50% of patients with ALGS demonstrating some response.9 Both liver transplant and SBD continue to require ongoing management and carry significant risks, including immunosuppression, allograft rejection, infection, surgical complications, and the potential stigmatization of a cutaneous stoma in SBD. Thus, avoiding or delaying these surgeries should be the primary goal of effective therapies developed for ALGS.
The predictive value of serum bilirubin and sBA in this cohort for long-term outcomes may provide meaningful insights into the pathophysiology of ALGS. Several studies have identified bilirubin as an important predictor of outcomes in ALGS. In a 2010 retrospective study, 33 children with ALGS and long-term follow-up (ie, at least 10 y) were categorized into a group with mild disease (ie, biochemical or bilirubin abnormalities with or without pruritus response to medications) and a group with severe disease (ie, intractable pruritus, evidence of cirrhosis or portal hypertension, or need for transplant or SBD).6 Patients with mild disease after 10 years of age had significantly lower median total bilirubin and conjugated bilirubin through the first 5 years of life compared with patients with severe disease. The optimal threshold of total bilirubin for children <5 years for differentiating between subsequent mild and severe disease outcomes was 6.5 mg/dL, the same threshold that was identified in this study for the prediction of TFS and EFS in patients with ALGS treated with MRX. Similar findings were observed in a multicenter prospective study of 144 children with ALGS by Mouzaki et al,19 which identified total bilirubin as the strongest laboratory predictor of worse outcomes. The study also reported that a decrease in serum bilirubin in early childhood predicted better outcomes, suggesting that bilirubin may be a surrogate indicator of disease progression. Finally, data from the Global ALagille Alliance also confirmed that mean bilirubin in infancy is predictive of liver transplant throughout childhood, as evidenced by children aged 6–12 months with total bilirubin < 5.0 mg/dL having lower rates of transplant and death.4
Elevated levels of sBA are associated with the cholestatic pruritus observed in patients with ALGS, hence the correlation between lowering of sBA levels and improvement in pruritus by IBATi therapy in ALGS.12 There is also emerging evidence that hepatic retention of bile acids may mediate hepatobiliary damage and activate inflammatory, oxidative stress, and fibrotic pathways in both animal models and human cholestatic liver disorders.20–22 For example, in children with genetic deficiency of bile salt export pump (progressive familial intrahepatic cholestasis [PFIC] type 2), the failure of canalicular secretion of conjugated bile acids impairs bile flow and causes hepatic accumulation of bile acids and elevated sBA,5 which is associated with severe pruritus. One strategy to mitigate the pruritus of both ALGS and PFIC is to create an SBD that removes bile acids from the enterohepatic circulation, by either providing for enterocutaneous stoma disposal of bile acids or increasing their fecal excretion. Research from the NAtural Course and Prognosis of PFIC and Effect of Biliary Diversion (NAPPED) study group showed that, for patients with PFIC2, the ability to reduce sBA following SBD predicted survival with the native liver. Specifically, 100% of individuals with sBA levels reduced to <102 µmol/L or by 75% of baseline levels survived with their native liver (without liver transplant) with up to 15 years of follow-up compared with <40% of individuals who failed to meet these criteria.23 In addition, a preliminary report of a meta-analysis of 24 reports of SBD in PFIC similarly demonstrated that absolute sBA levels or percent sBA reduction after SBD were strongly associated with the need for a liver transplant.24 In addition, in a phase 2 study with MRX, 5-year TFS was observed in patients who met the NAPPED-defined sBA response threshold.25 It is hypothesized that this apparent benefit of lowering sBA levels on hepatic outcomes may result from the proposed role of accumulated bile acids in promoting the development of inflammation, hepatocellular injury, and hepatic fibrosis.26–28
One important limitation of this study is that this analysis, while conducted using a sample size that may be considered large for rare diseases, is still limited and does not allow for the analysis to be conducted first in an exploratory dataset and then reassessed in a validation dataset. While this approach can be helpful in predictive analyses to avoid spurious associations, our initial set of potential predictors was selected based on a strong understanding of the biology of ALGS and cholestatic liver diseases. A second limitation of this study is the absence of liver histology or data from noninvasive techniques to assess hepatic fibrosis. Although most children with ALGS are transplanted for a combination of reasons that involve both pruritus and sequelae of chronic liver disease, it is not clear if the improvements in EFS and TFS observed in this analysis are solely the result of improvements in pruritus in individuals for whom that would be a major indication for transplant or if there was improvement or stabilization of hepatic fibrosis and consequent portal hypertension related to the reduction in hepatic retention of toxic bile acids. Since obtaining paired or follow-up liver biopsies in children with ALGS is generally not feasible, the performance of transient elastography or noninvasive markers of liver fibrosis might be helpful in the future to further elucidate the impact of IBATi on bile acid reduction in ALGS. It is also worth noting that, while an sBA threshold of 200 µmol/L at 48 weeks was an important predictor of EFS and TFS, this absolute value is appreciably high, and it may be reasonably understood that patients with this degree of elevated sBA would have a poor outcome regardless. In addition, this threshold may be impractical for informing clinical guidance for patients who do have such elevations of sBA >200 µmol/L. For this reason, and because percent reduction was considered a response criterion in the MRX pivotal study, the ICONIC trial, we also explored a 50% reduction in sBA, which demonstrated similar significance in predicting EFS and TFS. A third limitation of this analysis is that only individuals who were perceived to receive some benefit—improved pruritus, laboratory parameters, or nutrition—were maintained on MRX for sufficient follow-up that allowed for examination of long-term outcomes, such as EFS and TFS. Consequently, caution should be exercised in applying these findings to patients who do not seem to have any appreciable benefit from the drug. Likewise, all patients in this analysis received MRX, and there was no long-term placebo arm of the study. Thus, improvements in EFS and TFS observed in this cohort can not necessarily be attributed to MRX but rather may have been the result of improvements in these parameters that could occur as part of the natural history of ALGS. Comparison of our findings to an appropriate control cohort could help elucidate how EFS and TFS observed in our study compare with the overall natural history of ALGS. A natural history comparison has recently been conducted and suggests improved EFS and TFS with MRX treatment over the standard of care.29 Finally, all individuals in this analysis were participants in clinical trials, which represents a controlled patient population with specific standards of follow-up that may not be reflective of the broader practices for patients with ALGS in a real-world setting.
In conclusion, this study identifies the value of several clinical parameters (sBA levels, total serum bilirubin, and change in pruritus from baseline) obtained after 48 weeks of MRX therapy as potential predictors of subsequent TFS and EFS. These predictors of disease progression may help guide clinical decisions and prognostication in patients with ALGS treated with MRX. Inhibition of IBAT and its consequent reduction in sBA represent a new therapeutic pathway for the treatment of cholestatic pruritus that has led to the development of the first FDA-approved therapies for ALGS and PFIC.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the clinical trial participants, their families, and investigators for their participation in the MRX clinical trials. Editorial support was provided by Helen Clough, PhD, of Health Interactions, funded by Mirum Pharmaceuticals Inc.
Footnotes
Abbreviations: aHR, adjusted HR; ALGS, Alagille syndrome; C-statistic, concordance statistic; EFS, event-free survival; IBATi, ileal bile acid transporter inhibitor; ItchRO(Obs), Itch-Reported Outcome (Observer); LT, liver transplantation; MRX, maralixibat; PedsQL, Peds Quality of Life; PFIC, progressive familial intrahepatic cholestasis; sBA, serum bile acid; SBD, surgical biliary diversion; TFS, transplant-free survival; W48, week 48.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.hepjournal.com.
Contributor Information
Ronald J. Sokol, Email: ronald.sokol@childrenscolorado.org.
Emmanuel M. Gonzales, Email: emmanuel.gonzales@aphp.fr.
Binita M. Kamath, Email: binita.kamath@sickkids.ca.
Alastair Baker, Email: alastair.baker@nhs.net.
Pamela Vig, Email: pvig@mirumpharma.com.
Douglas B. Mogul, Email: douglas.mogul@mirumpharma.com.
Will Garner, Email: WGarner@mirumpharma.com.
Bettina E. Hansen, Email: b.hansen@erasmusmc.nl.
Emmanuel Jacquemin, Email: emmanuel.jacquemin@aphp.fr.
Richard J. Thompson, Email: richard.j.thompson@kcl.ac.uk.
DATA AVAILABILITY STATEMENT
Beginning 6 months and ending 5 years after publication, deidentified participant information from the data meta-analysis might be made available to investigators whose proposed use of the data has been approved by a review committee, including the primary authors. Proposals should be directed to medinfo@mirumpharma.com. Before being granted access, data requesters will be required to sign a data access agreement.
AUTHOR CONTRIBUTIONS
All authors contributed to the design of the study, data collection, analysis and interpretation, and writing and review of the manuscript. All authors had access to and verified the data and were responsible for the decision to submit the manuscript. All authors confirm the fidelity of the study to the protocol, accuracy, and completeness of data, and approved manuscript publication. The corresponding author had final responsibility for manuscript submission.
FUNDING INFORMATION
This study was supported by Mirum Pharmaceuticals Inc.
CONFLICTS OF INTEREST
Ronald J. Sokol advises and consults for Albireo. He advises Mirum. He consults for Alexion. Emmanuel M. Gonzales consults for and advises Albireo and Mirum. He consults for Laboratoires CTRS and Vivet Therapeutics. Binita M. Kamath consults for and received grants from Mirum and Albireo. She consults for Audentes. Alastair Baker received grants from Mirum and Albireo. Pamela Vig owns stock in and is employed by Mirum. Douglas B. Mogul owns stock in and is employed by Mirum. Will Garner owns stock in and is employed by Mirum. Bettina E. Hansen consults for and received grants from Intercept, Calliditas, and Cymabay. She consults for Ipsen, Alberio, Mirum, Enyo, and Eiger. Emmanuel Jacquemin consults for Laboratoire CTRS and Vivet Therapeutics. Richard J. Thompson consults for and owns stock in GenerationBio and Rectify Therapeutics. He consults for Mirum and Albireo.
REFERENCES
- 1.Alagille D, Odièvre M, Gautier M, Dommergues JP. Hepatic ductular hypoplasia associated with characteristic facies, vertebral malformations, retarded physical, mental, and sexual development, and cardiac murmur. J Pediatr. 1975;86:63–71. [DOI] [PubMed] [Google Scholar]
- 2.Saleh M, Kamath BM, Chitayat D. Alagille syndrome: Clinical perspectives. Appl Clin Genet. 2016;9:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamath BM, Ye W, Goodrich NP, Loomes KM, Romero R, Heubi JE, et al. Outcomes of childhood cholestasis in Alagille syndrome: results of a multicenter observational study. Hepatol Commun. 2020;4:387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vandriel SM, Li L-T, She H, Wang JS, Gilbert MA, Jankowska I, et al. Natural history of liver disease in a large international cohort of children with Alagille syndrome: Results from the GALA study. Hepatology. 2023;77:512–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamath BM, Stein P, Houwen RHJ, Verkade HJ. Potential of ileal bile acid transporter inhibition as a therapeutic target in Alagille syndrome and progressive familial intrahepatic cholestasis. Liver Int. 2020;40:1812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kronsten V, Fitzpatrick E, Baker A. Management of cholestatic pruritus in paediatric patients with Alagille syndrome: the King’s College Hospital experience. J Pediatr Gastroenterol Nutr. 2013;57:149–54. [DOI] [PubMed] [Google Scholar]
- 7.Bergasa NV.Carstens E, Akiyama T. Chapter 6: Pruritus of Cholestasis. Itch: Mechanisms and Treatment. CRC Press/Taylor & Francis; 2014:61–88. [Google Scholar]
- 8.Lemoine C, Superina R. Surgical diversion of enterohepatic circulation in pediatric cholestasis. Semin Pediatr Surg. 2020;29:150946. [DOI] [PubMed] [Google Scholar]
- 9.Wang KS, Tiao G, Bass LM, Hertel PM, Mogul D, Kerkar N, et al. Analysis of surgical interruption of the enterohepatic circulation as a treatment for pediatric cholestasis. Hepatology. 2017;65:1645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitington PF, Whitington GL. Partial external diversion of bile for the treatment of intractable pruritus associated with intrahepatic cholestasis. Gastroenterology. 1988;95:130–6. [DOI] [PubMed] [Google Scholar]
- 11.Mirum Pharmaceuticals Inc. LIVMARLI® (maralixibat) [prescribing information]. 2023. Accessed May 1, 2023. https://files.mirumpharma.com/livmarli/livmarli-prescribinginformation.pdf?_ga=2.264585739.542484716.1646698894-2002651408.1625676440. [Google Scholar]
- 12.Gonzales E, Hardikar W, Stormon M, Baker A, Hierro L, Gliwicz D, et al. Efficacy and safety of maralixibat treatment in patients with Alagille syndrome and cholestatic pruritus (ICONIC): A randomised phase 2 study. Lancet. 2021;398:1581–92. [DOI] [PubMed] [Google Scholar]
- 13.Kamath BM, Abetz-Webb L, Kennedy C, Hepburn B, Gauthier M, Johnson N, et al. Development of a novel tool to assess the impact of itching in pediatric cholestasis. Patient. 2018;11:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shneider BL, Spino C, Kamath BM, Magee JC, Bass LM, Setchell KD, et al. Placebo-controlled randomized trial of an intestinal bile salt transport inhibitor for pruritus in Alagille syndrome. Hepatol Commun. 2018;2:1184–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization (WHO). The WHO Child Growth Standards: Weight-for-length/height. Accessed February 27, 2023. https://www.who.int/tools/child-growth-standards/standards/weight-for-length-height.
- 16.Centers for Disease Control and Prevention (CDC). February 27, 2023. Clinical Growth Charts. https://www.cdc.gov/growthcharts/clinical_charts.htm.
- 17.Hosmer DW, Lemeshow S. Applied Logistic Regression, Second Edition. John Wiley & Sons, Inc.; 2000. [Google Scholar]
- 18.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–44. [DOI] [PubMed] [Google Scholar]
- 19.Mouzaki M, Bass LM, Sokol RJ, Piccoli DA, Quammie C, Loomes KM, et al. Early life predictive markers of liver disease outcome in an international, multicentre cohort of children with Alagille syndrome. Liver Int. 2016;36:755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fickert P, Wagner M. Biliary bile acids in hepatobiliary injury—what is the link? J Hepatol. 2017;67:619–31. [DOI] [PubMed] [Google Scholar]
- 21.Li T, Apte U. Bile acid metabolism and signaling in cholestasis, inflammation, and cancer. Adv Pharmacol. 2015;74:263–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yerushalmi B, Dahl R, Devereaux MW, Gumpricht E, Sokol RJ. Bile acid-induced rat hepatocyte apoptosis is inhibited by antioxidants and blockers of the mitochondrial permeability transition. Hepatology. 2001;33:616–26. [DOI] [PubMed] [Google Scholar]
- 23.van Wessel DBE, Thompson RJ, Gonzales E, Jankowska I, Sokal E, Grammatikopoulos T, et al. Genotype correlates with the natural history of severe bile salt export pump deficiency. J Hepatol. 2020;73:84–93. [DOI] [PubMed] [Google Scholar]
- 24.Verkade HJ, Thompson RJ, Arnell H, Fischler B, Gillberg PG, Mattsson JP, et al. Systematic literature review of the effect of partial external biliary diversion surgery on clinical and biochemical outcomes in progressive familial intrahepatic cholestasis patients. J Pediatr Gastroenterol Nutr. 2018;67(suppl 1):S209–10; Abstract 353. [Google Scholar]
- 25.Loomes KM, Squires RH, Kelly D, Rajwal S, Soufi N, Lachaux A, et al. Maralixibat for the treatment of PFIC: Long-term, IBAT inhibition in an open-label, phase 2 study. Hepatol Commun. 2022;6:2379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mousa OY, Juran BD, McCauley BM, Vesterhus MN, Folseraas T, Turgeon CT, et al. Bile acid profiles in primary sclerosing cholangitis and their ability to predict hepatic decompensation. Hepatology. 2021;74:281–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nimer N, Choucair I, Wang Z, Nemet I, Li L, Gukasyan J, et al. Bile acids profile, histopathological indices and genetic variants for non-alcoholic fatty liver disease progression. Metabolism. 2021;116:154457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poupon R, Lindor K, Cauch-Dudek K, Dickson E, Poupon R, Heathcote E. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology. 1997;113:884–90. [DOI] [PubMed] [Google Scholar]
- 29.Hansen BE, Vandriel SM, Vig P, Garner W, Li LT, She H, et al. Application of real-world evidence analytics: A 6-year event-free survival analysis in Alagille syndrome of the GALA clinical research database and maralixibat-treated patients. Hepatology. 2021;74:1387A–1389A. [Google Scholar]
- 30.ClinicalTrials.gov. Safety and Efficacy Study of LUM001 in the Treatment of Cholestatic Liver Disease in Patients With Alagille Syndrome (IMAGO). March 7, 2023. https://clinicaltrials.gov/ct2/show/NCT01903460.
- 31.ClinicalTrials.gov. An Extension Study to Evaluate the Long-Term Safety and Durability of Effect of LUM001 in the Treatment of Cholestatic Liver Disease in Subjects With Alagille Syndrome (ALGS) (IMAGINE). March 7, 2023. https://clinicaltrials.gov/ct2/show/NCT02047318.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Beginning 6 months and ending 5 years after publication, deidentified participant information from the data meta-analysis might be made available to investigators whose proposed use of the data has been approved by a review committee, including the primary authors. Proposals should be directed to medinfo@mirumpharma.com. Before being granted access, data requesters will be required to sign a data access agreement.
AUTHOR CONTRIBUTIONS
All authors contributed to the design of the study, data collection, analysis and interpretation, and writing and review of the manuscript. All authors had access to and verified the data and were responsible for the decision to submit the manuscript. All authors confirm the fidelity of the study to the protocol, accuracy, and completeness of data, and approved manuscript publication. The corresponding author had final responsibility for manuscript submission.
FUNDING INFORMATION
This study was supported by Mirum Pharmaceuticals Inc.