ABSTRACT
Depressive disorders are more prevalent and severe in women; however, our knowledge of the underlying factors contributing to female vulnerability to depression remains limited. Additionally, females are notably underrepresented in studies seeking to understand the mechanisms of depression. Various animal models of depression have been devised, but only recently have females been included in research. In this comprehensive review, we aim to describe the sex differences in the prevalence, pathophysiology, and responses to drug treatment in patients with depression. Subsequently, we highlight animal models of depression in which both sexes have been studied, in the pursuit of identifying models that accurately reflect female vulnerability to depression. We also introduce explanations for the neural basis of sex differences in depression. Notably, the medial prefrontal cortex and the nucleus accumbens have exhibited sex differences in previous studies. Furthermore, other brain circuits involving the dopaminergic center (ventral tegmental area) and the serotonergic center (dorsal raphe nucleus), along with their respective projections, have shown sex differences in relation to depression. In conclusion, our review covers the critical aspects of sex differences in depression, with a specific focus on female vulnerability in humans and its representation in animal models, including the potential underlying mechanisms. Employing suitable animal models that effectively represent female vulnerability would benefit our understanding of the sex-dependent pathophysiology of depression.
KEYWORDS: Sex difference, animal model, depression, stress, vulnerability
Abbreviations
- AAT:
active avoidance test
- aLH:
acute learned helplessness
- CUMS:
chronic unpredictable mild stress
- CVS:
chronic variable stress
- Dnmt:
DNA (cytosine-5)-methyltransferase
- DRN:
dorsal raphe nucleus
- EEG:
electroencephalography
- EPM:
elevated plus maze
- fMRI:
functional magnetic resonance imaging
- FST:
forced swim test
- GIRK:
G protein-gated inwardly rectifying potassium channel
- LHb:
lateral habenula
- MAOI:
monoamine oxidase inhibitor
- MDD:
major depressive disorder
- mPFC:
medial prefrontal cortex
- NAc:
nucleus accumbens
- NSF:
novelty suppressed feeding
- OFT:
open field test
- PET:
positron emission tomography
- SCVS:
subchronic variable stress
- SIS:
social instability stress
- SPT:
sucrose preference test
- SSRI:
selective serotonin reuptake inhibitor
- TCA:
tricyclic antidepressant
- TST:
tail suspension test
- VGLUT:
vesicular glutamate transporter
- vHPC:
ventral hippocampus
- VTA:
ventral tegmental area
Introduction
Major depressive disorder (MDD) is a clinically diagnosed mental disorder characterized by continuous pathological depression that can significantly compromise an individual’s capacity to handle daily life (WHO, 2017). Since the first study to shed light on sex differences in depression (Weissman and Klerman 1977), it has consistently been reported that women suffer from depressive disorders more than men (Angst et al. 2002; Romans et al. 2007; Salk et al. 2017; Hasin et al. 2018; Hapke et al. 2019). Moreover, studies have reported sex differences in comorbidity rates with other disorders and symptoms, indicating that females and males respond differently to stressors (Kim and Chung 2021). In other words, some stress response factors are sensitive to sex (Melartin et al. 2002; Oquendo et al. 2007; Marcus et al. 2008; Altemus et al. 2014; Salk et al. 2017). In addition to these asymptotic differences, the treatment responses differ. Many studies have reported differences in antidepressant efficacy (e.g. tricyclic antidepressants [TCA], monoamine oxidase inhibitors [MAOI], and selective serotonin reuptake inhibitors [SSRI]) between men and women (Kornstein et al. 2000; Martenyi et al. 2001; Young et al. 2009; Sramek et al. 2016). Although females are at a higher risk of depressive disorders with distinct responsiveness to antidepressants, many previous studies utilizing animal models primarily used male subjects to elucidate the biological mechanisms of depression (Zucker and Beery 2010; LeGates et al. 2019; Bangasser and Cuarenta 2021; Sur and Lee 2022). Recently, a growing body of research aims to elucidate the neural mechanisms underlying sex differences in depression. Animal models are used in various paradigms of chronic stress (e.g. chronic unpredictable mild stress [CUMS], chronic variable stress [CVS], and social instability stress [SIS]), and acute stress (e.g. acute learned helplessness [aLH], and subchronic variable stress [SCVS]). In addition, some of these paradigms have been applied early in life to mimic maternal separation, which can induce depression. With these stress protocols, numerous behavioral tests are carried out to measure anxiety (e.g. open field test [OFT], elevated plus maze test [EPM], novelty suppressed feeding behavior test [NSF]), anhedonic behavior (e.g. sucrose preference test [SPT]), and despair behavior (e.g. forced swim test [FST], tail suspension test [TST], and active avoidance test [AAT]). However, the results are not always consistent (Franceschelli et al. 2014; Ma et al. 2019) and sometimes do not adequately explain the directional patterns or neural mechanisms of stress reactivity. Variability in depression susceptibility also arises from sex differences (Krishnan et al. 2007). Therefore, to explain sex differences in depression and gain deeper insights, we focused on different patterns of depressive behaviors across both sexes and highlighted the possible mechanisms underlying these differences.
Sex differences in the pathophysiology of depression-linked symptoms
With a higher prevalence of depression in females, which has been continuously reported across different nations and age groups (Weissman and Klerman 1977; Romans et al. 2007; Salk et al. 2017; Hasin et al. 2018), studies have reported higher comorbidity rates with other disorders, such as anxiety, alcohol abuse, and personality disorder, as well as more frequent suicidal acts in women (Melartin et al. 2002; Oquendo et al. 2007; Marcus et al. 2008; Altemus et al. 2014; Salk et al. 2017). These findings demonstrate clear sex-related differences in depressive disorders, indicating greater female vulnerability.
Men and women exhibit distinct patterns of depressive symptoms. While men with depression are more likely to display aggression, risk-taking behaviors, and substance use, women with depression tend to manifest appetite disturbance, depressed mood, and sleep impairment (Romans et al. 2007; Marcus et al. 2008; Ogrodniczuk and Oliffe 2011; Cavanagh et al. 2016). In a study by Ogrodniczuk and Oliffe (2011), male patients with depression were reported to be more likely to engage in escape behaviors such as over-involvement at work and increased sexual activity. In contrast, depressed females were more frequently observed to have depressive symptoms, including excessive fatigue and oversleeping, throughout their lives (Smith et al. 2008). However, in contrast to previous reports, Herreen et al. observed that depressed men tended to die from suicide more often than women (Herreen et al. 2022), which contributes to stigmatization and misperception of male depression (Oliffe, Hannan-Leith, et al. 2016; Oliffe, Ogrodniczuk, et al. 2016). These reports suggest that men remain relatively resilient and steadfast to factors that trigger depression, yet they may break rather than bend under severe conditions, as in the case of suicide. However, women appear to bend more easily because of factors that trigger depression.
Human studies have proposed neural mechanisms that mediate depressive symptoms specifically in each sex. Anatomically, women with early stage depression have a larger volume of habenula white matter (Carceller-Sindreu et al. 2015) and a larger anterior cingulate cortex (Ancelin et al. 2019). Functionally increased amplitudes of low-frequency fluctuations were observed in the bilateral caudate nucleus and posterior cingulate gyrus (Mei et al. 2022), left middle frontal gyrus, and left precuneus of female but not male patients with MDD (Sun et al. 2022).
Furthermore, transcriptional networks in the brains of patients with MDD show sex differences (Labonte et al. 2017). Interestingly, transcriptional differences have been observed across corticolimbic regions in males and females (Seney et al. 2018). The medial prefrontal cortex (mPFC) has distinct functional and transcriptional features in females that align with increased spontaneous neuronal activity in the left mPFC (Zhang X et al. 2016). In addition, glutamatergic genes were found to be expressed in a sex-specific manner in the dorsolateral PFC in post-mortem patients with MDD (Gray et al. 2015).
Clinical interventions considering sex differences in depression treatment
Adding to the research showing different pathophysiology of depression between men and women, men and women are reported to respond differently to depression treatments (Kokras et al. 2011; Sramek et al. 2016). Some studies suggest that women tend to respond more favorably to SSRIs, such as sertraline and fluvoxamine, whereas men may exhibit better responses to TCAs, such as imipramine (Kornstein et al. 2000; Martenyi et al. 2001; Hildebrandt et al. 2003; Young et al. 2009). Recent developments in depression therapy have introduced ketamine as a potential novel treatment (Zanos and Gould 2018; Corriger and Pickering 2019; Jelen and Stone 2021). Intriguingly, repeated ketamine treatment over 21 days induced antidepressant-like effects in male mice, while eliciting anxiety and depressive behaviors in their female counterparts (Thelen et al. 2016).
Sex-specific distinctions extend to combinatorial drug therapies as well. In patients with treatment-resistant depression, the addition of antipsychotics or mood stabilizers along with antidepressants has shown greater improvement in women than men (Moderie et al. 2022). Amidst these differences in depression prevalence rate, pathophysiology, and treatment responses between the sexes, we suspect that there may be fundamental differences in the pathogenic mechanisms underlying depression in males and females.
Animal models and behavioral tests used to study the sex-specificity of depression
Owing to the limitations inherent in human studies, animal models, especially rodents, have played a pivotal role in research seeking the underlying mechanisms of depression. They are a valuable means to comprehend the molecular and neural underpinnings and identify potential therapeutic targets. However, despite the significant prevalence and severity of depression in women, preclinical research has predominantly employed male animals (Zucker and Beery 2010).
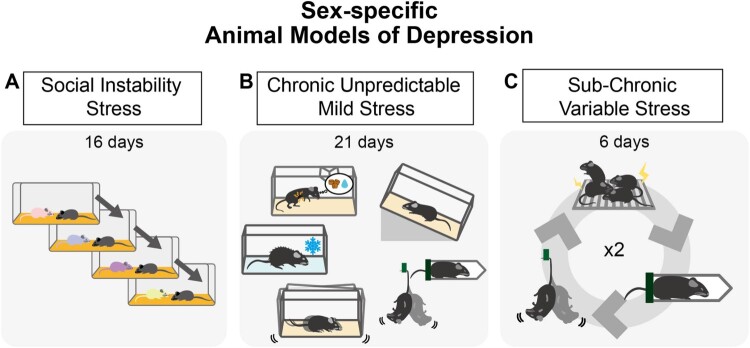
Although various approaches have been used to establish animal models of depression, a notable gap exists in employing these paradigms in both sexes. Among the limited number of studies that have examined depression in both sexes, only a handful have shown sex differences in depressive behavior. Paradigms such as the SIS, CUMS, and SCVS have been proposed as models that may reflect female vulnerability to depression (Figure 1), although not all studies have yielded consistent results (Table 1). In this review, we aim to shed light on the phenomenon of female vulnerability to depression and discuss its reproducibility and validity.
Figure 1.
Animal models of depression exhibiting female vulnerability A. Social instability stress (SIS) is based on experiencing an unstable social hierarchy. The animal is exposed to novel cagemates or sometimes isolated. B. Chronic unpredictable mild stress (CUMS) is composed of numerous types of stressors. The stressor types and durations vary. The stressors include food or water restriction, temperature stress (e.g. heat, cold), disturbance of housing (e.g. cage shaking or tilting, lighting), and direct physical stress (e.g. tail suspension or restraint). C. Subchronic variable stress (SCVS) shows prominent female specificity in depression. This paradigm consists of footshock, tail suspension, and restraint, and is repeated twice.
Table 1.
Results of behavioral tests to measure different aspects of depression conducted in females.
| Test | Reference | Animal | Depression Model | Results of female models | Sex |
|---|---|---|---|---|---|
| Despair Behavior | |||||
| FST | (Dion-Albert et al., 2022) | C57BL/6 | CLDN5 KD | Higher immobility | M |
| (Jones & Lucki, 2005) | 129sv Background | 5-HT1b KO | Female 5-HT1b KO showed a decrease of immobility M/F | M/F | |
| (Leussis & Andersen, 2008) | Sprague Dawley | Social Isolation | Higher immobility | M/F | |
| (Marco et al., 2017) | Wistar Han | CMS | Higher immobility | M/F | |
| (Zhu et al., 2014) | C57BL/6 | CMS | Higher immobility | F | |
| (Johnson, Rainville, Rivero-Ballon, Dhimitri, & Hodes, 2021) | C57BL/6 | SCVS | Female stress showed decreased latency to immobility | F | |
| TST | (Jones & Lucki, 2005) | 129sv Background | 5-HT1b KO | Female 5-HT1b KO showed a decrease of immobility | M/F |
| (Leussis & Andersen, 2008) | Sprague Dawley | Social Isolation | Increased immobility bout number | M/F | |
| (Iniguez et al., 2018) | C57BL/6 | Vicarious Defeat Stress | Increased immobility | F | |
| Anhedonic Behavior | |||||
| SIT (Sucrose Intake Test) | (Dalla et al., 2005) | Wistar rats | CMS | Females show less decrease of sucrose intake | M/F |
| SPT | (Dion-Albert et al., 2022) | C57BL/6 | SCVS | Decreased sucrose preference | F |
| (Dion-Albert et al., 2022) | C57BL/6 | CLDN5 KD | Decreased sucrose preference | F | |
| (Karisetty, Joshi, Kumar, & Chakravarty, 2017) | C57BL/6 | CVMS | Decreased sucrose preference | M/F | |
| (Zhu et al., 2014) | C57BL/6 | CMS | Lower sucrose consumption | F | |
| (Hodes et al., 2015) | C57BL/6 | SCVS | Decreased sucrose preference in females only | M/F | |
| (Williams et al., 2020) | C57BL/6 | SCVS | Decreased sucrose preference in females only | M/F | |
| Anxiety-Like Behavior | |||||
| OFT | (Dalla et al., 2005) | Wistar rats | CMS | M/F | |
| (Zhu et al., 2014) | C57BL/6 | CMS | Less time in center | F | |
| (Nowacka-Chmielewska, Kasprowska-Liskiewicz, Barski, Obuchowicz, & Malecki, 2017) | Sprague Dawley | SIS | Less rearing time | F | |
| (Dao et al., 2010) | C57BL/6 | CACNA1C haploinsufficiency +/- | Less time in the center | M/F | |
| EPM | (Dion-Albert et al., 2022) | C57BL/6 | CLDN5 KD | Less time in open arms | F |
| (Dion-Albert et al., 2022) | C57BL/6 | SCVS | More time in closed arms | F | |
| (Zhu et al., 2014) | C57BL/6 | CMS | Less time in open arms | F | |
| (Grippo, Wu, Hassan, & Carter, 2008) | Prairie vole | Social Isolation | Less time in open arms | F | |
| (Dao et al., 2010) | C57BL/6 | CACNA1C haploinsufficiency +/- | Less time in open-arm | M/F | |
| NSF | (Zhu et al., 2014) | C57BL/6 | CMS | Longer latency to eat | F |
| (Johnson et al., 2021) | C57BL/6 | SCVS | Longer latency to eat (males also) | M/F | |
| (Hodes et al., 2015) | C57BL/6 | SCVS | Longer latency to eat | M/F | |
| (Goodwill et al., 2019) | C57BL/6N | Early Life Stress | Longer latency to eat (adult) | M/F | |
| Social Behavior | |||||
| Social interaction | (Haller, Baranyi, Bakos, & Halasz, 2004) | Wistar rats | SIS | Less social investigation, more agonistic behaviors | F |
| (Baranyi, Bakos, & Haller, 2005) | Wistar Han | SIS | Higher agonistic interaction | F | |
To test the validity of the depression model or the therapeutic effects of potential drugs, various behavioral tests are exploited in animal models. Based on the innate, characteristic behaviors of rodents, several tests were designed. FST, and TST are used to measure the despair behavior of rodent models. Upon forced swimming or tail suspension, the immobility of rodents is measured and considered as a level of helplessness. Depressed animals show higher immobility. Another symptom of depression is anhedonia, which is defined as the inability to experience pleasure (Sternat & Katzman, 2016). SPT is used to measure anhedonia exploiting the rodent’s innate preference to sweets (Der-Avakian & Markou, 2012; Liu et al., 2018). OFT and EPM are for testing anxiety since rodents tend to avoid open spaces (Kraeuter, Guest, & Sarnyai, 2019; Knight et al., 2021). NSF is another measurement of anxiety, based on the conflicting situation of rodents’ motivation for eating after food restriction versus fear of novelty. Depressed animals often show higher anxiety. This table summarizes the results of these behavioral tests performed in female rodent models of depression.
Social instability stress
SIS induces depressive phenotypes in female rodents (Herzog et al. 2009; Dadomo et al. 2018). Dadomo et al. reported that SIS induces anhedonia-like behaviors in female mice (Dadomo et al. 2018). However, recent research has shown that SIS can also occur in male mice and elicits depression-like behaviors and increased corticosterone levels in both male and female mice (Yohn et al. 2019). Male rats exposed to the SIS paradigm exhibit reduced social interaction (Asgari et al. 2021). Although there have been suggestions in the past that SIS may be more stressful for females (Haller et al. 1999), a major limitation of research employing SIS is the scarcity of reports involving both sexes. Studies typically focus on single-sex groups and do not include both sexes under the same experimental conditions. This limitation poses challenges when considering SIS as a robust model to study sex differences in rodents. A complete study involving both men and women is essential to comprehensively investigate the mechanisms underlying sex differences in response to socially stressful situations.
Chronic unpredictable mild stress
The CUMS paradigm is one of the most extensively used stress models for inducing depression in rodents (Figure 1). Compared to other stress models, CUMS possesses a distinct advantage in its ability to replicate chronic stressful life events in humans. Notably, CUMS is well-known for its ability to induce anhedonia (Willner 2017; Antoniuk et al. 2019). Although the CUMS paradigm has predominantly been applied to male subjects, studies have reported sexually dimorphic behavioral and physiological changes. For instance, Dalla et al. reported that, following CUMS, males appeared to be more affected in sucrose consumption, whereas females exhibited decreased dopaminergic activity in the PFC and reduced serotonergic activity in the hippocampus and hypothalamus (Dalla et al. 2005; Dalla et al. 2008). Another study reported increased serum corticosterone levels in women with CUMS (Xing et al. 2013). Intriguingly, one report that considered the social hierarchy within animal groups revealed that dominant females displayed reduced anxiety-like behavior compared with subordinate males (Karamihalev et al. 2020). Furthermore, while CUMS induced anhedonic behavior in both male and female mice, the antidepressant effect of ketamine persisted for a longer duration only in CUMS-exposed males (Franceschelli et al. 2015), reflecting the sex difference in the efficacy of antidepressants.
The endocannabinoid system has been implicated in addiction and depression (Patel and Hillard 2009; Huang et al. 2016), and related molecules may serve as molecular substrates that mediate sex differences. The expression of hippocampal CB1 receptors was also observed to differ between baseline and after CUMS in rats. Males exhibited higher baseline expression of CB1 receptors, which decreased after CUMS, whereas females showed lower baseline expression of CB1 receptors, which increased following CUMS. This is a prominent example of the divergence of working principles for the same molecule in males and females, and further investigation is required to fully understand its role in the sex differences in depression. The dysfunction of CB1 receptors is believed to play a role in mood disorders, and the upregulation observed in females suggests a female-specific mechanism to protect against depression following CUMS (Reich et al. 2009).
Notably, some of these inconsistencies may arise from variability in the stress protocol itself, differences in the animals used, or a combination of both (Figure 1B). Many studies have employed CUMS as a stress paradigm, inclduing food and/or water deprivation, and sleep deprivation (Jung and Noh 2021), and female rodents are known to be more vulnerable to them. CUMS-exposed females often exhibit a more pronounced decrease in sucrose preference, slower weight gain, and greater despair-like behavior in the FST than CUMS-exposed males (Kamper et al. 2009). Conversely, other researchers have reported that only CUMS-exposed males show a consistent and significant reduction in sucrose preference. The validity of the CUMS paradigm is questionable because of its poor reproducibility and excessive variability (Markov and Novosadova 2022). In a meta-analysis of CUMS studies, the authors acknowledged the heterogeneity of animal responses but argued that the CUMS protocol is a robust animal model for depression (Antoniuk et al. 2019). Based on previous studies employing CUMS, it is necessary to establish a standardized CUMS protocol to systematically uncover the potential sex differences resulting from sustained exposure to mild stressors.
Subchronic variable stress
SCVS induces female-specific depression-like behaviors (Figure 1). This protocol entails daily exposure to foot shocks, tail suspension, or restraint stress, which is repeated twice daily for 6 days. In comparison with CUMS, the duration of stress is shorter with less variability, and generally, the intensity of each stressor is higher (Lopez and Bagot 2021).
One significant advantage of utilizing SCVS is the ability to modify stress intensity. Typically, only females are vulnerable to SCVS, demonstrating higher levels of anhedonia-like, despair, and anxiety-like behaviors than males. Researchers have introduced variations to the SCVS protocol, allowing the examination of the specific effects of various factors on stress responses. For instance, one sequence of 3-day stress can be utilized to assess the impact of target factors on the stress response (Labonte et al. 2017), whereas seven sequences, for a total of 21 days of stress, were used to induce depressive behavior in both male and female mice (Bittar et al. 2021). This modifiability of the stress intensity provides control over the unintended effects of the application of other stressors, offering convenience to experimenters through a well-considered strategy. SCVS effectively reflects the higher vulnerability of females to depression, rendering it a valuable model for investigating the biological mechanisms underlying sex differences (Figure 1C).
The validity of these animal models is often evaluated using behavioral tests that measure despair, helplessness, anhedonia, and anxiety (Table 1). In addition to behavioral tests, physiological changes and alterations in neural circuits have been analyzed in rodent models.
Preclinical clues of neural substrates to explain the sex difference in depressive disorders
With the advancement of neuroimaging techniques such as functional magnetic resonance imaging (fMRI), electroencephalography (EEG), and positron emission tomography (PET), it is now possible to explore changes in brain activity among patients with depression with higher temporal and spatial resolution. However, there are technological constraints in controlling experimental conditions as well as ethical concerns inherent to human-based studies. These limitations persist despite the potential of these techniques to provide insights into the neural underpinnings of depression. To address these challenges, many studies have focused on the preclinical phase, using animal models that exhibit sex-specific differences in depressive disorders.
Numerous studies have proposed various potential circuit mechanisms of depression; however, not all proposed circuits show sex specificity. By revealing sex differences in brain areas and linking their circuitry with other areas, we will take a step closer to identifying the neural mechanisms of sex differences in depression.
Medial prefrontal cortex
The mPFC is a hub region involved in emotional processing and stress responses (Duman and Aghajanian 2012; Hare and Duman 2020; Bittar and Labonte 2021). The mPFC is highly affected in patients with MDD and in animal models of chronic stress (Bittar and Labonte 2021). Previous reports have shown that patients with MDD have reduced gray matter volume in the PFC (Grieve et al. 2013). Similar results in animal models showed that exposure to CUS decreased the spine density of PFC layer V pyramidal cells (Li N et al. 2011).
In addition, dopamine receptors in the mPFC are involved in depression. Inhibition of the mPFC-projecting ventral tegmental area (VTA) dopamine neurons promotes susceptibility to social defeat stress (Chaudhury et al. 2013). Some studies have reported sex-related differences in various neurotransmitters in the mPFC. Jankovic et al. reported that after CUS, only male rats, but not females, showed decreased expression of β2-adrenoceptors and D1 receptors in the mPFC (Jankovic et al. 2022). A baseline difference in the expression of synaptosomal GluA1 and GluA2 between sexes has been reported, with females showing higher expression levels (Knouse et al. 2022). Velasco et al. reported a sex difference in GABAB receptor-GIRK (G protein-gated inwardly rectifying potassium channel) signaling, showing that GABABR-dependent GIRK currents were larger in the prelimbic cortex in adolescent male mice than in age-matched females (Marron Fernandez de Velasco et al. 2015).
D1-D2 heteromer activation was previously reported to induce depression-like and anxiety-like behaviors in male rats (Shen et al. 2015). A follow-up study reported higher expression of D1-D2 heteromers in female rats, which may significantly increase their predisposition to depressive and anxious behaviors (Hasbi et al. 2020). The role of microglia has been highlighted in the mPFC, as their density was reported to be increased in females, and gonadal hormones were suggested to participate (Bollinger et al. 2019).
Transcriptional features of the mPFC have also drawn attention. For example, downregulation of lncRNA LINC00473 in the mPFC mediates susceptibility to depression in female but not male mice (Issler et al. 2020). Another study reported downregulation of the tight junction protein Claudin-5 expression in the mPFC, which is thought to promote anxiety and depression-like behaviors in females (Dion-Albert et al. 2022). Overall, previous studies have pointed out sex differences in the mPFC from multiple perspectives, and these differences were observed in both baseline and depressed states.
Nucleus accumbens
The nucleus accumbens (NAc) is known for its roles in reward processing, addiction, motivation, mood, and depression. There is a negative association between anhedonia and NAc responses, and the size of the NAc was negatively associated with anhedonia in humans (Wacker et al. 2009). In a rodent study, activation of NFκB, a stress-related transcription factor, in the NAc blocked stress susceptibility in female mice (LaPlant et al. 2009).
Transcriptional differences have also been observed in the NAc. Increased Dnmt3a (DNA (cytosine-5)-methyltransferase 3a) in the NAc, which was previously suggested to contribute to social stress susceptibility and drug abuse, appears to be responsible for the susceptibility of female mice to SCVS. Overexpression of Dnmt3a causes male mice to become more susceptible to SCVS (Hodes et al. 2015). Another study reported that the transcriptional profile of the NAc 21 days after CVS differed between male and female mice (Labonte et al. 2017). Vesicular glutamate transporters (VGLUTs) are markers of neuroplasticity. Different isoforms of VGLUTs segregate in different brain regions. VGLUT1 plays a role in loading glutamate vesicles and is mainly expressed in the cerebral cortex, hippocampus, basolateral amygdala, and cerebellar cortex, whereas VGLUT2 is primarily expressed in the thalamus, brainstem, and deep cerebellar nuclei. In the NAc of SCVS-susceptible female mice, VGLUT1 levels were decreased whereas VGLUT2 levels were increased (Brancato et al. 2017). These observations indicate that neuroplastic changes due to glutamate input into the NAc may be input-specific. Interestingly, despite the extensive role of dopamine in the NAc, the number of VGLUT2 and tyrosine hydroxlase (TH) co-expressing punctae was not quantitatively different, suggesting that the alteration may not have originated from the VTA (Brancato et al. 2017).
Transcriptional regulation of neuromodulators occurs in the NAc. When neonatal mice were exposed to predator odor, mRNA levels of μ- and κ-opioid receptors were decreased specifically in the NAc of females. Given that the endogenous opioid system plays an important role in mood regulation and is dysregulated in patients with MDD (Pecina et al. 2019; Jelen et al. 2022), sex-specific expression of opioid receptors may contribute to sex differences in depression.
Brain circuits of interest
To investigate possible changes in the neural network underlying sex differences in depression, it is essential to examine the circuitry between brain regions that show sex differences. Fine-tuning the interaction between brain regions via a wide spectrum of electrochemical signaling enables the brain to flexibly manage the various unpredictable situations that are encountered every day. Circuit-level studies in rodents offer advantages in mechanistic approaches because it is feasible to manipulate the activity of given circuits in various transgenic rodents using optogenetics and chemogenetics.
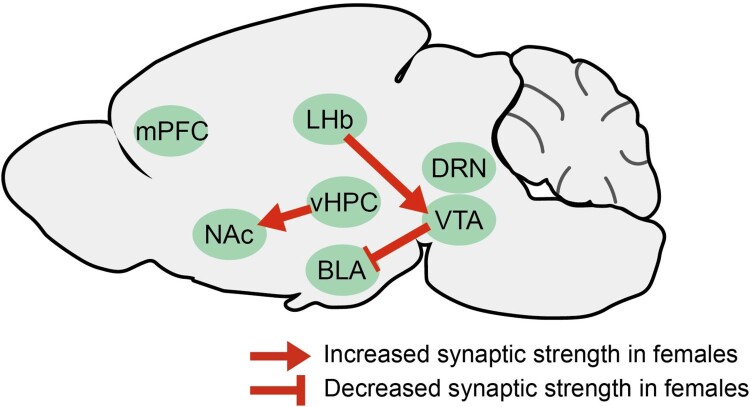
The dopaminergic system of the VTA has been well studied and is critical for the brain’s reward and motivation processing circuitry (Nestler and Carlezon 2006; Lammel et al. 2014; Grace 2016). The projections from the VTA to the BLA are sexually dimorphic. The bouton densities of the VTA dopaminergic projections to the BLA are lower in females than in males, whereas axon densities are comparable (Manion et al. 2022). One of the highlighted areas to which the VTA is connected is the lateral habenula (LHb). It is reported to be activated in ‘disappointing’ situations, encodes negative values, and is thought to be potentiated in depression (Li B et al. 2011; Yang et al. 2018; Hu et al. 2020). The LHb-VTA circuit is reported to show a sex difference after SCVS exposure; only female mice exposed to SCVS showed enhanced LHb-VTA circuitry (Zhang S et al. 2018).
Williams et al. reported that female mice show increased vHPC-NAc circuit excitability after SCVS. Ovariectomized female mice experiencing SCVS showed a similar trend of reduction; however, when they were treated with testosterone, the trend disappeared, and the excitability of vHPC-NAc neurons decreased. Moreover, when the vHPC-NAc pathway was stimulated, males became more susceptible to SCVS. In contrast, when this pathway was inhibited, females are no longer susceptible to SCVS (Williams et al. 2020). These studies suggest that males and females utilize distinct actions or modulatory mechanisms in defined neural circuitries (Figure 2).
Figure 2.
Brain circuits mediating female vulnerability of depression Fewer dopaminergic synaptic boutons from the VTA to the BLA were observed in females. In addition, the LHb → VTA and vHPC → NAc projections have been reported to increase selectively in females after SCVS. All indicated areas are of potential interest for investigating sex differences in depression. BLA, basolateral amygdala; LHb, lateral habenula; NAc, nucleus accumbens; SCVS, subchronic variable stress; vHPC, ventral hippocampus; VTA, ventral tegmental area.
Future perspectives
Although recent efforts to include female subjects in depression research in animal models have shown promising results, inconsistencies and conflicting outcomes have emerged, challenging the reproducibility of the findings (Table 1). The variability observed in depression phenotypes among female subjects may be influenced by differences in stress protocols or inherent individual susceptibilities that cannot be disregarded. Therefore, the standardization and reproducibility of female-specific (or vulnerable) depression models are essential for advancing our understanding of depression in females. Additionally, considering the dynamic nature of female hormonal fluctuations and their potential impact on depression, incorporating these factors into preclinical research is crucial to generate more relevant and applicable results. Human studies have provided preliminary evidence for distinct brain activation patterns in men and women with depression. This highlights the importance of investigating sex-specific neural circuitry to unravel the neurobiological underpinnings of depression in a sex-specific context. In conclusion, this review underscores the need to incorporate females more comprehensively in preclinical depression research. By developing standardized and reproducible animal models of sex-specific depression and exploring the distinct neural circuitry in both sexes, we can advance our understanding of the pathophysiology of depression and contribute to the development of personalized and effective treatment strategies for individuals of all sexes affected by this debilitating disorder.
Funding Statement
This work was supported by National Research Foundation of Korea: [Grant Number NRF-2019M3C7A1031742 and NRF-2020R1A2C2005868].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Altemus M, Sarvaiya N, Neill Epperson C.. 2014. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 35(3):320–330. doi: 10.1016/j.yfrne.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancelin ML, Carriere I, Artero S, Maller J, Meslin C, Ritchie K, Ryan J, Chaudieu I.. 2019. Lifetime major depression and grey-matter volume. J Psychiatry Neurosci. 44(1):45–53. doi: 10.1503/jpn.180026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst J, Gamma A, Gastpar M, Lepine JP, Mendlewicz J, Tylee A, Depression Research in European Society S . 2002. Gender differences in depression. Epidemiological findings from the European DEPRES I and II studies. Eur Arch Psychiatry Clin Neurosci. 252(5):201–209. [DOI] [PubMed] [Google Scholar]
- Antoniuk S, Bijata M, Ponimaskin E, Wlodarczyk J.. 2019. Chronic unpredictable mild stress for modeling depression in rodents: meta-analysis of model reliability. Neurosci Biobehav Rev. 99:101–116. doi: 10.1016/j.neubiorev.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Asgari P, McKinney G, Hodges TE, McCormick CM.. 2021. Social instability stress in adolescence and social interaction in female rats. Neuroscience. 477:1–13. doi: 10.1016/j.neuroscience.2021.09.022. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Cuarenta A.. 2021. Sex differences in anxiety and depression: circuits and mechanisms. Nat Rev Neurosci. 22(11):674–684. doi: 10.1038/s41583-021-00513-0. [DOI] [PubMed] [Google Scholar]
- Baranyi J, Bakos N, Haller J.. 2005. Social instability in female rats: the relationship between stress-related and anxiety-like consequences. Physiol Behav. 84(4):511–518. doi: 10.1016/j.physbeh.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Bittar TP, Labonte B.. 2021. Functional contribution of the medial prefrontal circuitry in major depressive disorder and stress-induced depressive-like behaviors. Front Behav Neurosci. 15:699592. doi: 10.3389/fnbeh.2021.699592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar TP, Pelaez MC, Silva JCH, Quessy F, Lavigne AA, Morency D, Blanchette LJ, Arsenault E, Cherasse Y, Seigneur J, Timofeev I.. 2021. Chronic stress induces Sex-specific functional and morphological alterations in corticoaccumbal and corticotegmental pathways. Biol Psychiatry. 90(3):194–205. doi: 10.1016/j.biopsych.2021.02.014. [DOI] [PubMed] [Google Scholar]
- Bollinger JL, Salinas I, Fender E, Sengelaub DR, Wellman CL.. 2019. Gonadal hormones differentially regulate sex-specific stress effects on glia in the medial prefrontal cortex. J Neuroendocrinol. 31(8):e12762. doi: 10.1111/jne.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancato A, Bregman D, Ahn HF, Pfau ML, Menard C, Cannizzaro C, Russo SJ, Hodes GE.. 2017. Sub-chronic variable stress induces sex-specific effects on glutamatergic synapses in the nucleus accumbens. Neuroscience. 350:180–189. doi: 10.1016/j.neuroscience.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carceller-Sindreu M, de Diego-Adelino J, Serra-Blasco M, Vives-Gilabert Y, Martin-Blanco A, Puigdemont D, Alvarez E, Perez V, Portella MJ.. 2015. Volumetric MRI study of the habenula in first episode, recurrent and chronic major depression. Eur Neuropsychopharmacol. 25(11):2015–2021. doi: 10.1016/j.euroneuro.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Cavanagh A, Wilson CJ, Caputi P, Kavanagh DJ.. 2016. Symptom endorsement in men versus women with a diagnosis of depression: a differential item functioning approach. Int J Soc Psychiatry. 62(6):549–559. doi: 10.1177/0020764016653980. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR.. 2013. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 493(7433):532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriger A, Pickering G.. 2019. Ketamine and depression: a narrative review. Drug Des Devel Ther. 13:3051–3067. doi: 10.2147/DDDT.S221437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadomo H, Gioiosa L, Cigalotti J, Ceresini G, Parmigiani S, Palanza P.. 2018. What is stressful for females? Differential effects of unpredictable environmental or social stress in CD1 female mice. Horm Behav. 98:22–32. doi: 10.1016/j.yhbeh.2017.11.013. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z.. 2005. Chronic mild stress impact: are females more vulnerable? Neuroscience. 135(3):703–714. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Kokras N, Drossopoulou G, Papathanasiou G, Bekris S, Daskas S, Papadopoulou-Daifoti Z.. 2008. Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiol Behav. 93(3):595–605. doi: 10.1016/j.physbeh.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Dao DT, Mahon PB, Cai X, Kovacsics CE, Blackwell RA, Arad M, Shi J, Zandi PP, O'Donnell P, Knowles JA, Weissman MM.. 2010. Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol Psychiatry. 68(9):801–810. doi: 10.1016/j.biopsych.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A.. 2012. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 35(1):68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion-Albert L, Cadoret A, Doney E, Kaufmann FN, Dudek KA, Daigle B, Parise LF, Cathomas F, Samba N, Hudson N, Lebel M.. 2022. Vascular and blood-brain barrier-related changes underlie stress responses and resilience in female mice and depression in human tissue. Nat Commun. 13(1):164. doi: 10.1038/s41467-021-27604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK.. 2012. Synaptic dysfunction in depression: potential therapeutic targets. Science. 338(6103):68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschelli A, Herchick S, Thelen C, Papadopoulou-Daifoti Z, Pitychoutis PM.. 2014. Sex differences in the chronic mild stress model of depression. Behav Pharmacol. 25(5-6):372–383. doi: 10.1097/FBP.0000000000000062. [DOI] [PubMed] [Google Scholar]
- Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM.. 2015. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and “depressed” mice exposed to chronic mild stress. Neuroscience. 290:49–60. doi: 10.1016/j.neuroscience.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Goodwill HL, Manzano-Nieves G, Gallo M, Lee HI, Oyerinde E, Serre T, Bath KG.. 2019. Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model. Neuropsychopharmacology. 44(4):711–720. doi: 10.1038/s41386-018-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. 2016. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 17(8):524–532. doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray AL, Hyde TM, Deep-Soboslay A, Kleinman JE, Sodhi MS.. 2015. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol Psychiatry. 20(9):1057–1068. doi: 10.1038/mp.2015.91. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM.. 2013. Widespread reductions in gray matter volume in depression. Neuroimage Clin. 3:332–339. doi: 10.1016/j.nicl.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, Carter CS.. 2008. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depress Anxiety. 25(6):E17–E26. doi: 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Baranyi J, Bakos N, Halasz J.. 2004. Social instability in female rats: effects on anxiety and buspirone efficacy. Psychopharmacology (Berl). 174(2):197–202. doi: 10.1007/s00213-003-1746-x. [DOI] [PubMed] [Google Scholar]
- Haller J, Fuchs E, Halasz J, Makara GB.. 1999. Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain Res Bull. 50(1):33–39. doi: 10.1016/S0361-9230(99)00087-8. [DOI] [PubMed] [Google Scholar]
- Hapke U, Cohrdes C, Nubel J.. 2019. Depressive symptoms in a European comparison – results from the European Health Interview Survey (EHIS) 2. J Health Monit. 4(4):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare BD, Duman RS.. 2020. Prefrontal cortex circuits in depression and anxiety: contribution of discrete neuronal populations and target regions. Mol Psychiatry. 25(11):2742–2758. doi: 10.1038/s41380-020-0685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, Nguyen T, Rahal H, Manduca JD, Miksys S, Tyndale RF, Madras BK, Perreault ML, George SR.. 2020. Sex difference in dopamine D1-D2 receptor complex expression and signaling affects depression- and anxiety-like behaviors. Biol Sex Differ. 11(1):8. doi: 10.1186/s13293-020-00285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, Grant BF.. 2018. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 75(4):336–346. doi: 10.1001/jamapsychiatry.2017.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreen D, Rice S, Zajac I.. 2022. Brief assessment of male depression in clinical care: validation of the Male Depression Risk Scale short form in a cross-sectional study of Australian men. BMJ Open. 12(3):e053650. doi: 10.1136/bmjopen-2021-053650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog CJ, Czeh B, Corbach S, Wuttke W, Schulte-Herbruggen O, Hellweg R, Flugge G, Fuchs E.. 2009. Chronic social instability stress in female rats: a potential animal model for female depression. Neuroscience. 159(3):982–992. doi: 10.1016/j.neuroscience.2009.01.059. [DOI] [PubMed] [Google Scholar]
- Hildebrandt MG, Steyerberg EW, Stage KB, Passchier J, Kragh-Soerensen P.. 2003. Are gender differences important for the clinical effects of antidepressants? Am J Psychiatry. 160(9):1643–1650. doi: 10.1176/appi.ajp.160.9.1643. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, Magida J, Brancato A, Takahashi A, Flanigan ME, Ménard C.. 2015. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J Neurosci. 35(50):16362–16376. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Cui Y, Yang Y.. 2020. Circuits and functions of the lateral habenula in health and in disease. Nat Rev Neurosci. 21(5):277–295. doi: 10.1038/s41583-020-0292-4. [DOI] [PubMed] [Google Scholar]
- Huang WJ, Chen WW, Zhang X.. 2016. Endocannabinoid system: role in depression, reward and pain control (review). Mol Med Rep. 14(4):2899–2903. doi: 10.3892/mmr.2016.5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez SD, Flores-Ramirez FJ, Riggs LM, Alipio JB, Garcia-Carachure I, Hernandez MA, Sanchez DO, Lobo MK, Serrano PA, Braren SH, et al. 2018. Vicarious social defeat stress induces depression-related outcomes in female mice. Biol Psychiatry. 83(1):9–17. doi: 10.1016/j.biopsych.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issler O, van der Zee YY, Ramakrishnan A, Wang J, Tan C, Loh YE, Purushothaman I, Walker DM, Lorsch ZS, Hamilton PJ, et al. 2020. Sex-specific role for the long non-coding RNA LINC00473 in depression. Neuron. 106(6):912–926. doi: 10.1016/j.neuron.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic M, Spasojevic N, Ferizovic H, Stefanovic B, Dronjak S.. 2022. Sex specific effects of the fatty acid amide hydrolase inhibitor URB597 on memory and brain β2-adrenergic and D1-dopamine receptors. Neurosci Lett. 768:136363. doi: 10.1016/j.neulet.2021.136363. [DOI] [PubMed] [Google Scholar]
- Jelen LA, Stone JM.. 2021. Ketamine for depression. Int Rev Psychiatry. 33(3):207–228. doi: 10.1080/09540261.2020.1854194. [DOI] [PubMed] [Google Scholar]
- Jelen LA, Stone JM, Young AH, Mehta MA.. 2022. The opioid system in depression. Neurosci Biobehav Rev. 140:104800. doi: 10.1016/j.neubiorev.2022.104800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Rainville JR, Rivero-Ballon GN, Dhimitri K, Hodes GE.. 2021. Testing the limits of sex differences using variable stress. Neuroscience. 454:72–84. doi: 10.1016/j.neuroscience.2019.12.034. [DOI] [PubMed] [Google Scholar]
- Jones MD, Lucki I.. 2005. Sex differences in the regulation of serotonergic transmission and behavior in 5-HT receptor knockout mice. Neuropsychopharmacology. 30(6):1039–1047. doi: 10.1038/sj.npp.1300664. [DOI] [PubMed] [Google Scholar]
- Jung T, Noh J.. 2021. Alteration of fear behaviors in sleep-deprived adolescent rats: increased fear expression and delayed fear extinction. Anim Cells Syst (Seoul). 25(2):83–92. doi: 10.1080/19768354.2021.1902854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamper EF, Chatzigeorgiou A, Tsimpoukidi O, Kamper M, Dalla C, Pitychoutis PM, Papadopoulou-Daifoti Z.. 2009. Sex differences in oxidant/antioxidant balance under a chronic mild stress regime. Physiol Behav. 98(1-2):215–222. doi: 10.1016/j.physbeh.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Karamihalev S, Brivio E, Flachskamm C, Stoffel R, Schmidt MV, Chen A.. 2020. Social dominance mediates behavioral adaptation to chronic stress in a sex-specific manner. Elife. 9. doi: 10.7554/eLife.58723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karisetty BC, Joshi PC, Kumar A, Chakravarty S.. 2017. Sex differences in the effect of chronic mild stress on mouse prefrontal cortical BDNF levels: A role of major ovarian hormones. Neuroscience. 356:89–101. doi: 10.1016/j.neuroscience.2017.05.020. [DOI] [PubMed] [Google Scholar]
- Kim W, Chung C.. 2021. Brain-wide cellular mapping of acute stress-induced activation in male and female mice. FASEB J. 35(12):e22041. [DOI] [PubMed] [Google Scholar]
- Knight P, Chellian R, Wilson R, Behnood-Rod A, Panunzio S, Bruijnzeel AW.. 2021. Sex differences in the elevated plus-maze test and large open field test in adult Wistar rats. Pharmacol Biochem Behav. 204:173168. doi: 10.1016/j.pbb.2021.173168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knouse MC, McGrath AG, Deutschmann AU, Rich MT, Zallar LJ, Rajadhyaksha AM, Briand LA.. 2022. Sex differences in the medial prefrontal cortical glutamate system. Biol Sex Differ. 13(1):66. doi: 10.1186/s13293-022-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokras N, Dalla C, Papadopoulou-Daifoti Z.. 2011. Sex differences in pharmacokinetics of antidepressants. Expert Opin Drug Metab Toxicol. 7(2):213–226. doi: 10.1517/17425255.2011.544250. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Davis SM, Harrison WM, Keller MB.. 2000. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 157(9):1445–1452. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- Kraeuter AK, Guest PC, Sarnyai Z.. 2019. The elevated plus maze test for measuring anxiety-like behavior in rodents. Methods Mol Biol. 1916:69–74. doi: 10.1007/978-1-4939-8994-2_4. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, et al. 2007. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Labonte B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, Scarpa JR, Moy G, Loh YE, Cahill M, et al. 2017. Sex-specific transcriptional signatures in human depression. Nat Med. 23(9):1102–1111. doi: 10.1038/nm.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC.. 2014. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 76:351–359. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlant Q, Chakravarty S, Vialou V, Mukherjee S, Koo JW, Kalahasti G, Bradbury KR, Taylor SV, Maze I, Kumar A, et al. 2009. Role of nuclear factor κB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol Psychiatry. 65(10):874–880. doi: 10.1016/j.biopsych.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Kvarta MD, Thompson SM.. 2019. Sex differences in antidepressant efficacy. Neuropsychopharmacology. 44(1):140–154. doi: 10.1038/s41386-018-0156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leussis MP, Andersen SL.. 2008. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 62(1):22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R.. 2011. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 470(7335):535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS.. 2011. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 69(8):754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX, Chen H, Zhu DY, Zhou QG.. 2018. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc. 13(7):1686–1698. doi: 10.1038/s41596-018-0011-z. [DOI] [PubMed] [Google Scholar]
- Lopez J, Bagot RC.. 2021. Defining valid chronic stress models for depression with female rodents. Biol Psychiatry. 90(4):226–235. doi: 10.1016/j.biopsych.2021.03.010. [DOI] [PubMed] [Google Scholar]
- Ma L, Xu Y, Wang G, Li R.. 2019. What do we know about sex differences in depression: a review of animal models and potential mechanisms. Prog Neuropsychopharmacol Biol Psychiatry. 89:48–56. doi: 10.1016/j.pnpbp.2018.08.026. [DOI] [PubMed] [Google Scholar]
- Manion MTC, Glasper ER, Wang KH.. 2022. A sex difference in mouse dopaminergic projections from the midbrain to basolateral amygdala. Biol Sex Differ. 13(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EM, Ballesta JA, Irala C, Hernandez MD, Serrano ME, Mela V, Lopez-Gallardo M, Viveros MP.. 2017. Sex-dependent influence of chronic mild stress (CMS) on voluntary alcohol consumption; study of neurobiological consequences. Pharmacol Biochem Behav. 152:68–80. doi: 10.1016/j.pbb.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Marcus SM, Kerber KB, Rush AJ, Wisniewski SR, Nierenberg A, Balasubramani GK, Ritz L, Kornstein S, Young EA, Trivedi MH.. 2008. Sex differences in depression symptoms in treatment-seeking adults: confirmatory analyses from the Sequenced Treatment Alternatives to Relieve Depression study. Compr Psychiatry. 49(3):238–246. doi: 10.1016/j.comppsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov DD, Novosadova EV.. 2022. Chronic unpredictable mild stress model of depression: possible sources of poor reproducibility and latent variables. Biology (Basel). 11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marron Fernandez de Velasco E, Hearing M, Xia Z, Victoria NC, Lujan R, Wickman K.. 2015. Sex differences in GABABR-GIRK signaling in layer 5/6 pyramidal neurons of the mouse prelimbic cortex. Neuropharmacology. 95:353–360. doi: 10.1016/j.neuropharm.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martenyi F, Dossenbach M, Mraz K, Metcalfe S.. 2001. Gender differences in the efficacy of fluoxetine and maprotiline in depressed patients: a double-blind trial of antidepressants with serotonergic or norepinephrinergic reuptake inhibition profile. Eur Neuropsychopharmacol. 11(3):227–232. doi: 10.1016/S0924-977X(01)00089-X. [DOI] [PubMed] [Google Scholar]
- Mei L, Wang Y, Liu C, Mou J, Yuan Y, Qiu L, Gong Q.. 2022. Study of Sex differences in unmedicated patients with major depressive disorder by using resting state brain functional magnetic resonance imaging. Front Neurosci. 16:814410. doi: 10.3389/fnins.2022.814410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melartin TK, Rytsala HJ, Leskela US, Lestela-Mielonen PS, Sokero TP, Isometsa ET.. 2002. Current comorbidity of psychiatric disorders among DSM-IV major depressive disorder patients in psychiatric care in the Vantaa Depression Study. J Clin Psychiatry. 63(2):126–134. doi: 10.4088/JCP.v63n0207. [DOI] [PubMed] [Google Scholar]
- Moderie C, Nunez N, Fielding A, Comai S, Gobbi G.. 2022. Sex differences in responses to antidepressant augmentations in treatment-resistant depression. Int J Neuropsychopharmacol. 25(6):479–488. doi: 10.1093/ijnp/pyac017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA Jr. 2006. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nowacka-Chmielewska MM, Kasprowska-Liskiewicz D, Barski JJ, Obuchowicz E, Malecki A.. 2017. The behavioral and molecular evaluation of effects of social instability stress as a model of stress-related disorders in adult female rats. Stress. 20(6):549–561. doi: 10.1080/10253890.2017.1376185. [DOI] [PubMed] [Google Scholar]
- Ogrodniczuk JS, Oliffe JL.. 2011. Men and depression. Can Fam Physician. 57(2):153–155. [PMC free article] [PubMed] [Google Scholar]
- Oliffe JL, Hannan-Leith MN, Ogrodniczuk JS, Black N, Mackenzie CS, Lohan M, Creighton G.. 2016. Men’s depression and suicide literacy: a nationally representative Canadian survey. J Ment Health. 25(6):520–526. doi: 10.1080/09638237.2016.1177770. [DOI] [PubMed] [Google Scholar]
- Oliffe JL, Ogrodniczuk JS, Gordon SJ, Creighton G, Kelly MT, Black N, Mackenzie C.. 2016. Stigma in male depression and suicide: a Canadian sex comparison study. Community Ment Health J. 52(3):302–310. doi: 10.1007/s10597-015-9986-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Bongiovi-Garcia ME, Galfalvy H, Goldberg PH, Grunebaum MF, Burke AK, Mann JJ.. 2007. Sex differences in clinical predictors of suicidal acts after major depression: a prospective study. Am J Psychiatry. 164(1):134–141. doi: 10.1176/ajp.2007.164.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Hillard CJ.. 2009. Role of endocannabinoid signaling in anxiety and depression. Curr Top Behav Neurosci. 1:347–371. doi: 10.1007/978-3-540-88955-7_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina M, Karp JF, Mathew S, Todtenkopf MS, Ehrich EW, Zubieta JK.. 2019. Endogenous opioid system dysregulation in depression: implications for new therapeutic approaches. Mol Psychiatry. 24(4):576–587. doi: 10.1038/s41380-018-0117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich CG, Taylor ME, McCarthy MM.. 2009. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res. 203(2):264–269. doi: 10.1016/j.bbr.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romans SE, Tyas J, Cohen MM, Silverstone T.. 2007. Gender differences in the symptoms of major depressive disorder. J Nerv Ment Dis. 195(11):905–911. doi: 10.1097/NMD.0b013e3181594cb7. [DOI] [PubMed] [Google Scholar]
- Salk RH, Hyde JS, Abramson LY.. 2017. Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol Bull. 143(8):783–822. doi: 10.1037/bul0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney ML, Huo Z, Cahill K, French L, Puralewski R, Zhang J, Logan RW, Tseng G, Lewis DA, Sibille E.. 2018. Opposite molecular signatures of depression in Men and women. Biol Psychiatry. 84(1):18–27. doi: 10.1016/j.biopsych.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MY, Perreault ML, Bambico FR, Jones-Tabah J, Cheung M, Fan T, Nobrega JN, George SR.. 2015. Rapid anti-depressant and anxiolytic actions following dopamine D1-D2 receptor heteromer inactivation. Eur Neuropsychopharmacol. 25(12):2437–2448. doi: 10.1016/j.euroneuro.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Kyle S, Forty L, Cooper C, Walters J, Russell E, Caesar S, Farmer A, McGuffin P, Jones I, et al. 2008. Differences in depressive symptom profile between males and females. J Affect Disord. 108(3):279–284. doi: 10.1016/j.jad.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Sramek JJ, Murphy MF, Cutler NR.. 2016. Sex differences in the psychopharmacological treatment of depression. Dialogues Clin Neuro. 18(4):447–457. English. doi: 10.31887/DCNS.2016.18.4/ncutler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternat T, Katzman MA.. 2016. Neurobiology of hedonic tone: the relationship between treatment-resistant depression, attention-deficit hyperactivity disorder, and substance abuse. Neuropsychiatr Dis Treat. 12:2149–2164. doi: 10.2147/NDT.S111818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Luo Y, Ma Y, Guo C, Du Z, Gao S, Chen L, Wang Z, Li X, Xu K, et al. 2022. Sex differences of the functional brain activity in treatment-resistant depression: a resting-state functional magnetic resonance study. Brain Sci. 12(12):1604. doi: 10.3390/brainsci12121604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur B, Lee B.. 2022. Luteolin reduces fear, anxiety, and depression in rats with post-traumatic stress disorder. Anim Cells Syst (Seoul). 26(4):174–182. doi: 10.1080/19768354.2022.2104925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen C, Sens J, Mauch J, Pandit R, Pitychoutis PM.. 2016. Repeated ketamine treatment induces sex-specific behavioral and neurochemical effects in mice. Behav Brain Res. 312:305–312. doi: 10.1016/j.bbr.2016.06.041. [DOI] [PubMed] [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA.. 2009. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 46(1):327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Klerman GL.. 1977. Sex differences and the epidemiology of depression. Arch Gen Psychiatry. 34(1):98–111. doi: 10.1001/archpsyc.1977.01770130100011. [DOI] [PubMed] [Google Scholar]
- WHO.. 2017. Depression and other common mental disorders: Global health estimates. Geneva: World Health Organization. [Google Scholar]
- Williams ES, Manning CE, Eagle AL, Swift-Gallant A, Duque-Wilckens N, Chinnusamy S, Moeser A, Jordan C, Leinninger G, Robison AJ.. 2020. Androgen-dependent excitability of mouse ventral hippocampal afferents to nucleus accumbens underlies sex-specific susceptibility to stress. Biol Psychiatry. 87(6):492–501. doi: 10.1016/j.biopsych.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. 2017. The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol Stress. 6:78–93. doi: 10.1016/j.ynstr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, He J, Hou J, Lin F, Tian J, Kurihara H.. 2013. Gender differences in CMS and the effects of antidepressant venlafaxine in rats. Neurochem Int. 63(6):570–575. doi: 10.1016/j.neuint.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang H, Hu J, Hu H.. 2018. Lateral habenula in the pathophysiology of depression. Curr Opin Neurobiol. 48:90–96. doi: 10.1016/j.conb.2017.10.024. [DOI] [PubMed] [Google Scholar]
- Yohn CN, Ashamalla SA, Bokka L, Gergues MM, Garino A, Samuels BA.. 2019. Social instability is an effective chronic stress paradigm for both male and female mice. Neuropharmacology. 160:107780. doi: 10.1016/j.neuropharm.2019.107780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Kornstein SG, Marcus SM, Harvey AT, Warden D, Wisniewski SR, Balasubramani GK, Fava M, Trivedi MH, John Rush A.. 2009. Sex differences in response to citalopram: a STAR∗D report. J Psychiatr Res. 43(5):503–511. doi: 10.1016/j.jpsychires.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Gould TD.. 2018. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 23(4):801–811. doi: 10.1038/mp.2017.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhang H, Ku SM, Juarez B, Morel C, Tzavaras N, Montgomery S, Hodes GE, Brancato A, Russo SJ, et al. 2018. Sex differences in the neuroadaptations of reward-related circuits in response to subchronic variable stress. Neuroscience. 376:108–116. doi: 10.1016/j.neuroscience.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tang Y, Maletic-Savatic M, Sheng J, Zhang X, Zhu Y, Zhang T, Wang J, Tong S, Wang J, et al. 2016. Altered neuronal spontaneous activity correlates with glutamate concentration in medial prefrontal cortex of major depressed females: An fMRI-MRS study. J Affect Disord. 201:153–161. doi: 10.1016/j.jad.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Zhu S, Wang J, Zhang Y, Li V, Kong J, He J, Li XM.. 2014. Unpredictable chronic mild stress induces anxiety and depression-like behaviors and inactivates AMP-activated protein kinase in mice. Brain Res. 1576:81–90. doi: 10.1016/j.brainres.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Zucker I, Beery AK.. 2010. Males still dominate animal studies. Nature. 465(7299):690. doi: 10.1038/465690a. [DOI] [PubMed] [Google Scholar]