Abstract
From boon molecules to molecules contributing to rising concern has been the sojourn of antibiotics. The problem of antibiotic contamination has gotten worse due to antibiotics’ pervasive use in every aspect of the environment. One such consequence of pollution is the increase in infections with antibiotic resistance. All known antimicrobials being used for human benefit lead to their repetitive and routine release into the environment. The misuse of antibiotics has aggravated the situation to a level that we are short of antibiotics to treat infections as organisms have developed resistance against them. Overconsumption is not just limited to human health care, but also occurs in other areas such as aquaculture, livestock, and veterinary applications for the purpose of improving feed and meat products. Due to their harmful effects on non-target species, the trace level of antibiotics in the aquatic ecosystem presents a significant problem. Since the introduction of antibiotics into the environment is more than their removal, they have been given the status of persistent pollutants. The buildup of antibiotics in the environment threatens aquatic life and may lead to bacterial strains developing resistance. As newer organisms are becoming resistant, there exists a shortage of antibiotics to treat infections. This has presented a very critical problem for the health-care community. Another rising concern is that the development of newer drug molecules as antibiotics is minimal. This review article critically explains the cause and nature of the pollution and the effects of this emerging trend. Also, in the latter sections, why we need newer antibiotics is questioned and discussed.
Keywords: Antibiotic pollution, Antibiotic resistance, Overconsumption, Antibiotic-resistant genes, Active pharmaceutical ingredients, Dwindling development
Introduction
Antibiotics were once considered a boon for saving the lives of not just humans, but also animals. The discovery of antibiotics is regarded as one of the most significant scientific achievements of the twentieth century, revolutionizing both human and veterinary medicine. From penicillin to third- generation last resort antibiotics, we have come a long way in the development of antibiotics, not only in terms of the health-care sector in the treatment of bacterial infections, but also there has been a lot of resistance development by bacterial pathogens t toward antibiotics. Antibiotics have been recently recognized as an emerging class of environmental contaminants since they have been massively administered in humans and animals and persist in the environment through a complex vicious cycle of biotransformation and bioaccumulation. Presence of high concentrations of different active forms of antibiotics and their metabolites have been shown in studies conducted by Kumar and Schweizer (2005), Maia et al. (2009), Marsoni et al. (2014) and Sodhi et al. (2021). The diffusion of antibiotics in the environment, particularly in natural water systems, contributes to the development and global dissemination of antibiotic resistance. There have been cases where certain micro-organisms were found in the environment or in clinical isolates, which showed resistance to even the last resort antibiotics (Li et al. 2019). Antibiotics have reached agricultural land using contaminated livestock waste in manure. The soil exhibits an inherent buffering capacity on antibiotics, ARGs (antibiotic resistance genes), and ARBs (antibiotic-resistant bacteria). This effect is attributed to existing microbiota that degrades antibiotics. The underlying mechanism of such resistance is not fully understood. This problem has arisen in the last two decades; especially from the years 2011 to 2020, there have been lots of clinical infections caused by antibiotic-resistant organisms, for example multidrug-resistant tuberculosis. This is one of the most important challenges to the health-care sector in the twenty-first century; correlating this subject to antibiotic consumption and their dynamic behavior in the environment, the acquired insights provide an improved understanding of pollution by antibiotics.
Due to links between the emergence and quick spread of antibiotic resistance and their overall consumption and prevalence in the environment, antibiotics have drawn particular attention among pharmaceuticals since the late 1990s, because substantial amounts (30% to up to 90%) of antibiotics administered to humans and animals are excreted into the waste stream via urine and feces, unmetabolized. Municipal, agricultural, and industrial wastewater are the major sources of antibiotics in the environment. Conventional wastewater and recycling water treatments are only partially effective in their removal. As a result, depending on their mobility and persistence in the soil–water environment, antibiotics and their by-products may reach surface waters, groundwaters, and potentially drinking water. Depending on the aqueous environment matrices, the detected concentrations range from ngL−1 to gL−1. The current situation necessitates determining antibiotic contamination levels along with their by-products, which will improve the knowledge of source, pathways, fate, transport, and effects on the environment.
One of the major setback to this situation of antibiotic resistance is that the development of newer antibiotics is dwindling. Since the year 2003 after Salinosporamide, no new antibiotic class has come up which could effectively treat the resistant infections. Even the ones which are in the final stages of clinical trials are failing (Hay et al. 2014). This review article aims to address the current situation of antibiotic resistance across various domains of environment. How has the abuse of antibiotic over a small span of time led us to this grave situation where we are short of medicines to treat certain infectious diseases? This question raises concerns over consumption of antibiotic drugs across nations. The current scenario of antibiotic development is also discussed in the latter section of the manuscript.
Emerging pollution
Emerging pollutants or contaminants of emerging concern are the chemical entities capable of potentially affecting different ecosystems, which shows their detectable presence in different environmental matrices. There are different contaminants which are classified as emerging pollutants, but the compounds of major concern are majorly antibiotics and other endocrine disrupting chemicals (EDCs). Antibiotics are found in various matrices of the environment in exceedingly high concentrations far more than the recommended values. This is alarming as the concentration of antibiotics plays an active role in the selection of resistant populations. The gradual increase in the use of antibiotics over time has resulted in the development of various environmental and health concerns, which are alarming to many nations, especially developing countries like BRICS nations. The misuse and overutilization of antibiotics have led to the certain complications including antibiotic pollution, development of ARB, and presence of ARGs in the environmental streams. All these complications lead to heavy pressure on the health-care system which are:
Difficult to treat pathogenic microbial infections,
Increased mortality rate.
The above-mentioned health issues complement each other in that the increase in the level or intensity of environmental complications leads to an increase in health issues. One of the major problems is the presence of antibiotics in sub-detection limits, which presents a weak selective pressure over the microbial population. This in turn favored the selective growth of certain lineages, hence conferring an increase in genetic diversity (Lee et al. 2018). The minimal selective concentration (MSC), which is often predicted to vary between 1/4 and 1/230 of the minimum inhibitory concentration (MIC) values, is the lowest antibiotic level that promotes the selection of a resistant mutant over the wild-type cells (Bengtsson-Palme and Larsson 2016). Such concentrations are present widely across various environmental domains.
Consumption pattern
One of the recent exhaustive analyses of human antibiotic consumption estimated that between 2000 and 2015, antibiotic consumption, expressed in defined daily doses (DDD), increased by 65%, with a particularly rapid increase in the use of the last resort antibiotics such as glycyclines, oxazolidinones, carbapenems, and polymyxins. We could expect an increase of up to 200% by 2030 if no policy changes are made.
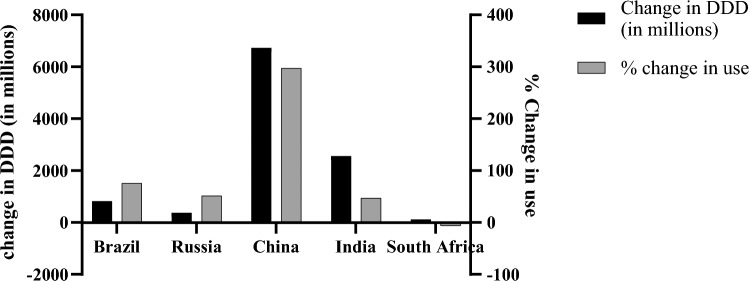
In India alone, the DDD of antibiotics was 4950 per thousand population in 2015. There has been a significant increase in the consumption of antibiotics in BRICS countries from the year 2000 to 2010, of which the top three are South Africa (219%), Brazil (68%), and India (66%) (CDDEP 2015). The updated data of consumption of antibiotics in BRICS countries is given in Fig. 1 from the year 2010 to 2020 (CDDEP, 2021). From the year 2000 to 2015, the cumulative increase is a whopping 77% consumption rate, which is quite alarming (Klein et al. 2018a). If raw numbers are taken into consideration, India, China, and the USA top the list of highest antibiotic consumers across the globe. A comparison of India and USA shows that quinolones are among the top prescribed antibiotics accounting for 34% of the total prescribed antibiotics in India, also tetracycline is the preferred choice of antibiotics by clinicians (71%) (Bombaywala et al. 2021). The scale and use of non-prescription antibiotics in LMICs are unknown, as bypassing the prescription for such antimicrobials is common in these nations. Prescription of antibiotics peaks during the winter season, which is largely related to influenza occurrences. A recent study conducted by Klein et al. (2018a) indicates the decreased use of antibiotics (6.5% reduction) as a result of increase (10% rise) in influenza vaccines. The COVID-19 pandemic has also encouraged the empirical use of antibiotics among patients. This can be attributed partially to misinformation about antibiotics’ benefits (Hsu 2020; Arshad et al. 2020). A data published in 2021 indicates an antibiotic administration in 71% of the patients in New York despite 3.6% coinfection cases (Nori et al. 2021).
Fig. 1.
Consumption pattern of antibiotics in BRICS nations (2010–2020), (CDDEP, 2021), of which China tops change in DDD and % change in use
Non-therapeutic use accounts for more than 70% of antibiotic use in veterinary and animal husbandry (Roe and Pillai 2003). Most antibiotics are used in feed to prevent diseases and as growth promoters for the sole purpose of meeting the demands in the dairy and meat industry across the globe. Many antibiotics are used as therapeutics for human medicine and veterinary application (Kumar et al. 2019). Some of the common examples include tetracycline, oxytetracycline, penicillin G, sulfamethazine, etc. (García-Fernández et al. 2018). Some others, such as monensin (as a growth promoter and feed enhancer for heifers) (Baile et al. 1982) and virginiamycin (as growth promoter) are exclusively designed for animal rearing purposes. China tops the usage of antibiotics in 2010 for livestock which is no less than 15,000 tons (CDDEP 2015) and in absolute terms is 318 mg/PCU (a Kg of animal product) (Klein et al. 2020). The Asia Pacific region accounts for 93% of the global antimicrobial consumption in aquaculture, of which China contributes 57.9% making it the largest consumer of antimicrobials in the aquaculture sector also. India accounts for a total of 3% of global antibiotic consumption in animals, whereas in 2010, livestock consumption of antibiotics was 63,151 tons and is predicted to rise by 67% by 2030 (Van Boeckel et al. 2015a). As per Klein et al. (2018) the global consumption of antibiotics for food animals was 131,109 tons in 2013, but if such a trend continues it is projected to rise to 200,235 tons by 2030.
It has been observed that unnecessary antibiotic use is related to patients, practitioners, and health-care systems issues (e.g., expectation that a physician visit will lead to antibiotic prescription, poor knowledge of microbiology, underuse of available guidelines, lack of availability of local guidelines, or lack of access to diagnostic tools, etc.). Also, numerous social and cultural factors drive the misuse of antibiotics and influence attitudes toward prescribing and use of antibiotics. However, improved hygiene, sanitation, vaccination, and access to diagnostic tools remain the main measures that should be implemented for responsible and rational use of antibiotics. Other necessary measures are required to limit the use of broad-spectrum antibiotics and critically important antibiotics, and to prohibit the discharge of antibiotic waste into the environment.
Contribution of pharmaceutical industries to antibiotic pollution
The pharmaceutical industry is one of the leading suppliers and consumers of antibiotics and its precursors, respectively. As the demand for antibiotics has ramped up due to better accessibility options, there has been an increase in the industry's pharmaceutical effluent as a result of the expansion in antibiotic production capability.
There are two types of pharmaceutical industries:
Manufacturing units that produce active pharmaceutical ingredients (APIs).
Units that produce finished pharmaceutical products (FPPs) which are products of APIs.
There are antibiotic residues in the effluents produced by both API and FPP. The effluent from the API-producing plants is still anticipated to have much greater levels of residues. The concentration of API and intermediates in the effluent from API production facilities makes these facilities hotspots for environmental pollution and the development of AMR (antimicrobial resistance). India and China have been the primary suppliers of APIs for the pharmaceutical industry during the past three decades. India has positioned itself as the center for processing the APIs into final pharmaceutical goods or formulations, despite China being the primary provider of APIs (Arnum 2013). The concentration of API and intermediates in the effluent from pharmaceutical production facilities, particularly API manufacturing plants, makes these facilities hotspots for environmental pollution and the development of AMR. By the end 2023, the Asia Pacific area was projected to have the second-largest pharmaceutical market (24.07%) (Gandra et al. 2017) and the graph is progressing toward such figures. There is enough gray literature across the web, where it is evidently shown that the industrially hit areas, especially villages, have higher health issues, few of them are cancer, miscarriages, and death of the livestock. The lack of concrete evidence is giving a lot of leverage to pharmaceutical industries with regard to evading law enforcement.
Clause in Environment (Protection) Rules 1986 under Indian Constitution does not address the problem of AMR/antibiotics flushing into the environmental waters. Standards for pharmaceutical industries include parameters such as BOD, COD, suspended solid, grease, and oil, with no mention about the bacterial load or antibiotic concentration. To curb this issue, The Indian Ministry of Environment, Forest, and Climate Change (MoEF&CC) laid down certain stringent measures and standards on 23 June 2020, to curtail the antibiotic pollution in the water bodies, especially downstream. This step was the first and one of its kind across the globe. It was taken in response to increasing antibiotic resistance. Under this Environmental (Protection) amendment rules, 2019, the ministry has set limits on 121 antibiotics in the treated wastewater discharge. The draft notification was taken back 7 months later where all effluent was regarded as ‘hazardous waste’.
Pharmaceutical effluent contains a mixture of antibiotics and ARGs found in the environment in huge amounts (Larsson and Flach 2021). Various studies have been conducted to confirm the presence of antibiotic residues in the treated wastewater of pharmaceutical industries. Research on quantifying ARGs in pharmaceutical WWTPs (wastewater treatment plants) predicted that 1012–1014 ARG copies might be released daily (Guo et al. 2018). It was demonstrated that the multidrug-resistant (MDR) bacteria were selected for and amplified due to the strong antibiotic selection pressure frequently present in pharmaceutical WWTPs. The majority (86%) of the bacteria discovered from the treatment facility at the Indian pharmaceutical WWTP, which collected waste from around 90 regional bulk medicine producers, were resistant to 20 or more antibiotics (Marathe et al. 2013). One of the main pharmaceutical hubs in India is the Baddi–Barota–Nalagarh industrial center, which is situated in the Solan district of Himachal Pradesh. Its 670 manufacturing facilities account for more than 35% of Asia's pharmaceutical output. The health of the waters of the Sirsa and Sutlej rivers near the pharmaceutical center was examined by The Veterans Forum for Transparency in Public Life. According to the investigation's results, the water sources were contaminated with antibiotic residue. Ciprofloxacin, an antibiotic used to treat bacterial infections, has a concentration of 296 μg/L, which is more than 1500 times the recommended limit. A similar study conducted by Kristiansson et al. (2011) have assessed water streams in Patancheruvu, Hyderabad, which receives input from 90 bulk drug manufacturers. The concentration of ciprofloxacin was found out to be 103 times more than the blood plasma of patients under treatment. Thai et al. (2018) has found out higher concentration of sulfonamides and quinolones in the effluent of pharmaceutical plants in Vietnam. In addition to the above, the studies conducted in Pakistan (Khan et al. 2013), Korea (Sim et al. 2011), and Taiwan (Lin and Tsai 2009) showed similar results. The above discussion points toward the non-compliance of these industries with good manufacturing practices.
In India, 130,000 instances of multidrug resistance to tuberculosis were found in 2018. Due to the overuse of antibiotics, the COVID-19 pandemic has exacerbated the AMR problem in the nation. If the current course is continued, it will cause 10 million fatalities globally by 2050. There are reports suggesting that wastewater from pharmaceutical companies in the,European Union (Cardoso et al. 2014), the USA (Phillips et al. 2010) and India (Larsson 2014) are not treated as the concentration of APIs have increased in the receiving waters. The found amount is usually of the order of mg/L, which is far more than MSC (minimum selective concentration). One solution to this rising problem of antibiotic pollution is efficient treatment of effluent from the pharmaceutical industries at source, which will limit the spread of antibiotics into the receiving waters.
Contribution of the aquaculture sector to antibiotic resistance and pollution
Food security has been a major issue these days with the rise in climatic variation, global warming, etc. The traditional farming system has moved from extensive to more intensive practices. These practices are causing a disbalance in the microenvironments. Intensive practices are more inclined toward production of produce comprising animal health and biosecurity (Bergeret 2017). This in turn increases the use of antimicrobials to combat illness.
Several studies support the fact that to sustain human and environmental health, shifting human diet toward fish and seafood consumption could be a solution (Froehlich et al. 2018). A cumulative 27% increase in the consumption of fish and sea food is expected by the end of 2030, which can be sustained by the aquaculture sector, pointing toward a 67% increase during the said period. To battle infections and illness that endanger productivity, however, fish farming significantly relies on the usage of antibiotics. As global temperature rises, more emerging infectious diseases are anticipated (Vezzulli et al. 2016), for example, streptococcosis, Edwardsiellosis and acute hepatopancreatic necrosis (Reverter et al. 2020). This scenario is directing to increase antimicrobial usage in coming years. This case is much worse in LMICs where intensified procedures are practiced meeting the increasing demand for food and flesh (Van Boeckel et al. 2015; Schar et al. 2018).
The cumulative effect of both anthropogenic contamination from land and use of antibiotics in aquaculture causes natural selection, spread, and emergence of resistant pathogens. A study conducted by Su et al. (2017) has shown that 14 ARGs were detected from intestinal tract samples of shrimp reared in a pond which was contaminated with resistant genes (Su et al. 2017). A similar study by Jang et al. (2018) also points toward the trend where 22 ARGs were found in the coastal aquacultural establishments in South Korea (Jang et al. 2018). The aquatic environment acts as a natural reservoir for antibiotic residues, and ARGs and ARBs in addition to another contribution from the aquaculture industry make this problem more aggravated.
Antibiotic resistance: a menance
Antimicrobial-resistant infections take the lives of 58,000 infants in India annually. There are a number of studies conducted across several parts of the globe deducing that antibiotic resistance bacteria bypass purification and breeding. As per the Centre for Disease Control (CDC) 2019 report, 2.8 million people got infected by antibiotic-resistant bacteria only in the USA and almost 35,000 die each year (2019). This number is far away from the expectation when it comes to more populous countries like India and China. On extrapolation, by 2050, the total death toll will reach 10 million by resistant infections (O’Neill 2016). On a rough estimate, multidrug-resistant tuberculosis kills 250,000 people across the globe. Similarly, 54,500 Vancomycin-resistant infections (VREs) were reported among hospitalized patients including 5400 deaths in the USA alone. In addition, there are many more infectious pathogens on the continuous move that are likely to develop resistance.
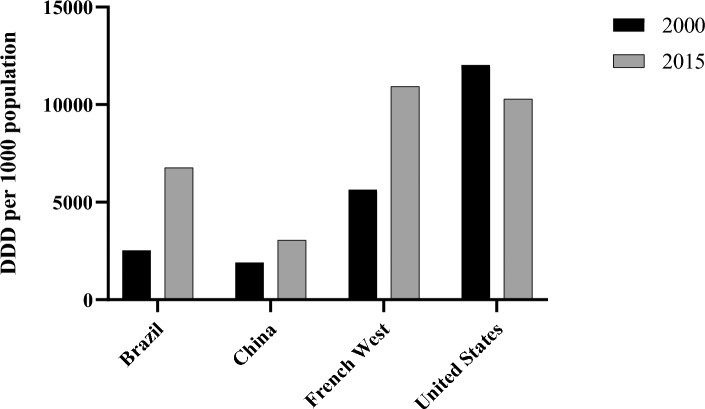
Pathogens causing malaria, typhoid fever, and HIV are now becoming increasingly resistant to first-line antimicrobials. This is threatening the global progress on health, especially in low- and middle-income countries (LMICs). The use of critically important antibiotics increased 91% worldwide and 165% in LMICs between 2000 and 2015. There is a higher DRI (drug resistance index) (Liu et al. 2016) value in LMICs. This value dictates low antibiotic effectiveness due to limited access of effective antibiotics. This is another issue why resistance poses an elevated danger in such nations. The comparison between LMICs and the USA of the rise in DDD (defined daily dose) per 100 population between the year 2000 and 2015 is shown in Fig. 2. Higher rates of occurrence of infectious diseases along with the population density have resulted in high antibiotic consumption rates and this puts a high selective pressure on the emergence of antibiotic-resistant bacteria. Out of the 140 largest pharmaceutical companies, only 12% are members of the Pharmaceutical Supply Chain Initiative. This initiative was set up to demand better environmental conditions in locations where pharmaceuticals are supplied. This situation indicates mismanagement and negligence toward environmental health.
Fig. 2.
DDD per 1000 population rise from 2000 to 2015 comparison between LMICs and the USA
Of the two leading causes for antibiotic resistance in India are sludge loaded with improperly treated antimicrobials being used in agro-applications due to the improper treatment of wastewater and misuse of antimicrobial drugs, due to the over-the-counter availability of all antibiotics from pharmacies. There are antibiotic-resistant organisms and their genes, which are commonly found in waterbodies in India. Being culturally and socio-economically diverse makes this problem more aggravated, and hence the containment of the resistance more difficult.
A major problem combating antibiotic resistance is the poor data regarding antibiotic pollution in different domains of the environment (Singer et al. 2016). No study talks exclusively of antibiotic pollution/contamination in different domains of the environment. Although there are various rules and limits laid down by the government to combat antibiotic pollution in receiving waters, lack of data and information poses a serious threat. India has emerged as a country with a maximum number of antimicrobial resistance cases. The scoping report on antimicrobial resistance in India 2017 by the collaboration of CDDEP and DBT India clearly explains the extent of antibiotic resistance in India.
Out of all broad-spectrum antibiotics, fluoroquinolones and cephalosporins are ineffective against most of Acinetobacter baumannii, Klebsiella pneumoniae, Escherichia coli, and nearly all of Pseudomonas aeruginosa. Similarly, variable rates of resistance were found against the last resorts carbapenem and colistin by similar organisms. Genes encoding carbapenem-resistant Gram-negative bacteria are ndm-1, oxa-48, kpc, vim and imp and colistin-resistant genes are mcr-1, mcr-2, mcr-3, mcr-4, mcr-5, and icr-Mo (plasmid-mediated genes) (Li et al. 2019). mcr-1 is also reported in K. pneumoniae and Enterobacter species. In poultry, the presence of extended-spectrum β-lactamase (ESBL) producing E. coli and various Salmonella species resistant to multiple antibiotics has been found (Naik et al. 2015). Bhattacharyya et al. (2016) found VRSA (vancomycin-resistant Staphylococcus aureus) in milk samples from cows with mastitis in a study conducted in 2016. ESBL+ Gram-negative bacteria were found in meat products, livestock and, in fewer cases, companion animals. Fecal samples from pigs were found contaminated with ESBLs producing E. coli (nearly half of Enterobacteriaceae isolates from Tapia fish were found to be ESBL producers (Marathe et al. 2016). Ampicilin-resistant Vibrio was isolated from shrimp, shellfish, and clamps, but they were sensitive to chloramphenicol (Sudha et al. 2014). Similar trends were seen in various environmental samples where hospital waste was found to be contaminated with third-generation cephalosporin-resistant E. coli (Akiba et al. 2015). In Cauvery, all the E. coli isolates were resistant to third-generation cephalosporin (Skariyachan et al. 2015). In River Yamuna, 17.4% of isolates were ESBL producers (Azam et al. 2016).
Mechanisms involved in the resistance which protects the bacteria from antibiotics are the following.
Preventing the entry of antimicrobials
Gram-negative bacteria possess the innate capability to resist certain molecules. The lipopolysaccharide layer is responsible for resistance to certain large antimicrobial entities (Blair et al. 2014). Hydrophilic antibiotics such as aminoglycosides, glycopeptides, colistin, and β-lactams have blocked access to the mycobacterial cell because of the presence of a lipid-rich outer membrane (Kumar and Schweizer 2005). Similarly, mycoplasma becomes intrinsically resistant to the glycopeptides and β-lactams, which attack the cell wall. During the development of antibiotic resistance, the cells regulate the thickness of their outer membrane or cell wall to resist the entry of antibiotic molecules. This mechanism was seen in vancomycin-resistant Staphylococcus aureus (Miller et al. 2014). Porin proteins also play a major role in selectively modulating the entry of antimicrobials via mutation for, e.g., Enterobacteriaceae aerogenes gain resistance against cephalosporins and imipenem and Neisseria gonorrhoeae against tetracycline and β-lactams (Thiolas et al. 2004). Sometimes, the cell intrinsically decreases the number of proin channels for, e.g., some members of Enterobacteriaceae become resistant to carbapenems.
The biofilm matrix contains thick sticky polysaccharide and proteins from the bacterial community, which makes it very difficult for the antibiotics to cross through. Also, the resident bacterial community consists of sessile bacteria which are resistant to antibiotics. Since the microbes are close to the biofilm, horizontal gene transfer becomes easy facilitating the transfer of resistance genes (Van Acker et al. 2014).
Modification of the antibiotic target
Gram-positive bacteria alter the structure and number of penicillin-binding transpeptidases (PBTs or PBPs) inhibiting the β-lactams from enteing the cell (Beceiro et al. 2013), for e.g., change in the structure of PBT is mediated by the acquisition of mecA gene in Staphylococcus aureus.
Changes in the charge of the cell membrane (via mutation in mprF gene) to positive inhibits the binding of calcium, thus inhibiting daptomycin from entering the cell (Yang et al. 2009).
Activation of van genes causes structural changes in the precursors of peptidoglycan inhibiting the binding of vancomycin (Beceiro et al. 2013).
Mutation in gyrA and grlA causes structural changes in the DNA gyrase and topoisomerase IV which protects them against the attack of fluoroquinolones (Redgrave et al. 2014).
Mutations in the genes encoding for various metabolically important enzymes changes the active site such that only the target drug is inhibited, but the metabolic machinery keeps functioning (Vedantam et al. 1998). Generally, the target enzyme’s substrates are structural analogs of the antibiotics, hence causing their inhibition, but selective mutation in the active site renders them more specific to their natural substrate.
Drug inactivation
Majorly, there are two classes of enzymes which cause antibiotic inactivation.
Hydrolases
-
A.
β-Lactamases
These enzymes fracture the β-lactam ring via hydrolysis of the amide bond. β-Lactamase superfamily (approx. 2000 member enzymes) consists of four subclasse,s i.e., A, B, C, and D, of which class B are metalloenzymes (Menard and Dondorp 2017).
Examples of class A β-lactamases are CTX-M, TEM, SHV, and KPC lactamases. The mutation in the genetic makeup of these enzymes increases the volume of enzymes and hydrolyzing capability; hence, they now can easily hydrolyze cephalosporins of second to fourth generations. Such mutants are called extended spectrum β-lactamase (ESBL) (Brown et al. 2010).
Class B metallo-β-lactamases (MBL) contain two Zn2+ in their active site facilitating the hydrolysis of nearly all β-lactam antibiotics except for monobactams. The new MBL variant NDM-type-1 carbapenemases with co-expressed serine β-lactamase majorly found in Enterobacteriaceae is resistant to all the existing β-lactams (Nordmann et al. 2011).
-
A
Esterases.
The esterases, although being involved in a number of resistance mechanisms, are less studied enzymes which provide resistance against macrolides. The enzyme cleaves the macrolactone ring by the Ere class of enzymes called erythromycin esterases. The gene encoding Ere enzymes are majorly found on the plamsids of pathogens excluding Ere D (Egorov et al. 2018).
Ere B, D can act upon 14-, 15-, and 16-membered macrolides with some exceptions where the hydrophobic group in the macrolide is absent, for e.g., tulathromycin and spiramycin (Egorov et al. 2018).
Transferases
There are several antibiotic-modifying enzymes for several antibiotics such as phenicols, streptogramins, rifamycins, lincosamides, and aminoglycosides.
Acetyl transferases modify both phenicols and streptogramins. Chloramphenicol acetyltransferase prevents the binding of chloramphenicol to ribosome via transferring the acetyl group to coenzyeme-A (Liu et al. 2020). Streptogramin antibiotics are classified into two groups: Group A binds to peptidyl transferase center and Group B binds to peptide exit tunnel. Virginiamycin acetyltransferase acetylates alcohol of streptogramin, resulting in conformational change which causes reduction in the activity of antibiotics (Li et al. 2020).
Nucleotidyltransferases modify lincosamides by the addition of the phosphate group. This enzyme is encoded by lnu genes (Feßler et al. 2018) which appears on mobile genetic elements and hence can be disseminated across community (Zhu et al. 2017).
Aminoglycosides can be modified by both the enzymes discussed in the above points. They modify the affinity of antibiotics to the drug target by modificaion of the amino/hydroxyl group (Szychowski et al. 2011). Both Gram-positive and Gram-negative bacteria possess aminoglycoside-modifying enzymes on mobile genetic elements. One prominent example is ApmA, inactivating apramycin (Bordeleau et al. 2021).
Drug efflux
There are multiple genes for efflux pumps that are chromosomally encoded in bacteria. They transport a wide range of chemicals, primarily hazardous molecules out from the cellular environment. The availability of carbon source affects the capacity of the pumps to impart resistance (Blair et al. 2014). Five types of transporters are described in Table 1:
Table 1.
Types of transporters involved in drug efflux
| Sr. no. | Type of transporter | Organism | Genes | Function | Energy source | Antibiotics | Other molecules | References |
|---|---|---|---|---|---|---|---|---|
| 1 | ABC transporter | Vibrio cholerae | VcaM | Uptake and efflux | ATP | Fluoroquinolones and tetracyclines | Drugs, ions, sugars, polysaccharides, proteins | Lubelski et al. (2007) |
| 2 | MATE transporter | Vibrio parahaemolyticus, Neisseria gonorrhoeae, N. meningitidis | NorM | Efflux | Na+ ions | Fluoroquinolones and aminoglycosides | Cationic dyes | Kuroda and Tsuchiya (2009) |
| 3 | SMR transporter | Staphylococcus epidermidis, E. coli | EmeR | Efflux | Proton-motive force H+ | β-Lactams, aminoglycosides | Lipophilic cations | Bay et al. (2008) |
| 4 | MFS transporter | Acinetobacter baumannii | SmvA | Efflux | Solute/cation (H+ or Na+) Symport or solute/H+ antiport | Erythromycin | Anions, metabolites and sugars | Collu and Cascella (2013) |
| CraA, CmlA | Chloramphenicol | |||||||
| MefB | Macrolide | |||||||
| E. coli | QepA | Fluoroquinolone | ||||||
| Fsr | Trimethoprim | |||||||
| Staphylococcus aureus | NorA | Fluoroquinolones | ||||||
| LmrS | Linezolid | |||||||
| 5 |
RND transporter, MDR transporter |
Pseudomonas aeruginosa | MecAB-OprM | Efflux (Tet pump- tetracyclines, Mef pump-macrolides) | Sub/H+ antiport | All antibiotics | Dyes, Detergents, Heavy metals, solvents | Deak et al. (2016) |
| E. coli | AcrAB-TolC |
Bacterial enzymes which confer antibiotic resistance are:
β-Llactamases (narrow spectrum)—argets first-generation cephalosporins and penicillins. For e.g., OXA type enzymes, SHV-1, TEM-1 and -2, and cephalosporinases.
ESBL (extended spectrum β-lactamases)—targets all generations of cephalosporins and penicillins. For e.g., SHV-2, 5, 7, 12; TEM-10, 12, 26; CTX-M; OXA-type ESBLs.
Carbapenemases—target all generations of cephalosporins, penicillins, and carbapenems. For e.g., KPC, NDM-1, VIM, and IMP carbapenemases, OXA-type carbapenemases.
Toxic effect of antibiotics on various life forms
Effect on plants
Entry of antibiotics into the agroecosystem occurs via farmyard manure, sewage sludge, digestates, or animal by-products. The contamination of manure and fertilizers occurs via animal urine and feces which contain 90% of the unmetabolized antibiotics. The use of animal waste as manure is 65,000 tons per year worldwide, which is contaminated with antibiotics and their metabolites (van Boeckel et al. 2015).
Many factors are responsible for the alteration of the physiological properties of antibiotics in soil and water, such as sorption, degradation, leaching, etc. (Carvalho and Santos 2016) The combination of a different class of antibiotics has aggravated toxicity upon the environment. Also, the presence of toxic pollutants such as heavy metals and organics has increased the cumulative toxicity of antibiotics. Tetracyclines and fluoroquinolones are quite stable in soil (Lillenberg et al. 2010) and sediments (Samuelsen et al. 1994), but they photodegrade quite rapidly in surface waters. The by-products as a result of photodegradation can be toxic (Guo and Chen 2012). Some common problems plants encounter include difficulties in chloroplast gene expression and cell proliferation, and oxidative stress response in plants (Wang et al. 2015). Some of the general effects of antibiotics on lower and higher plants are listed in Table 2.
Table 2.
Effects of different classes of antibiotics on plants
| Sr. no. | Antibiotics | Effect on plants | References |
|---|---|---|---|
| 1 | Cephalosporins | Reducs the net somatic conductivity and inhibit the net assimilation | Opris et al. (2013) |
| 2 | β-Llactams | Affect division of plastids in lower plants | Wang et al. (2015) |
| 3 | Fluoroquinolones | Interfere with photosynthetic processes and cause deformities along with reduced somatic conductivity | Wang et al. (2015) |
| 4 | Tetracyclines | Cause reduction in the chlorophyll and carotenoids as a result of chromosomal aberrations | Larramendy et al. (2015) |
In a study conducted by Hamscher and Mohring (2012), high concentrations of sulfadiazine and tetracycline, 235 mg/L FW and 66 mg/L FW, respectively, have a direct impact on the germination and root development of the plant. A study by Yang et al. (2021) showed that antibiotics ciprofloxacin, norfloxacin, and tetracycline have a derogatory effect on the root development in Brassica chinensis L. The binary mixtures of these antibiotics have a more aggravated effect on root elongation. Sun et al. (2018) deduced that a mix of 17 PPCPs in the range of 5–50 µg/L could cause growth complications in cucumber plants such as burnt leaf edges, oxidative stress, and reduction in both chlorophyll a and b. There are certain plant species where the effect concentration (EC50) value is quite high. The tolerance level of such plant species is high, e.g., for Lactuca sativa L. EC50 is 453 mg/L for tetracycline and > 1000 mg/L for sulfamethoxazole (Pino et al. 2016). EC50 at 109 mg/L tetracyclines in the case of Sinapis alba L. causes stunted root development (Timmerer et al. 2020). Emofloxacin shows a very strong absorption to the soil matrix and organic matter. Also, a similar study showed quite a high toxicity of enrofloxacin which is 5 mg/L, where the growth of root was reduced by 25%. A total of 50% reduction in root growth of Oryza sativa L. occurred when the nutrient solution was contaminated with tetracycline (69 mg/L) (Liu et al. 2009). Stress-related studies in Alfalfa were studied by Christou et al. (2016) where a mixture of pharmaceuticals, sulfamethoxazole, trimethoprim, diclofenac, etc., was used to deduce the synergistic effect of antibiotics when in combination.
In addition to this, several side effects of antibiotics have been exemplified by streptomycin-resistant plant pathogens like E.amylovora, Pectobacterium carotovora, Pseudomonas chichorii, Pseudomonas syringae pv. papulans, Pseudomonas syringae pv. syringae, Pseudomonas lachrymans, and Xanthomonas dieffenbachiae etc. (Mann et al. 2021).
Effects on algae
Aquatic ecosystems receive a mixture of antibiotics, and these in combination may interact synergistically or antagonistically. A study by Carusso et al. (2018) showed that even isolating Ankistrodesmus fusiformis from a very polluted river and characterized as heavy metal tolerant yet the minor concentration of various antibiotics alone or in combination was toxic to the development of microalgae. Antibiotics affect the growth and development of algal species. Extensively studied are Raphidocelis subcapitata and Ankistrodesmus fusiformis (Carusso et al. 2018). Typically found antibiotics such as chlortetracycline, oxytetracycline, and enrofloxacin reduce the growth of the algae in varied concentrations. Their effect is enhanced when they are present in combination. The risk of these antibiotics alone or in combination depends upon the organism which is encountering them. There are also reports where degradation products pose enhanced toxicity to various algal species, for e.g., by-products of chlortetracycline degradation exhibit higher toxicity Scenedesmus obliquus (Sharma et al. 2021). Also, the presence of inorganic nutrients such as nitrogen in excess causes toxicity of spiramycin to Microcystis aeruginosa (Liu et al. 2015).
Effects on animals and humans
Non-therapeutic antibiotics in livestock feed have a negative impact on the farm animals' normal microbiome, resulting in dysbiosis and the development of antibacterial resistance (Chattopadhyay et al. 2014). It also has several serious consequences for human health, as antibiotic-resistant strains such as Campylobacter coli/jejuni, Salmonella enterica, Yersinia enterocolitica, and MRSA CC398 (Wegener 2012) propagate and spread under selective pressure, either directly or indirectly through the food chain, water, soil, or fertilizers contaminated with animal excreta (Marshall and Levy 2011) or genetic transfer between resistant bacteria in animals and humans.
The human gut microbiota is the most frequent non-pathogenic bacterium that is affected by antibiotic administration among these beneficial bacteria. A persistent condition called dysbiosis is frequently brought on by the disruptions of the gut microorganisms brought on by excessive antibiotic use. It is defined as a change in the microbial ecosystem's capacity for resistance outweighing an alteration in the capacity of microbial ecosystem for composition and function. Type II diabetes, rheumatoid arthritis, inflammatory bowel disease, atopy, and other diseases, all causes gut dysbiosis. Antibiotic-associated diarrhea (AAD), which affects 5–35% of patients because of low antimicrobial peptide release, Clostridium difficile-associated diarrhea, increased susceptibility to Salmonella typhimurium, infections caused by Helicobacter pylori,and Citrobacter rodentium, and development of chronic conditions like cardiovascular disease, cancer, and even premature death are some of the long-term effects of antibiotic use in humans (Heianza et al. 2020; Ramirez et al. 2020).
Antibiotics also have a direct impact on the CNS of humans, which are linked to certain pre-existing health complications as listed in Table 3. Much less recognition is given to the toxic effect of antimicrobials on the CNS. Ototoxicity, nonspecific encephalopathy, seizures, etc., are some of the complications related to at-risk patients among many groups of antibiotics (Grill and Maganti 2011). Majorly aged, critically ill, and patients with renal dysfunction are most likely to be at high risk of antibiotic-induced neurotoxicity.
Table 3.
CNS neurotoxicity induced by antibiotics
| Sr. no. | Class of antibiotics | Antibiotics | Complications | References |
|---|---|---|---|---|
| 1 | Aminoglycosides | Gentamicin |
Peripheral neuropathy and encephalopathy Lesions in the pons and mesencephalon |
Watanabe et al. (1978) |
| 2 | Aminoglycosides | Amikacin, tobramycin, neomycin, kanamycin | Needle muscular and autonomic transmission blockade | – |
| 3 | Cephalosporin | Cefazolin, cefuroxin, ceftazidime, cefepime |
Non-convulsive status epilepticus Truncal asterixis Renal failure Myoclonus |
Grill and Maganti (2008) |
| 4 | Penicillin | Piperacillin |
Tardive seizures Tonic–clonic seizures |
|
| Ampicillin |
Low birth-weight neonates Renal immaturity Neonatal seizure without clinical manifestations |
|||
| 5 | Carbapenem | Luripenem | Renal insufficiency | Lamoth et al. (2009) |
| 6 | Tetracyclines | – |
Cranial nerve toxicity Neuromuscular blockade |
Thomas (1994) |
| 7 | Macrolides | – |
Ototoxicity with damage to the cochlea Hearing impairment |
– |
| 8 | Quinolones | – |
Myoclonus, toxic psychosis Oro-facial dyskinesias Acute delirium with psychotic features |
Kiangkitiwan et al. (2008) |
| 9 | Vancomycin | – |
Ventriculitis CSF-pleocytosis Eosinophilia |
– |
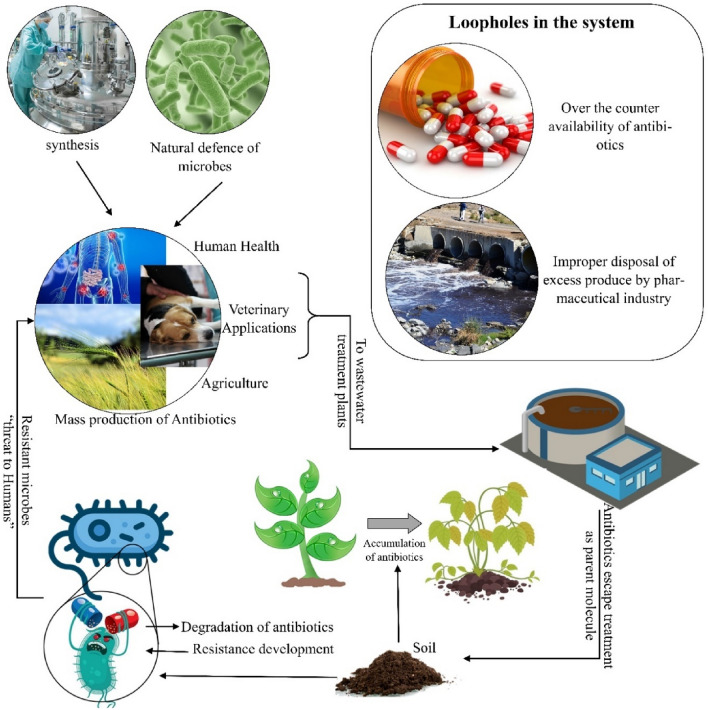
Box: Antibiotics: from shelves to surroundings (Fig. 3).
Fig. 3.
Representative flow of antibiotics in the environment causing antibiotic pollution and resistance
With the advent of antibiotics, there was a transformation in the way microbial infections were treated. But within decades, the problem of antibiotic resistance emerged, which has set off alarm today. Antibiotics have increased so much that it has taken the shape of abuse. There are few considerations that lead to the abuse of antibiotics. The first, the easy over-the-counter availability, especially in low- to middle-income countries, and the insufficient knowledge among common people about the use and consumption of antibiotics, is the leading cause of reckless use. As mentioned in the text, not just human medicine, but animal farming and agriculture are also the major consumers of antibiotics.
With the growing needs, antibiotic production has also increased to a great extent. For this purpose, the industrial mass production of antibiotic has boomed, resulting in antibiotic waste accumulating worldwide. The overproduced and mismanaged products either mix with the soil and leach where it contaminates the groundwater or go directly into the wastewater treatment plant where the antibiotics escape conventional treatments and again reach soil as sludge and waterbodies as “treated" water and again the cycle is repeated. This kind of long-term exposure of micro-organisms with antibiotics puts selective pressure on the community and selects for the resistant organisms over time.
Fate of antibiotics
Antibiotics are administered as medicine for human health and veterinary purposes. Antibiotics, majorly fluoroquinolones, sulfamethoxazole, trimethoprim, and tetracyclines, show good absorption in the digestive tract, while there are some others that show poor bioavailability (Levison and Levison 2009). More than 40–90% of the administered antibiotic is directly excreted as active compounds (Polianciuc et al. 2020). The conventional wastewater treatment plants are inefficient for the elimination of antibiotic pollutants; hence, the final sludge/effluent from WWTP contains a considerable quantity of antibiotics as active compounds or as products of degradation (Wang et al. 2017; Kuppusamy et al. 2018). The process of deconjugation strips off the metabolites from their antibiotic counterparts, making them more bioavailable post-wastewater treatment. In the environment, these compounds can contaminate the biota, including different trophic levels (Jechalke et al. 2014). Antibiotics naturally degrade in the environment which is largely affected by various biotic and abiotic factors. Table 4 represents the fate of the antibiotics in the environment, especially water.
Table 4.
Fate of antibiotics in the environment
| Sr. no. | Antibiotic | Fate | References |
|---|---|---|---|
| 1. | Meropenem | Susceptible to hydrolysis (at neutral pH, the half-life is extended at 52 h) | Al-Ahmad et al. (1999) |
| 2. | Ceftiofur | Susceptible to hydrolysis (at neutral pH, the half-life is extended to 8 days) | Gartiser et al. (2007) |
| 3. | Amoxicillin | Susceptible to hydrolysis (at neutral pH, the half-life is extended to > 5 days) | Andreozzi et al. (2004) |
| Photodegraded by UV light (UV light cannot penetrate deeply through the water surface) | Burhenne et al. (1997) | ||
| 4. | Quinolones | Photodegraded by UV light (UV light cannot penetrate deeply through the water surface) | Andreozzi et al. (2004) |
| 5. | Tetracyclines | Huber et al. (2005) | |
| 6. | Macrolides | Half-life varies from 5 to 120 days in soil and photodegrade in water between 0.2 and 200 min | Voigt and Jaeger (2017) |
| 7. | Fluoroquinolones | Chemically and thermally stable, adsorbed rapidly from water to soil particles | Frade et al. (2014) |
| 8. | Cephalosporins | Hydrolyze in > 2 weeks in surface waters and > 8 days in alkaline systems | Li and Zhang (2010) |
The organisms which are at the lower hierarchical level are more susceptible to the effects of antibiotics, especially in the aquatic environment (Grenni et al. 2018). The bioavailability of antibiotics and how they interact with other environmental factorssuch as pH, the amount of organic carbon in the soil, the kind of water present, and the type of organism present determine the biological activity of antibiotics in various environmental matrices. Therefore, it is both necessary and extremely difficult to offer information about the bioavailable fractions and how these interacts with the environment, especially when it comes to analytical results (Aga et al. 2016). Fluoroquinolones remain unchanged, and the recovery rate in urine is 70% of the administered dose (Marx et al. 2015). Vancomycin was found in very high concentration in urinary excretions, which is almost 90% of the administered dose, pointing toward 10% bioavailability (Levison and Levison 2009). Sulfamethoxazole occurs as its metabolites at an 80% administered dose. There exist discrepancies between the clinical treatment and antimicrobial susceptibility testing for infections caused by opportunistic pathogens (Brandt et al. 2015).
As evident from Table 4, β-lactam antibiotics may be susceptible to hydrolysis; still, they escape the WWTPs as the usual hydrolytic retention time of WWTPs is almost 8–20 h which is insufficient for its removal (Zhang and Li 2011).
Halt in antibiotic development
The discovery of the first-ever antibiotic penicillin in 1928 was a great stepping stone for future discoveries of more and more antibiotics (Moloney 2016). Based on Flemings’ discovery principle, Waksman followed a more refined and systematized approach. His experimentation led to an important discovery of streptomycin effective against Gram-negative and Gram-positive bacteria (Jones et al. 1944). Following the Waksman protocols and approaches, many clinically significant antibiotics were discovered shortly after 1943 over a brief period of 20 years, referred to as the 'Golden Period' of antibiotic development (Lyddiard et al. 2016). The majority of antibiotics of clinical use are natural products or their semi-synthetic derivatives were discovered during this period (Mohr 2016). Prominent examples which are still in use today are vancomycin, daptomycin, tetracycline, clindamycin, and rifampicin (Wright 2014). Over the past 20 years, there are only two new classes of antibiotics, lipopeptides and oxazolidinones (Luepke et al. 2017).
The time-consuming and labor-intensive job of drug development does not always ensure a fruitful result. Only one out of every five drugs for infectious diseases receives approval from the FDA. This number is relatively low in an era where disease-causing organisms are evolving faster than the drugs against them.
WHO considers carbapenem-resistant/extended-spectrum β-lactamase producing Enterobacteriacea, Pseudomonas, and Acinetobacter baumannnii as critical threats as these are resistant to nearly all approved antibiotics (Hay et al. 2014). Neisseria gonorrhoeae and Clostridium difficile are considered urgent threats to public health by CDC (Lessa et al. 2015). Neisseria gonorrhoeae is resistant to azithromycin and ceftriaxone. Klebsiella pneumoniae and Escherichia coli can cause fatal infections in immune-compromised patients, including newborns. There exists a huge knowledge gap in action against resistant protein NDM-1 (New Delhi-metallo-β-lactamase-1). There are only three antibiotics currently in development against this protein. This protein makes bacteria highly resistant to all antibiotics including the last resort carbapenem family of antibiotics.
Some other infections include:
Factors affecting the development of antibiotics
As per WHO, a large number of products (antibiotic drugs) in development has little positive effect on the existing treatment strategies. Two major players in dwindling antibiotic development are declining investment and lack of innovation. 95% of the drugs in development are taken care of by small companies, of which 70% of companies are pre-revenue.
A huge amount of investment goes into the development of new antibiotics and ultimately to bring it to market, but the recovery of the cost and profit making are difficult in this case. This is because of the reason that certain drugs, mainly the last resort antibiotics, though very potent, have very limited use and hence not sold in large volumes. This is one of the main reasons for less investment in new drug development.
Certain infections impact clinicians, medical practitioners, and the pharma industry all over the globe, and changes in the statistical data of morbidity, mortality, etc., will make it difficult for the governments to form task plans/working action plans to formulate policies.
The Infectious Disease Society of America (IDSA) has proposed a few points which include the following:
Economic reformation including incentives for market failure.
Regulatory approaches to facilitate antimicrobial development.
Enhancement of antimicrobial resistance surveillance systems (the STAAR act).
Investment in antimicrobial-focused research.
To check the non-judicious use of antibiotics for animals, plants, and the marine environment.
Despite many efforts and action plans, there has been a continuous rise in antibiotic resistance and infection arising from such organisms. The problem is more aggravated as the common public is entirely unaware of the gravity of the situation (Spellberg et al. 2008) and also no new antibiotic class has been discovered since the 1980s.
Current scenario of development of newer antibiotics
The recent discovery of teixobactin (Fiers et al. 2017), using a modified Waksman approach, gave promising results. The method primarily includes using metagenomic analysis, which also considers uncultured bacteria (Nichols et al. 2010). In the year 2021, there were only two novel drug application antibiotics that are submitted for approval. One of the two belongs to class oxazolidinone-contezolid (MRX-1)/contezolis acefosamil (MRX-4) by MicroRx Pharmaceuticals Inc. (China). It targets the 50 s ribosomal subunits of skin and skin structure infection-causing E. faecium and S. aureus. Others belong to thiopenem β-lactam antibiotics sulopenem/sulopenem etzadroxil–probenecid by Heum Therapeutics PLC (U.S.). It targets penicillin-binding proteins of K. pneumoniae, Enterobacter spp., and drug-resistant N. gonorrhoeae.
As per Dec 2020 data, there are 43 antibiotics in the development phase, out of which 60% of drugs entered phase 3 clinical trials. Out of these, 19 antibiotics have the potential to treat Gram-negative pathogenic infections. The issue with the development of new antibiotics that are effective against severe infections is that: there are fewer individuals that contract these infections. Hence the sample size becomes really small for traditional clinical trials. One another phenomenon which is in continuous motion is the development of resistant organisms. The resistance development process has outcasted the development or repurposing of drugs for such infections.
Conclusion
The discovery of antibiotics brought a complete change in the disease treatment scenario. These molecules, despite attaining the status of persistent pollutants, are still important for the treatment of various deadly infections. But we are now in a situation where antibiotics are rendered ineffective by micro-organisms via evolution by various means such as mutation, horizontal transfer of ARGs. The resistance has aggravated to such an extent that it has become a global issue and some of the pathogens have developed resistance even against the third generation of antibiotics.
Our conventional treatment processes are inefficient against remediation of antibiotics. Many studies are being conducted in this area to address the issues of antibiotic pollution and antibiotic resistance, but with little success as of yet. All the issues discussed in the manuscript, including pollution as well as halt in the development, indicate that antibiotic resistance is continuously increasing and we are in desperate need of newer targets or newer antibiotics.
There are two basic questions which we need to ask ourselves. Do we really need antibiotics? Or do we need antibiotic resistance? Sincerely, taking in antibiotics is all we need to do as far as public health is concerned. But for researchers, there are two avenues which can be focused upon: first is the environmental pollution of antibiotics and the other is antibiotic resistance. As antibiotic pollution has gained the status of persistent pollutants, there is a need to develop efficient processes, which in conjuction with our existing WWTP can remediate antibiotic contamination. There is ample research in this field, but none has been upscaled to make visible change. Regarding antibiotic resistance, we need to search for alternate targets which might be helpful in combating pathogens in a more robust way. Also, there is a newer avenue of research where non-antibiotic targets are used. Hence, directing the flow of research in such fields might prove beneficial and may help in relieving this issue if not completely, maybe on a small scale.
Acknowledgements
The authors acknowledge the Indian Institute of Technology Guwahati, Assam, India, for providing research fellowship and infrastructural resources for carrying out the present work.
Funding
The authors acknowledge the Indo-EU Horizon 2020 project (BT/IN/EU-WR/60/SP/2018) funded by the Department of Biotechnology, New Delhi, for providing financial support.
Data availability
The authors confirm that the data supporting the findings of this study are available within this article.
Declarations
Conflict of interest
The authors declare no conflict of interest regarding the publication of this review article.
References
- Aga DS, Lenczewski M, Snow D, et al. Challenges in the measurement of antibiotics and in evaluating their impacts in agroecosystems: a critical review. J Environ Qual. 2016;45:407–419. doi: 10.2134/JEQ2015.07.0393. [DOI] [PubMed] [Google Scholar]
- Akiba M, Senba H, Otagiri H, et al. Impact of wastewater from different sources on the prevalence of antimicrobial-resistant Escherichia coli in sewage treatment plants in South India. Ecotoxicol Environ Saf. 2015;115:203–208. doi: 10.1016/J.ECOENV.2015.02.018. [DOI] [PubMed] [Google Scholar]
- Al-Ahmad A, Daschner FD. Kümmerer K (1999) Biodegradability of Cefotiam, Ciprofloxacin, Meropenem, Penicillin G, and Sulfamethoxazole and Inhibition of Waste Water Bacteria. Archiv Environ Contamin Toxicol. 1999;37:158–163. doi: 10.1007/S002449900501. [DOI] [PubMed] [Google Scholar]
- Andersson DI. Hughes D (2014) Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol. 2014;12:465–478. doi: 10.1038/nrmicro3270. [DOI] [PubMed] [Google Scholar]
- Andreozzi R, Caprio V, Ciniglia C, et al. Antibiotics in the environment: occurrence in Italian STPs, fate, and preliminary assessment on algal toxicity of amoxicillin. Environ Sci Technol. 2004;38:6832–6838. doi: 10.1021/ES049509A/SUPPL_FILE/ES049509ASI20040324_101936.PDF. [DOI] [PubMed] [Google Scholar]
- Arnum PV (2013) The Weaknesses and Strengths of the Global API Market. PTSM: Pharmaceutical Technology Sourcing and Management 9. https://www.pharmtech.com/view/weaknesses-and-strengths-global-api-market. Accessed 10 Nov 2023
- Arshad M, Mahmood SF, Khan M, Hasan R. Covid -19, misinformation, and antimicrobial resistance. BMJ. 2020 doi: 10.1136/BMJ.M4501. [DOI] [PubMed] [Google Scholar]
- Azam M, Jan AT, Haq QMR. blaCTX-M-152, a Novel Variant of CTX-M-group-25, Identified in a Study Performed on the Prevalence of Multidrug Resistance among Natural Inhabitants of River Yamuna. India Front Microbiol. 2016;7:176. doi: 10.3389/FMICB.2016.00176/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baile CA, McLaughlin CL, Chalupa WV, Snyder DL, Pendlum LC, Potter EL. Effects of Monensin Fed to Replacement Dairy Heifers During the Growing and Gestation Period upon Growth, Reproduction, and Subsequent Lactation. J Dairy Sci. 1982;65(10):1941–1944. doi: 10.3168/JDS.S0022-0302(82)82442-9. [DOI] [PubMed] [Google Scholar]
- Bay DC, Rommens KL, Turner RJ. Small multidrug resistance proteins: A multidrug transporter family that continues to grow. Biochimica Et Biophysica Acta (BBA) Biomembranes. 2008;1778:1814–1838. doi: 10.1016/J.BBAMEM.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Beceiro A, Tomás M, Bou G. Antimicrobial resistance and virulence: a successful or deleterious association in the bacterial world? Clin Microbiol Rev. 2013;26:185–230. doi: 10.1128/CMR.00059-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson-Palme J, Larsson DGJ. Concentrations of antibiotics predicted to select for resistant bacteria: Proposed limits for environmental regulation. Environ Int. 2016;86:140–149. doi: 10.1016/J.ENVINT.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Bergeret P (2017) The future of food and agriculture: trends and challenges [ Note de lecture ]. 3
- Bhattacharyya D, Banerjee J, Bandyopadhyay S, et al (2016) First Report on Vancomycin-Resistant Staphylococcus aureus in Bovine and Caprine Milk. https://home.liebertpub.com/mdr 22:675–681. 10.1089/MDR.2015.0330. Accessed 30 May 2023 [DOI] [PubMed]
- Bischoff A, Meier C, Roth F. Gentamicin neurotoxicity (polyneuropathy–encephalopathy) Schweiz Med Wochenschr. 1977;107:3–8. [PubMed] [Google Scholar]
- Blair JMA, Richmond GE, Piddock LJV. Multidrug efflux pumps in gram-negative bacteria and their role in antibiotic resistance. Fut Microbiol. 2014;9:1165–1177. doi: 10.2217/FMB.14.66. [DOI] [PubMed] [Google Scholar]
- Bombaywala S, Mandpe A, Paliya S, Kumar S. Antibiotic resistance in the environment: a critical insight on its occurrence, fate, and eco-toxicity. Environ Sci Pollut Res. 2021;28:24889–24916. doi: 10.1007/S11356-021-13143-X. [DOI] [PubMed] [Google Scholar]
- Bordeleau E, Stogios PJ, Evdokimova E, et al. ApmA is a unique aminoglycoside antibiotic acetyltransferase that inactivates apramycin. Mbio. 2021;12:1–11. doi: 10.1128/MBIO.02705-20/SUPPL_FILE/MBIO.02705-20-SF008.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt KK, Amézquita A, Backhaus T, et al. Ecotoxicological assessment of antibiotics: A call for improved consideration of microorganisms. Environ Int. 2015;85:189–205. doi: 10.1016/J.ENVINT.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Brown NG, Pennington JM, Huang W, et al. Multiple Global Suppressors of Protein Stability Defects Facilitate the Evolution of Extended-Spectrum TEM β-Lactamases. J Mol Biol. 2010;404:832–846. doi: 10.1016/J.JMB.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burhenne J, Ludwig M, Nikoloudis P, Spiteller M. Primary photoproducts and half-lives. Environ Sci Pollut Res. 1997;4:10–15. doi: 10.1007/BF02986257. [DOI] [PubMed] [Google Scholar]
- Cardoso O, Porcher JM, Sanchez W. Factory-discharged pharmaceuticals could be a relevant source of aquatic environment contamination: Review of evidence and need for knowledge. Chemosphere. 2014;115:20–30. doi: 10.1016/J.CHEMOSPHERE.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Carusso S, Juárez AB, Moretton J, Magdaleno A. Effects of three veterinary antibiotics and their binary mixtures on two green alga species. Chemosphere. 2018;194:821–827. doi: 10.1016/J.CHEMOSPHERE.2017.12.047. [DOI] [PubMed] [Google Scholar]
- Carvalho IT, Santos L. Antibiotics in the aquatic environments: A review of the European scenario. Environ Int. 2016;94:736–757. doi: 10.1016/J.ENVINT.2016.06.025. [DOI] [PubMed] [Google Scholar]
- CDC (2019) Antibiotic resistance threats in the United States, U.S. Department of Health and Human Services, CDC, Atlanta, GA 2019
- Center for Disease Dynamics, Economics & Policy (2015) State of the World’s Antibiotics, 2015. CDDEP, Washington, D.C
- Chambers HF. Community-Associated MRSA — Resistance and Virulence Converge. N Engl J Med. 2005;352:1485–1487. doi: 10.1056/NEJME058023. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay MK, Zurek L, Nosanchuk JD. Use of antibiotics as feed additives: a burning question. Front Microbiol. 2014 doi: 10.3389/fmicb.2014.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou A, Antoniou C, Christodoulou C, et al. Stress-related phenomena and detoxification mechanisms induced by common pharmaceuticals in alfalfa (Medicago sativa L.) plants. Sci Total Environ. 2016;557:652–664. doi: 10.1016/J.SCITOTENV.2016.03.054. [DOI] [PubMed] [Google Scholar]
- Collu F, Cascella M. Multidrug resistance and efflux pumps: insights from molecular dynamics simulations. Curr Top Med Chem. 2013;13:3165–3183. doi: 10.2174/15680266113136660224. [DOI] [PubMed] [Google Scholar]
- Deak D, Outterson K, Powers JH, Kesselheim AS. Progress in the fight against multidrug-resistant bacteria? A review of U.S. food and drug administration-approved antibiotics, 2010–2015. Ann Intern Med. 2016;165:363–372. doi: 10.7326/M16-0291. [DOI] [PubMed] [Google Scholar]
- Douglas MW, Mulholland K, Denyer V, Gottlieb T. Multi-drug resistant Pseudomonas aeruginosa outbreak in a burns unit — an infection control study. Burns. 2001;27:131–135. doi: 10.1016/S0305-4179(00)00084-X. [DOI] [PubMed] [Google Scholar]
- Du L, Liu W. Occurrence, fate, and ecotoxicity of antibiotics in agro-ecosystems. A Review. Agron Sustain Dev. 2012;32:309–327. doi: 10.1007/S13593-011-0062-9/TABLES/5. [DOI] [Google Scholar]
- Eady EA, Cove JH. Staphylococcal resistance revisited: community-acquired methicillin resistant Staphylococcus aureus–an emerging problem for the management of skin and soft tissue infections. Curr Opin Infect Dis. 2003;16:103–124. doi: 10.1097/00001432-200304000-00007. [DOI] [PubMed] [Google Scholar]
- Egorov AM, Ulyashova MM, Rubtsova MY. Bacterial Enzymes and Antibiotic Resistance. Acta Naturae. 2018;10:33. doi: 10.32607/20758251-2018-10-4-33-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feßler AT, Wang Y, Wu C, Schwarz S. Mobile lincosamide resistance genes in Staphylococci. Plasmid. 2018;99:22–31. doi: 10.1016/J.PLASMID.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Fiers WD, Craighead M, Singh I. Teixobactin and Its Analogues: A New Hope in Antibiotic Discovery. ACS Infect Dis. 2017;3:688–690. doi: 10.1021/ACSINFECDIS.7B00108/ASSET/IMAGES/LARGE/ID-2017-001088_0002.JPEG. [DOI] [PubMed] [Google Scholar]
- File TM. Streptococcus pneumoniae and community-acquired pneumonia: A cause for concern. Am J Med Suppl. 2004;117:39–50. doi: 10.1016/J.AMJMED.2004.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File TM. Clinical implications and treatment of multiresistant Streptococcus pneumoniae pneumonia. Clin Microbiol Infect. 2006;12:31–41. doi: 10.1111/J.1469-0691.2006.01395.X. [DOI] [PubMed] [Google Scholar]
- Frade VMF, Dias M, Teixeira ACSC, Palma MSA. Environmental contamination by fluoroquinolones. Braz J Pharm Sci. 2014;50:41–54. doi: 10.1590/S1984-82502011000100004. [DOI] [Google Scholar]
- Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-Resistant Staphylococcus aureus Disease in Three Communities. N Engl J Med. 2005;352:1436–1444. doi: 10.1056/NEJMOA043252/SUPPL_FILE/1436SA1.PDF. [DOI] [PubMed] [Google Scholar]
- Froehlich HE, Runge CA, Gentry RR, et al. Comparative terrestrial feed and land use of an aquaculture-dominant world. Proc Natl Acad Sci USA. 2018;115:5295–5300. doi: 10.1073/PNAS.1801692115/SUPPL_FILE/PNAS.1801692115.SAPP.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandra S, Joshi J, Trett A, Sankhil Lamkang A (2017) Suggested citation. Scoping Report on Antimicrobial Resistance in India
- García-Fernández A, Dionisi AM, Arena S, et al. Human campylobacteriosis in Italy: Emergence of multi-drug resistance to ciprofloxacin, tetracycline, and erythromycin. Front Microbiol. 2018;9:1906. doi: 10.3389/FMICB.2018.01906/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartiser S, Urich E, Alexy R, Kümmerer K. Anaerobic inhibition and biodegradation of antibiotics in ISO test schemes. Chemosphere. 2007;66:1839–1848. doi: 10.1016/J.CHEMOSPHERE.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Gordon KA, Biedenbach DJ, Jones RN. Comparison of Streptococcus pneumoniae and Haemophilus influenzae susceptibilities from community-acquired respiratory tract infections and hospitalized patients with pneumonia: five-year results for the SENTRY antimicrobial surveillance program. Diagn Microbiol Infect Dis. 2003;46:285–289. doi: 10.1016/S0732-8893(03)00087-7. [DOI] [PubMed] [Google Scholar]
- Granich RM, Oh P, Lewis B, et al. Multidrug Resistance Among Persons With Tuberculosis in California, 1994–2003. JAMA. 2005;293:2732–2739. doi: 10.1001/JAMA.293.22.2732. [DOI] [PubMed] [Google Scholar]
- Grant GR, Lederman JA, Brandstetter RD. T.G. Heaton, tuberculosis, and artificial pneumothorax: Once again, back to the future? Chest. 1997;112:7–8. doi: 10.1378/chest.112.1.7. [DOI] [PubMed] [Google Scholar]
- Grenni P, Ancona V, Barra Caracciolo A. Ecological effects of antibiotics on natural ecosystems: A review. Microchem J. 2018;136:25–39. doi: 10.1016/J.MICROC.2017.02.006. [DOI] [Google Scholar]
- Grill MF, Maganti R. Cephalosporin-induced neurotoxicity: Clinical manifestations, potential pathogenic mechanisms, and the role of electroencephalographs monitoring. Ann Pharmacother. 2008;42:1843–1850. doi: 10.1345/aph.1L307. [DOI] [PubMed] [Google Scholar]
- Grill MF, Maganti RK. Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol. 2011;72:381. doi: 10.1111/J.1365-2125.2011.03991.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo RX, Chen JQ. Phytoplankton toxicity of the antibiotic chlortetracycline and its UV light degradation products. Chemosphere. 2012;87:1254–1259. doi: 10.1016/J.CHEMOSPHERE.2012.01.031. [DOI] [PubMed] [Google Scholar]
- Guo X, Yan Z, Zhang Y, et al. Behavior of antibiotic resistance genes under extremely high-level antibiotic selection pressures in pharmaceutical wastewater treatment plants. Sci Total Environ. 2018;612:119–128. doi: 10.1016/J.SCITOTENV.2017.08.229. [DOI] [PubMed] [Google Scholar]
- Hakko E, Mete B, Ozaras R, et al. Levofloxacin-induced delirium. Clin Neurol Neurosurg. 2005;107:158–159. doi: 10.1016/J.CLINEURO.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Hamscher G, Mohring SAI. Übersichtsbeitrag. Chem Ing Tec. 2012;7:1052–1061. doi: 10.1002/CITE.201100255. [DOI] [Google Scholar]
- Hay M, Thomas DW, Craighead JL, et al. Clinical development success rates for investigational drugs. Nat Biotechnol. 2014;32:40–51. doi: 10.1038/NBT.2786. [DOI] [PubMed] [Google Scholar]
- Heianza Y, Ma W, Li X, et al. Duration and Life-Stage of Antibiotic Use and Risks of All-Cause and Cause-Specific Mortality: Prospective Cohort Study. Circ Res. 2020;126:364–373. doi: 10.1161/CIRCRESAHA.119.315279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J. How covid-19 is accelerating the threat of antimicrobial resistance. BMJ. 2020 doi: 10.1136/BMJ.M1983. [DOI] [PubMed] [Google Scholar]
- Huber MM, Göbel A, Joss A, et al. Oxidation of pharmaceuticals during ozonation of municipal wastewater effluents: A pilot study. Environ Sci Technol. 2005;39:4290–4299. doi: 10.1021/ES048396S/ASSET/IMAGES/LARGE/ES048396SF00009.JPEG. [DOI] [PubMed] [Google Scholar]
- Hyde TB, Gay K, Stephens DS, et al. Macrolide Resistance Among Invasive Streptococcus pneumoniae Isolates. JAMA. 2001;286:1857–1862. doi: 10.1001/JAMA.286.15.1857. [DOI] [PubMed] [Google Scholar]
- Jang HM, Kim YB, Choi S, et al. Prevalence of antibiotic resistance genes from effluent of coastal aquaculture, South Korea. Environ Pollut. 2018;233:1049–1057. doi: 10.1016/J.ENVPOL.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Jayalakshmi K, Paramasivam M, Sasikala M, Sumithra A. Review on antibiotic residues in animal products and its impact on environments and human health. J Entomol Zool Studies. 2017;5:1446–1451. [Google Scholar]
- Jechalke S, Heuer H, Siemens J, et al. Fate and effects of veterinary antibiotics in soil. Trends Microbiol. 2014;22:536–545. doi: 10.1016/J.TIM.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Jones D, Metzger HJ, Schatz A, Waksman SA. Control of gram-negative bacteria in experimental animals by streptomycin. Science. 1944;100:103–105. doi: 10.1126/SCIENCE.100.2588.103. [DOI] [PubMed] [Google Scholar]
- Justice SS, Hunstad DA, Cegelski L, Hultgren SJ. Morphological plasticity as a bacterial survival strategy. Nat Rev Microbiol. 2008;6:162–168. doi: 10.1038/nrmicro1820. [DOI] [PubMed] [Google Scholar]
- Katz L, Baltz RH. Natural product discovery: past, present, and future. J Ind Microbiol Biotechnol. 2016;43:155–176. doi: 10.1007/S10295-015-1723-5. [DOI] [PubMed] [Google Scholar]
- Kaushik P, Anjay KS, et al. Isolation and prevalence of salmonella from chicken meat and cattle milk collected from local markets of Patna, India. Vet World. 2014;7:62–65. doi: 10.14202/VETWORLD.2014.62-65. [DOI] [Google Scholar]
- Kays MB, Smith DW, Wack MF, Denys GA. Levofloxacin Treatment Failure in a Patient with Fluoroquinolone-Resistant Streptococcus pneumoniae Pneumonia. Pharmacother J Hum Pharmacol Drug Ther. 2002;22:395–399. doi: 10.1592/PHCO.22.5.395.33185. [DOI] [PubMed] [Google Scholar]
- Kazakova SV, Hageman JC, Matava M, et al. A Clone of Methicillin-Resistant Staphylococcus aureus among Professional Football Players. N Engl J Med. 2005;352:468–475. doi: 10.1056/NEJMOA042859. [DOI] [PubMed] [Google Scholar]
- Keeney KM, Yurist-Doutsch S, Arrieta MC, Finlay BB. Effects of Antibiotics on Human Microbiota and Subsequent Disease. Annu Rev Microbiol. 2014;68:217–235. doi: 10.1146/ANNUREV-MICRO-091313-103456. [DOI] [PubMed] [Google Scholar]
- Khan GA, Berglund B, Khan KM, et al. Occurrence and Abundance of Antibiotics and Resistance Genes in Rivers, Canal and near Drug Formulation Facilities—A Study in Pakistan. PLoS ONE. 2013;8:e62712. doi: 10.1371/JOURNAL.PONE.0062712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiangkitiwan B, Doppalapudi A, Fonder M, et al. Levofloxacin-induced delirium with psychotic features. Gen Hosp Psychiatry. 2008;30:381–383. doi: 10.1016/J.GENHOSPPSYCH.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Kir A, Tahaoǧlu K, Okur E, Hatipoǧlu T. Role of surgery in multi-drug-resistant tuberculosis: results of 27 cases. Eur J Cardiothorac Surg. 1997;12:531–534. doi: 10.1016/S1010-7940(97)00230-3. [DOI] [PubMed] [Google Scholar]
- Klein EY, Schueller E, Tseng KK, et al. The Impact of Influenza Vaccination on Antibiotic Use in the United States, 2010–2017. Open Forum Infect Dis. 2020 doi: 10.1093/OFID/OFAA223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018;115:E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtesi C, Ball AR, Huang Y-Y, et al. Suppl 1: Microbial Efflux Systems and Inhibitors: Approaches to Drug Discovery and the Challenge of Clinical Implementation. Open Microbiol J. 2013;7:34. doi: 10.2174/1874285801307010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansson E, Fick J, Janzon A, et al. Pyrosequencing of Antibiotic-Contaminated River Sediments Reveals High Levels of Resistance and Gene Transfer Elements. PLoS ONE. 2011;6:e17038. doi: 10.1371/JOURNAL.PONE.0017038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Schweizer HP. Bacterial resistance to antibiotics: Active efflux and reduced uptake. Adv Drug Deliv Rev. 2005;57:1486–1513. doi: 10.1016/J.ADDR.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Kumar M, Jaiswal S, Sodhi KK, et al. Antibiotics bioremediation: Perspectives on its ecotoxicity and resistance. Environ Int. 2019;124:448–461. doi: 10.1016/J.ENVINT.2018.12.065. [DOI] [PubMed] [Google Scholar]
- Kumar S, Mukherjee MM, Varela MF. Modulation of Bacterial Multidrug Resistance Efflux Pumps of the Major Facilitator Superfamily. Int J Bacteriol. 2013;2013:1–15. doi: 10.1155/2013/204141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümmerer K. Antibiotics in the aquatic environment – A review – Part I. Chemosphere. 2009;75:417–434. doi: 10.1016/J.CHEMOSPHERE.2008.11.086. [DOI] [PubMed] [Google Scholar]
- Kuppusamy S, Kakarla D, Venkateswarlu K, et al. Veterinary antibiotics (VAs) contamination as a global agro-ecological issue: A critical view. Agric Ecosyst Environ. 2018;257:47–59. doi: 10.1016/J.AGEE.2018.01.026. [DOI] [Google Scholar]
- Kuroda T, Tsuchiya T. Multidrug efflux transporters in the MATE family. Biochimica Et Biophysica Acta (BBA)-Proteins Proteom. 2009;1794:763–768. doi: 10.1016/J.BBAPAP.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Kushner JM, Peckman HJ, Snyder CR. Seizures associated with fluoroquinolones. Ann Pharmacother. 2001;35:1194–1198. doi: 10.1345/aph.10359. [DOI] [PubMed] [Google Scholar]
- Lambert PA. Cellular impermeability and uptake of biocides and antibiotics in Gram-positive bacteria and mycobacteria. J Appl Microbiol. 2002;92:46S–54S. doi: 10.1046/J.1365-2672.92.5S1.7.X. [DOI] [PubMed] [Google Scholar]
- Lamoth F, Erard V, Asner S, et al. High imipenem blood concentrations associated with toxic encephalopathy in a patient with mild renal dysfunction. Int J Antimicrob Agents. 2009;34:386–388. doi: 10.1016/J.IJANTIMICAG.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Larramendy ML, Soloneski S, Larramendy ML, Soloneski S. Emerging pollutants in the environment-current and further implications. Emerg Pollutants Environ-Curr Further Implications. 2015 doi: 10.5772/59332. [DOI] [Google Scholar]
- Larsson DGJ. Pollution from drug manufacturing: Review and perspectives. Philos Transact R Soc b: Biol Sci. 2014 doi: 10.1098/RSTB.2013.0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson DGJ, Flach CF. Antibiotic resistance in the environment. Nat Rev Microbiol. 2021;20:257–269. doi: 10.1038/s41579-021-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Savage VM, Yeh PJ. Intermediate Levels of Antibiotics May Increase Diversity of Colony Size Phenotype in Bacteria. Comput Struct Biotechnol J. 2018;16:307–315. doi: 10.1016/J.CSBJ.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile Infection in the United States. N Engl J Med. 2015;372:825–834. doi: 10.1056/NEJMOA1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin AS, Barone AA, Penço J, et al. Intravenous Colistin as Therapy for Nosocomial Infections Caused by Multidrug-Resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin Infect Dis. 1999;28:1008–1011. doi: 10.1086/514732. [DOI] [PubMed] [Google Scholar]
- Levison ME, Levison JH. Pharmacokinetics and Pharmacodynamics of Antibacterial Agents. Infect Dis Clin North Am. 2009;23:791–815. doi: 10.1016/j.idc.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K. Platforms for antibiotic discovery. Nat Rev Drug Discovery. 2013;12:371–387. doi: 10.1038/nrd3975. [DOI] [PubMed] [Google Scholar]
- Li B, Zhang T. Biodegradation and adsorption of antibiotics in the activated sludge process. Environ Sci Technol. 2010;44:3468–3473. doi: 10.1021/ES903490H/SUPPL_FILE/ES903490H_SI_001.PDF. [DOI] [PubMed] [Google Scholar]
- Lillenberg M, Litvin SV, Nei L, et al. Enrofloxacin and ciprofloxacin uptake by plants from soil. Agron Res. 2010;8:807–814. [Google Scholar]
- Lin AYC, Tsai YT. Occurrence of pharmaceuticals in Taiwan’s surface waters: Impact of waste streams from hospitals and pharmaceutical production facilities. Sci Total Environ. 2009;407:3793–3802. doi: 10.1016/J.SCITOTENV.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Ling LL, Schneider T, Peoples AJ, et al. A new antibiotic kills pathogens without detectable resistance. Nature. 2015;517:455–459. doi: 10.1038/nature14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Pellegrino J, Lee DJ, et al. Synthetic group A streptogramin antibiotics that overcome Vat resistance. Nature. 2020;586:145–150. doi: 10.1038/s41586-020-2761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CG, Green SI, Min L, et al. Phage-antibiotic synergy is driven by a unique combination of antibacterial mechanism of action and stoichiometry. Mbio. 2020;11:1–19. doi: 10.1128/MBIO.01462-20/SUPPL_FILE/MBIO.01462-20-SF005.TIF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Ying GG, Tao R, et al. Effects of six selected antibiotics on plant growth and soil microbial and enzymatic activities. Environ Pollut. 2009;157:1636–1642. doi: 10.1016/J.ENVPOL.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang F, Chen X, et al. Cellular responses and biodegradation of amoxicillin in Microcystis aeruginosa at different nitrogen levels. Ecotoxicol Environ Saf. 2015;111:138–145. doi: 10.1016/J.ECOENV.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Liu YY, Wang Y, Walsh TR, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- Li Z, Cao Y, Yi L, et al. Emergent Polymyxin Resistance: End of an Era? Open Forum Infect Dis. 2019 doi: 10.1093/OFID/OFZ368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubelski J, Konings WN, Driessen AJM. Distribution and Physiology of ABC-Type Transporters Contributing to Multidrug Resistance in Bacteria. Microbiol Mol Biol Rev. 2007;71:463–476. doi: 10.1128/MMBR.00001-07/ASSET/98294B98-F500-4BDD-8A00-B3890B9E967E/ASSETS/GRAPHIC/ZMR0030721590003.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luepke KH, Suda KJ, Boucher H, et al. Past, Present, and Future of Antibacterial Economics: Increasing Bacterial Resistance, Limited Antibiotic Pipeline, and Societal Implications. Pharmacotherapy. 2017;37:71–84. doi: 10.1002/PHAR.1868. [DOI] [PubMed] [Google Scholar]
- Lyddiard D, Jones GL, Greatrex BW. Keeping it simple: lessons from the golden era of antibiotic discovery. FEMS Microbiol Lett. 2016;363:84. doi: 10.1093/FEMSLE/FNW084. [DOI] [PubMed] [Google Scholar]
- Maia PP, da Silva EC, Rath S, Reyes FGR. Residue content of oxytetracycline applied on tomatoes grown in open field and greenhouse. Food Control. 2009;20:11–16. doi: 10.1016/J.FOODCONT.2008.01.007. [DOI] [Google Scholar]
- Mann A, Nehra K, Rana JS, Dahiya T. Antibiotic resistance in agriculture: Perspectives on upcoming strategies to overcome upsurge in resistance. Curr Res Microb Sci. 2021;2:100030. doi: 10.1016/J.CRMICR.2021.100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marathe NP, Gaikwad SS, Vaishampayan AA, et al. Mossambicus tilapia (Oreochromis mossambicus) collected from water bodies impacted by urban waste carries extended-spectrum beta-lactamases and integron-bearing gut bacteria. J Biosci. 2016;41:341–346. doi: 10.1007/S12038-016-9620-2/FIGURES/2. [DOI] [PubMed] [Google Scholar]
- Marathe NP, Regina VR, Walujkar SA, et al. A Treatment Plant Receiving Waste Water from Multiple Bulk Drug Manufacturers Is a Reservoir for Highly Multi-Drug Resistant Integron-Bearing Bacteria. PLoS ONE. 2013;8:e77310. doi: 10.1371/JOURNAL.PONE.0077310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall BM, Levy SB. Food animals and antimicrobials: Impacts on human health. Clin Microbiol Rev. 2011;24:718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsoni M, De Mattia F, Labra M, et al. Uptake and effects of a mixture of widely used therapeutic drugs in Eruca sativa L. and Zea mays L. plants. Ecotoxicol Environ Saf. 2014;108:52–57. doi: 10.1016/J.ECOENV.2014.05.029. [DOI] [PubMed] [Google Scholar]
- Marx C, Mühlbauer V, Schubert S, et al. Representative input load of antibiotics to WWTPs: Predictive accuracy and determination of a required sampling quantity. Water Res. 2015;76:19–32. doi: 10.1016/J.WATRES.2015.02.049. [DOI] [PubMed] [Google Scholar]
- McDonald LC. Trends in Antimicrobial Resistance in Health Care-Associated Pathogens and Effect on Treatment. Clin Infect Dis. 2006;42:S65–S71. doi: 10.1086/499404. [DOI] [PubMed] [Google Scholar]
- McGowan JE. Resistance in nonfermenting gram-negative bacteria: Multidrug resistance to the maximum. Am J Infect Control. 2006;34:S29–S37. doi: 10.1016/J.AJIC.2006.05.226. [DOI] [PubMed] [Google Scholar]
- McManus PS, Stockwell VO, Sundin GW, Jones AL. Antibiotic use in plant agriculture. Annu Rev Phytopathol. 2002;40:443–465. doi: 10.1146/ANNUREV.PHYTO.40.120301.093927. [DOI] [PubMed] [Google Scholar]
- Menard D, Dondorp A. Antimalarial Drug Resistance: A Threat to Malaria Elimination. Cold Spring Harb Perspect Med. 2017;7:a025619. doi: 10.1101/CSHPERSPECT.A025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing Fasciitis Caused by Community-Associated Methicillin-Resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–1453. doi: 10.1056/NEJMOA042683. [DOI] [PubMed] [Google Scholar]
- Miller WR, Munita JM, Arias CA. Mechanisms of antibiotic resistance in enterococci. Expert Rev Anti-Infective Ther. 2014;12:1221–1236. doi: 10.1586/14787210.2014.956092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza SH, Beeching NJ, Hart CA. Multi-drug resistant typhoid: A global problem. J Med Microbiol. 1996;44:317–319. doi: 10.1099/00222615-44-5-317/CITE/REFWORKS. [DOI] [PubMed] [Google Scholar]
- Mohr KI. History of Antibiotics Research. Curr Top Microbiol Immunol. 2016;398:237–272. doi: 10.1007/82_2016_499. [DOI] [PubMed] [Google Scholar]
- Moloney MG. Natural Products as a Source for Novel Antibiotics. Trends Pharmacol Sci. 2016;37:689–701. doi: 10.1016/J.TIPS.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Moran GJ, Amii RN, Abrahamian FM, Talan DA. Methicillin-resistant Staphylococcus aureus in Community-acquired Skin Infections. Emerg Infect Dis. 2005;11:928. doi: 10.3201/EID1106.040641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-Resistant S. aureus Infections among Patients in the Emergency Department. N Engl J Med. 2006;355:666–674. doi: 10.1056/NEJMOA055356. [DOI] [PubMed] [Google Scholar]
- Nachega JB, Chaisson RE. Tuberculosis Drug Resistance: A Global Threat. Clin Infect Dis. 2003;36:S24–S30. doi: 10.1086/344657. [DOI] [PubMed] [Google Scholar]
- Naik VK, Shakya S, Patyal A, et al. Isolation and molecular characterization of Salmonella spp. from chevon and chicken meat collected from different districts of Chhattisgarh, India. Vet World. 2015;8:702. doi: 10.1420/VETWORLD.2015.702-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettleman MD. Multidrug-Resistant Tuberculosis: News From the Front. JAMA. 2005;293:2788–2790. doi: 10.1001/JAMA.293.22.2788. [DOI] [PubMed] [Google Scholar]
- Nichols D, Cahoon N, Trakhtenberg EM, et al. Use of ichip for high-throughput in situ cultivation of “uncultivable microbial species”. Appl Environ Microbiol. 2010;76:2445–2450. doi: 10.1128/AEM.01754-09/ASSET/E834948C-1F80-490D-B7D3-ABA0397D5ABE/ASSETS/GRAPHIC/ZAM9991008580004.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann P, Poirel L, Walsh TR, Livermore DM. The emerging NDM carbapenemases. Trends Microbiol. 2011;19:588–595. doi: 10.1016/J.TIM.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Nori P, Cowman K, Chen V, et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect Control Hosp Epidemiol. 2021;42:84–88. doi: 10.1017/ICE.2020.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J (2016) Tackling drug-resistant infections globally: final report and recommendations
- Opris O, Copaciu F, Loredana Soran M, et al. Influence of nine antibiotics on key secondary metabolites and physiological characteristics in Triticum aestivum: Leaf volatiles as a promising new tool to assess toxicity. Ecotoxicol Environ Saf. 2013;87:70–79. doi: 10.1016/J.ECOENV.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Paterson DL. The Epidemiological Profile of Infections with Multidrug-Resistant Pseudomonas aeruginosa and Acinetobacter Species. Clin Infect Dis. 2006;43:S43–S48. doi: 10.1086/504476. [DOI] [PubMed] [Google Scholar]
- Paterson GK, Harrison EM, Holmes MA. The emergence of mecC methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2014;22:42–47. doi: 10.1016/J.TIM.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PJ, Smith SG, Kolpin DW, et al. Pharmaceutical formulation facilities as sources of opioids and other pharmaceuticals to wastewater treatment plant effluents. Environ Sci Technol. 2010;44:4910–4916. doi: 10.1021/ES100356F/SUPPL_FILE/ES100356F_SI_001.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino MR, Muñiz S, Val J, Navarro E. Phytotoxicity of 15 common pharmaceuticals on the germination of Lactuca sativa and photosynthesis of Chlamydomonas reinhardtii. Environ Sci Pollut Res. 2016;23:22530–22541. doi: 10.1007/S11356-016-7446-Y/TABLES/2. [DOI] [PubMed] [Google Scholar]
- Polianciuc SI, Gurzău AE, Kiss B, et al. Antibiotics in the environment: causes and consequences. Med Pharm Rep. 2020;93:231. doi: 10.15386/MPR-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J, Guarner F, Bustos Fernandez L, et al. Antibiotics as major disruptors of gut microbiota. Front Cell Infect Microbiol. 2020 doi: 10.3389/FCIMB.2020.572912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave LS, Sutton SB, Webber MA, Piddock LJV. Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014;22:438–445. doi: 10.1016/J.TIM.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Reverter M, Sarter S, Caruso D, et al. Aquaculture at the crossroads of global warming and antimicrobial resistance. Nat Commun. 2020;11:1–8. doi: 10.1038/s41467-020-15735-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhomberg PR, Fritsche TR, Sader HS, Jones RN. Clonal occurrences of multidrug-resistant Gram-negative bacilli: report from the Meropenem Yearly Susceptibility Test Information Collection Surveillance Program in the United States (2004) Diagn Microbiol Infect Dis. 2006;54:249–257. doi: 10.1016/J.DIAGMICROBIO.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Roe MT, Pillai SD. Monitoring and identifying antibiotic resistance mechanisms in bacteria. Poult Sci. 2003;82:622–626. doi: 10.1093/PS/82.4.622. [DOI] [PubMed] [Google Scholar]
- Rouquette-Loughlin C, Dunham SA, Kuhn M, et al. The NorM efflux pump of Neisseria gonorrhoeae and Neisseria meningitidis recognizes antimicrobial cationic compounds. J Bacteriol. 2003;185:1101–1106. doi: 10.1128/JB.185.3.1101-1106.2003/ASSET/96CCE6FF-C099-46B9-BEEB-65FEB2884809/ASSETS/GRAPHIC/JB0330838003.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen OB, Lunestad BT, Ervik A, Fjelde S. Stability of antibacterial agents in an artificial marine aquaculture sediment studied under laboratory conditions. Aquaculture. 1994;126:283–290. doi: 10.1016/0044-8486(94)90044-2. [DOI] [Google Scholar]
- Sattler CA, Mason EO, Kaplan SL. Prospective comparison of risk factors and demographic and clinical characteristics of community-acquired, methicillin-resistant versus methicillin-susceptible Staphylococcus aureus infection in children. Pediatr Infect Dis J. 2002;21:910–916. doi: 10.1097/00006454-200210000-00005. [DOI] [PubMed] [Google Scholar]
- Saxer G, Doebeli M, Travisano M. The Repeatability of Adaptive Radiation During Long-Term Experimental Evolution of Escherichia coli in a Multiple Nutrient Environment. PLoS ONE. 2010;5:e14184. doi: 10.1371/JOURNAL.PONE.0014184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schar D, Sommanustweechai A, Laxminarayan R, Tangcharoensathien V. Surveillance of antimicrobial consumption in animal production sectors of low- and middle-income countries: Optimizing use and addressing antimicrobial resistance. PLoS Med. 2018;15:e1002521. doi: 10.1371/JOURNAL.PMED.1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüsener MP, Bester K. Persistence of antibiotics such as macrolides, tiamulin and salinomycin in soil. Environ Pollut. 2006;143:565–571. doi: 10.1016/J.ENVPOL.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Sharma L, Siedlewicz G, Pazdro K. The Toxic Effects of Antibiotics on Freshwater and Marine Photosynthetic Microorganisms: State of the Art. Plants. 2021;10:1–15. doi: 10.3390/PLANTS10030591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AC, Shaw H, Rhodes V, Hart A. Review of antimicrobial resistance in the environment and its relevance to environmental regulators. Front Microbiol. 2016;7:1728. doi: 10.3389/FMICB.2016.01728/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim WJ, Lee JW, Lee ES, et al. Occurrence and distribution of pharmaceuticals in wastewater from households, livestock farms, hospitals and pharmaceutical manufactures. Chemosphere. 2011;82:179–186. doi: 10.1016/J.CHEMOSPHERE.2010.10.026. [DOI] [PubMed] [Google Scholar]
- Skariyachan S, Mahajanakatti AB, Grandhi NJ, et al. Environmental monitoring of bacterial contamination and antibiotic resistance patterns of the fecal coliforms isolated from Cauvery River, a major drinking water source in Karnataka, India. Environ Monit Assess. 2015;187:1–13. doi: 10.1007/S10661-015-4488-4/FIGURES/6. [DOI] [PubMed] [Google Scholar]
- Sodhi KK, Kumar M, Balan B, et al. Perspectives on the antibiotic contamination, resistance, metabolomics, and systemic remediation. SN Appl Sci. 2021;3:1–25. doi: 10.1007/S42452-020-04003-3/FIGURES/2. [DOI] [Google Scholar]
- Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: A call to action for the medical community from the infectious diseases society of America. Clin Infect Dis. 2008;46:155–164. doi: 10.1086/524891/2/46-2-155-FIG002.GIF. [DOI] [PubMed] [Google Scholar]
- Springmann M, Godfray HCJ, Rayner M, Scarborough P. Analysis and valuation of the health and climate change cobenefits of dietary change. Proc Natl Acad Sci U S A. 2016;113:4146–4151. doi: 10.1073/PNAS.1523119113/SUPPL_FILE/PNAS.1523119113.SAPP.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell VO, Duffy B. Use of antibiotics in plant agriculture. Rev Sci Tech. 2012;31:199–210. doi: 10.20506/RST.31.1.2104. [DOI] [PubMed] [Google Scholar]
- Sudha S, Mridula C, Silvester R, Hatha AAM. Prevalence and antibiotic resistance of pathogenic Vibrios in shellfishes from Cochin market. Indian J Geo-Marine Sci. 2014;43:815–824. [Google Scholar]
- Su H, Liu S, Hu X, et al. Occurrence and temporal variation of antibiotic resistance genes (ARGs) in shrimp aquaculture: ARGs dissemination from farming source to reared organisms. Sci Total Environ. 2017;607–608:357–366. doi: 10.1016/J.SCITOTENV.2017.07.040. [DOI] [PubMed] [Google Scholar]
- Sui Q, Huang J, Deng S, et al. Occurrence and removal of pharmaceuticals, caffeine and DEET in wastewater treatment plants of Beijing, China. Water Res. 2010;44:417–426. doi: 10.1016/J.WATRES.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Sun C, Dudley S, Trumble J, Gan J. Pharmaceutical and personal care products-induced stress symptoms and detoxification mechanisms in cucumber plants. Environ Pollut. 2018;234:39–47. doi: 10.1016/J.ENVPOL.2017.11.041. [DOI] [PubMed] [Google Scholar]
- Sung SW, Kang CH, Kim YT, et al. Surgery increased the chance of cure in multi-drug resistant pulmonary tuberculosis. Eur J Cardiothorac Surg. 1999;16:187–193. doi: 10.1016/S1010-7940(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Szychowski J, Kondo J, Zahr O, et al. Inhibition of Aminoglycoside-Deactivating Enzymes APH(3′)-IIIa and AAC(6′)-Ii by Amphiphilic Paromomycin O2″-Ether Analogues. ChemMedChem. 2011;6:1961. doi: 10.1002/CMDC.201100346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai PK, Ky LX, Binh VN, et al. Occurrence of antibiotic residues and antibiotic-resistant bacteria in effluents of pharmaceutical manufacturers and other sources around Hanoi, Vietnam. Sci Total Environ. 2018;645:393–400. doi: 10.1016/J.SCITOTENV.2018.07.126. [DOI] [PubMed] [Google Scholar]
- Thilsted SH, Thorne-Lyman A, Webb P, et al. Sustaining healthy diets: The role of capture fisheries and aquaculture for improving nutrition in the post-2015 era. Food Policy. 2016;61:126–131. doi: 10.1016/J.FOODPOL.2016.02.005. [DOI] [Google Scholar]
- Thiolas A, Bornet C, Davin-Régli A, et al. Resistance to imipenem, cefepime, and cefpirome associated with mutation in Omp36 osmoporin of Enterobacter aerogenes. Biochem Biophys Res Commun. 2004;317:851–856. doi: 10.1016/J.BBRC.2004.03.130. [DOI] [PubMed] [Google Scholar]
- Thomas RJ. Neurotoxicity of antibacterial therapy. South Med J. 1994;87:869–874. doi: 10.1097/00007611-199409000-00001. [DOI] [PubMed] [Google Scholar]
- Timmerer U, Lehmann L, Schnug E, Bloem E. Toxic Effects of Single Antibiotics and Antibiotics in Combination on Germination and Growth of Sinapis alba L. Plants. 2020;9:107. doi: 10.3390/PLANTS9010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Acker H, Van Dijck P, Coenye T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014;22:326–333. doi: 10.1016/j.tim.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Van Boeckel TP, Brower C, Gilbert M, et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci USA. 2015;112:5649–5654. doi: 10.1073/PNAS.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedantam G, Guay GG, Austria NE, et al. Characterization of mutations contributing to sulfathiazole resistance in Escherichia coli. Antimicrob Agents Chemother. 1998;42:88–93. doi: 10.1128/AAC.42.1.88/ASSET/01CBDE71-6151-4A2C-B7FB-E2ACC87E4D99/ASSETS/GRAPHIC/AC0180370002.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzulli L, Grande C, Reid PC, et al. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proc Natl Acad Sci USA. 2016;113:E5062–E5071. doi: 10.1073/PNAS.1609157113/SUPPL_FILE/PNAS.201609157SI.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt M, Jaeger M. On the photodegradation of azithromycin, erythromycin and tylosin and their transformation products—a kinetic study. Sustain Chem Pharm. 2017;5:131–140. doi: 10.1016/J.SCP.2016.12.001. [DOI] [Google Scholar]
- Wang X, Ryu D, Houtkooper RH, Auwerx J. Antibiotic use and abuse: A threat to mitochondria and chloroplasts with impact on research, health, and environment. BioEssays. 2015;37:1045–1053. doi: 10.1002/BIES.201500071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Shen W, Yan L, et al. Stepwise impact of urban wastewater treatment on the bacterial community structure, antibiotic contents, and prevalence of antimicrobial resistance. Environ Pollut. 2017;231:1578–1585. doi: 10.1016/J.ENVPOL.2017.09.055. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Hodges GR, Dworzack DL, et al. Neurotoxicity of intrathecal gentamicin: A case report and experimental study. Ann Neurol. 1978;4:564–572. doi: 10.1002/ANA.410040618. [DOI] [PubMed] [Google Scholar]
- Wegener HC (2012) A15 Antibiotic resistance—linking human and animal health. In: Institute of Medicine (US). Improving Food Safety through a One Health Approach: Workshop Summary. National Academies Press, Washington, DC, USA, pp 331–349) [PubMed]
- Wohde M, Berkner S, Junker T, et al. Occurrence and transformation of veterinary pharmaceuticals and biocides in manure: a literature review. Environ Sci Eur. 2016;28:1–25. doi: 10.1186/S12302-016-0091-8/FIGURES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GD. Something old, something new: Revisiting natural products in Antibiotic drug discovery. Can J Microbiol. 2014;60:147–154. doi: 10.1139/CJM-2014-0063/ASSET/IMAGES/LARGE/CJM-2014-0063F1.JPEG. [DOI] [PubMed] [Google Scholar]
- Yang L, Feng YX, Zhang H, Yu XZ. Estimating the synergistic and antagonistic effects of dual antibiotics on plants through root elongation test. Ecotoxicology. 2021;30:1598–1609. doi: 10.1007/S10646-020-02308-Y/FIGURES/4. [DOI] [PubMed] [Google Scholar]
- Yang SJ, Kreiswirth BN, Sakoulas G, et al. Enhanced expression of dltABCD is associated with the development of daptomycin nonsusceptibility in a clinical endocarditis isolate of Staphylococcus aureus. J Infect Dis. 2009;200:1916–1920. doi: 10.1086/648473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerushalmi H, Lebendiker M, Schuldiner S. EmrE, an Escherichia coli 12-kDa Multidrug Transporter, Exchanges Toxic Cations and H+ and Is Soluble in Organic Solvents. J Biol Chem. 1995;270:6856–6863. doi: 10.1074/JBC.270.12.6856. [DOI] [PubMed] [Google Scholar]
- Zazueta-Beltran J, Muro-Amador S, Flores-Gaxiola A, et al. High rates of multidrug-resistant Mycobacterium tuberculosis in Sinaloa State, Mexico. J Infect. 2007;54:411–412. doi: 10.1016/j.jinf.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Zhang T, Li B. Occurrence, Transformation, and Fate of Antibiotics in Municipal Wastewater Treatment Plants. Crit Rev Environ Sci Technol. 2011;41:951–998. doi: 10.1080/10643380903392692. [DOI] [Google Scholar]
- Zhu XQ, Wang XM, Li H, et al. Novel lnu(G) gene conferring resistance to lincomycin by nucleotidylation, located on Tn6260 from Enterococcus faecalis E531. J Antimicrob Chemother. 2017;72:993–997. doi: 10.1093/JAC/DKW549. [DOI] [PubMed] [Google Scholar]
- Zignol M, Hosseini MS, Wright A, et al. Global Incidence of Multidrug-Resistant Tuberculosis. J Infect Dis. 2006;194:479–485. doi: 10.1086/505877. [DOI] [PubMed] [Google Scholar]
- Understanding the chemical basis of drug stability and degradation - The Pharmaceutical Journal. https://pharmaceutical-journal.com/article/ld/understanding-the-chemical-basis-of-drug-stability-and-degradation. Accessed 5 Nov 2022
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within this article.