ABSTRACT
Successful pathogens must be able to adapt to a multitude of stressors imposed by their host. Acinetobacter baumannii has emerged as a major global health threat due to its exceptional ability to adapt to hostile environments and skyrocketing rates of multidrug resistance. Recent studies have begun to explore the importance of tRNA methylation in the regulation of bacterial stress responses, including adaptation to antibiotic and oxidative stresses. However, tRNA methyltransferases (trms) have not been investigated in A. baumannii. Bioinformatic analyses revealed nine putative, SAM-dependent trms conserved across clinical A. baumannii isolates and laboratory strains. We generated eight trm mutants in a modern, colistin-resistant clinical isolate, ARC6851, and analyzed the mutants’ stress responses. One mutant, ΔtrmB, was vastly more sensitive to oxidative stress and displayed a growth defect at low pH. Accordingly, ΔtrmB was unable to replicate in J774A.1 macrophages and had decreased virulence in an acute pneumonia murine model. Subsequently, we showed that A. baumannii TrmB makes the m7G tRNA modification. A proteomic analysis revealed that ARC6851 significantly upregulates a siderophore biosynthesis and uptake cluster, acinetobactin, under oxidative stress. In contrast, the upregulation of the acinetobactin proteins in ΔtrmB was only modest, which impacted its ability to withstand iron deprivation under oxidative stress. qRT-PCR data showed that TrmB-dependent regulation of acinetobactin is post-transcriptional. Our results indicate that TrmB-mediated stress responses are important for A. baumannii pathogenesis, highlighting the therapeutic potential of targeting trms to combat the rise of multidrug-resistant A. baumannii.
IMPORTANCE
As deficiencies in tRNA modifications have been linked to human diseases such as cancer and diabetes, much research has focused on the modifications’ impacts on translational regulation in eukaryotes. However, the significance of tRNA modifications in bacterial physiology remains largely unexplored. In this paper, we demonstrate that the m7G tRNA methyltransferase TrmB is crucial for a top-priority pathogen, Acinetobacter baumannii, to respond to stressors encountered during infection, including oxidative stress, low pH, and iron deprivation. We show that loss of TrmB dramatically attenuates a murine pulmonary infection. Given the current efforts to use another tRNA methyltransferase, TrmD, as an antimicrobial therapeutic target, we propose that TrmB, and other tRNA methyltransferases, may also be viable options for drug development to combat multidrug-resistant A. baumannii.
KEYWORDS: tRNA modification, Acinetobacter, iron acquisition, pneumonia, oxidative stress, macrophages
INTRODUCTION
Acinetobacter baumannii is an opportunistic, Gram-negative pathogen that causes a wide range of nosocomial infections, including catheter-associated urinary tract infections (CAUTIs), endocarditis, meningitis, wound and burn infections, and, most commonly, ventilator-associated pneumonia and bacteremia (1). Notably, A. baumannii is acquiring multidrug resistance (MDR) phenotypes at unprecedented rates (2). In response, the World Health Organization has categorized carbapenem-resistant A. baumannii as a critical priority for research and development of new antibiotics (3). Unfortunately, this crisis has worsened during the COVID-19 pandemic, with rates of carbapenem-resistant A. baumannii rising 78% from 2019 to 2020 (4).
Over the past decade, research has failed to reveal one specific toxin or molecular mechanism that can explain the virulence potential of a particular A. baumannii strain (5). Therefore, our current understanding of A. baumannii pathogenesis relies on the pathogen’s ability to survive in and adapt to a wide variety of adverse conditions encountered during infection and in a healthcare environment, such as desiccation, disinfectants, oxidative stress, and iron deprivation (6). Unfortunately, the regulation of A. baumannii stress responses is still poorly understood.
To adapt to stressful conditions, several bacteria have recently been reported to use tRNA methylation to post-transcriptionally regulate the translation of transcripts that are enriched with specific mRNA codons (7 – 9). However, our understanding of the role of tRNA modifications in bacterial physiology and stress responses has barely scratched the surface. In fact, the full set of genes encoding tRNA modification enzymes has only been identified in Escherichia coli and Mycoplasma capricolum (10, 11). Furthermore, these identified genes are not conserved across all bacterial species, and it has been estimated that only 70%–80% of tRNA modifications can be predicted using homology to known tRNA modification enzymes (12). Additional diversity in the variety of tRNA modifications, or the tRNA “modificome,” stems from the identification of new modifications in other species that are not present in E. coli, such as the acetylated 3-(3-amino-3-carboxypropyl)uridine modification found in Vibrio cholerae (13). Furthermore, homologous tRNA modification enzymes may have different RNA targets in different species, and different species may use non-orthologous enzymes to either make the same modification or perform a similar function through distinct mechanisms (14 – 18). Importantly, these modifications can also be dependent on cell physiology and environmental conditions, as organisms have evolved unique solutions and require particular modifications according to the stressors that they encounter in their niches (7).
Given the diversity of tRNA modifications across bacterial species and the dearth of tRNA modificome research in many prokaryotes, it is imperative to identify the tRNA modifications present in human pathogens and elucidate their role in the pathogens’ abilities to respond to environmental stressors. Many tRNA modification pathways include a methylation step, with tRNAs being the most heavily methylated molecules in all domains of life (19, 20). In fact, tRNA methylation has been shown to impact bacterial stress responses, including antibiotic stress responses (8). Masuda et al. have shown that increased m1G37 levels in E. coli and Salmonella enhance the translation of mRNAs for membrane-associated proteins involved in barrier and efflux activity, preventing the intracellular accumulation of antimicrobial drugs and promoting the development of resistance (8). This modification is performed by the essential, conserved tRNA methyltransferase (trm) TrmD, an enzyme that has been widely targeted by pharmaceutical companies for the development of antimicrobials (21 – 23). Post-transcriptional regulation via trms also contributes to other bacterial stress responses; in Pseudomonas aeruginosa, the m7G46 modification catalyzed by TrmB influences the oxidative stress response via regulation of the katA and katB catalases (9). Exposure to H2O2 leads to increased levels of m7G methylation and increased translation efficiency of the Phe- and Asp- enriched mRNAs, including katA and katB transcripts. Accordingly, inactivation of trmB sensitizes P. aeruginosa to oxidative stress. Another modification, 2′-O-methylation of tRNATyr catalyzed by E. coli TrmH may play a role in suppressing the host immune system response to bacterial tRNAs, potentially due to the modification reducing TLR7-mediated interferon release (24, 25).
While the field of bacterial tRNA modifications has begun to grow in recent years, the role of trms in A. baumannii remains elusive, and the A. baumannii tRNA modificome has not been identified. Several papers have suggested that methyltransferases could play a role in the rise of tigecycline resistance in A. baumannii clinical isolates; in the MDR A. baumannii isolate MDR-ZJ06, trmB was upregulated twofold in response to tigecycline stress (26). Additionally, two studies observed that disruption of another putative methyltransferase, A1S_2858, increased resistance to tigecycline in multiple strains (27, 28). However, these putative methyltransferases have not been functionally characterized in A. baumannii, and their roles in response to other stresses this bacterium may encounter during its pathogenesis and persistence have not been explored. Our lab has shown that A. baumannii utilizes unique pathways to respond to antibiotic and oxidative stresses; therefore, it is likely that A. baumannii may utilize its tRNA modificome in novel ways (29).
To explore the role of trms in A. baumannii stress responses and pathogenesis, we have identified nine, putative, SAM-dependent trms in the core A. baumannii genome and constructed deletion mutants. We report the mutants’ responses to antibiotic and oxidative stresses and demonstrate that TrmB is essential for complete oxidative and acid stress responses, macrophage infection, and virulence in a pneumonia murine model. We also provide whole-cell proteomic data under oxidative stress to gain insight into the role TrmB plays in A. baumannii physiology.
RESULTS
Nine putative SAM-dependent tRNA methyltransferases are conserved in A. baumannii
The presence, variety, and roles of trms in A. baumannii have not been explored. Basic Local Alignment Search Tool (BLAST) analyses using the sequences of trms from other bacteria revealed nine putative, SAM-dependent trms conserved across modern clinical A. baumannii isolates and laboratory strains (Table 1). Seven of these trms were annotated with functions previously identified in the literature. However, two putative trms did not have annotated targets, so we designated them trmZ1 and trmZ2; Phyre2 predicts that trmZ1 potentially catalyzes the formation of 5-methyluridine and trmZ2 may act as a 7-methylguanosine methyltransferase or a 2′-O-methyltransferase (30). Many of these trms have not been fully characterized in bacteria, and their activities and targets may be species-specific (12). Therefore, we generated unmarked trm deletion mutants in the modern clinical isolate ARC6851, a particularly virulent respiratory pathogen that is resistant to the last-resort antibiotic colistin (Table 1). Ultimately, we were able to generate unmarked mutants for all putative SAM-dependent A. baumannii trms except trmD, which catalyzes the essential m1G37 modification conserved across all domains of life (31).
TABLE 1.
ARC6851 has nine putative, SAM-dependent tRNA methyltransferases a
| Gene | Predicted modification | ARC6851 locus tag |
|---|---|---|
| trmA | m5U (54) | OB946_10490 |
| trmB | m7G (46) | OB946_12575 |
| mnmC | mnm5U (34) | OB946_02580 |
| trmD | m1G (37) | OB946_01600 |
| trmJ | 2′OmC/U | OB946_10840 |
| trmL | 2′OmC (34) | OB946_03595 |
| trmO | m6t6A (37) | OB946_06360 |
| trmZ1 | m5U | OB946_02910 |
| trmZ2 | m7G | OB946_16835 |
The prediction modifications are denoted as follows: m5U (54) (5-methyluridine at position 54), m7G (46) (7-methylguanosine at position 46), mnm5U (34) (5-methylaminomethyl uridine at position 34), m1G (37) (1-methylguanosine at position 37), 2′OmC/U (2′-O-methylcytidine or 2′-O-methyluridine), 2′OmC (34) (2′-O-methylcytidine at position 34), m6t6A (37) (N6-methyl-threonylcarbamoyladenosine at position 37), m5U (5-methyluridine), and m7G (7-methylguanosine).
Loss of single trms does not significantly impact ARC6851 antibiotic susceptibility
As trms mediate antibiotic resistance in several bacteria, we determined whether inactivation of the putative trms affected ARC6851 antibiotic susceptibility. In our screen, we included tigecycline and its ancestor tetracycline, as tigecycline was the only antibiotic associated with trm regulation in A. baumannii literature (26 – 28). In addition, we tested several quinolones that target DNA topoisomerases; chloramphenicol, erythromycin, and aminoglycosides that target protein synthesis; rifampicin which targets RNA polymerase; zeocin which cleaves DNA; beta-lactams that target cell-wall synthesis; and colistin which targets the cell membrane. Ultimately, we did not observe many significant alterations (defined here as >2-fold) in the minimum inhibitory concentrations (MICs) of this diverse array of antibiotics (Fig. 1A). Interestingly, ΔtrmA had a MIC 4-fold higher for rifampicin. This phenotype was not seen in the other mutants, further suggesting that trms can be involved in modulating the responses to different stressors. While ΔtrmB showed 2-fold lower MICs for several antibiotics, we did not observe any change in MIC to tigecycline or tetracycline, suggesting that A. baumannii TrmB may not play a role in tigecycline resistance. However, we did observe a 2-fold decrease in tigecycline susceptibility with the trmZ1 mutant, the putative methyltransferase whose absence was reported to increase tigecycline resistance in A. baumannii strains (27, 28). Overall, these trms do not appear to play a major role in determining antibiotic susceptibility in A. baumannii.
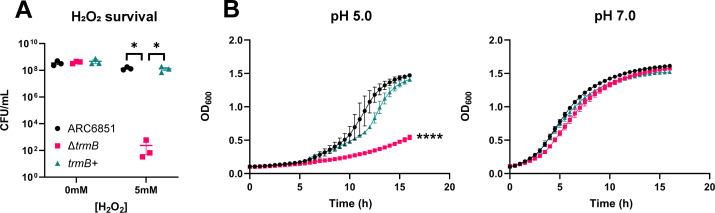
Fig 1.
Loss of trmB does not significantly impact antibiotic susceptibility, but renders ARC6851 more susceptible to oxidative stress (A) Antibiotic susceptibility of trm mutants. Eight ARC6851 trm deletion mutants were screened for changes in MICs to a variety of antibiotics using a 2-fold broth dilution method. Mid-exponential cultures were normalized at OD600 0.01 and grown for 16 h with shaking at 37°C. MIC was determined as <10% growth compared to a non-treated culture. Greater than 2-fold changes were considered significant. (B) Oxidative stress resistance of trm mutants. Strains were grown to mid-exponential phase before being treated with 0 mM or 5 mM H2O2 for 2 h. Survival was measured by serial dilution and quantification of the recoverable CFU/mL. Fold changes were calculated against the wild-type strain.
TrmB is essential for resistance to oxidative and acid stresses
Next, we assessed the roles of the trms in resistance to another environmental and host stressor, oxidative stress. A previous report in P. aeruginosa suggested that PaTrmB plays a role in its oxidative stress response (9). To investigate if trms mediate the A. baumannii response to oxidative stress, cells were treated with 5 mM H2O2. Strikingly, the ΔtrmB mutant was almost entirely killed, while the other mutants behaved similarly to wild-type bacteria at this concentration (Fig. 1B). As opposed to P. aeruginosa, we did not observe the same phenotype in the ΔtrmJ mutant, suggesting A. baumannii oxidative stress responses may differ (32). Given the ΔtrmB mutant’s inability to survive oxidative stress, and the lack of data on the role of bacterial TrmB in vivo and in other stressful conditions, we decided to further investigate the role of TrmB in A. baumannii pathogenesis.
We next complemented the ΔtrmB mutant by chromosomally inserting a copy of the wild-type allele to validate our screening results, trmB+. Indeed, we found that the increased susceptibility to H2O2 was due to the loss of TrmB (Fig. 2A). Subsequently, we tested another major stressor encountered during pathogenesis, acid stress. As a byproduct of its metabolism, A. baumannii secretes large amounts of ammonia, resulting in the alkalization of its environment, and our lab recently discovered that A. baumannii also alkalizes its vacuole within macrophages during intracellular replication (33). At pH 7.0, we observed that the mutant is significantly shorter in length than the wild-type cells; however, we did not observe a growth defect (Fig S1; Fig. 2B). Therefore, we subjected the mutant to grow in a non-buffered, acidic, rich media. The mutant presented a growth defect that was successfully complemented (Fig. 2B; Fig S2). This defect suggests that the ΔtrmB mutant may be at a disadvantage in vivo because replicative strains must be able to grow quickly at an acidic pH to produce ammonia that can neutralize the acidic vacuole, ultimately avoiding degradation.
Fig 2.
TrmB is essential for resistance to oxidative and acid stresses. (A) H2O2 killing of ARC6851 strains. Wild-type, ΔtrmB, and trmB+ strains were grown to mid-exponential phase before being treated with 0 mM or 5 mM H2O2 for 2 h. Survival was measured by serial dilution and quantification of recoverable CFU/mL. Points represent technical replicates from at least three biological replicates, with a horizontal line representing the mean, and errors bars representing the standard error of the mean (SEM). *P < 0.05; one-way ANOVA, Tukey’s test for multiple comparisons. (B and C) Representative growth curves of wild type, ΔtrmB, and trmB+ in non-buffered LB at pH 5.0 and pH 7.0. Mid-exponential cultures were normalized to OD600 = 0.01 and grown for 16 h with shaking at 37°C. ****P < 0.0001, unpaired t tests at 16 h for ΔtrmB compared to wild type and trmB+.
TrmB plays a significant role in bacterial replication within macrophages
As pathogens encounter both oxidative and acid stressors imposed by the host during infection, these data suggest that trmB-deficient ARC6851 may be at a disadvantage during pathogenesis. Previous work on bacterial TrmB has solely been performed in vitro, but the impact in vivo and in A. baumannii remains unknown. Macrophages contribute to host defense against A. baumannii infections, but our lab has recently shown that multiple new clinical A. baumannii isolates, unlike domesticated lab strains, can replicate inside spacious vacuoles within macrophages (34). Therefore, we first confirmed that ARC6851 can replicate inside J774A.1 macrophages and found that ARC6851 wild type doubles in J774A.1 macrophages by 4 h post-infection (Fig. 3A). Remarkably, the ΔtrmB mutant had a replication defect in the macrophages, only persisting within the cells for 4 h. Using confocal microscopy, we also observed less bacteria per A. baumannii-containing vacuole (ACV) compared to the wild-type and complemented strains (Fig. 3B and C), while observing similar rates of infection and ACVs per cell (Fig S3). Altogether these data suggest that ΔtrmB was able to be phagocytosed at similar rates as wild type, but was unable to replicate once inside its vacuole, potentially due to its inability to respond to the stresses imposed by the macrophages (i.e., oxidative and pH).
Fig 3.
TrmB plays a significant role in replication within macrophages (A) J774A.1 macrophages were infected with mid-exponential phase ARC6851 wild-type, ΔtrmB, and trmB+ strains. Intracellular CFU were determined at 2, 4, and 6 h post-infection. *P < 0.05, ****P < 0.0001, mixed effects model, Tukey’s test for multiple comparisons. (B) Infected macrophages were fixed at 4 h post-infection. Number of bacteria per ACV were determined with at least 14 representative confocal microscopy images and two biological replicates per strain. (C) The samples were stained to detect cell nuclei (blue), A. baumannii (green), and actin (red). Bars 20 µM. Insets (13 µM) are a higher magnification of the area denoted in the white box of the corresponding image. Representative ACVs are indicated with pink (>six bacteria/ACV) and yellow (one to two bacteria/ACV) arrows.
ARC6851 ΔtrmB is attenuated in an acute murine pneumonia model
Given the role of macrophages during A. baumannii pneumonia infections and the mutant’s defect in J774A.1 macrophages, we next assessed whether trmB is required for virulence in an acute murine pneumonia model, as previously described (35). Briefly, mice were intranasally inoculated with wild-type, ΔtrmB, and trmB+ strains, and at 24 h post-infection, the lungs, spleens, and kidneys were harvested and processed to determine bacterial burden. Ultimately, we observed that the ΔtrmB mutant had a significant reduction (>10,000-fold) in recovered CFU as compared to the wild-type and complemented ARC6851 strains in all tested organs (Fig. 4).
Fig 4.
ARC6851 ΔtrmB is attenuated in an acute murine pneumonia model. C57BL/6 mice were infected with ~5 × 107 CFU of mid-exponential ARC6851 wild-type, ΔtrmB, and trmB+ strains. At 24 h post-infection, the lungs, kidneys, and spleens were harvested, and the bacterial load present in each tissue was determined with serial dilutions. Each symbol represents an individual mouse, and the horizontal bar represents the median. Data collected from three independent experiments. ****P < 0.0001, Kruskal-Wallis test.
While A. baumannii is most commonly associated with pneumonia, we recently found that one-fifth of isolates come from UTIs (36). Given that the lung and urinary tract environments significantly differ, we also decided to assess the role of TrmB in our recently developed CAUTI model (36). However, ARC6851 does not establish CAUTI in this model, so we generated ΔtrmB and trmB+ strains in another modern MDR clinical isolate, Ab04. We found that Ab04 ΔtrmB (ACX61_12415) is not attenuated in our murine CAUTI model, suggesting that the role of trmB may be specific to the stresses imposed by a pneumonia infection (Fig. S4).
A. baumannii TrmB performs the m7G tRNA modification
As the sequence homology of TrmB is not sufficient to determine its function, we verified that TrmB makes the m 7 G modification in A. baumannii similarly to its homologs in other bacteria (9, 37, 38). For ARC6851, we used LC-MS to assess m7G levels in wild-type, ΔtrmB, and trmB+ strains. As predicted, we found that ΔtrmB lacks m7G, and the presence of m7G is restored in the complemented strain (Fig. 5A). For Ab04, we employed a different technique, thin-layer chromatography (TLC), to measure m7G levels. Similarly, we observed decreased levels of m7G in the mutant strain, demonstrating that TrmB does indeed make the m7G modification in multiple clinical A. baumannii isolates (Fig. 5B). The Ab04 phenotype was partially complemented; however, all strains have been whole genome sequenced to avoid additional mutations. Therefore, this partial complementation is likely due to the restored gene being located at a different site in the chromosome and lacking all of its native regulatory sequences.
Fig 5.
A. baumannii TrmB performs the m7G tRNA modification. (A) m7G levels in ARC6851 wild-type, ΔtrmB, and trmB+ strains were determined with LC-MS. ΔtrmB m7G levels were below detection limit (ND). ****P < 0.0001, one-way ANOVA, Tukey’s test for multiple comparisons. (B) m7G levels in Ab04 wild-type, ΔtrmB, and trmB+ strains were determined with thin-layer chromatography and (C) analyzed with ImageQuant. *P < 0.05, one-way ANOVA, Tukey’s test for multiple comparisons.
TrmB plays a significant regulatory role in ARC6851 under oxidative stress
tRNA modifications can affect the translation efficiency of mRNAs enriched for specific codons, which can be used to post-transcriptionally regulate the translation of stress-related factors (8, 9). To identify proteins affected by the presence or absence of m7G in ARC6851, we performed whole-cell proteomics of wild-type, ΔtrmB, and trmB+ strains grown with and without H2O2 treatment. Notably, the principal component analysis showed distinct grouping of the wild-type and complemented strains with and without stress, while the mutant clustered separately in both conditions (Fig. 6). This analysis indicates that the ΔtrmB mutant has global changes in its translation profiles in the presence or absence of H2O2 as compared to the wild-type and complemented strains.
Fig 6.
TrmB plays a significant regulatory role in ARC6851 under oxidative stress. (A) Principal component analysis of ARC6851 wild-type, ΔtrmB, and trmB+ proteomes with and without H2O2 treatment. Mid-exponential cultures were treated with 0 mM or 5 mM H2O2 for 2 h before being pelleted, lysed, and acetone precipitated. Digested proteome samples were analyzed with reverse phase liquid chromatography-mass spectrometry. (B) (left) Volcano plot of Table 2 data showing differentially expressed proteins in ARC6851 wild type in H2O2 treatment versus non-treated. (right) Volcano plot of Table 3 data showing differentially expressed proteins in ARC6851 ΔtrmB in H2O2 treatment versus non-treated. Red dots are more than 2.5-fold upregulated, and blue dots are more than 2.5-fold downregulated. Large dots represent hits used for further analysis.
TABLE 3.
Differentially expressed proteins in ARC6851 ΔtrmB in H2O2 treatment vs non-treated
| Accession | Fold change a | Annotated protein |
|---|---|---|
| UYC78524.1 | 7.32 | TetR/AcrR family transcriptional regulator |
| UYC76661.1 | 6.74 | Organic hydroperoxide resistance protein |
| UYC78132.1 | 5.65 | Type I-F CRISPR-associated helicase Cas3f |
| UYC76254.1 | 5.38 | TonB-dependent siderophore receptor |
| UYC75768.1 | 5.37 | Alkyl hydroperoxide reductase subunit F |
| UYC78695.1 | 4.53 | GTP 3,8-cyclase MoaA |
| UYC76045.1 | 4.51 | Cold-shock protein |
| UYC78239.1 | 4.46 | Hypothetical protein OB946_05355 |
| UYC78349.1 | 4.38 | Hypothetical protein OB946_05940 |
| UYC77774.1 | 4.15 | Acyl-CoA desaturase |
| UYC76578.1 | 3.60 | NADH-quinone oxidoreductase subunit M |
| UYC76099.1 | 3.59 | Ribonuclease HII |
| UYC76290.1 | 3.55 | Poly-beta-1,6-N-acetyl-D-glucosamine biosynthesis protein PgaD |
| UYC78650.1 | 3.54 | ATP-binding cassette domain-containing protein |
| UYC78900.1 | 3.48 | TetR/AcrR family transcriptional regulator |
| UYC78238.1 | 3.41 | Hypothetical protein OB946_05350 |
| UYC76938.1 | 3.08 | TRAP transporter large permease subunit |
| UYC77398.1 | 3.02 | DUF1737 domain-containing protein |
| UYC75593.1 | 2.96 | Acetyltransferase |
| UYC78982.1 | 2.91 | Uracil-DNA glycosylase family protein |
| UYC78065.1 | 2.85 | Sulfate ABC transporter ATP-binding protein |
| UYC78208.1 | 2.73 | TonB-dependent siderophore receptor BauA |
| UYC77179.1 | 2.66 | GntR family transcriptional regulator |
| UYC76950.1 | 2.59 | Preprotein translocase subunit SecE |
| UYC79112.1 | 2.57 | Hypothetical protein OB946_12705 |
| UYC79050.1 | 2.55 | Acinetobactin non-ribosomal peptide synthetase subunit BasB |
| UYC77830.1 | 0.40 | YegP family protein |
| UYC75549.1 | 0.40 | Thiamine pyrophosphate-dependent dehydrogenase E1 component subunit alpha |
| UYC76443.1 | 0.40 | Gamma-glutamyltransferase |
| UYC77151.1 | 0.40 | Enoyl-CoA hydratase |
| UYC78302.1 | 0.40 | Alpha/beta hydrolase |
| UYC75929.1 | 0.39 | 1,2-phenylacetyl-CoA epoxidase subunit A |
| UYC78495.1 | 0.39 | SRPBCC family protein |
| UYC75928.1 | 0.39 | 1,2-phenylacetyl-CoA epoxidase subunit B |
| UYC78130.1 | 0.39 | DUF962 domain-containing protein |
| UYC77150.1 | 0.39 | Enoyl-CoA hydratase/isomerase family protein |
| UYC78788.1 | 0.38 | Hypothetical protein OB946_08315 |
| UYC75753.1 | 0.38 | Tautomerase family protein |
| UYC75847.1 | 0.38 | Class I SAM-dependent methyltransferase |
| UYC76799.1 | 0.38 | RNA-binding protein |
| UYC75632.1 | 0.38 | Hypothetical protein OB946_10145 |
| UYC76150.1 | 0.38 | Cache domain-containing protein |
| UYC78477.1 | 0.38 | DUF2171 domain-containing protein |
| UYC78379.1 | 0.37 | Hypothetical protein OB946_06090 |
| UYC78033.1 | 0.37 | Rhombotarget A |
| UYC77971.1 | 0.36 | Diacylglycerol kinase family protein |
| UYC77152.1 | 0.36 | Acyl-CoA dehydrogenase family protein |
| UYC78876.1 | 0.36 | Hydrolase |
| UYC78647.1 | 0.35 | OmpW family protein |
| UYC77692.1 | 0.33 | Ribonuclease I |
| UYC77169.1 | 0.33 | SDR family NAD(P)-dependent oxidoreductase |
| UYC75548.1 | 0.32 | Alpha-ketoacid dehydrogenase subunit beta |
| UYC78437.1 | 0.31 | YeaC family protein |
| UYC77309.1 | 0.31 | DNA-3-methyladenine glycosylase I |
| UYC75917.1 | 0.30 | PaaI family thioesterase |
| UYC78152.1 | 0.29 | Thiamine pyrophosphate-binding protein |
| UYC79075.1 | 0.28 | Acyl-CoA dehydrogenase family protein |
| UYC77165.1 | 0.27 | Ester cyclase |
| UYC78031.1 | 0.27 | AI-2E family transporter |
| UYC76604.1 | 0.27 | TetR/AcrR family transcriptional regulator |
| UYC78839.1 | 0.26 | Flavin reductase family protein |
| UYC76941.1 | 0.24 | YMGG-like glycine zipper-containing protein |
| UYC77154.1 | 0.22 | 3-hydroxyisobutyrate dehydrogenase |
| UYC77153.1 | 0.20 | AMP-binding protein |
| UYC78778.1 | 0.20 | Rieske (2Fe-2S) protein |
| UYC77155.1 | 0.19 | CoA-acylating methylmalonate-semialdehyde dehydrogenase |
| UYC78838.1 | 0.18 | PDR/VanB family oxidoreductase |
| UYC78767.1 | 0.17 | Hypothetical protein OB946_08205 |
| UYC78848.1 | 0.15 | Muconolactone Delta-isomerase |
| UYC77084.1 | 0.14 | RidA family protein |
Fold change cutoff: 2.5-fold, bolded if P value < 0.05, Student’s unpaired t test.
Functional analysis of the proteomic data revealed that under H2O2-imposed stress ARC6851 upregulates many proteins involved in inorganic ion transport and metabolism, while the ΔtrmB mutant upregulates more proteins involved in transcription and replication, potentially to replace damaged proteins (Fig S5). Under oxidative stress, we observed a putative catalase (UYC76832.1) was upregulated 2.6-fold in the wild type but only 1.8-fold in the mutant (Supplemental data set). While this minor difference may contribute to the ΔtrmB mutant’s large increase in susceptibility to hydrogen peroxide, it is unlikely to be the sole contributor to this phenotype. Notably, we observed that in response to H2O2, wild-type ARC6581 upregulates several members of a siderophore cluster, acinetobactin, previously reported to be required for Acinetobacter virulence (Table 2) (39 – 41). Strikingly, BasB, a putative acinetobactin biosynthesis protein, was upregulated ~200-fold in wild-type ARC6851 under oxidative stress but was only upregulated 2.6-fold in the ΔtrmB mutant (Table 2; Supplemental data set). The other members of the cluster that were upregulated in wild-type ARC6851 include BauA (12.7-fold in wild type vs 2.7-fold in the ΔtrmB mutant), BauB (6.2-fold vs 1.8-fold), and BasE (5.1-fold vs 1.0-fold). Juttukonda et al. reported that several acinetobactin biosynthesis genes are transcriptionally upregulated in the model, “domesticated” A. baumannii strain Ab17978 in response to H2O2-imposed stress, so we aimed to determine whether the large differences we observed in wild-type compared to the ΔtrmB mutant in the ARC6851 clinical isolate were due to transcriptional or post-transcriptional regulation (42). We performed qRT-PCR on wild-type, ΔtrmB, and complemented strains exposed to 2 mM H2O2 for 10 min to see immediate changes and 2 h to match the proteomic conditions. Surprisingly, we observed that the acinetobactin cluster genes basB, basE, bauA, and bauB were not more transcriptionally upregulated in wild type compared to the mutant at either 10 min or 2 h of oxidative stress, indicating that the large differences observed in the proteomics data set were due to post-transcriptional regulation (Fig. 7A).
TABLE 2.
Differentially expressed proteins in ARC6851 wild type in H2O2 treatment vs non-treated
| Accession | Fold change a | Annotated protein |
|---|---|---|
| UYC79050.1 | 226.13 | Acinetobactin non-ribosomal peptide synthetase subunit BasB |
| UYC78208.1 | 12.74 | TonB-dependent siderophore receptor BauA |
| UYC76661.1 | 8.05 | Organic hydroperoxide resistance protein |
| UYC79121.1 | 6.57 | TonB-dependent receptor |
| UYC75768.1 | 6.47 | Alkyl hydroperoxide reductase subunit F |
| UYC78207.1 | 6.21 | Siderophore-binding periplasmic lipoprotein BauB |
| UYC76254.1 | 6.20 | TonB-dependent siderophore receptor |
| UYC78211.1 | 5.14 | (2,3-Dihydroxybenzoyl)adenylate synthase BasE |
| UYC77454.1 | 4.52 | Amino acid permease |
| UYC75593.1 | 4.32 | Acetyltransferase |
| UYC78132.1 | 4.03 | Type I-F CRISPR-associated helicase Cas3f |
| UYC76981.1 | 3.80 | Ferrous iron transport protein A |
| UYC76313.1 | 3.52 | Biliverdin-producing heme oxygenase |
| UYC78228.1 | 3.40 | Xanthine dehydrogenase molybdopterin binding subunit |
| UYC76709.1 | 2.72 | RidA family protein |
| UYC75664.1 | 2.71 | PilZ domain-containing protein |
| UYC78065.1 | 2.68 | Sulfate ABC transporter ATP-binding protein |
| UYC76832.1 | 2.64 | Catalase/peroxidase HPI |
| UYC78212.1 | 2.63 | Acinetobactin biosynthesis bifunctional isochorismatase/aryl carrier protein BasF |
| UYC78043.1 | 2.60 | Protein TolR |
| UYC78131.1 | 2.58 | Type I-F CRISPR-associated endonuclease Cas1f |
| UYC77811.1 | 0.40 | RtcB family protein |
| UYC77797.1 | 0.40 | Hypothetical protein OB946_02925 |
| UYC78848.1 | 0.39 | Muconolactone Delta-isomerase |
| UYC78838.1 | 0.38 | PDR/VanB family oxidoreductase |
| UYC79075.1 | 0.36 | Acyl-CoA dehydrogenase family protein |
| UYC77864.1 | 0.35 | Asp-tRNA(Asn)/Glu-tRNA(Gln) amidotransferase subunit GatC |
| UYC78708.1 | 0.35 | Hypothetical protein OB946_07885 |
| UYC79007.1 | 0.32 | MFS transporter |
| UYC76575.1 | 0.31 | Cold-shock domain-containing protein |
| UYC78839.1 | 0.30 | Flavin reductase family protein |
| UYC78097.1 | 0.28 | MFS transporter |
| UYC78900.1 | 0.28 | TetR/AcrR family transcriptional regulator |
| UYC76833.1 | 0.26 | MBL fold metallo-hydrolase |
Fold change cutoff: 2.5-fold, bolded if P value < 0.05, Student’s unpaired t test.
Fig 7.
ARC6851 ΔtrmB fails to post-transcriptionally upregulate acinetobactin and is more susceptible to iron deprivation under H2O2 stress. (A) Relative gene expression of basB, basE, bauA, bauB, and OB946_15780 (OHRP) genes in ARC6851 wild-type (W), ΔtrmB (Δ), and trmB+ (+) strains grown in 2 mM H2O2 for 10 min (top) or 2 h (bottom) compared to 0 mM, as determined by qRT-PCR. Dotted line represents 2-fold change. (B) H2O2 killing of ARC6851 wild-type, ΔtrmB, and trmB+ strains in grown in LB, Tris minimal succinate (TMS) media, or Chelex-100-treated TMS (cTMS) with treatment in 3 mM H2O2 for 2 h compared to 0 mM. Survival was measured by serial dilution and quantification of recoverable CFU/mL. Points represent technical replicates from at least four biological replicates, with a horizontal line representing the mean, and errors bars representing the standard error of the mean (SEM). **P < 0.01; one-way ANOVA, Tukey’s test for multiple comparisons.
As a proof of principle, we also tested the transcriptional levels of the most upregulated protein in wild type that was similarly upregulated in the mutant, an annotated organic hydroperoxide resistance protein (OHRP, UYC76661.1). Its corresponding gene, OB946_15780, was highly upregulated at 10 min for both wild type and the mutant, which may indicate that this response to the imposed stress was highly induced at the transcriptional level, in contrast to the acinetobactin cluster (Fig. 7A). This finding supports the hypothesis that the difference in TrmB-dependent oxidative stress responses is due to post-transcriptional regulation, while responses that occur in both wild-type and the ΔtrmB mutant may involve transcriptional regulation.
These data led us to hypothesize that the ΔtrmB mutant would be more susceptible to iron deprivation during H2O2-imposed stress since the strain is unable to efficiently upregulate key members of the acinetobactin cluster. To test this, we performed H2O2 killing assays at a sublethal concentration in rich or Tris-minimal succinate (TMS) media. When the ΔtrmB mutant was exposed to H2O2 in TMS media treated with a chelating resin (cTMS), it was almost entirely killed, indicating that loss of trmB disrupted its ability to respond to iron-limiting conditions when also exposed to low levels of oxidative stress (Fig. 7B). As bacterial pathogens must withstand oxidative stressors and possess efficient mechanisms of iron acquisition to survive within a host and establish infection, the ΔtrmB mutant’s increased susceptibility to iron deprivation during oxidative stress may explain its dramatic fitness defect during pneumonia.
DISCUSSION
tRNA modifications are important for translation, and therefore to the physiology of pathogens that are prominent global health threats; however, research has been largely focused on eukaryotes, as tRNA modifications have been linked to a multitude of human diseases (43). It is now increasingly clear that different bacterial species use tRNA modifications in distinct and diverse ways. This form of post-transcriptional regulation quickly modulates the translation efficiency of proteins, particularly those that may be used to respond to stressors encountered during infection (12, 44, 45). As the genetic code is inherently degenerate and codon bias is unique to each species, it is likely that the tRNA species targeted by the trms can vary between species, impacting the translation of modified tunable transcripts (MoTTs) that are enriched for the corresponding codons and/or are sensitive to alteration in levels of tRNA modifications. While TrmB is important for the P. aeruginosa oxidative stress response, it is critical that we investigate the role of trmB in A. baumannii, given the pathogen’s unique virulence factors and stress response pathways.
A previous study has reported that A. baumannii Ab17978 transcriptionally upregulates the acinetobactin siderophore cluster, potentially in response to iron-sulfur clusters being oxidized during H2O2 treatment (42). In ARC6851, this upregulation appears to be post-transcriptional, and the ΔtrmB mutant cannot upregulate the members of the acinetobactin cluster involved in biosynthesis and uptake to the same degree as the wild-type strain. As acinetobactin has also been shown to be required for A. baumannii virulence, the mutant’s inability to post-transcriptionally upregulate acinetobactin to appropriate levels may be one of the mechanisms by which loss of trmB leads to decreased pathogenesis, especially as iron acquisition is key to a pathogen’s success within a host (39 – 41). Interestingly, a recent report described a role for queuosine tRNA modifications during multimetal starvation in A. baumannii, further supporting the importance of exploring the impact tRNA modifications have on A. baumannii pathobiology (46).
Although the mechanism of TrmB in A. baumannii remains unknown, it is tempting to speculate that the presence of m7G may impact the stability of other modifications on the same tRNA. For example, the m7G46 modification has been shown to be critical for tRNA stability in several organisms, whereby bases G46, C13, and G22 make a base triple pair to maintain the canonical L structure (47 – 52). When a Thermus thermophilus ΔtrmB mutant was exposed to high temperatures, many nucleotide positions became hypomodified and the strain had a severe growth defect (50). Therefore, while the absence of m7G alone may not be sufficient to force the tRNA structure away from its favored conformation, it is possible that this equilibrium could be disturbed under stress, exposing other modifications to an unfavorable environment or preventing their corresponding trms from making the modification. One potential example of an affected modification could be the Gm18 TrmH modification that was shown to reduce the immunostimulatory properties of bacterial tRNA in E. coli by suppressing TLR7-mediated interferon release (25, 50). Even if the other modifications on the tRNA are not affected by a disturbed equilibrium state in the absence of m7G stabilization, the very nature of a change in tRNA folding may be enough of a stressor to disrupt essential processes and reduce pathogenic potential.
Notably, the regulation of bacterial TrmB remains largely unknown, and its catalytic mechanism has still not been definitively proven, though several mechanisms have been proposed and catalytic residues have been identified (53). Furthermore, TrmB has been reported to dimerize in some bacteria and may require a partner protein in yeast, highlighting the mechanistic variation between species (38, 54). Potentially, bioinformatic analyses on the differentially regulated proteins could reveal that their transcripts are enriched for particular codons that would suggest putative tRNA species targeted by A. baumannii TrmB. This information would facilitate the identification of potential MoTTs that are regulated in response to the presence of certain stressors. Moreover, we aim to investigate whether trmB is being regulated pre or post-transcriptionally and if TrmB itself is regulating MoTTs post-transcriptionally. These analyses are imperative to increase our understanding of the role TrmB plays in A. baumannii pathogenicity.
In this paper, we discovered that TrmB is essential for A. baumannii oxidative and acid stress responses in vitro, replication within macrophages, and full virulence in a murine pneumonia model. As the A. baumannii vacuole is still being characterized, the physiologically relevant concentration of hydrogen peroxide in the vacuole is unknown. In vitro hydrogen peroxide killing assays with A. baumannii have been performed by multiple groups at concentrations ranging from 5 to 175 mM. In this work, we used 2–5 mM for our assays, which is at the lowest end of this range (29, 42, 55 – 57). To examine the role of TrmB in vivo, we utilized an additional clinical isolate, Ab04, to determine if TrmB is important for murine CAUTI and found that the presence of TrmB did not affect virulence in this model. This suggests that TrmB may play a bigger role in response to stressors imposed during pneumonia infections as compared to CAUTIs. In murine pneumonia models, macrophages play an integral role in eliminating A. baumannii infections, and modern clinical isolates can infect alveolar macrophages and replicate within vacuoles in vivo (33, 58). However, the role of macrophages in CAUTIs is still unknown, and no evidence has been found that A. baumannii is internalized in macrophages during CAUTIs. Therefore, TrmB may either be more important for A. baumannii-induced pneumonia or may regulate different virulence factors in clinical isolates that establish CAUTIs.
Beyond TrmB, we identified eight other putative SAM-dependent trms in the core genome of A. baumannii. Of these nine, two putative trms, TrmZ1 and TrmZ2, do not have an annotated modification; Phyre2 fold recognition analysis predicted TrmZ1 as a uracil-5 methyltransferase and TrmZ2 as either a 2′-O-methyltransferase or a guanine-N7 methyltransferase. However, as discussed previously, homology does not guarantee function. It is possible that there are more modifications and nonorthologous enzymes in A. baumannii that our analysis did not detect. In the future, we aim to use mass spectrometry to identify the full tRNA modificome of A. baumannii and elucidate its role in A. baumannii pathobiology.
A. baumannii is categorized at the highest threat level by the Centers for Disease Control and Prevention and the World Health Organization due to its increasing rates of MDR that far outpace other prominent Gram-negative pathogens (2, 3, 59). The ability of A. baumannii to successfully detect and respond to stress is integral to its success as a pathogen and to its survival in the environment, including on medical surfaces. While some recent studies have begun to explore the role of the tRNA modificome in bacteria, most research is still performed in model, domesticated strains, which have been shown to possess less or different stress response factors than modern clinical isolates (5, 60). A. baumannii in particular has a large accessory genome, with clinical strains only possessing part of a massive array of virulence factors (61). Therefore, the MoTTs that are impacted by a particular tRNA modification can vary between isolates. While the affected stress factors may vary, if all isolates are affected by the loss of trms in some aspect, then trms are still a viable drug target. Several groups are currently attempting to target TrmD therapeutically, as the modification that it catalyzes is essential to all domains of life (21 – 23). In fact, while many tRNA modifications are conserved across kingdoms, the catalytic mechanisms of the trms may not be, which is ideal for targeting bacterial trms without disturbing the function of the human trm equivalents. While TrmB is not essential in ideal conditions, we have shown that it is critical to A. baumannii virulence in a pneumonia model, which is one of the most common forms of A. baumannii infection in clinical settings (5). Ultimately, characterization of A. baumannii trms may prove to be a valuable tool for developing novel therapeutics to fight the meteoric rise of MDR A. baumannii.
MATERIALS AND METHODS
Bacterial strains and growth conditions
The strains and plasmids used in this study are described in Tables S1 and S2. Cultures were grown at 37°C and 200 rpm using lysogeny broth (LB) medium. When appropriate, E. coli strains were grown in the following antibiotic concentrations: 30 µg/mL apramycin or 50 µg/mL zeocin; and A. baumannii strains were grown in 50 µg/mL apramycin, 300 µg/mL hygromycin B, or 50 µg/mL zeocin. For phenotypic assays, strains were struck out on solid LB plates, grown overnight without antibiotics, subcultured and grown to mid-exponential phase (1 OD of mid-exponential culture ~4 × 108 CFU/mL). Iron deprivation killing assays were performed with TMS medium or Chelex-100 treated TMS (cTMS) as previously described (62, 63).
Construction of trmB mutants and complemented strains
The primers used in this study are listed in Table S3. The ARC6851 and Ab04 deletion mutants were constructed as previously described (64). DNA assembly was performed with the NEBuilder HiFi DNA Assembly Cloning Kit. Briefly, 1 kb regions upstream and downstream of genes to be deleted were amplified from ARC6851 genomic DNA (or Ab04 genomic DNA for Ab04 ΔtrmB). Overlap extension PCR was used to fuse the fragments with an FRT site-flanked apramycin resistance cassette that was amplified from a variant of pKD4 (65, 66). The purified linear DNA product was electroporated into the proper wild-type strain containing the pAT04 plasmid that encodes an IPTG-inducible copy of RecAB recombinase, and mutants were selected using resistance to apramycin treatment (64). To excise the antibiotic cassette, a plasmid encoding an IPTG-inducible copy of FLP recombinase, PAT03, was transformed into each of the mutants (64). Clean mutants were confirmed via return of apramycin sensitivity and whole-genome sequencing. Genetic complementation of the mutants was performed with a mini-Tn7 system as previously described (67, 68). ARC6851 ΔtrmB was complemented using pUCT18T-miniTn7-Zeo, and Ab04 ΔtrmB was complemented using pUCT18T-miniTn7-Apra (69). Briefly, the plasmids were amplified and fused with the appropriate ARC6851 or Ab04 alleles along with ~500 bp upstream that contains the putative promoter region (65). The copy of the gene and the antibiotic resistance cassette were introduced into the chromosome of their respective mutant strains with four-parental conjugation and confirmed with PCR analyses (67 – 70). Complemented strains were confirmed using PCR and whole-genome sequencing.
Bioinformatic analyses
BLAST was used to identify putative SAM-dependent trms using homology to known trms in other bacterial species, including E. coli, P. aeruginosa, Pyrococcus abyssi, and Methanopyrus kandleri. The locus tags of the identified trms are listed in Table 1.
Antibiotic susceptibility assays
MICs were determined using the 2-fold broth dilution microtiter assay as previously described (71, 72). Overnight cultures were subcultured into 10 mL LB at an OD600 of 0.05 and grown for 3 h at 37°C and 200 rpm. About 105 cells/mL of mid-exponential phase cells were used to inoculate a 96-well microtiter plate (Corning 3788) containing 2-fold dilutions of the appropriate antibiotics. Microtiter plates were incubated at 37°C under shaking conditions for 16 h before MICs were determined using OD600. MICs were defined as <10% growth compared to the non-treated controls. Experiments were performed at least five independent times.
H2O2 killing assays
H2O2 killing assays were performed as previously described (42). Briefly, bacterial cultures were started from LB agar plates, inoculated in LB broth, and grown for 16 h at 37°C and 200 rpm. Overnight cultures were subcultured in 10 mL LB at OD600 0.05 and incubated at 37°C and 200 rpm for 3 h to mid-exponential phase. Cultures were normalized to the lowest OD600, and 1 mL was aliquoted into 14 mL polypropylene round-bottom tubes (Falcon 352059). Cultures were treated with 0 or 5 mM H2O2 (Supelco HX0635-3) for 2 h at 37°C and 200 rpm. Killing was determined by samples being serially diluted and plated on LB agar, incubating the plates overnight, and enumerating CFU. In the iron deprivation experiments, mid-exponential cultures were normalized, washed two times in either LB broth, TMS, or cTMS, and treated with 0, 2, or 3 mM H2O2 as previously described (Fig S6). Experiments were performed at least five independent times.
Growth curve assays
Growth curves were performed in sterile, round-bottom, polystyrene, 96-well plates (Corning 3788). Bacterial cultures were started from LB agar plates, inoculated in LB broth, and grown 16 h at 37°C and 200 rpm. Overnight cultures were subcultured in 10 mL LB at OD600 0.05 and incubated at 37°C and 200 rpm for 3 h to mid-exponential phase. Cultures were diluted in fresh medium to an OD600 of 0.01 and inoculated into 96-well plates at a final volume of 150 µL. For acidic growth conditions, non-buffered LB was adjusted to pH 5.0 using HCl. Plates were incubated at 37°C in shaking conditions for 16 h in a BioTek microplate reader, with OD600 values measured at 30 min intervals. All experiments were performed on at least five independent days with at least three wells per strain per condition. Endpoint pH measurements were obtained as described previously (33).
Cell culture conditions
The J774A.1 mouse macrophage cell line (ATCC TIB-67) was cultured in Dulbecco’s modified Eagle medium (DMEM) High Glycose (Hyclone, SH30022.01) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Corning) at 37°C and 5% CO2.
Intracellular replication assays
J774A.1 cells were seeded in 48-well plates 16 h before the experiment at 3 × 105 cells/well and incubated at 37°C and 5% CO2. Bacterial cultures were started from LB agar plates, inoculated in LB broth, and grown for 16 h at 37°C and 200 rpm. Overnight cultures were subcultured in 10 mL LB at OD600 0.05 and incubated at 37°C and 200 rpm for 3 h to mid-exponential phase. Cultures were washed two times with phosphate-buffered saline (PBS) and an appropriate volume was added to each well with DMEM + 10% FBS media to reach a multiplicity of infection (MOI) of 10. Plates were centrifuged 10 min at 200 × g to enhance bacterial contact with the host cells and incubated for 1 h at 37°C and 5% CO2. Wells were washed three times with PBS and fresh DMEM + 10% FBS supplemented with 30 µg/mL apramycin was added to the cells to eliminate extracellular bacteria. At 2, 4, and 6 h post-infection, the cells were washed three times with PBS and lysed with 500 µL PBS containing 0.05% Triton X-100. Lysates were serially diluted and plated on LB plates supplemented with chloramphenicol to determine CFU.
Immunofluorescence staining
A total of 1.5 × 105 J774A.1 cells were plated onto glass coverslips in 24-well plates and incubated 16 h at 37°C and 5% CO2. Bacterial inocula was prepared as described for intracellular replication assays. Once the bacterial suspensions were added to the wells at an MOI of 10, the plates were centrifuged at 200 × g and incubated at 37°C and 5% CO2 for 1 h. Then, the cells were washed three times with PBS and extracellular bacteria were killed with DMEM + 10% FBS supplemented with 50 µg/mL colistin. At 4 h, samples were washed with PBS and fixed with 4% paraformaldehyde for 15 min at 37°C and then incubated for 30 min shaking at room temperature in permeabilizing and blocking solution (PBS + 0.1% saponin + 0.5% BSA [Fisher BioReagents, BP9706100] and 10% FBS [Corning]). Then, the glass coverslips were incubated overnight at 4°C with primary antibodies developed against the insoluble fraction of A. baumannii and produced in rabbit. The next day, the coverslips were washed three times with washing solution (PBS + 0.1% saponin + 0.5% BSA) and incubated with DAPI, Alexa Fluor 555 phalloidin (0.33 µM; CST, #8943) at a 1:100 dilution, and a goat anti-rabbit secondary antibody Alexa Fluor 647 (Invitrogen, A-21244) at a 1:250 dilution for 1 h at 37°C. Afterward, coverslips were washed three times in PBS with washing solution, rinsed with water, and mounted on a glass slide in Invitrogen ProLong Gold Antifade Mountant (Invitrogen, P36930). Stained samples were analyzed by confocal microscopy.
Confocal microscopy
Stained samples were analyzed with a Zeiss LSM880 laser scanning confocal microscope (Carl Zeiss Inc.) equipped with 405 nm diose, 488 nm, Argon, 543 nm HeNe, and 633 nm HeNe lasers. A Plan-Apochromat 63× (NA 1.4) DIC objective and ZEN black 2.1 SP3 software were used for image acquisition. Images were analyzed using ImageJ software (NIH, USA).
Transmission electron microscopy
For quantitation of length, two biological replicates of each strain were subcultured and grown to mid-exponential growth as described previously. For negative staining and analysis by transmission electron microscopy, bacterial suspensions were allowed to absorb for 10 min onto freshly glow-discharged Formvar/carbon-coated copper grids. The grids were washed in distilled water and stained for 1 min with 1% aqueous uranyl acetate (Ted Pella Inc., Redding, CA, USA). Excess liquid was gently removed, and grids were air-dried. Samples were viewed on a JEOL 1200EX transmission electron microscope (JEOL USA, Peabody, MA, USA) equipped with an AMT 8-MP digital camera (Advanced Microscopy Techniques, Woburn, MA, USA). Forty bacteria for each strain and replicate were chosen randomly and images were taken at a magnification of 3,000×. The length of each bacterium was determined using ImageJ 1.38 g (National Institutes of Health, USA, customized for AMT images).
Murine pneumonia model
All animal experiments were approved by the Washington University Animal Care and Use Committee, and we have complied with all relevant ethical regulations. The murine pneumonia infections were performed as previously described for A. baumannii (35). Briefly, overnight cultures of bacteria were subcultured into 100 mL of OD600 0.05 and grown at 37°C and shaking for 3 h to mid-exponential growth. Cultures were washed two times and resuspended in PBS. Six- to 8-week-old female C57BL/6 mice (Charles River Laboratories, Wilmington, MA, USA) were anesthetized by inhalation of 4% isoflurane, and then intranasally inoculated with 5 × 107 CFU of resuspended bacteria. At 24 h post-infection, the mice were sacrificed, and the lungs, kidneys, and spleens were aseptically removed. The bacterial load present in each tissue was determined by homogenizing each organ in PBS and plating serial dilutions on LB agar supplemented with chloramphenicol.
Murine CAUTI model
The CAUTI infections were performed as previously described for A. baumannii (36). Briefly, bacteria were grown in static conditions at 37°C for 48 h, passaging once at 24 h. Six- to 8-week-old female C57BL/6 mice (Charles River Laboratories, Wilmington, MA, USA) were anesthetized by inhalation of 4% isoflurane, and a 4- to 5-mm piece of silicone tubing (catheter) was transplanted transurethrally. Cultures were washed two times and resuspended in PBS. Mice were infected immediately following implant placement with 50 µL containing ~1 × 108 CFU of the bacterial suspension. At 24 h post-infection, the mice were sacrificed, and the catheters, bladders, and kidneys were harvested. The bacterial load present in each tissue was determined as previously described. Bacterial burden in catheters was determined by sonicating the catheters in PBS and plating as previously described.
Measuring m7G levels in ARC6851
ARC6851 wild-type, ΔtrmB, and trmB+ cultures were grown in LB for 16 h at 37°C and 200 rpm and then subcultured into 10 mL LB with an OD600 of 0.05. Three individual 10 mL culture biological replicates were prepared for each strain. Subcultures were grown for 3 h until midexponential. Samples were harvested by pelleting 2 mL OD600 1.0, resuspending in Qiagen RNAprotect, incubating for 5 min, pelleting, removing the supernatant, and snap freezing. Samples were stored at −80°C until processing. Total RNA was extracted using the Zymo Research Quick-RNA Miniprep Kit. Sample quality was confirmed with Qubit RNA Broad Range and IQ Kits, and sample concentrations were normalized to 0.5 µg total RNA for digestion.
Free adenosine, guanosine, and cytosine standards were purchased from Acros Organics, uridine was purchased from Tokyo Chemical Industry, and m7G was purchased from Carbosynth. Samples were digested using nuclease P1 (Millipore Sigma, 10 units) at 50°C overnight (73). Afterwards, Tris pH 7.5 was added to a final concentration of 100 mM to adjust the pH, 10 units of CIP was added, and samples were incubated at 37°C for 90 min. After CIP treatment, samples were filtered using a 0.22-µM pore size syringe filter. To run each sample, 10 µL was loaded onto a Zorbax Eclipse Plus C18 column (2.1 × 50 mM, 1.8 µM) paired with an Agilent 6490 QQQ triple-quadrupole LC mass spectrometer. Runs were analyzed using multiple-reaction monitoring in positive-ion mode. The transitions used were: 268.1→136 (A), 244.1→112 (C), 284.2→152 (G), 245.1→113 (U), and 298→166 (m7G). Standard calibration curves were generated for each nucleoside by fitting the signal intensities against concentrations of pure-nucleoside preparations. The curves were used to determine the concentration of each respective nucleoside in the samples.
Measuring m7G levels in Ab04
Total tRNA was purified from wild type, ΔtrmB, and trmB+. Following purification, total tRNA was digested with ribonuclease T2 to generate 3′-monophosphate nucleotides and then vacuum-dried. The resulting pellet was resuspended in water and 5′-labeled with γ32P-ATP using T4 polynucleotide kinase to yield 3′,5′-diphosphate nucleotides, where the 5′-phospate was radioactive. Excess ATP was hydrolyzed using apyrase. The 3′-phosphate was cleaved using Nuclease P1 overnight (18 h) then vacuum-dried under high heat. The resulting pellet was resuspended in water then spotted on a cellulose TLC plate. The digested, 5′-labeled monophosphate nucleosides were separated in two dimensions. The first dimension was developed in solvent A {isobutyric acid/concentrated ammonia/water [66/1/33 (vol/vol/vol)]}. Once completely air-dried, the plate was rotated 90° and ran in solvent B {phosphate buffer/NH4 sulfate/n-propanol [100/60/2 (vol/wt/vol)]}. The dried plate was exposed to a PhosphorImager Screen overnight. Results were visualized using Typhoon FLA 9000 (GE) and analyzed using ImageQuant. Fraction of m7G was calculated using the signal from the pm7G spot divided by the total pm7G + pG signal (pm7G/pG + pm7G). Published two-dimensional TLC maps as well as all above methods are detailed further elsewhere (74, 75).
Preparation of whole-cell pellets for comparative proteomics
ARC6851 wild-type, ΔtrmB, and trmB+ cultures were grown in LB for 16 h at 37°C and 200 rpm. These overnight cultures were then subcultured into 10 mL LB at an OD600 of 0.05. Four individual 10 mL culture biological replicates were prepared for each condition: wild type (±H2O2), ΔtrmB (±H2O2), and trmB+ (±H2O2). The subcultures were grown 3 h to mid-exponential and then either subjected to 2 mM H2O2 treatment or left untreated for 2 h growth at 37°C and 200 rpm. Whole cells were harvested by pelleting the 10 mL cultures at 4°C, washing with ice-cold PBS, and pelleting again before resuspending them in 4% SDS, 100 mM Tris pH 8.5.
Sample preparation for proteomic analysis
Resuspended protein samples were solubilized by boiling them for 10 min at 95°C. The protein concentrations were then assessed by bicinchoninic acid protein assays (Thermo Fisher Scientific) and 200 µg of each biological replicate was prepared for digestion using Micro S-traps (Protifi, USA) according to the manufacturer’s instructions. Briefly, samples were reduced with 10 mM DTT for 10 min at 95°C and then alkylated with 40 mM IAA in the dark for 1 h. Samples were acidified to 1.2% phosphoric acid and diluted with seven volumes of S-trap wash buffer (90% methanol, 100 mM tetraethylammonium bromide, pH 7.1) before being loaded onto S-traps and washed three times with S-trap wash buffer. Samples were then digested with 2.5 µg of Trypsin overnight at 37°C before being collected by centrifugation with washes of 100 mM Tetraethylammonium bromide, followed by 0.2% formic acid, followed by 0.2% formic acid/50% acetonitrile. Samples were dried down and further cleaned up using C18 Stage tips to ensure the removal of any particulate matter (76, 77).
Reverse phase liquid chromatography–mass spectrometry
C18 enriched proteome samples were resuspended in Buffer A × (2% acetonitrile, 0.01% trifluoroacetic acid) and separated using a two-column chromatography setup composed of a PepMap100 C18 20 mm by 75 µM trap (Thermo Fisher Scientific) and a PepMap C18 500 mm by 75 µm analytical column (Thermo Fisher Scientific) using a Dionex Ultimate 3000 UPLC (Thermo Fisher Scientific). Samples were concentrated onto the trap column at 5 µL/min for 6 min with Buffer A (0.1% formic acid, 2% DMSO) and then infused into an Orbitrap 480 (Thermo Fisher Scientific) at 300 nL/min via the analytical columns. Peptides were separated by altering the buffer composition from 3% Buffer B (0.1% formic acid, 77.9% acetonitrile, and 2% DMSO) to 23% B over 89 min, then from 23% B to 40% B over 10 min and then from 40% B to 80% B over 5 min. The composition was held at 80% B for 5 min before being returned to 3% B for 10 min. The Orbitrap 480 Mass Spectrometer was operated in a data-dependent mode automatically switching between the acquisition of a single Orbitrap MS scan (300–2,000 m/z, maximal injection time of 25 ms, an Automated Gain Control (AGC) set to a maximum of 300% and a resolution of 120k) and 3 s of Orbitrap MS/MS HCD scans of precursors (Stepped NCE of 25;30;45%, a maximal injection time of 80 ms, a AGC of 500% and a resolution of 30k).
Proteomic data analysis
Identification and LFQ analysis were accomplished using MaxQuant (v1.6.17.0) using the ARC6851 (NCBI GCA_025677625.1/ASM2567762v1) with Carbamidomethyl (C) allowed as a fixed modification and Acetyl (Protein N-term) as well as Oxidation (M) allowed as variable modifications with the LFQ and “Match Between Run” options enabled (78). The resulting data files were processed using Perseus (version 1.6.0.7) with missing values imputed based on the total observed protein intensities with a range of 0.3 σ and a downshift of 1.8 σ (78). Statistical analysis was undertaken in Perseus using two-tailed unpaired t tests and ANOVAs. Matching of protein homologs between the strain ARC6851 and UPAB1 (NCBI GCF_006843645.1/ASM684364v1) and was undertaken using the proteome comparison tool within PATRIC, the bacterial bioinformatics database and analysis resource (79). Functional analysis was performed with eggNOG-mapper v2 (80).
Reverse transcription-quantitative PCR
ARC6851 wild-type, ΔtrmB, and trmB+ cultures were grown in LB for 16 h at 37°C and 200 rpm. These overnight cultures were then subcultured into 10 mL LB at an OD600 of 0.05 and grown for 3 h to mid-exponential phase. Cultures were treated with 0 mM or 2 mM H2O2 for 10 min or 2 h before being quickly pelleted, treated with RNAprotect (Qiagen, Inc.), and flash frozen. RNA was extracted from thawed samples using a TRIzol-chloroform extraction in conjunction with the Qiagen RNeasy Mini Kit. To remove contaminating DNA, both the Qiagen on-column DNase treatment and an off-column rigorous DNase treatment using the TURBO DNA-free kit were used. For reverse transcription (RT)-PCR, cDNA was prepared from 1 µg RNA using a high-capacity RNA-to-cDNA kit (Applied Biosystems), according to the manufacturer’s protocol. The cDNA was diluted to 20 ng/µL, and 1 µL was used as template for quantitative PCR (qPCR) using PowerUp SYBR green master mix (Applied Biosciences) on a ViiA7 real-time PCR machine (Applied Biosystems), following the manufacturer’s suggested protocol. The A. baumannii rpoB and recA genes were used as reference genes. All primers used for qPCR were designed using IDT PrimerQuest and are listed in Table S3. Threshold cycle (CT ) values were normalized to the average of rpoB and recA, and fold changes and log2(fold changes) were calculated using the ΔΔCT method.
Statistical methods
All statistical analyses were performed using GraphPad Prism version 9.
ACKNOWLEDGMENTS
We thank Entasis Therapeutics for the ARC6851 isolate. We thank the Melbourne Mass Spectrometry and Proteomics Facility of The Bio21 Molecular Science and Biotechnology Institute for access to MS instrumentation. We thank Dakota Hall for technical support and the members of the Feldman lab for critical reading of the manuscript. We thank the imaging laboratory of the Molecular Microbiology Department at Washington University in St. Louis. We want to thank Wandy Beatty for her collaboration in obtaining the electron microscopy images shown in this work.
This work was supported by funding to M.F.F. (R01AI144120 and R01AI166359), C.J.L. (T32AI007172), H.S.Z. (R00GM094210), and J.D.A. and A.A.Z. (GM084065-11) through the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. N.E.S. is supported by an Australian Research Council Future Fellowship (FT200100270), and J.M.L. is supported by an ARC Discovery Project Grant (DP210100362).
The authors declare that they have no conflicts of interest with the contents of this article.
AFTER EPUB
[This article was published on 17 August 2023 with a typographical error in Results. The error was corrected in the current version, posted on 23 August 2023.]
Contributor Information
Mario F. Feldman, Email: mariofeldman@wustl.edu.
M. Stephen Trent, University of Georgia, Athens, Georgia, USA .
DATA AVAILABILITY
The mass spectrometry proteomics data have been deposited in the Proteome Xchange Consortium via the PRIDE partner repository with the data set identifier (81) PXD040002. This article contains supporting information (65, 69, 70, 82).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/mbio.01416-23.
Plasmids and strains used in this study.
Figures S1 to S6.
Legend for the proteomic data set.
Proteomic data set.
Primers used in this study.
Differentially expressed proteins in ARC6851 ΔtrmB vs. wild type in LB.
Differentially expressed proteins in ARC6851 ΔtrmB vs. wild type in H2O2.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, Edwards JR, Sievert DM. 2016. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2011-2014. Infect Control Hosp Epidemiol 37:1288–1301. doi: 10.1017/ice.2016.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Giammanco A, Calà C, Fasciana T, Dowzicky MJ, Bradford PA. 2017. Global assessment of the activity of tigecycline against multidrug-resistant gram-negative pathogens between 2004 and 2014 as part of the tigecycline evaluation and surveillance trial. mSphere 2:e00310-16. doi: 10.1128/mSphere.00310-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, WHO Pathogens Priority List Working Group . 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. doi: 10.1016/S1473-3099(17)30753-3 [DOI] [PubMed] [Google Scholar]
- 4. CDC . 2022. COVID-19: U.S. Impact on Antimicrobial Resistance, SPECIAL REPORT 2022
- 5. Harding CM, Hennon SW, Feldman MF. 2018. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat Rev Microbiol 16:91–102. doi: 10.1038/nrmicro.2017.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. 2017. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 30:409–447. doi: 10.1128/CMR.00058-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chionh YH, McBee M, Babu IR, Hia F, Lin W, Zhao W, Cao J, Dziergowska A, Malkiewicz A, Begley TJ, Alonso S, Dedon PC. 2016. tRNA-mediated codon-biased translation in mycobacterial hypoxic persistence. Nat Commun 7:13302. doi: 10.1038/ncomms13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Masuda I, Matsubara R, Christian T, Foster LJ, Huang KC, Hou Y-M. 2019. tRNA methylation is a global determinant of bacterial multi-drug resistance. Cell Syst 8:302–314. doi: 10.1016/j.cels.2019.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thongdee N, Jaroensuk J, Atichartpongkul S, Chittrakanwong J, Chooyoung K, Srimahaeak T, Chaiyen P, Vattanaviboon P, Mongkolsuk S, Fuangthong M. 2019. TrmB, a tRNA m7G46 methyltransferase, plays a role in hydrogen peroxide resistance and positively modulates the translation of katA and katB mRNAs in Pseudomonas aeruginosa. Nucleic Acids Res 47:9271–9281. doi: 10.1093/nar/gkz702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Crécy-Lagard V, Marck C, Brochier-Armanet C, Grosjean H. 2007. Comparative RNomics and modomics in mollicutes: prediction of gene function and evolutionary implications. IUBMB Life 59:634–658. doi: 10.1080/15216540701604632 [DOI] [PubMed] [Google Scholar]
- 11. Björk GR, Hagervall TG. 2014. Transfer RNA modification: presence, synthesis, and function. EcoSal Plus 6. doi: 10.1128/ecosalplus.ESP-0007-2013 [DOI] [PubMed] [Google Scholar]
- 12. de Crécy-Lagard V, Jaroch M. 2021. Functions of bacterial tRNA modifications: from ubiquity to diversity. Trends Microbiol 29:41–53. doi: 10.1016/j.tim.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kimura S, Dedon PC, Waldor MK. 2020. Comparative tRNA sequencing and RNA mass spectrometry for surveying tRNA modifications. Nat Chem Biol 16:964–972. doi: 10.1038/s41589-020-0558-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lartigue C, Lebaudy A, Blanchard A, El Yacoubi B, Rose S, Grosjean H, Douthwaite S. 2014. The flavoprotein Mcap0476 (RlmFO) catalyzes m5U1939 modification in Mycoplasma capricolum 23S rRNA. Nucleic Acids Res 42:8073–8082. doi: 10.1093/nar/gku518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Urbonavicius J, Skouloubris S, Myllykallio H, Grosjean H. 2005. Identification of a novel gene encoding a flavin-dependent tRNA:m5U methyltransferase in bacteria--evolutionary implications. Nucleic Acids Res 33:3955–3964. doi: 10.1093/nar/gki703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ryu H, Grove TL, Almo SC, Kim J. 2018. Identification of a novel tRNA wobble uridine modifying activity in the biosynthesis of 5-methoxyuridine. Nucleic Acids Res 46:9160–9169. doi: 10.1093/nar/gky592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taniguchi T, Miyauchi K, Sakaguchi Y, Yamashita S, Soma A, Tomita K, Suzuki T. 2018. Acetate-dependent tRNA acetylation required for decoding fidelity in protein synthesis. Nat Chem Biol 14:1010–1020. doi: 10.1038/s41589-018-0119-z [DOI] [PubMed] [Google Scholar]
- 18. Chatterjee K, Blaby IK, Thiaville PC, Majumder M, Grosjean H, Yuan YA, Gupta R, de Crécy-Lagard V. 2012. The archaeal COG1901/DUF358 SPOUT-methyltransferase members, together with pseudouridine synthase Pus10, catalyze the formation of 1-methylpseudouridine at position 54 of tRNA. RNA 18:421–433. doi: 10.1261/rna.030841.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang W, Foo M, Eren AM, Pan T. 2022. tRNA modification dynamics from individual organisms to metaepitranscriptomics of microbiomes. Mol Cell 82:891–906. doi: 10.1016/j.molcel.2021.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hori H. 2014. Methylated nucleosides in tRNA and tRNA methyltransferases. Front Genet 5:144. doi: 10.3389/fgene.2014.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Malasala S, Polomoni A, Ahmad MN, Shukla M, Kaul G, Dasgupta A, Chopra S, Nanduri S. 2021. Structure based design, synthesis and evaluation of new thienopyrimidine derivatives as anti-bacterial agents. J Mol Struct 1234:130168. doi: 10.1016/j.molstruc.2021.130168 [DOI] [Google Scholar]
- 22. Zhong W, Pasunooti KK, Balamkundu S, Wong YH, Nah Q, Gadi V, Gnanakalai S, Chionh YH, McBee ME, Gopal P, Lim SH, Olivier N, Buurman ET, Dick T, Liu CF, Lescar J, Dedon PC. 2019. Thienopyrimidinone derivatives that inhibit bacterial tRNA (guanine37-N1)-methyltransferase (TrmD) by restructuring the active site with a tyrosine-flipping mechanism. J Med Chem 62:7788–7805. doi: 10.1021/acs.jmedchem.9b00582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhong W, Koay A, Ngo A, Li Y, Nah Q, Wong YH, Chionh YH, Ng HQ, Koh-Stenta X, Poulsen A, Foo K, McBee M, Choong ML, El Sahili A, Kang C, Matter A, Lescar J, Hill J, Dedon P. 2019. Targeting the bacterial Epitranscriptome for antibiotic development: Discovery of novel tRNA-(N 1 G37) methyltransferase (Trmd) inhibitors. ACS Infect. Dis 5:326–335. doi: 10.1021/acsinfecdis.8b00275 [DOI] [PubMed] [Google Scholar]
- 24. Galvanin A, Vogt L-M, Grober A, Freund I, Ayadi L, Bourguignon-Igel V, Bessler L, Jacob D, Eigenbrod T, Marchand V, Dalpke A, Helm M, Motorin Y. 2020. Bacterial tRNA 2′-O-methylation is dynamically regulated under stress conditions and modulates innate immune response. Nucleic Acids Res 48:12833–12844. doi: 10.1093/nar/gkaa1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rimbach K, Kaiser S, Helm M, Dalpke AH, Eigenbrod T. 2015. 2'-O-methylation within bacterial RNA acts as suppressor of TLR7/TLR8 activation in human innate immune cells. J Innate Immun 7:482–493. doi: 10.1159/000375460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hua X, Chen Q, Li X, Yu Y. 2014. Global transcriptional response of Acinetobacter baumannii to a subinhibitory concentration of tigecycline. Int J Antimicrob Agents 44:337–344. doi: 10.1016/j.ijantimicag.2014.06.015 [DOI] [PubMed] [Google Scholar]
- 27. Trebosc V, Gartenmann S, Royet K, Manfredi P, Tötzl M, Schellhorn B, Pieren M, Tigges M, Lociuro S, Sennhenn PC, Gitzinger M, Bumann D, Kemmer C. 2016. A novel genome-editing platform for drug-resistant Acinetobacter baumannii reveals an AdeR-unrelated tigecycline resistance mechanism. Antimicrob Agents Chemother 60:7263–7271. doi: 10.1128/AAC.01275-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen Q, Li X, Zhou H, Jiang Y, Chen Y, Hua X, Yu Y. 2014. Decreased susceptibility to tigecycline in Acinetobacter baumannii mediated by a mutation in trm encoding SAM-dependent methyltransferase. J Antimicrob Chemother 69:72–76. doi: 10.1093/jac/dkt319 [DOI] [PubMed] [Google Scholar]
- 29. Hooppaw AJ, McGuffey JC, Di Venanzio G, Ortiz-Marquez JC, Weber BS, Lightly TJ, van Opijnen T, Scott NE, Cardona ST, Feldman MF, Trent MS. 2022. The phenylacetic acid catabolic pathway regulates antibiotic and oxidative stress responses in Acinetobacter. mBio 13:e0186321. doi: 10.1128/mbio.01863-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. 2015. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858. doi: 10.1038/nprot.2015.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hou YM, Masuda I, Gamper H. 2018. Codon-specific translation by m1G37 methylation of tRNA. Front. Genet 9:1–8. doi: 10.3389/fgene.2018.00713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jaroensuk J, Atichartpongkul S, Chionh YH, Wong YH, Liew CW, McBee ME, Thongdee N, Prestwich EG, DeMott MS, Mongkolsuk S, Dedon PC, Lescar J, Fuangthong M. 2016. Methylation at position 32 of tRNA catalyzed by TrmJ alters oxidative stress response in Pseudomonas aeruginosa. Nucleic Acids Res 44:10834–10848. doi: 10.1093/nar/gkw870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Distel JS, Di Venanzio G, Mackel JJ, Rosen DA, Feldman MF. 2023. Replicative Acinetobacter baumannii strains interfere with phagosomal maturation by modulating the vacuolar pH. PLoS Pathog 19:e1011173. doi: 10.1371/journal.ppat.1011173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sycz G, Di Venanzio G, Distel JS, Sartorio MG, Le N-H, Scott NE, Beatty WL, Feldman MF. 2021. Modern Acinetobacter baumannii clinical isolates replicate inside spacious vacuoles and egress from macrophages. PLoS Pathog 17:e1009802. doi: 10.1371/journal.ppat.1009802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Palmer LD, Green ER, Sheldon JR, Skaar EP. 2019. Assessing Acinetobacter baumannii virulence and persistence in a murine model of lung infection. Methods Mol Biol 1946:289–305. doi: 10.1007/978-1-4939-9118-1_26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Di Venanzio G, Flores-Mireles AL, Calix JJ, Haurat MF, Scott NE, Palmer LD, Potter RF, Hibbing ME, Friedman L, Wang B, Dantas G, Skaar EP, Hultgren SJ, Feldman MF. 2019. Urinary tract colonization is enhanced by a plasmid that regulates uropathogenic Acinetobacter baumannii chromosomal genes. Nat Commun 10:2763. doi: 10.1038/s41467-019-10706-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Bie LGS, Roovers M, Oudjama Y, Wattiez R, Tricot C, Stalon V, Droogmans L, Bujnicki JM. 2003. The yggH gene of Escherichia coli encodes a tRNA (m7G46) methyltransferase. J Bacteriol 185:3238–3243. doi: 10.1128/JB.185.10.3238-3243.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zegers I, Gigot D, van Vliet F, Tricot C, Aymerich S, Bujnicki JM, Kosinski J, Droogmans L. 2006. Crystal structure of Bacillus subtilis TrmB, the tRNA (m7G46) methyltransferase. Nucleic Acids Res 34:1925–1934. doi: 10.1093/nar/gkl116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sheldon JR, Skaar EP. 2020. Acinetobacter baumannii can use multiple siderophores for iron acquisition, but only acinetobactin is required for virulence. PLoS Pathog 16:e1008995. doi: 10.1371/journal.ppat.1008995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gaddy JA, Arivett BA, McConnell MJ, López-Rojas R, Pachón J, Actis LA. 2012. Role of acinetobactin-mediated iron acquisition functions in the interaction of Acinetobacter baumannii strain ATCC 19606T with human lung epithelial cells, Galleria mellonella caterpillars, and mice. Infect Immun 80:1015–1024. doi: 10.1128/IAI.06279-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Penwell WF, Arivett BA, Actis LA. 2012. The Acinetobacter baumannii entA gene located outside the acinetobactin cluster is critical for siderophore production, iron acquisition and virulence. PLoS One 7:e36493. doi: 10.1371/journal.pone.0036493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Juttukonda LJ, Green ER, Lonergan ZR, Heffern MC, Chang CJ, Skaar EP, Whiteley M. 2019. Acinetobacter baumannii OxyR regulates the transcriptional response to hydrogen peroxide. Infect Immun 87:e00413-18. doi: 10.1128/IAI.00413-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Torres AG, Batlle E, Ribas de Pouplana L. 2014. Role of tRNA modifications in human diseases. Trends Mol Med 20:306–314. doi: 10.1016/j.molmed.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 44. Taylor RC, Webb Robertson BJM, Markillie LM, Serres MH, Linggi BE, Aldrich JT, Hill EA, Romine MF, Lipton MS, Wiley HS. 2013. Changes in translational efficiency is a dominant regulatory mechanism in the environmental response of bacteria. Integr Biol (Camb) 5:1393–1406. doi: 10.1039/c3ib40120k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shippy DC, Fadl AA. 2014. tRNA modification enzymes GidA and MnmE: potential role in virulence of bacterial pathogens. Int J Mol Sci 15:18267–18280. doi: 10.3390/ijms151018267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jordan MR, Gonzalez-Gutierrez G, Trinidad JC, Giedroc DP. 2022. Metal retention and replacement in QueD2 protect queuosine-tRNA biosynthesis in metal-starved Acinetobacter baumannii. Proc Natl Acad Sci U S A 119:e2213630119. doi: 10.1073/pnas.2213630119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Giegé R, Frugier M. 2013. Transfer RNA structure and identity [Google Scholar]
- 48. Lorenz C, Lünse CE, Mörl M. 2017. tRNA modifications: impact on structure and thermal adaptation. Biomolecules 7:35. doi: 10.3390/biom7020035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lin S, Liu Q, Lelyveld VS, Choe J, Szostak JW, Gregory RI. 2018. Mettl1/Wdr4-mediated m 7 G tRNA methylome is required for normal mRNA translation and embryonic stem cell self-renewal and differentiation. Mol Cell 71:244–255. doi: 10.1016/j.molcel.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tomikawa C, Yokogawa T, Kanai T, Hori H. 2010. N7-methylguanine at position 46 (m7G46) in tRNA from thermus thermophilus is required for cell viability at high temperatures through a tRNA modification network. Nucleic Acids Res 38:942–957. doi: 10.1093/nar/gkp1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM. 2006. Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell 21:87–96. doi: 10.1016/j.molcel.2005.10.036 [DOI] [PubMed] [Google Scholar]
- 52. Blersch KF, Burchert J-P, August S-C, Welp L, Neumann P, Köster S, Urlaub H, Ficner R. 2021. Structural model of the M7G46 methyltransferase TrmB in complex with tRNA. RNA Biol 18:2466–2479. doi: 10.1080/15476286.2021.1925477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tomikawa C. 2018. 7-methylguanosine modifications in transfer RNA (tRNA). Int J Mol Sci 19:4080. doi: 10.3390/ijms19124080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alexandrov A, Grayhack EJ, Phizicky EM. 2005. tRNA m7G methyltransferase Trm8p/Trm82p: evidence linking activity to a growth phenotype and implicating Trm82p in maintaining levels of active Trm8p. RNA 11:821–830. doi: 10.1261/rna.2030705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Green ER, Juttukonda LJ, Skaar EP. 2020. The manganese-responsive transcriptional regulator MumR protects Acinetobacter baumannii from oxidative stress. Infect Immun 88:e00762-19. doi: 10.1128/IAI.00762-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aranda J, Bardina C, Beceiro A, Rumbo S, Cabral MP, Barbé J, Bou G. 2011. Acinetobacter baumannii RecA protein in repair of DNA damage antimicrobial resistance, general stress response, and virulence. J Bacteriol 193:3740–3747. doi: 10.1128/JB.00389-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sato Y, Unno Y, Miyazaki C, Ubagai T, Ono Y. 2019. Multidrug-resistant Acinetobacter baumannii resists reactive oxygen species and survives in macrophages. Sci Rep 9:17462. doi: 10.1038/s41598-019-53846-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee HH, Aslanyan L, Vidyasagar A, Brennan MB, Tauber MS, Carrillo-Sepulveda MA, Dores MR, Rigel NW, Martinez LR, Torres VJ. 2020. Depletion of alveolar macrophages increases pulmonary neutrophil infiltration, tissue damage, and sepsis in a murine model of Acinetobacter baumannii pneumonia. Infect Immun 88:e00128-20. doi: 10.1128/IAI.00128-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. CDC . 2019. Antibiotic resistance threats in the United States
- 60. Jackson-Litteken CD, Di Venanzio G, Le N-H, Scott NE, Djahanschiri B, Distel JS, Pardue EJ, Ebersberger I, Feldman MF. 2022. InvL, an invasin-like adhesin, is a type II secretion system substrate required for Acinetobacter baumannii uropathogenesis. mBio 13:e0025822. doi: 10.1128/mbio.00258-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Antunes LCS, Visca P, Towner KJ. 2014. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis 71:292–301. doi: 10.1111/2049-632X.12125 [DOI] [PubMed] [Google Scholar]
- 62. Sebulsky MT, Speziali CD, Shilton BH, Edgell DR, Heinrichs DE. 2004. FhuD1, a Ferric hydroxamate-binding lipoprotein in Staphylococcus aureus: a case of gene duplication and lateral transfer. J Biol Chem 279:53152–53159. doi: 10.1074/jbc.M409793200 [DOI] [PubMed] [Google Scholar]
- 63. Chen C, Hooper DC. 2019. Intracellular accumulation of staphylopine impairs the fitness of Staphylococcus aureus cntE mutant. FEBS Lett 593:1213–1222. doi: 10.1002/1873-3468.13396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tucker AT, Nowicki EM, Boll JM, Knauf GA, Burdis NC, Trent MS, Davies BW. 2014. Defining gene-phenotype relationships in Acinetobacter baumannii through one-step chromosomal gene inactivation. mBio 5:e01313–14. doi: 10.1128/mBio.01313-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Le N-H, Peters K, Espaillat A, Sheldon JR, Gray J, Di Venanzio G, Lopez J, Djahanschiri B, Mueller EA, Hennon SW, Levin PA, Ebersberger I, Skaar EP, Cava F, Vollmer W, Feldman MF. 2020. Peptidoglycan editing provides immunity to Acinetobacter baumannii during bacterial warfare. Sci Adv 6:eabb5614. doi: 10.1126/sciadv.abb5614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Harding CM, Tracy EN, Carruthers MD, Rather PN, Actis LA, Munson RS. 2013. Acinetobacter baumannii strain M2 produces type IV pili which play a role in natural transformation and twitching motility but not surface-associated motility. mBio 4:e00360-13. doi: 10.1128/mBio.00360-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Carruthers MD, Nicholson PA, Tracy EN, Munson RS. 2013. Acinetobacter baumannii utilizes a type VI secretion system for bacterial competition. PLoS One 8:e59388. doi: 10.1371/journal.pone.0059388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ducas-Mowchun K, De Silva PM, Crisostomo L, Fernando DM, Chao T-C, Pelka P, Schweizer HP, Kumar A, Liu S-J. 2019. Next generation of Tn7-based single-copy insertion elements for use in multi- and pan-drug-resistant strains of Acinetobacter baumannii. Appl Environ Microbiol 85:e00066-19. doi: 10.1128/AEM.00066-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kumar A, Dalton C, Cortez-Cordova J, Schweizer HP. 2010. Mini-Tn7 vectors as genetic tools for single copy gene cloning in Acinetobacter baumannii. J Microbiol Methods 82:296–300. doi: 10.1016/j.mimet.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 71. Leus IV, Weeks JW, Bonifay V, Smith L, Richardson S, Zgurskaya HI. 2018. Substrate specificities and efflux efficiencies of RND efflux pumps of Acinetobacter baumannii. J Bacteriol 200:e00049-18. doi: 10.1128/JB.00049-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Leus IV, Adamiak J, Trinh AN, Smith RD, Smith L, Richardson S, Ernst RK, Zgurskaya HI. 2020. Inactivation of AdeABC and AdeIJK efflux pumps elicits specific nonoverlapping transcriptional and phenotypic responses in Acinetobacter baumannii. Mol Microbiol 114:1049–1065. doi: 10.1111/mmi.14594 [DOI] [PubMed] [Google Scholar]
- 73. Yan LL, Simms CL, McLoughlin F, Vierstra RD, Zaher HS. 2019. Oxidation and alkylation stresses activate ribosome-quality control. Nat Commun 10:5611. doi: 10.1038/s41467-019-13579-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Spears JL, Gaston KW, Alfonzo JD. 2011. Analysis of tRNA editing in native and synthetic substrates. Methods Mol Biol 718:209–226. doi: 10.1007/978-1-61779-018-8_13 [DOI] [PubMed] [Google Scholar]
- 75. Grosjean H, Droogmans L, Roovers M, Keith G. 2007. Detection of enzymatic activity of transfer RNA modification enzymes using radiolabeled tRNA substrates. Methods Enzymol 425:55–101. doi: 10.1016/S0076-6879(07)25003-7 [DOI] [PubMed] [Google Scholar]
- 76. Rappsilber J, Ishihama Y, Mann M. 2003. Stop and go extraction tips for matrix-assisted laser desorption/Ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal Chem 75:663–670. doi: 10.1021/ac026117i [DOI] [PubMed] [Google Scholar]
- 77. Rappsilber J, Mann M, Ishihama Y. 2007. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat Protoc 2:1896–1906. doi: 10.1038/nprot.2007.261 [DOI] [PubMed] [Google Scholar]
- 78. Cox J, Mann M. 2008. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26:1367–1372. doi: 10.1038/nbt.1511 [DOI] [PubMed] [Google Scholar]
- 79. Wattam AR, Abraham D, Dalay O, Disz TL, Driscoll T, Gabbard JL, Gillespie JJ, Gough R, Hix D, Kenyon R, Machi D, Mao C, Nordberg EK, Olson R, Overbeek R, Pusch GD, Shukla M, Schulman J, Stevens RL, Sullivan DE, Vonstein V, Warren A, Will R, Wilson MJC, Yoo HS, Zhang C, Zhang Y, Sobral BW. 2014. PATRIC, the bacterial bioinformatics database and analysis resource. Nucl. Acids Res 42:D581–D591. doi: 10.1093/nar/gkt1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cantalapiedra CP, Hernández-Plaza A, Letunic I, Bork P, Huerta-Cepas J. 2021. eggNOG-mapper V2: functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol Biol Evol 38:5825–5829. doi: 10.1093/molbev/msab293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Perez-Riverol Y, Bai J, Bandla C, García-Seisdedos D, Hewapathirana S, Kamatchinathan S, Kundu DJ, Prakash A, Frericks-Zipper A, Eisenacher M, Walzer M, Wang S, Brazma A, Vizcaíno JA. 2022. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res 50:D543–D552. doi: 10.1093/nar/gkab1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ahmed-Bentley J, Chandran AU, Joffe AM, French D, Peirano G, Pitout JDD. 2013. Gram-negative bacteria that produce carbapenemases causing death attributed to recent foreign hospitalization. Antimicrob Agents Chemother 57:3085–3091. doi: 10.1128/AAC.00297-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plasmids and strains used in this study.
Figures S1 to S6.
Legend for the proteomic data set.
Proteomic data set.
Primers used in this study.
Differentially expressed proteins in ARC6851 ΔtrmB vs. wild type in LB.
Differentially expressed proteins in ARC6851 ΔtrmB vs. wild type in H2O2.
Data Availability Statement
The mass spectrometry proteomics data have been deposited in the Proteome Xchange Consortium via the PRIDE partner repository with the data set identifier (81) PXD040002. This article contains supporting information (65, 69, 70, 82).