Abstract
Plants can reorient their organs in response to changes in environmental conditions. In some species, ethylene can induce resource-directed growth by stimulating a more vertical orientation of the petioles (hyponasty) and enhanced elongation. In this study on Arabidopsis (Arabidopsis thaliana), we show significant natural variation in ethylene-induced petiole elongation and hyponastic growth. This hyponastic growth was rapidly induced and also reversible because the petioles returned to normal after ethylene withdrawal. To unravel the mechanisms behind the natural variation, two contrasting accessions in ethylene-induced hyponasty were studied in detail. Columbia-0 showed a strong hyponastic response to ethylene, whereas this response was almost absent in Landsberg erecta (Ler). To test whether Ler is capable of showing hyponastic growth at all, several signals were applied. From all the signals applied, only spectrally neutral shade (20 μmol m−2 s−1) could induce a strong hyponastic response in Ler. Therefore, Ler has the capacity for hyponastic growth. Furthermore, the lack of ethylene-induced hyponastic growth in Ler is not the result of already-saturating ethylene production rates or insensitivity to ethylene, as an ethylene-responsive gene was up-regulated upon ethylene treatment in the petioles. Therefore, we conclude that Ler is missing an essential component between the primary ethylene signal transduction chain and a downstream part of the hyponastic growth signal transduction pathway.
Since plants are normally confined to one location, they must respond rapidly and appropriately to changes in local conditions if they are to survive in a variable and challenging habitat. Among other traits, this involves the reorientation of growth to optimize the location of plant organs toward resources (Ball, 1969; Kang, 1979). A clear example of reorientation of growth is the phototropic response in which differential growth directs organs to the light source (Firn and Digby, 1980; Ballaré et al., 1992; Hangarter, 1997; Ahmad et al., 1998; Friml et al., 2002). Also, readjustment of the direction of leaves and growth rate of petioles and shoots is clearly demonstrated during shade and submergence avoidance (Voesenek and Blom, 1989; Clúa et al., 1996; Gautier et al., 1997; Ballaré, 1999; Cox et al., 2003; Pierik et al., 2003, 2004). During these responses, growth is directed toward resources such as light and air, respectively. If plants are exposed to low light or submergence, the resource-directed growth is achieved via 2 distinguishable processes: (1) an upward movement of petioles (hyponasty); and (2) enhanced elongation of these petioles (Voesenek and Blom, 1989; Cox et al., 2003). Shade-induced hyponasty and petiole elongation are driven mainly by a decrease in the R to FR ratio and a reduction in blue light (Smith and Whitelam, 1997; Ballaré, 1999; Pierik et al., 2004). Recently, it was shown for tobacco (Nicotiana tabacum) plants that the gaseous phytohormone ethylene is also essential for well-timed shade avoidance responses (Pierik et al., 2003). Interestingly, ethylene is the main trigger for submergence-induced hyponastic growth and petiole elongation in semiaquatic plants such as Rumex palustris (Voesenek and Blom, 1989). Furthermore, an interaction between submergence-induced hyponasty and enhanced petiole elongation has been demonstrated for R. palustris (Cox et al., 2003). To achieve resource-directed growth, first a more vertical angle has to be reached before the enhanced petiole elongation will start, resulting in leaf growth toward the water surface in these submerged plants.
The stimulatory effect of ethylene on hyponastic growth and petiole elongation during shade (Pierik et al., 2003) and submergence (Voesenek and Blom, 1989; Cox et al., 2003; Voesenek et al., 2003) is in contrast with the generally accepted growth-inhibiting role of this plant hormone (Smalle and Van Der Straeten, 1997). However, there are several more examples of ethylene having a positive effect on elongation. For example, hypocotyl growth of Arabidopsis (Arabidopsis thaliana) seedlings in the light, grown on a low-nutrient medium, can be stimulated by ethylene (Smalle et al., 1997). Additionally, an ethylene concentration slightly higher than ambient can stimulate leaf extension in some Poa species (Fiorani et al., 2002).
In this study, we explore the extent to which the promoting effects of ethylene on rapid hyponastic growth and petiole elongation are also evident in the widely used model species, Arabidopsis. Since considerable quantitative differences exist between accessions of Arabidopsis (e.g. in phytochrome-mediated shade avoidance [Botto and Smith, 2002] and in seed dormancy behavior [Alonso-Blanco et al., 2003]), we included different accessions in our work. We selected nine accessions on the basis of the availability of potentially useful hormone mutants and recombinant inbred lines with the selected accession as background. We found that petiole elongation in Arabidopsis was not markedly stimulated by ethylene. However, a strong and rapid hyponastic response to ethylene was observed with considerable variation between the nine selected accessions. The accession Columbia-0 (Col-0) showed a strong and fast ethylene-induced hyponastic growth response, whereas the accession Landsberg erecta (Ler) showed only a weak response. The lack of a hyponastic response to ethylene in Ler does not reflect a general lack in the ability to show hyponastic growth since, by applying several other signals, we found that Ler is capable of hyponastic growth when spectrally neutral shaded. Furthermore, based on the up-regulation of an ethylene marker gene, we observed that Ler was not insensitive to ethylene and the endogenous ethylene production was not already saturating the response. Therefore, we conclude that Ler is missing an essential component between the primary ethylene signal transduction chain and the downstream part of the hyponastic growth signal transduction pathway.
RESULTS
Large Natural Variation in Ethylene-Induced Petiole Hyponastic Growth and Elongation
Quantitative measurements on petiole length and angle were performed on time lapse photographs. A typical example of these photographs is shown in Figure 1. The petiole angle (α) was measured between the horizontal and a line between an ink mark at the petiole-lamina junction and a fixed basal point. The length of the petiole was measured between the ink mark and the fixed basal point.
Figure 1.
Representative example of Arabidopsis accession Col-0 treated with air (A) or 5 μL L−1 ethylene (B) for 24 h. The petiole-leaf blade junction of the leaf under examination is marked with drawing ink (see arrows). A calibration object is placed in the same plane as the marked petiole. A black square on the calibration object has a dimension of 5 × 5 mm. α, Petiole angle.
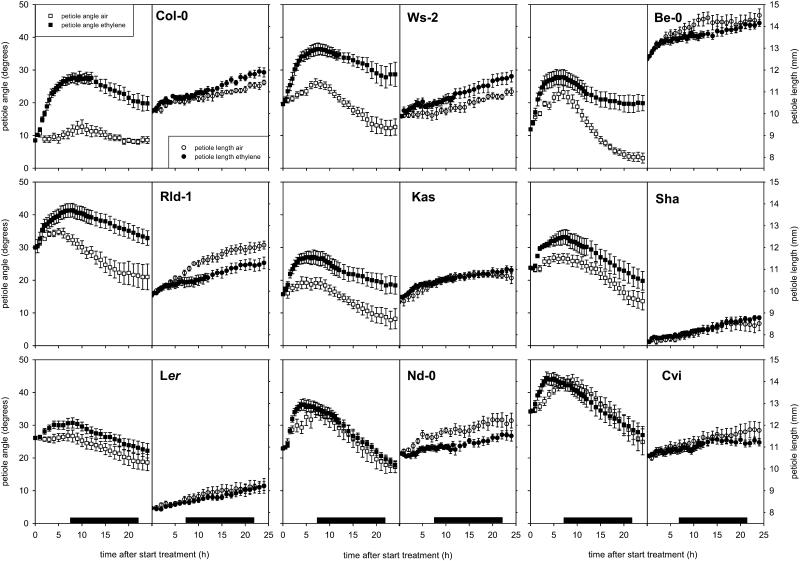
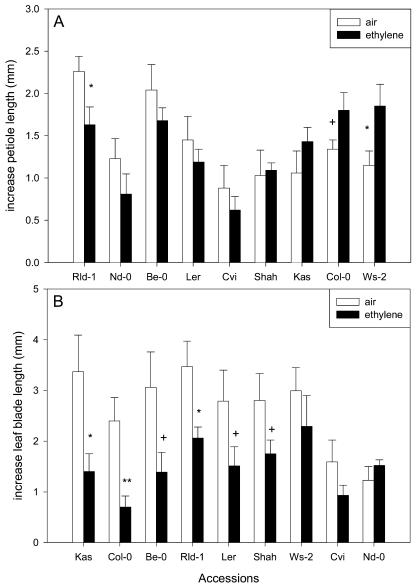
When the effect of ethylene on petiole and lamina elongation was examined in nine accessions of Arabidopsis, we observed significant natural variation (Figs. 2 and 3. After 24 h of treatment, ethylene promoted petiole length to a statistically significant extent only in Wassilewskija (Ws; P < 0.05; Fig. 3A), although a tendency toward stimulation was also seen in Col-0 (P < 0.1). However, this effect was not sustained after 48 or 72 h of ethylene treatment (data not shown). Only one of the accessions (Rschew-1 [Rld-1]) showed a significant inhibitory effect of ethylene on petiole elongation, whereas in the other accessions petiole length was not significantly affected. No stimulatory effect of ethylene on leaf blade elongation was found in any of the accessions used in this study (Fig. 3B). In some accessions (Kashmir [Kas], Col-0, and Rld-1), leaf blade elongation was inhibited, whereas in the others it was not significantly affected. There was no significant correlation between the effect of ethylene on elongation of the petiole and the leaf blade, indicating that ethylene can affect elongation of the petiole and of the leaf blade in an independent manner.
Figure 2.
Petioles angle (squares) and length (circles) of 9 Arabidopsis accessions treated with 5 μL L−1 ethylene or air. Plants were in continuous light during the experiment; the black bars represent the time when the night period would normally have taken place. Data are means of eight replicate plants from two separately grown batches per accession. Error bars = se.
Figure 3.
Effect of ethylene on the elongation of petioles (A) and leaf blades (B) of Arabidopsis accessions after 24 h. Data are means of eight replicate plants from two separately grown batches per accession. Error bars = se. +, P < 0.1; *, P < 0.05; **, P < 0.01. Note that the order in which the accessions are presented is based on the effect of the ethylene treatment.
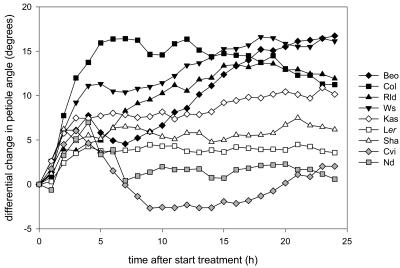
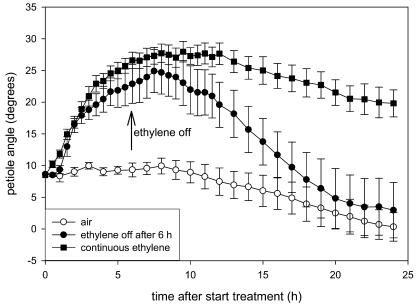
Next to affecting petiole elongation, ethylene also induced hyponastic growth (Figs. 2 and 4). This appears to be a reversible process, because switching off the ethylene after 6 h caused a decrease in angle after approximately 3 h, and after 10 h, the petiole angles had returned to control values (Fig. 5). Furthermore, large natural variation existed in ethylene-induced hyponastic growth (Figs. 2 and 4). Variation existed in the initial angle of the petiole at the start of the experiment, with particularly low initial angles in Col-0 and high angles in Cape Verde Island (Cvi). Additionally, this control petiole angle in a number of accessions changed considerably during 24-h experiments. In most accessions, ethylene induced a rapid (2 h) upward movement of the petioles. The variation between accessions is best visualized in Figure 4, where we corrected for changes in petiole angle of air-grown plants during the time course to give the net effect of ethylene by revealing the actual differences in petiole angle between ethylene-treated and control plants. These plots show that there is considerable variation between accessions in the kinetics of the hyponastic response.
Figure 4.
Differential change in petiole angle (mean ethylene − mean air; Fig. 2) for nine Arabidopsis accessions. The ethylene-induced hyponastic growth is divided into three groups: large (black symbols), intermediate (white symbols), and small (gray symbols) hyponastic response.
Figure 5.
The effect of switching off the ethylene supply after 6 h of treatment on the petiole angle of Arabidopsis Col-0. Following discontinuation of the ethylene supply, the ethylene concentration in the cuvette declined to ambient levels within 40 min. Approximately 2 h later, hyponasty started to reverse and, after approximately 10 h (= 16 h after start ethylene treatment), petiole angles had returned to control values. Data are means of four replicate plants. Error bars = se.
We divided the accessions into three groups based on their hyponastic response to ethylene. The first group (Bensheim-0 [Be-0], Col-0, Rld-1, and Ws-2; black symbols in Fig. 4) showed the strongest increase in petiole angle after 24 h in ethylene (more than 10 degrees compared to untreated plants). However, the way in which this ethylene-induced increase was reached differed considerably between these accessions. The maximum increase (compared to untreated plants) of Col-0 petioles was attained after only 6 h and declined slightly afterward. In contrast, in Be-0 and Rld-1, the maximum increase in angle, compared to untreated plants, was achieved no earlier than after approximately 15 h of treatment. Ws-2 was intermediate between these extremes. The second group (Kas, Ler, and Shakdara [Sha]; white symbols in Fig. 4) showed a weak hyponastic response, giving only a 5- to 10-degree increase in angle compared to untreated plants. Ler and Sha reached their maximum angle after approximately 5 h of ethylene treatment, while the similar process in Kas took 15 h. Cvi and Niederzenz-0 (Nd-1) were placed in the third group (gray symbols in Fig. 4), since almost no ethylene effect was discernible, with ethylene inducing only a transient 5-degree response that peaked within 5 h.
The leaf blade also showed natural variation in ethylene-induced hyponastic growth (data not shown). Both the petiole and the leaf blade showed a basal bending point. The average differential hyponastic response of the petiole and that of the leaf blade of the various accessions were positively correlated (Pearson 0.911; P < 0.001), indicating that a strong hyponastic petiole growth was accompanied by a strong hyponastic growth of the leaf blade. On average, the response of the leaf blade was almost twice as large as that of the petiole for all accessions (data not shown). This indicates that, at the basal end of both the petiole and the leaf blade, differential growth occurred to the same extent, resulting in a change in leaf blade angle that was twice as large as the change in petiole angle.
To gain more insight into the regulation of ethylene-induced hyponastic growth, a comparison was made between two accessions showing contrasting hyponastic responses to ethylene. These were Col-0, which had strong ethylene-induced hyponastic growth, and Ler, which showed almost no change in petiole angle upon ethylene treatment (Figs. 2 and 4). Although Nd-1 and Cvi responded even less to ethylene than Ler, they were not used because these accessions showed strong circadian petiole movements in air (Fig. 2).
By applying several other signals that can induce hyponasty, we tested whether the poor ethylene-responsive accession (Ler) is missing the capacity for hyponastic growth or whether elevated ethylene is not the appropriate signal in this accession to induce hyponastic growth. We tested the two selected accessions for their reaction to manipulation of the initial petiole angle, submergence, high temperature (30°C), and spectrally neutral shading.
Manipulation of Initial Petiole Angle Does Not Affect Hyponastic Growth
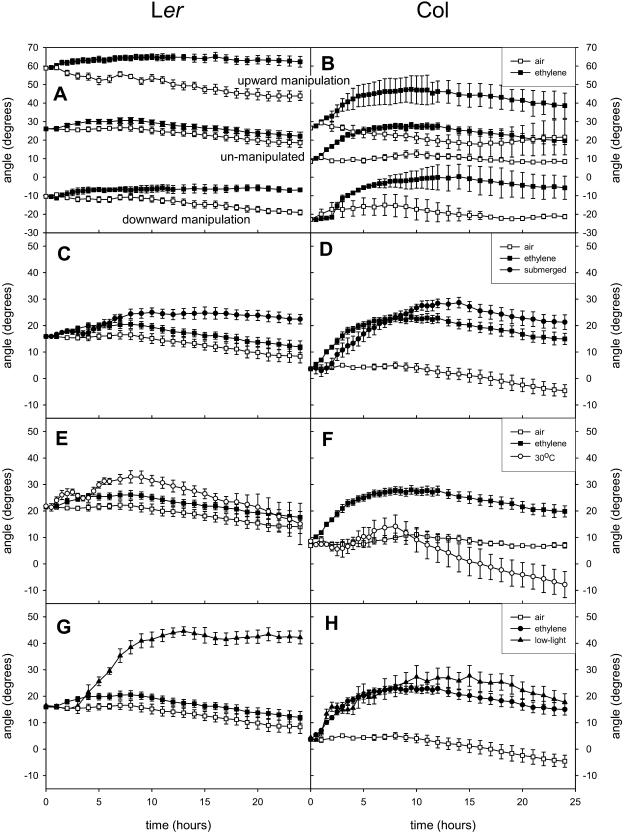
Before applying external signals, we tested whether an internal signal derived from the petiole angle is preventing the ethylene-induced hyponastic growth in Ler. The existence of such a putative signal is supported by the fact that Ler has a start angle comparable to the final angle in Col after ethylene addition. Furthermore, among the accessions tested, a significant negative correlation was found between the average differential response of the ethylene-treated petiole and the initial petiole angle relative to the horizontal (Pearson −0.696; P < 0.05). This suggested that accessions with low initial petiole angles (e.g. Col-0) give a more vigorous hyponastic response than accessions with a high start angle (e.g. Ler), a dependency that was also shown for submergence-induced hyponastic growth in R. palustris (Cox et al., 2003). To examine the impact of the initial angle on the extent of hyponasty, the angle was increased or decreased by tilting whole plants of Col-0 and Ler (Fig. 6, A and B). Unexpectedly, these tilting treatments had almost no effect on the kinetics and magnitude of the hyponastic response induced by ethylene. In Col-0, we observed a slight delay in the onset of hyponastic growth if the petioles were tilted downward at the start.
Figure 6.
Effect of angle manipulation (A and B), submergence (C and D), high temperature (E and F), and low light (G and H) on hyponastic growth in Ler (left) and Col-0 (right). Initial petiole angle is manipulated by tilting the pot at the start of the experiment. The high-temperature treatment is a shift from 20°C to 30°C. Low light is a spectrally neutral decrease in light intensity from 200 to 20 μmol m−2 s−1. Data are means of four replicate plants. Error bars = se.
For both accessions, increasing the initial angle at the start of the experiment resulted in a slight decrease of petiole angles of air-grown plants throughout the duration of the experiment, while lowering the angle induced almost no upward adjustment over 24 h. Upward and downward petiole growth in air was also unaffected by tilting the pots 90 degrees downward for several days (data not shown). These results show that hyponasty is not a readjustment by the leaves to a preferred angle relative to gravity (the so-called gravitropic set-point angle; Digby and Firn, 1995). This experiment also indicates that gravity has little influence on leaf angle in Arabidopsis because changing the initial angle caused only a small response in petioles.
Submergence- and Ethylene-Induced Hyponastic Growth Are Comparable
Submergence is known to induce hyponastic growth in several species (Ridge, 1987; Voesenek and Blom, 1989; Grimoldi et al., 1999; Cox et al., 2003). As expected, Col-0 plants showed a strong hyponastic response to submergence (Fig. 6D), which was very similar to that induced by ethylene. In contrast to Col-0, petioles of Ler showed little evidence of hyponastic growth under water, although the 10-degree change (Fig. 6C) was still twice that obtained by ethylene-treated Ler plants (P < 0.01). The kinetics of submergence-induced hyponastic growth was delayed compared to that in ethylene, as indicated by the time taken for the petioles to reach 90% of their maximum angle (P < 0.05). Since submergence-induced hyponasty is driven by endogenous ethylene accumulation (Voesenek and Blom, 1989, 1999), the slightly delayed response in submerged plants may be explained by the time taken for physiologically active concentrations of ethylene to accumulate within petioles once they are submerged. Similar differences between submergence- and ethylene-induced hyponastic growth patterns were observed in R. palustris (Cox, 2004).
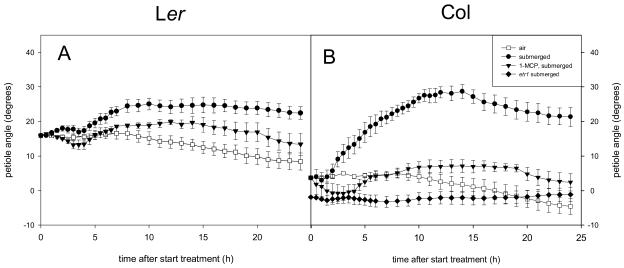
To further verify the importance of ethylene during submergence, both Col-0 and Ler were pretreated before submergence with the ethylene receptor antagonist 1-methylcyclopropene (1-MCP). Submergence-induced hyponasty was prevented with 1-MCP in Col-0 and reduced in the less-responding accession Ler (Fig. 7A). Moreover, the ethylene-insensitive receptor mutant etr1-1 in a Col-0 background showed no submergence-induced hyponasty (Fig. 7B). These data demonstrate that ethylene is a key player in the submergence-induced hyponastic growth process in Arabidopsis Col. In Ler, signals other than ethylene are possibly involved in the submergence-induced hyponastic growth.
Figure 7.
Ethylene dependency of submergence-induced hyponastic growth in Arabidopsis Ler (left) and Col-0 (right). Plants are pretreated with ethylene receptor antagonist 1-MCP (triangles) before submergence. The ethylene-insensitive receptor mutant etr1-1 in a Col-0 background (diamonds) was submerged. Data are means of four replicate plants. Error bars = se.
High Temperature Marginally Affects Hyponastic Growth
In Phaseolus vulgaris, both high light intensities and high temperature induce an upward movement of the leaves. This movement is achieved by the pulvinus and serves to optimize photosynthesis (Fu and Ehleringer, 1989; Yu and Berg, 1994). To study the impact of elevated temperatures on hyponastic growth of Arabidopsis, we exposed the accessions Col-0 and Ler to a quick temperature shift from 20°C to 30°C. Upon this treatment, Ler showed only a modest and transient increase in petiole angle of about 10 degrees (Fig. 6E). A small but transient positive effect, followed by a decrease in angle, was observed in Col-0 (Fig. 6F). Apparently, a shift to a higher temperature does not induce strong hyponastic growth in Arabidopsis.
Low Light Induces Strong Hyponastic Growth in Ler
Hyponastic growth is recognized as a shade avoidance trait in several species (Ballaré, 1999; Pierik et al., 2003, 2004). Therefore, we examined whether spectrally neutral shading (approximately 10% of the normal light level) could induce hyponastic petiole growth in two accessions of Arabidopsis. In Col-0, shading induced a change in petiole angle that was very similar to that of ethylene-treated plants (Fig. 6H). No additive effect of the two treatments was demonstrated apart from maintenance of a higher petiole angle in the last 5 h of the experiment in plants treated with both low light and ethylene (data not shown). Interestingly, petioles of Ler also showed a strong hyponastic response (20–25 degrees) upon spectrally neutral shading (Fig. 6G). This is in contrast to the very weak effect of ethylene on the petiole angle in this accession under normal light conditions (Fig. 6H), suggesting that, in Ler, low-light-induced hyponasty is not ethylene mediated. This is in correspondence with low-red-/far-red-induced hyponasty in tobacco, which also does not involve ethylene, although low-light- or low blue-light-induced hyponasty in this species does require ethylene (Pierik et al., 2004).
Ethylene Sensitivity
Since Ler showed a strong low-light-induced hyponastic growth, we concluded that Ler possesses all the downstream signal transduction components necessary for carrying out hyponastic growth. This raises the question of why Ler does not show the pronounced hyponastic response to ethylene as found for Col-0. The start angle of Ler is very similar to the final angle of ethylene-treated Col-0 plants. This could suggest that Ler has a higher ethylene production and/or is more sensitive to ambient ethylene concentrations, both resulting in a high default leaf angle and reduced hyponastic growth upon ethylene treatment. In contrast to this hypothesis, Ler showed a lower, rather than a higher, ethylene biosynthesis and a lower capacity of 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase, the last step of the ethylene biosynthesis, compared to Col-0 (Table I). Furthermore, enhanced sensitivity for ambient ethylene levels in Ler would predict a decrease of the leaf angle in air in this genotype upon blocking of ethylene perception with 1-MCP. There was, however, hardly a change in 1-MCP pretreated petiole angles of Ler after 24 h, while in Col-0 there was a marginal decrease of the petioles (about 10 degrees) after the 1-MCP pretreatment (Table I). These data strongly suggest that Ler is not producing ethylene concentrations to a level that already saturates hyponastic growth, nor more sensitive to ambient levels of ethylene than Col-0. To further test whether the ethylene signal transduction is operating to a lesser extent in Ler compared to Col-0, we measured the expression of an ethylene-responsive gene (ERS2) after 3 h of ethylene addition (Vriezen et al., 1997; Hua et al., 1998). The expression of this gene was measured with real-time reverse transcription (RT)-PCR. There was a significant up-regulation of ERS2 upon ethylene treatment in both Col-0 and Ler (Table I), suggesting that Ler does not have a general reduced responsiveness to ethylene.
Table I.
Overview of data showing that Ler is not insensitive to ethylene and that the endogenous ethylene production does not saturate hyponastic growth
The ethylene and ACC production in Col-0 was significantly higher (P < 0.001) compared to Ler, while the ethylene-induced ERS2 expression was higher (P < 0.05) in Ler compared to Col-0. The petiole angle is the difference in petiole angle between 1-MCP pretreated and nonpretreated plants. Data are means and se of four to eight replicate plants. FM, Fresh mass.
| Ethylene | ACC Oxidase Capacity | Petiole Angle 1-MCP/Air | ERS2 C2H4/Air | |
|---|---|---|---|---|
| pmol g−1 FM h−1 | pmol C2H4 g−1 FM h−1 | |||
| Col | 78 ± 7 | 842 ± 54 | −9.7 ± 1.0 | 4.7 ± 0.5 |
| Ler | 45 ± 4 | 320 ± 41 | 1.9 ± 1.0 | 50.1 ± 7.7 |
DISCUSSION
Ethylene-Induced Petiole Elongation and Hyponastic Growth
Natural variation was found in ethylene-induced petiole and leaf blade elongation, although the variation was less than that found for hyponastic growth (Figs. 2–4). In 2 accessions, we found a significant effect of ethylene treatment on petiole elongation after 24 h. In one of these (Rld-1), elongation was inhibited, whereas in another accession (Ws-2), stimulation was observed. Because ethylene is mostly associated with growth retardation in nonaquatic plants, such as Arabidopsis (Smalle and Van Der Straeten, 1997), it is surprising that only one of our nine accessions showed a significant retardation of petiole extension. In most accessions, elongation of the leaf blade showed a tendency toward inhibition by ethylene; no stimulatory effect of ethylene was found in any accession. Since there was no significant correlation between the effect of ethylene on petiole and leaf blade elongation, we conclude that the hormone acts independently on the petiole and the leaf blade. This indicates that organs such as petioles and leaf blades can exhibit different responses to ethylene. Furthermore, we tested whether other ethylene-induced processes were also impaired in accessions lacking ethylene-induced hyponastic growth. In this respect, a correlation was calculated between the data published in Peeters et al. (2002) describing ethylene-induced inhibition of hypocotyl growth in various accessions and the hyponastic growth measured in this study. There was no significant correlation between these two datasets. Also, the effect of ethylene on petiole and leaf blade growth (Fig. 3) showed no correlation and these characteristics also did not correlate with the effect of ethylene on hyponastic growth. This shows that the responsiveness to ethylene differs between organs and between processes within an organ.
In the nine accessions of Arabidopsis, a large variation in rapid ethylene-induced hyponastic growth was observed (Figs. 2 and 4). There are indications that differences between species and ecotypes in the effects of ethylene on growth may be related to the altitude of the habitat of origin. For example, a prairie ecotype of Stellaria longipes showed growth stimulation of the ramets at a slightly enhanced ethylene concentration, while this was not observed in an alpine ecotype (Emery et al., 1994). In contrast, two alpine species of the Poa genus (Poa alpine and Poa compressa) showed ethylene-induced shoot elongation at low, but enhanced, ethylene concentrations, as opposed to two other species from the same genus, which are lowland species (Poa annua and Poa trivialis). To check whether Arabidopsis accessions originating from a high altitude respond differently to those from a low altitude, we collected data from the original growth location from eight accessions (Rld is missing; Nottingham Arabidopsis Stock Centre [NASC]; www.arabidopsis.info browse catalog, Ecotypes, All Ecotypes). However, no correlation was found between the altitude (0–3,400 m) and the petiole hyponastic or elongation response.
In the strong responding accessions Col-0 and Ws-2, ethylene induced an upward movement within 2 h. This relatively fast response is in accordance with the time ranges for other hyponastic or epinastic growth processes described in the literature. For example, R. palustris petioles increased their angle relative to the horizontal within a few hours of submergence or ethylene treatment (Cox et al., 2003). In Lycopersicon esculentum, submergence or ethylene treatment promoted epinasty within a few hours as well (Jackson and Campbell, 1975; English et al., 1995; Grichko and Glick, 2001a). Similarly, stolons of Trifolium fragiferum and Arachis hypogaea showed ethylene-induced curvature after 2 h of treatment (Hansen and Bendixen, 1974; Ziv et al., 1976). It is therefore clear that in different species, ethylene induces hyponastic growth within only a few hours, which is in sharp contrast to the results of Vandenbussche et al. (2003) on hyponasty in Arabidopsis. In their system with agar-grown seedlings, ethylene or low-light treatments for 6 d were required to observe a change in leaf angle, rather than the few hours that we describe here. Therefore, it is unlikely that the two responses are based on the same mechanisms.
Contrary to our results, Arteca and Arteca (2001) failed to find hyponastic growth in ethylene-treated Arabidopsis Col-0. This may have been the outcome of constitutively high ethylene production in control plants caused by growing the plants on nonaerated hydroponics in their experiment. This is indicated by the high ethylene emission and ACC concentration in their control plants, which are comparable to the elevated values as measured in submerged R. palustris plants (Banga et al., 1996; Vriezen et al., 1999) or infected Arabidopsis leaves (Knoester et al., 1998; Grichko and Glick, 2001b). The high internal ethylene in the plants studied by Arteca and Arteca (2001) may thus have precluded any further response from additional exogenous ethylene.
We have shown that ethylene can induce hyponasty in Arabidopsis and that its presence is required for the maintenance of a high petiole angle. Therefore, hyponastic growth is a reversible process (Fig. 5). When ethylene supply to Col-0 was stopped, a downward movement of the petiole started after approximately 3 h to return leaf angles to normal within 10 h. Since the ethylene concentration in the cuvette reached ambient levels within 40 min, the extra delay in hyponasty reversal could reflect the time needed (1) for ethylene to dissociate from receptors (Bleecker, 1997); (2) to synthesize new receptor proteins (Hall et al., 2000); or (3) for the differential growth process that causes hyponasty to be reversed.
To unravel the mechanisms underlying hyponastic growth, two contrasting accessions were treated with several other signals with the aim of provoking hyponasty also in the Ler accession, which is lacking the ethylene-induced response. This would indicate whether this accession is able to display hyponastic growth at all. Manipulation of the initial petiole angle, submergence, and high temperature did not result in strong hyponastic growth in Ler. However, low light did induce hyponastic growth in Ler.
Ler Is Missing an Essential Step between Ethylene Signaling and Hyponastic Growth
It is well known that changes in the light environment can alter leaf angles (Ballaré, 1999; Pierik et al., 2003, 2004). In accordance with this, both Col-0 and Ler showed a clear hyponastic response after transfer to spectrally neutral low light (Fig. 6G). The kinetics of hyponastic growth in Col-0 was similar in ethylene and under low light (Fig. 6H). Also, for the first 20 h, there was no additive effect when ethylene and low light were given together (data not shown). We therefore hypothesize that a part of the downstream signal transduction pathway leading to hyponastic growth is shared by these two signals. It should be noted that, although Ler hardly responded to ethylene and relatively weakly to submergence and high temperature, low-light treatment did induce strong upward movement of the petiole, with a maximum increase in angle similar to that of Col-0 given ethylene or low light (Figs. 2, 4, and 6). These results show that Ler does contain signal transduction components that can lead to hyponastic growth, but that ethylene on its own is unable to switch on this cascade. The ethylene-induced hyponastic growth is not saturated in Ler because the endogenous ethylene production is lower compared to Col-0, and 1-MCP pretreatment has no influence on the petiole angle in air. Furthermore, to examine whether an elevated concentration of ethylene is sensed in the petioles of Ler and Col-0, we tested whether an ethylene-responsive gene was up-regulated in petioles after ethylene addition. The expression of ERS2 correlates positively with ethylene levels (Vriezen et al., 1997; Hua et al., 1998). In our experimental conditions, measured with real-time RT-PCR, ERS2 was up-regulated after 3 h of ethylene addition in both Col-0 (5 times) and, in particular, Ler (50 times). We therefore conclude that both Col-0 and Ler petioles perceive the ethylene applied. These results show that the lack of ethylene-induced hyponastic growth in Ler is caused by the inability of ethylene signaling to interact with downstream components of hyponastic growth rather than by a putative, reduced sensitivity to ethylene.
CONCLUSION
There was variation in the effect of ethylene on petiole and leaf blade elongation of a number of Arabidopsis accessions. Ethylene stimulated petiole elongation in Ws-2, whereas in the other accessions the hormone either inhibited petiole and leaf blade growth or there was no effect. Considerable variation in ethylene-stimulated hyponastic growth existed among the accessions studied. For example, Col-0 shows a strong and rapid increase in petiole angle upon ethylene treatment, while little effect was seen in Ler. In both these accessions, the extent of the hyponastic response does not depend on the initial angle. In contrast to ethylene, spectrally neutral shading induced hyponastic growth in both accessions. In addition, ethylene could induce the expression of an ethylene marker gene in both Col-0 and Ler. Taken together, we conclude that Ler possesses downstream signal transduction components required for hyponastic growth, but ethylene is unable to switch on this cascade in this accession.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Nine Arabidopsis (Arabidopsis thaliana) accessions were used: Be-0 (N964); Col-0 (N1092); Cvi (N902); Kas (N903); Ler (NW20); Nd-1 (N1636); Rld-1 (N913); Sha (N929); and Ws-2 (N1602). NASC accession numbers are shown in parentheses.
Seeds were sown on moistened filter paper in sealed petri dishes and cold stratified in the dark at 4°C for 4 d. Subsequently, the seeds were germinated for 4 d in a growth chamber with the following conditions: 20°C, 70% (v/v) relative humidity, 9-h photoperiod (200 μmol m−2 s−1 photosynthetically activated radiation photon flux density). Seedlings were transferred with a brush to pots (70 mL) containing a mixture of potting soil and perlite (1:2; v:v) enriched with 0.14 mg of MgOCaO (17%; Vitasol BV, Stolwijk, The Netherlands) and 0.14 mg of slow-release fertilizer (Osmocote Plus Mini; Scotts Europe, Heerlen, The Netherlands) per pot. Prior to seedling transfer, each pot was saturated with 20 mL nutrient solution containing 2.6 mm KNO3, 2.0 mm Ca[NO3]2, 0.6 mm KH2PO4, 0.9 mm MgSO4, 6.6 mm MnSO4, 2.8 mm ZnSO4, 0.5 mm CuSO4, 66 mm H3BO3, 0.8 mm Na2MoO4, and 134 mm Fe-EDTA, pH 5.8. All chemicals were pro analyze grade and obtained from Merck (Darmstadt, Germany). Following transplantation, plants were grown for 28 d in a growth chamber (conditions as described above). Pots with seedlings were kept in a glass-covered tray for the first 4 d following transplantation, after which they were transferred to irrigation mats (Maasmond-Westland, Utrecht, The Netherlands). The mats were automatically watered with tap water to saturation once a day (at the beginning of the light period), and the excess water was drained. Except where stated otherwise, petioles of 36-d-old plants (from sowing) were used. We examined the hyponastic response and elongation of one petiole on each plant. Table II shows for each accession the number of leaves on the plants at the time of study.
Table II.
Shoot characteristics of 36-d-old plants of 9 accessions of Arabidopsis at the time of the experiment
Min-Max represents the leaf number of the youngest and oldest measured petiole. Leaves were counted from the oldest leaf up to a leaf size of approximately 0.5 cm. Data are means of 16 replicate plants with se in parentheses.
| Accession | Total No. Leaves | Measured Leaf No. | Min-Max |
|---|---|---|---|
| Col-0 | 16.8 (1.1) | 11.3 (1.8) | 8–13 |
| Ws-2 | 16.1 (0.6) | 8.3 (1.4) | 6–11 |
| Be-0 | 20.3 (1.9) | 12.1 (1.1) | 10–13 |
| Rld-1 | 19.3 (1.4) | 10.1 (1.0) | 9–12 |
| Kas | 20.3 (2.4) | 10.9 (1.5) | 8–14 |
| Sha | 13.9 (1.2) | 9.1 (0.9) | 8–12 |
| Ler | 14.2 (1.8) | 8.5 (1.3) | 6–13 |
| Nd-1 | 15.1 (1.0) | 10.9 (1.0) | 9–10 |
| Cvi | 14.8 (3.0) | 8.4 (1.1) | 7–11 |
Computerized Digital Camera System and Image Analysis
To measure changes in petiole angle and length, a custom-built computerized digital camera system was used as described in Cox et al. (2003). To enable continuous photography, no dark period was included in the 24-h experimental period. On the day before the experiment, plants were placed in individual glass cuvettes (13.5 × 16.0 × 29.0 cm) with the petiole of study positioned perpendicular to the axis of the camera. To facilitate measurement, any leaves that were obscuring the petiole being photographed were removed. Additionally, the petiole was marked at the petiole-lamina junction with drawing ink. The number of leaves per plant and the leaf number of the marked petiole were recorded (Table II). Counting started at the oldest leaf, with the youngest leaf being approximately 0.5 cm long. A calibration object with known dimensions was placed in the soil in the same plane as the measured petiole (shown in Fig. 1). All these preparations did not influence the response of the petiole to ethylene. Experiments started the following day, at 10 am, to minimize variation induced by circadian rhythms.
Digital photographs (1,280 × 1,000 pixels) were taken every 10 min. The angle and length of the petiole and the leaf blade were measured on these digital photographs using a PC-based image analysis system with a macro developed in house using the KS400 (version 3.0) software package (Carl Zeiss Vision, Jena, Germany). Petiole angle was taken as the angle between the horizontal and a line drawn between an ink mark at the petiole-lamina junction and a fixed base of the petiole that was determined using 10 random photographs (Fig. 1). Petiole and leaf blade length was measured along the adaxial surface.
Data Analysis
Although care was taken to select plants that were in a similar developmental stage, the angle and length of the petiole at the start of the experiment varied between replicate plants within any one accession. To allow unbiased comparisons between treated and untreated plants, the change in angle or length compared to t = 0 h was calculated for each replicate. As a consequence, control and treated plants are depicted as starting with an initial angle/length of 0. Additionally, we calculated the average initial angle or length for untreated and treated plants together. This average initial value was added to the change in petiole angle or length for each individual plant, resulting in plots where the control and the treated plants of one accession started at a similar angle/length. These individual plots were used to calculate averages and se. Further, to take into account the changes in angle of control plants during the course of the experiments, we also calculated the differential response (the difference between the angle of treated and control plants for each time point; see Fig. 4), and the average differential response (over the 24 h of the experiment).
The program SPSS 10 (SPSS Benelux, Gorinchem, The Netherlands) was used to compute an analysis of variance and to calculate Pearson (two-tailed) correlations.
Reproducibility of the Hyponastic Growth Measurements
For all accessions, two independently grown batches of plants (36 d old) were treated with ethylene. In all cases, except one, the average angle (calculated over the 24 h of the experiment for both air- and ethylene-grown plants) did not differ significantly between the two batches. The exception was a batch in which some plants were already bolting. The data from this batch were not included.
Plants were used for the experiments when 36 d old. However, differences in age did not affect the results because plants that were 33 or 39 d old showed very similar ethylene-induced hyponastic responses compared to 36-d-old plants (data not shown). The time of day when an experiment started also has little effect on petiole angle, at least when tested with ethylene: a 12-h delay in starting treatment having minimal affect. As shown in Table II, accessions differed in developmental stage when they were used in the experiments. This was a consequence of their different intrinsic rates of growth and forced us to compare hyponasty in plants with different number of leaves. To test whether this influenced our results, a correlation between the average angle and the total number of leaves, or the number of the measured leaf, was calculated. No significant correlation between these developmental parameters and the hyponastic response was found, indicating that upward leaf movement is independent of the developmental stage of the accession, within the limited range of leaf number that we tested.
To summarize, ethylene-induced hyponastic growth in Arabidopsis is a robust response that is independent of plant batch, plant age, starting time of the experiment, measured leaf number, and total number of leaves per plant.
Experiments
Ethylene (100 μL L−1; Hoek Loos, Amsterdam) and air (70% relative humidity) were mixed using flow meters (Brooks Instruments, Veenendaal, The Netherlands) to generate a concentration of 5 μL L−1 ethylene, which was flushed continuously through glass cuvettes (13.5 × 16.0 × 29.0 cm) at 75 L h−1 and then vented to the outside of the building. This concentration saturates ethylene-induced hyponasty in Rumex palustris (Voesenek and Blom, 1989), Arabidopsis (R. Pierik, unpublished data), and tobacco (Nicotiana tabacum; Pierik et al., 2004), and represents the endogenous ethylene concentration during submergence (Voesenek and Blom, 1989). A concentration of 1 μL L−1 ethylene was reached in the cuvettes after approximately 10 min of starting treatment, and 5 μL L−1 was reached after 40 min. The ethylene concentration was checked regularly on a gas chromatograph (GC955; Synspec, Groningen, The Netherlands) and remained constant for the duration of the experiment. Control cuvettes were flushed with air (70% relative humidity) at the same flow rate.
The initial petiole angle with respect to the horizontal was increased or decreased by tilting the pot at the start of the experiment. The glass cuvettes were fitted with a metal ring holding each pot. This ring could be tilted to achieve the desired inclination without interfering with the free movement of the petiole.
To achieve complete plant submergence, the cuvette containing the plant was gently filled with tap water (20°C) to 10 cm above the soil surface. Ethylene receptors were blocked by pretreatment with 1-MCP; the gaseous 1-MCP being released from EthylBloc (Floralife, Walterboro, SC), a preparation containing 0.14% 1-MCP. To release 1-MCP, EthylBloc was first dissolved in water in an airtight container at 40°C for 12 min. 1-MCP gas was then collected from the headspace with a syringe and injected into an airtight cuvette (for 1 μL L−1, 1.6 g EthylBloc/m3). Plants were pretreated with 1 μL L−1 1-MCP for 3 h, after which the cuvettes were opened and submergence, ethylene, or a control air treatment was applied.
For the shading treatment, the light quantity was reduced by 90% to 15 to 20 μmol m−2 s−1 (photon flux density) at the start of the experiment. This was achieved by switching off a number of lamps in the growth chamber and by using a black spectrally neutral shade cloth. These alterations did not change the light quality when checked with a LI-COR 1800 spectroradiometer (LI-COR, Lincoln, NE; data not shown).
Real-Time RT-PCR
For one RNA sample, eight petioles of two plants were mixed and ground. Samples were taken after 3 h of ethylene treatment or from plants in air for 3 h. Total RNA was isolated from Arabidopsis petioles using the RNeasy Plant Mini kit (Qiagen, Leusden, The Netherlands). Genomic DNA was removed using the DNA-Free kit (Ambion, Cambridgeshire, UK). cDNA was synthesized using 3.3 μg of total RNA with SuperScript III RNase H− reverse transcriptase (Invitrogen, Breda, The Netherlands) using random-hexamer primers. Real-time PCR reactions were performed on a MyiQ Single-Color Real-Time PCR detection system and software using iQ SYBR Green Supermix fluorescein (Bio-Rad Laboratories, Veenendaal, The Netherlands).
For AtERS2 (At1g04310 and AtL8s15088), the following primers were used: 5′-ACGCTTGCCAAAACATTGTA-3′ and 5′-TGAGACGCTTTTCACCAAAC-3′, which gave a single product of 83 bp on cDNA. Real-time PCR was conducted (12.5 μL of SYBR Green Supermix fluorescein, 1 μL from each primer [100 pmol], 1 μL of cDNA, 9.5 μL of water) for 40 cycles with the following temperatures: 30-s 95°C denaturation, 30-s 60°C annealing, and 60-s 72°C extension. Melt curves showed single products for all samples. Relative mRNA values were calculated using the comparative threshold cycle method described by Livak and Schmittgen (2001), expressing mRNA values relative to actin (At5g09810) that are constitutively expressed in vegetative structures and are not influenced by the different treatments. For Col-0, the following primers have been used: 5′-TTCGTGGTGGTGAGTTTGTT-3′ and 5′-GCATCATCACAAGCATCCTAA-3′ (195 bp); for Ler, 5′-GGAGCTGAGAGATTCCGTTG-3′ and 5′-GGTGCAACCACCTT GATCTT-3′ (245 bp). Primer efficiency for both control primers was tested by running a dilution series of amplified cDNA fragments with the same PCR protocol as described above. All threshold cycle values were obtained from PCR reactions with efficiencies close to 2 (data not shown). Gene expression was calculated according to Livak and Schmittgen (2001).
ACC Oxidase Capacity
The ACC oxidase capacity was measured according to Ververidis and John (1991) with minor modifications. About 0.3 g of whole rosette tissue were ground to a fine powder (in liquid nitrogen). The powder was thawed on ice and 0.9 mL of ice-cold extraction buffer (0.3 m Tris-HCl, pH 7.2, 10% glycerol, and 30 mm Na-ascorbate) was added. Samples were vortexed and centrifuged at 20,000g for 20 min at 4°C. In a septum vial was added 1.7 mL of incubation buffer (0.1 m Tris-HCl, pH 7.2, 10% glycerol, and 30 mm Na-ascorbate), 50 μL of ACC (80 mm), 50 μL of FeSO4 (3 mm), 100 μL of NaHCO3 (1 m), and the mixture was complete with 200 μL of supernatant, after which the vials were sealed with septum and cap. The mixture was shaken in a water bath at 30°C for 60 min. After vortexing, a gas sample of the headspace was analyzed on a gas chromatograph (GC955; Synspec) to measure the ethylene concentration.
Ethylene Production
Whole rosettes of about 300 mg were placed in a syringe with a volume of 1.5 mL. Ethylene was allowed to accumulate in the syringe for 15 min, after which the air was analyzed on a gas chromatograph (GC955; Synspec). After harvesting Col-0 rosettes in the climate room, the ethylene production was constant for the first 25 min. After 25 min, the ethylene release increased more than 5-fold, suggesting that wounding-induced ethylene production takes place after 25 min.
Acknowledgments
We thank M. Terlou (Utrecht University) for developing the image analysis macro in KS400 for analysis of the photographs, M.B. Jackson for thoroughly reading the draft of this article, and Martijn van Zanten for obtaining the real-time RT-PCR data.
This work was supported by the Dutch Science Foundation (PIONIER grant no. 800–84–470).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.053967.
References
- Ahmad M, Jarillo JA, Smirnova O, Cashmore AR (1998) Cryptochrome blue-light photoreceptors of Arabidopsis implicated in phototropism. Nature 392: 720–723 [DOI] [PubMed] [Google Scholar]
- Alonso-Blanco C, Bentsink L, Hanhart CJ, Blankestijn-de Vries H, Koornneef M (2003) Analysis of natural allelic variation at seed dormancy loci of Arabidopsis thaliana. Genetics 164: 711–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteca JM, Arteca RN (2001) Brassinosteroid-induced exaggerated growth in hydroponically grown Arabidopsis plants. Physiol Plant 112: 104–112 [DOI] [PubMed] [Google Scholar]
- Ball NG (1969) Nastic responses. In MB Wilkins, ed, The Physiology of Plant Growth and Development. McGraw-Hill, London, pp 277–300
- Ballaré CL (1999) Keeping up with the neighbours: phytochrome sensing and other signaling mechanisms. Trends Plant Sci 4: 97–102 [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Scopel AL, Radosevich SR, Kendrik RE (1992) Phytochrome-mediated phototropism in de-etiolated seedlings. Plant Physiol 100: 170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga M, Blom CWPM, Voesenek LACJ (1996) Sensitivity to ethylene: the key factor in submergence-induced shoot elongation of Rumex. Plant Cell Environ 19: 1423–1430 [Google Scholar]
- Bleecker AB (1997) The ethylene binding site of the ETR1 protein. In A Kanellis, A Chang, H Kende, D Grierson, eds, Biology and Biotechnology of the Plant Hormone Ethylene. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 63–70
- Botto JF, Smith H (2002) Differential genetic variation in adaptive strategies to a common environmental signal in Arabidopsis accessions: phytochrome-mediated shade avoidance. Plant Cell Environ 25: 53–63 [Google Scholar]
- Clúa A, Bottini R, Brocchi GN, Bogino J, Luna V, Montaldi ER (1996) Growth habit of Lotus tenuis shoots and the influence of photosynthetic photon flux density, sucrose and endogenous levels of gibberellins A1 and A3. Physiol Plant 98: 381–388 [Google Scholar]
- Cox MCH (2004) Plant movement: kinetics and hormonal regulation of hyponastic growth and stimulated petiole elongation. PhD thesis, University of Nijmegen, Nijmegen, The Netherlands
- Cox MCH, Millenaar FF, Van Berkel YE, Peeters AJ, Voesenek LA (2003) Plant movement. Submergence-induced petiole elongation in Rumex palustris depends on hyponastic growth. Plant Physiol 132: 282–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digby J, Firn RD (1995) The gravitropic set-point angle (GSA): the identification of an important developmentally controlled variable governing plant architecture. Plant Cell Environ 18: 1434–1440 [DOI] [PubMed] [Google Scholar]
- Emery RJN, Reid DM, Chinnappa CC (1994) Phenotypic plasticity of stem elongation in two ecotypes of Stellaria longipes: the role of ethylene and response to wind. Plant Cell Environ 17: 691–700 [Google Scholar]
- English PJ, Lycett GW, Roberts JA, Jackson MB (1995) Increased 1-aminocyclopropane-1-carboxylic acid oxidase activity in shoots of flooded tomato plants raises ethylene production to physiological active levels. Plant Physiol 109: 1435–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorani F, Bögemann GM, Visser EJW, Lambers H, Voesenek LACJ (2002) Ethylene emission and responsiveness to applied ethylene vary among Poa species that inherently differ in leaf elongation rates. Plant Physiol 129: 1382–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firn RD, Digby J (1980) The establishment of tropic curvatures in plants. Annu Rev Plant Physiol 31: 131–148 [Google Scholar]
- Friml J, Wisniewska J, Benková E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Fu QA, Ehleringer JR (1989) Heliotropic leaf movement in common beans controlled by air temperature. Plant Physiol 91: 1162–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier H, Varlet-Grancher C, Baudry N (1997) Effects of blue light on the vertical colonization of space by white clover and their consequences for dry matter distribution. Ann Bot (Lond) 80: 665–671 [Google Scholar]
- Grichko VP, Glick BR (2001. a) Flooding tolerance of transgenic tomato plants expressing the bacterial enzyme ACC deaminase controlled by the 35S, rolD or PRB-1b promotor. Plant Physiol Biochem 39: 19–25 [Google Scholar]
- Grichko VP, Glick BR (2001. b) Amelioration of flooding stress by ACC deaminase-containing plant growth-promoting bacteria. Plant Physiol Biochem 39: 11–17 [Google Scholar]
- Grimoldi AA, Insausti P, Roitman GG, Soriano A (1999) Response to flooding intensity in Leontodon taraxacoides. New Phytol 141: 119–128 [Google Scholar]
- Hall AE, Findell JL, Schaller GE, Sisler EC, Bleecker AB (2000) Ethylene perception by the ERS1 protein in Arabidopsis. Plant Physiol 123: 1449–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangarter RP (1997) Gravity, light and plant form. Plant Cell Environ 20: 796–800 [DOI] [PubMed] [Google Scholar]
- Hansen DJ, Bendixen LE (1974) Ethylene-induced tropism of Trifolium fragiferum L. stolons. Plant Physiol 53: 80–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, Meyerowitz EM (1998) EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell 10: 1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Campbell DJ (1975) Ethylene and waterlogging effect in tomato. Ann Appl Biol 81: 102–105 [Google Scholar]
- Kang BG (1979) Epinasty. In W Haupt, ME Feinleib, eds, Encyclopedia of Plant Physiology, New Series, Vol 7, Physiology of Movements. Springer-Verlag, Berlin, pp 647–667
- Knoester M, van Loon LC, van den Heuvel J, Henning J, Bol JF (1998) Ethylene-insensitive tobacco lacks non-host resistance against soil-borne fungi. Proc Natl Acad Sci USA 95: 1933–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-rime quantitative PCR and the 2-ΔΔCT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Peeters AJM, Cox MCH, Benschop JJ, Vreeburg RAM, Bou J, Voesenek LACJ (2002) Submergence research using Rumex palustris as a model: looking back and going forward. J Exp Bot 53: 391–398 [DOI] [PubMed] [Google Scholar]
- Pierik R, Visser EJW, De Kroon H, Voesenek LACJ (2003) Ethylene is required in tobacco to successfully compete with proximate neighbours. Plant Cell Environ 26: 1229–1234 [Google Scholar]
- Pierik R, Whitelam GC, Voesenek LACJ, De Kroon H, Visser EJW (2004) Canopy studies on ethylene-insensitive tobacco identify ethylene as a novel element in blue light and plant-plant signaling. Plant J 38: 310–319 [DOI] [PubMed] [Google Scholar]
- Ridge I (1987) Ethylene and growth control in amphibious species. In RMM Crawford, ed, Plant Life in Aquatic and Amphibious Habitats. Blackwell Scientific Publications, Oxford, pp 53–76
- Smalle J, Haegman M, Kurepa J, Van Montagu M, Van Der Straeten D (1997) Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA 94: 2756–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, Van Der Straeten D (1997) Ethylene and vegetative development. Physiol Plant 100: 593–605 [Google Scholar]
- Smith H, Whitelam GC (1997) The shade avoidance syndrome: multiple responses mediated by multiple phytochromes. Plant Cell Environ 20: 840–844 [Google Scholar]
- Vandenbussche F, Vriezen WH, Smalle J, Laarhoven LJJ, Harren FJM, Van Der Straeten D (2003) Ethylene and auxin control the Arabidopsis response to decreased light intensity. Plant Physiol 133: 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ververidis P, John P (1991) Complete recovery in vitro of ethylene-forming enzyme activity. Phytochemistry 30: 725–727 [Google Scholar]
- Voesenek LACJ, Benschop JJ, Bou J, Cox MCH, Groeneveld HW, Millenaar FF, Vreeburg RAM, Peeters AJM (2003) Interaction between plant hormones regulates submergence-induced shoot elongation in the flooding-tolerant dicot Rumex palustris. Ann Bot (Lond) 91: 205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Blom CWPM (1989) Growth responses of Rumex species in relation to submergence and ethylene. Plant Cell Environ 12: 433–439 [Google Scholar]
- Voesenek LACJ, Blom CWPM (1999) Stimulated shoot elongation: a mechanism of semiaquatic plants to avoid submergence stress. In HR Lerner, ed, Plant Responses to Environmental Stresses: From Phytohormones to Genome Reorganization. Marcel Dekker, New York, pp 431–448
- Vriezen WH, Hulzink R, Mariani C, Voesenek LACJ (1999) 1-Aminocyclopropane-1-carboxylate oxidase activity limits ethylene biosynthesis in Rumex palustris during submergence. Plant Physiol 121: 189–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriezen WH, van Rijn CPE, Voesenek LACJ, Mariani C (1997) A homolog of the Arabidopsis thaliana ERS gene is actively regulated in Rumex palustris upon flooding. Plant J 11: 1265–1271 [DOI] [PubMed] [Google Scholar]
- Yu F, Berg VS (1994) Control of paraheliotropism in two Phaseolus species. Plant Physiol 106: 1567–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv M, Koller D, Halevy AH (1976) Ethylene and the geotropic response of lateral branches in peanuts (Arachis hypogaea L.). Plant Cell Physiol 17: 333–339 [Google Scholar]