Abstract
Background
The burden of non-small cell lung cancer (NSCLC) remains high in Spain, with lung cancer accounting for 20% of cancer-related deaths annually. Programs such as the Spanish Thoracic Tumour Registry (TTR) and the global I-O Optimise initiative have been developed to observe patients in clinical practice with the aim of improving outcomes. This analysis examined treatment patterns and survival in patients with stage III NSCLC from the TTR. These patients represent a heterogenous group with complex treatment pathways.
Methods
The TTR is an ongoing, observational, prospective, and retrospective cohort multicentre study (NCT02941458) that follows patients with thoracic cancer in Spain. Adults aged ≥18 years with stage IIIA/IIIB NSCLC enrolled in the TTR between 01 Jan 2010 and 31 Oct 2019 were included in this analysis. Initial treatment received was described by cancer stage and histology (squamous and non-squamous NSCLC). Kaplan-Meier estimates of progression-free survival (PFS) and overall survival (OS) were calculated over a 5-year period.
Results
A total of 1,838 patients were included in the cohort, including 1,082 with stage IIIA (58.9%) and 756 with stage IIIB (41.1%). Median follow-up was 18.3 months. The median age of patients was 66 years, and most had non-squamous NSCLC (54.0%), were male (81.2%), and were active or former smokers (93.4%). Overall, 26.3% of patients received surgical resection (37.0% for stage IIIA and 11.1% for stage IIIB). The most frequent initial treatment received was concurrent chemoradiotherapy for stage IIIA (30.2%) and stage IIIB (37.0%) patients. Median OS was lower in patients with stage IIIB than stage IIIA (28 vs. 37 months) disease and was lower for patients with squamous than non-squamous histology (19 vs. 26 months). Median PFS and OS varied when patients were stratified by initial treatment.
Conclusions
This TTR analysis describes the clinical reality surrounding the initial management and survival outcomes for stage III NSCLC in Spain and presents survival outcomes comparable with other real-world evidence. It provides insights into the diverse approaches used before the availability of immunotherapies and targeted treatments in the non-metastatic NSCLC setting.
Keywords: Lung cancer, non-small cell lung cancer (NSCLC), cancer registry, I-O optimise, Spain
Highlight box.
Key findings
• The Thoracic Tumour Registry (TTR) is an observational study following patients with thoracic disease in Spain; this analysis of TTR data highlights the diverse approaches adopted for the treatment of stage III non-small cell lung cancer (NSCLC) resulting in variable survival outcomes over 5 years.
What is known and what is new?
• Patients with stage III NSCLC represent a heterogenous group with complex treatment pathways.
• This manuscript provides insights into the management of these patients in real-world settings in Spain using data from Jan 2010 to Oct 2019 (prior to durvalumab approval in this setting).
What is the implication, and what should change now?
• These findings highlight gaps in our current treatment pathway of stage III NSCLC, and illustrate how overall survival was impacted by treatment choice (3.9–69.4 months); it will be important to monitor how new therapies and changes to guidelines improve survival in real-world settings.
Introduction
The disease burden associated with lung cancer remains high. In 2020, the number of new cases of lung cancer worldwide was 2,206,771 (11.4% of all new cancer cases) and the associated mortality was 1,796,144 (18.0% of cancer deaths) (1). In Europe, lung cancer accounted for 384,176 (21.4%) cancer deaths in 2020; the death rate was similarly high in Spain (20.3%; 22,930), highlighting the need for lung cancer initiatives in this country (2,3). Non-small cell lung cancer (NSCLC) accounts for most lung cancer cases (84%); it has a poor prognosis, with a 5-year overall survival (OS) of 34–61% (non-metastatic) and 7% (metastatic) (4). The 5-year survival in patients with NSCLC stage IIIA and IIIB is 26.2% and 17.3%, respectively (5). NSCLC is often diagnosed at a later stage due to lack of obvious symptoms (6), which can make the management of this disease challenging.
For the management of locally advanced stage III NSCLC, the European Society for Medical Oncology (ESMO)-guided treatment pathways recommend surgical resection wherever possible (7,8). The exact treatment pathway will depend on a multidisciplinary team assessment including positron emission tomography-computed tomography scans and pathological staging. For patients with resectable stage III disease, surgery with curative intent, with or without (neo)adjuvant treatments is recommended. At the time of this study, definitive concurrent chemoradiotherapy (usually with platinum-based chemotherapy with cisplatin) was the treatment of choice for patients with unresectable stage IIIA/IIIB disease, with a sequential approach as an alternative if needed (7). Programmed death ligand 1 (PD-L1) checkpoint inhibitors were being evaluated during this time as (neo)adjuvant treatment for patients with resectable disease or as consolidation therapy after chemoradiotherapy in those with unresectable disease, and they are now recommended for use in this setting (8). Durvalumab was also recently approved by the European Medicines Agency (EMA) in 2018 as maintenance therapy for unresectable stage III NSCLC tumours with PD-L1 expression ≥1% (9,10), and the ESMO guidelines were updated in 2021 to reflect this recommendation (8).
It is essential to monitor how treatment modalities are being implemented in clinical practice in relation to outcomes. To address this, the I-O Optimise initiative was developed in 2017. I-O Optimise is a multinational collaborative research framework that is providing insights into the real-world management of thoracic malignancies, including NSCLC, with the aim of improving long-term clinical and safety outcomes (11). To date, an I-O Optimise network has been established with thoracic data sources from Europe (Denmark, France, Germany, Norway, Portugal, Sweden, and the UK) and Canada. The Spanish Lung Cancer Group (Grupo Español de Cáncer de Pulmón; GECP) has recently collaborated with the I-O Optimise network in association with the ongoing nationwide Thoracic Tumour Registry (TTR) study from Spain.
To gain a greater understanding of the management of stage III NSCLC, we performed further analysis of data from the TTR. The primary aim of this analysis was to observe treatment patterns and survival outcomes in patients with stage III NSCLC in Spain. These patients represent a heterogenous group of patients presenting with resectable and unresectable disease, and the treatment pathways are complex. A greater understanding of how these patients are being treated in real-world settings may help to tailor treatment pathways and improve outcomes. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-176/rc).
Methods
Study design and TTR overview
This is an observational study based on the TTR cohort. Patients with stage IIIA and IIIB NSCLC diagnosed between 01 Jan 2010 and 31 Oct 2019 were included. All patients were followed from their initial NSCLC diagnosis (index date) until 31 Oct 2020 (interim study end), death, or known exit from the TTR (whichever occurred first).
The TTR is an ongoing, observational, prospective and retrospective, cohort, multicentre study (ClinicalTrials.gov identifier NCT02941458) that includes patients with thoracic cancers (12). As of Jul 2022, 25,018 patients with thoracic cancers from 88 centres have been included in the TTR. The TTR was initially created in 2016 by the Spanish Lung Cancer Group (GECP), a multidisciplinary oncology group that was established in 1991 to promote lung cancer research. The GECP consists of more than 400 specialists from a network of 160 hospitals in Spain.
Ethics
The TTR was approved in 2016 by the Spanish Agency for Medicines and Medical Devices (AEMPS) and the institutional review board at each study site (12). The current analysis of the TTR was performed in accordance with the principles of the Declaration of Helsinki [2013] (13), and the International Society for Pharmacoepidemiology (ISPE) Guidelines for Good Pharmacoepidemiology Practices (GPP) (14). The study was approved by the GECP ethics committee (IRB Number PI 148.15). For prospective enrolment, informed consent was taken from all individual participants. For retrospective enrolment, individual consent for this retrospective analysis was waived.
Patient population and inclusion criteria
Men and women aged ≥18 years with a diagnosis of incident stage IIIA/IIIB NSCLC based on the 7th [2012] and 8th [2016] tumor-node-metastasis (TNM) classification (as this study was performed over a 9-year period it captured both versions depending on year of diagnosis) and clinical staging system from Jan 2010 to Jan 2019 (using ICD-10 codes C33.0, C34.0, C34.1, C34.2, C34.3, C34.8, and C34.9) who were enrolled in the TTR were included in this analysis. Stage IIIC was introduced in the 8th TNM staging edition in 2017, as such no patient records with acceptable data quality were available at the time of this study. Only data from contributing centres with large populations (n≥100) and robust linkage to mortality data were included from the TTR; patients diagnosed outside of the study period or with poor-quality data were excluded.
Design and data collection
Data on patient diagnosis were collected from 01 Jan 2010 to 31 Oct 2019 (latest data available at the time of the extraction) and follow-up data for treatments, disease progression, and death were collected until 31 Oct 2020; this timeframe was deemed sufficient to provide an overview of clinical care in Spain.
Extracted data were recorded in an electronic case report form (eCRF); these were maintained in accordance with updates from participating study sites. The following variables were recorded in the eCRF: sociodemographic and clinical characteristics including histology, Eastern Cooperative Oncology Group (ECOG) performance status, biomarker tests performed, NSCLC diagnosis (index date), initial treatments received, and death (date and cause) or last documented entry. All variables were entered in the eCRF by one reviewer and quality control was undertaken by a second independent reviewer.
Statistical analysis
Descriptive statistics were performed; continuous data were summarised as mean, first and third quartiles (Q1 and Q3), standard deviation (SD), median, minimum, and maximum, and categorical variables were described using percentages. No imputation methods were used to handle missing data except for date of birth; however, this was not required as all dates were complete.
Initial treatment was defined as the first date of radiotherapy administration, surgery, or first dose or prescription of systemic anticancer therapy (SACT) within 6 months of an NSCLC stage III diagnosis (index date). The proportion of patients receiving treatment and the frequency of treatment types were stratified by cancer stage (overall, IIIA, or IIIB NSCLC) and histology (squamous and non-squamous NSCLC). The initial treatments received were categorised into seven groups, which are regarded as the main therapeutic approaches in this setting: (I) neoadjuvant SACT then surgery, (II) surgery with adjuvant SACT, (III) surgery alone, (IV) sequential chemoradiotherapy, (V) concurrent chemoradiotherapy, (VI) SACT ± palliative radiotherapy, or (VII) radiotherapy alone. The rules applied to define these categories using TTR longitudinal data regarding treatment exposure are defined in Table S1. The list of SACT recorded as initial therapy is summarised in Table S2.
OS and progression-free survival (PFS) for treated patients stratified by cancer stage (overall, IIIA, or IIIB), histology, and initial treatment received were described using Kaplan-Meier methods. Kaplan-Meier estimates of PFS, OS, and treatment duration were calculated over a 5-year period. OS was defined as the time from NSCLC diagnosis (index date) to death due to any cause. PFS was defined as the time from the treatment start until the earliest date based on progression (as recorded in the database), start of next line of therapy, death, lost to follow-up (censored), or study end (censored).
The clinical characteristics associated with surgical resection and use of neoadjuvant therapy were also described. Treatment response in patients treated with chemoradiotherapy (i.e., any treatment group containing SACT) was also recorded and defined using complete response, partial response, stable disease, progressive disease, not evaluable, or not performed.
Univariate analyses were performed to identify sociodemographic and clinical covariates with a significant association with neoadjuvant treatment and surgical resection. These covariates, and any that were of clinical interest, were then entered into a multivariable selection model that used LASSO (least absolute shrinkage and selection operator) to reduce and select the final covariates. These covariates, together with sex and TN staging, were included in a multivariate regression model that examined their association with neoadjuvant therapy use (planned and actual) and surgical resection.
All statistical analyses were performed using R (version 4.1.2) and R Studio (version 2021.09.1+372) software.
Results
Patient cohort
Overall, 3,751 patients were diagnosed with stage III NSCLC and 1,838 patients met the inclusion criteria from which 1,082 (58.9%) were diagnosed at stage IIIA and 756 (41.1%) at stage IIIB NSCLC and were followed up for a median of 18.3 months (Table S3 and Table 1). Most patients had non-squamous (adenocarcinoma, large-cell carcinoma, specified/non-specified) NSCLC (54.0%), were male (81.2%), with a median age of 66 years at diagnosis, and were active or former smokers (93.4%). Most patients had ECOG performance status 0 (fully active; 47.1%) or 1 (strenuous physical activity restricted; 44.2%). PD-L1 status was obtained in 29.4% of patients, with slightly more testing for stage IIIB (32.4%) than stage IIIA (27.4%) NSCLC. The PD-L1 status was ≥1% in 53.8% of patients who were tested (Table 1).
Table 1. Patient demographics and clinical characteristics by stage.
| Patient demographics and clinical characteristics | Total | Stage IIIA NSCLC | Stage IIIB NSCLC |
|---|---|---|---|
| Patients, n (%) | 1,838 (100.0) | 1,082 (58.9) | 756 (41.1) |
| Age (years) at NSCLC diagnosis, median (Q1–Q3) | 66.3 (59.2–73.0) | 66.5 (59.8–73.0) | 65.8 (58.0–73.1) |
| Male, n (%) | 1,493 (81.2) | 889 (82.2) | 604 (79.9) |
| Ethnicity, n (%) | |||
| Caucasian | 1,827 (99.4) | 1,076 (99.4) | 751 (99.3) |
| Latin | 7 (0.4) | 4 (0.4) | 3 (0.4) |
| African | 2 (0.1) | 0 | 2 (0.3) |
| Other | 2 (0.1) | 2 (0.2) | 0 |
| Smoking status, n (%) | |||
| Active smoker | 761 (41.4) | 440 (40.7) | 321 (42.5) |
| Former smoker (not smoked within 12 months) | 956 (52.0) | 567 (52.4) | 389 (51.5) |
| Never smoker (≤100 cigarettes ever smoked) | 112 (6.1) | 69 (6.4) | 43 (5.7) |
| Missing | 9 (0.5) | 6 (0.6) | 3 (0.4) |
| T stage | |||
| T0 | 1 (0.1) | 0 | 1 (0.1) |
| T1 | 179 (9.7) | 122 (11.3) | 57 (7.5) |
| T2 | 387 (21.1) | 304 (28.1) | 83 (11.0) |
| T3 | 448 (24.4) | 281 (26.0) | 167 (22.1) |
| T4 | 736 (40.0) | 330 (30.5) | 406 (53.7) |
| Tx | 78 (4.2) | 42 (3.9) | 36 (4.8) |
| Missing | 9 (0.5) | 3 (0.3) | 6 (0.8) |
| N stage, n (%) | |||
| N0 | 239 (13.0) | 239 (22.1) | 0 |
| N1 | 193 (10.5) | 192 (17.7) | 1 (0.1) |
| N2 | 1,064 (57.9) | 621 (57.4) | 443 (58.6) |
| N3 | 288 (15.7) | 0 | 288 (38.1) |
| Nx | 46 (2.5) | 27 (2.5) | 19 (2.5) |
| Missing | 8 (0.4) | 3 (0.3) | 5 (0.7) |
| M stage, n (%) | |||
| M0 | 1,746 (95.0) | 1,038 (95.9) | 708 (93.7) |
| M1 | 3 (0.2) | 1 (0.1) | 2 (0.3) |
| Mx | 81 (4.4) | 40 (3.7) | 41 (5.4) |
| Missing | 8 (0.4) | 3 (0.3) | 5 (0.7) |
| ECOG performance status, n (%) | |||
| 0 | 865 (47.1) | 550 (50.8) | 315 (41.7) |
| 1 | 812 (44.2) | 442 (40.9) | 370 (48.9) |
| 2 | 131 (7.1) | 74 (6.8) | 57 (7.5) |
| 3 | 27 (1.5) | 14 (1.3) | 13 (1.7) |
| 4 | 2 (0.1) | 1 (0.1) | 1 (0.1) |
| Missing | 1 (0.1) | 1 (0.1) | 0 |
| Histology, n (%) | |||
| Non-squamous cell and others† | 993 (54.0) | 588 (54.3) | 405 (53.6) |
| Squamous-cell | 845 (46.0) | 494 (45.7) | 351 (46.4) |
| PD-L1 test performed‡, n (%) | |||
| Yes | 541 (29.4) | 296 (27.4) | 245 (32.4) |
| No | 1,297 (70.6) | 786 (72.6) | 511 (67.6) |
| PD-L1 status (in those tested) | |||
| Positive | 291 (53.8) | 159 (53.7) | 132 (53.9) |
| Negative | 220 (40.7) | 123 (41.6) | 97 (39.6) |
| Unknown | 1 (0.2) | 1 (0.3) | 0 |
| Missing | 29 (5.4) | 13 (4.4) | 16 (6.5) |
| Time (days) from diagnosis to initial treatment, median (Q1–Q3) | 37.0 (22.0–62.0) | 36.0 (20.0–62.0) | 37.0 (23.0–61.0) |
| Time (months) from diagnosis to end of follow-up, median (Q1–Q3) | 18.3 (9.2–34.3) | 20.3 (10.7–36.8) | 15.7 (7.5–28.5) |
†, includes adenocarcinoma, large cell carcinoma, other NSCLC (specified or non-specified); ‡, biomarker test results recorded closest to initial treatment, if unavailable, tests performed at diagnosis were used. NSCLC, non-small cell lung cancer; Q, quartile; ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death ligand 1.
Initial treatment patterns
The initial treatments received in patients diagnosed with stage III NSCLC are shown in Table 2. In total, 26.3% of patients received surgical resection (37.0% of patients with stage IIIA NSCLC and 11.1% with stage IIIB NSCLC). The most common procedure was (bi)lobectomy or pneumonectomy (18.7% overall). The breakdown of surgical intervention by initial treatment group is shown in Table S1.
Table 2. Treatment patterns in patients with stage III NSCLC.
| Treatment pattern | Total stage III NSCLC (N=1,838) | Stage IIIA NSCLC (n=1,082) | Stage IIIB NSCLC (n=756) |
|---|---|---|---|
| Surgery, n (%) | 484 (26.3) | 400 (37.0) | 84 (11.1) |
| (Bi)lobectomy or pneumonectomy | 344 (18.7) | 295 (27.3) | 49 (6.5) |
| Other and unspecified procedures | 88 (4.8) | 62 (5.7) | 26 (3.4) |
| Other and unspecified resection | 19 (1.0) | 14 (1.3) | 5 (0.7) |
| Other lung or tracheobronchial resection | 19 (1.0) | 16 (1.5) | 3 (0.4) |
| Thoraco-mediastinal resection | 0 | 0 | 0 |
| Missing | 14 (0.8) | 13 (1.2) | 1 (0.1) |
| Any radiotherapy, n (%) | 1,095 (59.6) | 609 (56.3) | 486 (64.3) |
| Planned neoadjuvant treatment, n (%) | 281 (15.3) | 216 (20.0) | 65 (8.6) |
| Planned neoadjuvant treatment and went on to receive surgery, n (%) | 138 (7.5) | 113 (10.4) | 25 (3.3) |
| Initial treatment received, n (%) | |||
| Neoadjuvant SACT then surgery | 133 (7.2) | 105 (9.7) | 28 (3.7) |
| Surgery with adjuvant SACT | 254 (13.8) | 211 (19.5) | 43 (5.7) |
| Surgery alone | 92 (5.0) | 80 (7.4) | 12 (1.6) |
| Sequential chemoradiotherapy | 193 (10.5) | 91 (8.4) | 102 (13.5) |
| Concurrent chemoradiotherapy | 607 (33.0) | 327 (30.2) | 280 (37.0) |
| SACT ± palliative radiotherapy | 373 (20.3) | 168 (15.5) | 205 (27.1) |
| Radiotherapy alone | 80 (4.4) | 45 (4.2) | 35 (4.6) |
| No treatment | 92 (5.0) | 45 (4.2) | 47 (6.2) |
| Other treatments† | 14 (0.8) | 10 (0.9) | 4 (0.5) |
| Time (days) from start of neoadjuvant SACT to surgery | |||
| n (%) | 124 (6.7) | 100 (9.2) | 24 (3.2) |
| Median (Q1–Q3) | 96.5 (82.0–118.0) | 98.5 (85.0–121.0) | 83.0 (68.0–111.0) |
| Completeness of resection, n (%) | |||
| R0 | 75 (60.5) | 62 (62.0) | 13 (54.2) |
| R1 | 7 (5.6) | 5 (5.0) | 2 (8.3) |
| R2 | 7 (5.6) | 5 (5.0) | 2 (8.3) |
| Missing | 35 (28.2) | 28 (28.0) | 7 (29.2) |
| Time (days) from start of surgery to adjuvant SACT | |||
| n (%) | 254 (13.8) | 211 (19.5) | 43 (5.7) |
| Median (Q1–Q3) | 43.5 (35.0–54.0) | 43.0 (35.0–54.0) | 46.0 (35.0–55.0) |
†, includes surgery with (neo)adjuvant radiotherapy, curative SACT with radiotherapy, concurrent chemoradiotherapy with immunotherapies, and other SACT with radiotherapy. NSCLC, non-small cell lung cancer; SACT, systemic anticancer therapy; Q, quartile.
Concurrent chemoradiotherapy was the most common initial treatment for patients with both stage IIIA (30.2%) and IIIB (37.0%) NSCLC. The next most used initial treatment was surgery with adjuvant SACT for patients at stage IIIA (19.5%) and SACT ± palliative radiotherapy for those at stage IIIB (27.1%). When SACT was used in the adjuvant setting (13.8%), treatment was started within a median of 43.5 days post surgery. When SACT was used in the neoadjuvant setting (7.2%), surgery was performed within a median of 96.5 days from start of preoperative/neoadjuvant SACT (98.5 days for stage IIIA and 83.0 days for stage IIIB). Of the patients who were intended to receive neoadjuvant therapy followed by surgery, 52% (n=113/216) with stage IIIA and 38% (n=25/65) with stage IIIB NSCLC went on to receive surgery. Among patients receiving neoadjuvant SACT, R0 resection was achieved (62.0% for stage IIIA and 54.2% for stage IIIB).
OS and PFS
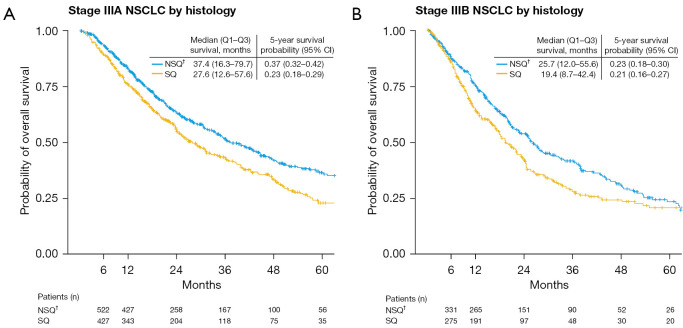
Median (Q1 to Q3) OS was lower in patients with stage IIIB than stage IIIA NSCLC and was also lower in patients with squamous than non-squamous NSCLC [27.6 (12.6 to 57.6) vs. 37.4 (16.3 to 79.7) months for stage IIIA and 19.4 (8.7 to 42.4) vs. 25.7 (12.0 to 55.6) months for stage IIIB, respectively] (Figure 1).
Figure 1.
Kaplan-Meier estimates of overall survival in patients with stage (A) IIIA or (B) IIIB NSCLC by histology. †, includes adenocarcinoma, large cell carcinoma, other NSCLC (specified or non-specified). NSCLC, non-small cell lung cancer; Q, quartile; NSQ, non-squamous; SQ, squamous; CI, confidence interval.
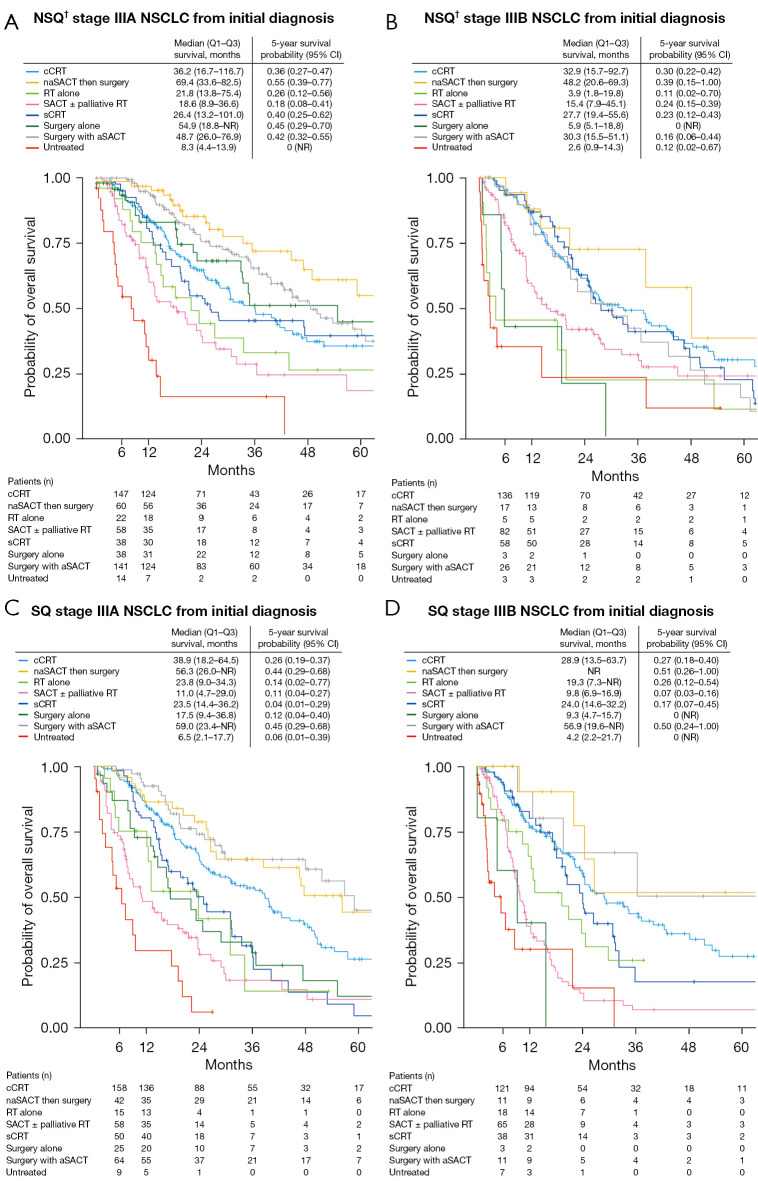
Median OS also varied when patients were stratified by initial treatment use, and ranged from 3.9 months (stage IIIB, non-squamous, radiotherapy alone) to 69.4 months (stage IIIA, non-squamous, neoadjuvant SACT then surgery) (Figure 2). Median OS for patients receiving neoadjuvant SACT then surgery was 69.4 (non-squamous) and 56.3 months (squamous) for stage IIIA and 48.2 months (non-squamous) for stage IIIB NSCLC (OS was not reached in patients with stage IIIB squamous histology). Median OS for patients receiving surgery with adjuvant SACT was 48.7 (non-squamous) and 59.0 months (squamous) for stage IIIA and 30.3 (non-squamous) and 56.9 months (squamous) for stage IIIB NSCLC. Median OS for patients receiving chemoradiotherapy ranged from 28.9 [stage IIIB (squamous)] to 38.9 months [stage IIIA (squamous)] (concurrent) and from 23.5 [stage IIIA (squamous)] to 27.7 months [stage IIIB (non-squamous)] (sequential) (Figure 2).
Figure 2.
Kaplan-Meier estimates of overall survival in patients with (A) NSQ† stage IIIA, (B) NSQ† stage IIIB, (C) SQ stage IIIA, and (D) SQ stage IIIB NSCLC from diagnosis. †, includes adenocarcinoma, large cell carcinoma, other NSCLC (specified or non-specified). NSQ, non-squamous; NSCLC, non-small cell lung cancer; Q, quartile; CI, confidence interval; cCRT, concurrent chemoradiotherapy; naSACT, neoadjuvant systemic anticancer therapy; RT, radiotherapy; SACT, systemic anticancer therapy; sCRT, sequential chemoradiotherapy; NR, not reached; aSACT, adjuvant systemic anticancer therapy; SQ, squamous.
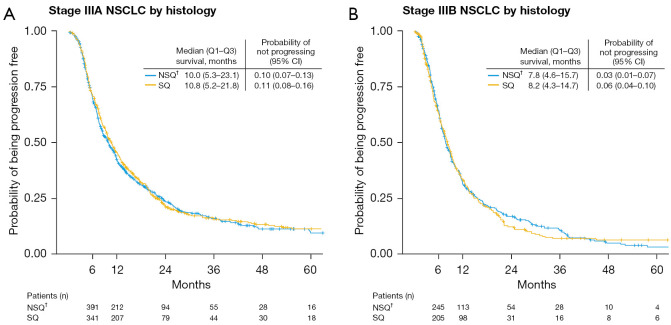
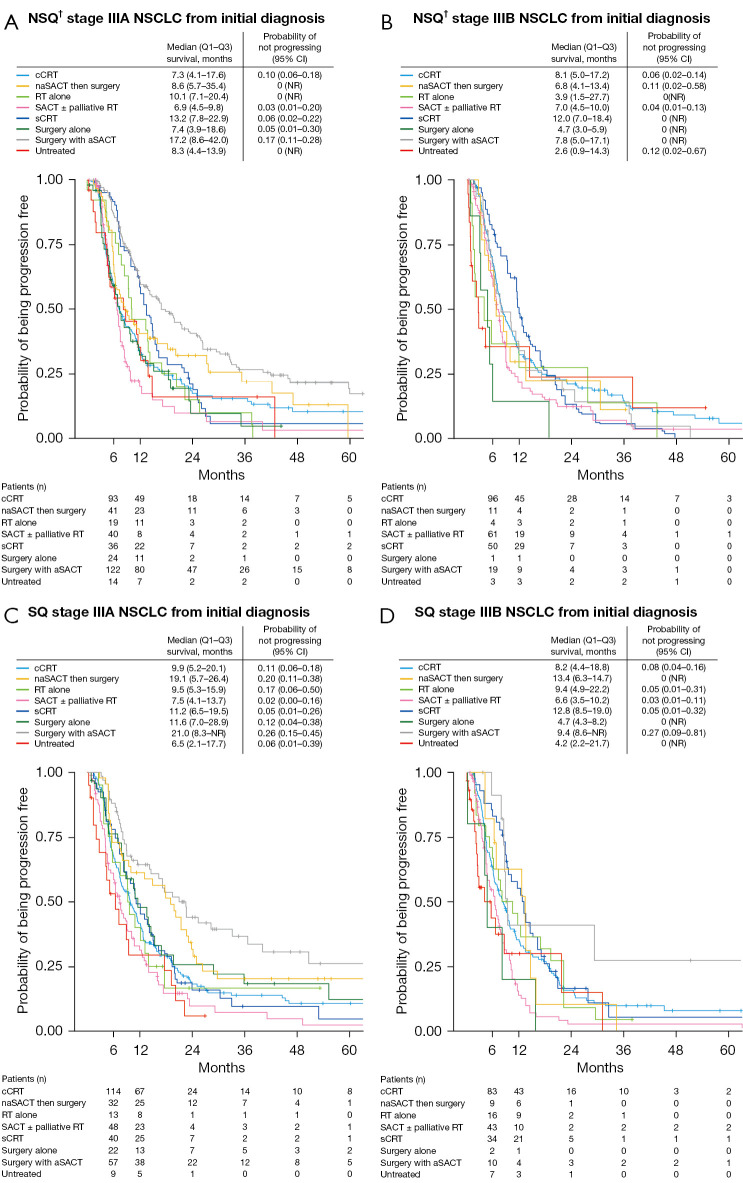
Median PFS was approximately 10 months in stage IIIA NSCLC and 8 months in stage IIIB NSCLC and was similar in patients with non-squamous and squamous histologies (Figure 3). Median PFS varied widely when patients were stratified by initial treatment used, and ranged from 3.9 (stage IIIB, non-squamous, radiotherapy alone) to 21.0 months (stage IIIA, squamous, surgery then adjuvant SACT) (Figure 4).
Figure 3.
Kaplan-Meier estimates of progression-free survival in patients with stage (A) IIIA or (B) IIIB NSCLC by histology. †, includes adenocarcinoma, large cell carcinoma, other NSCLC (specified or non-specified). NSCLC, non-small cell lung cancer; Q, quartile; NSQ, non-squamous; SQ, squamous; CI, confidence interval.
Figure 4.
Kaplan-Meier estimates of progression-free survival in patients with (A) NSQ† stage IIIA, (B) NSQ† stage IIIB, (C) SQ stage IIIA, and (D) SQ stage IIIB NSCLC from diagnosis. †, includes adenocarcinoma, large cell carcinoma, other NSCLC (specified or non-specified). NSQ, non-squamous; NSCLC, non-small cell lung cancer; Q, quartile; CI, confidence interval; cCRT, concurrent chemoradiotherapy; naSACT, neoadjuvant systemic anticancer therapy; NR, not reached; RT, radiotherapy; SACT, systemic anticancer therapy; sCRT, sequential chemoradiotherapy; aSACT, adjuvant systemic anticancer therapy; SQ, squamous.
Chemoradiotherapy treatment response
Treatment response for stage III NSCLC patients who received chemoradiotherapy is shown in Table 3. Of the patients with evaluable chemoradiotherapy data, 7.0% had complete response and 37.9% had partial response. Complete response rate was slightly higher among patients with stage IIIA NSCLC (9.1%) than stage IIIB NSCLC (4.1%).
Table 3. Treatment response in patients with stage III NSCLC treated with chemotherapy.
| Treatment response | Total stage III NSCLC with chemotherapy (N=1,757) | Stage IIIA NSCLC (n=1,024) | Stage IIIB NSCLC (n=733) |
|---|---|---|---|
| Patients treated with chemotherapy, n (%) | 1,757 (100.0) | 1,024 (100.0) | 733 (100.0) |
| Complete response | 123 (7.0) | 93 (9.1) | 30 (4.1) |
| Partial response | 666 (37.9) | 394 (38.5) | 272 (37.1) |
| Stable disease | 225 (12.8) | 109 (10.6) | 116 (15.8) |
| Progressive disease | 194 (11.0) | 98 (9.6) | 96 (13.1) |
| Not listed† | 549 (31.2) | 330 (32.2) | 219 (29.9) |
†, includes not evaluable, not performed, and missing data. NSCLC, non-small cell lung cancer.
Factors associated with surgical resection and neoadjuvant therapy
Multivariate regression analyses revealed that patients were more likely to receive surgical resection if they were younger, had lower TN staging, had lower ECOG performance status, or had squamous histology (Figure S1). Patients were more likely to receive (or have planned receipt of) neoadjuvant treatment in stage IIIA NSCLC if they had higher TN staging and squamous histology (Figure S2) compared with all other patients receiving surgery as their initial treatment. There were no factors significantly associated with neoadjuvant treatment choice in stage IIIB NSCLC.
Discussion
This analysis of the TTR study provides insight into the diverse approaches adopted for the treatment of stage III NSCLC in Spain from 2010 to 2019 and their impact on survival outcomes. Consistent with previous studies and recently reported incidence figures (12,15), the majority of patients in the TTR were male (81.2%). Concurrent chemoradiotherapy was found to be the most common initial treatment used for patients with stages IIIA and IIIB NSCLC in Spain during this period irrespective of stage or histology. (Neo)adjuvant therapy was more common in patients with stage IIIA NSCLC, and SACT ± palliative radiotherapy was more commonly used in patients with stage IIIB.
Survival outcomes were affected by several factors, including initial treatment choice. Concurrent chemoradiotherapy was associated with survival of 29–39 months. The OS in patients receiving surgery with (neo)adjuvant SACT was high: up to 69 months. Similar findings were observed with PFS, although the effects were less pronounced. Furthermore, patients receiving palliative radiotherapy ± SACT had lower survival in our study; this is not the recommended treatment for stage III and its use may reflect those patients with poorer prognosis whereas patients receiving surgical resection would be those with a better prognosis, i.e., patients who were younger, had smaller tumours, and had less metastatic spread.
These findings are consistent with published data. One study showed that only 20–25% of patients with stage III NSCLC treated with concurrent chemoradiotherapy will survive more than 5 years (16). Survival has been shown to be higher in patients with resectable NSCLC receiving surgery with neoadjuvant therapy. Neoadjuvant chemotherapy was associated with a 13% reduction in the risk of death, resulting in a 5-year survival improvement of 5% (from 40% to 45%) in a meta-analysis of 15 randomised controlled trials (2,385 resectable patients with stage IB to IIIA NSCLC) (17). A more recent study in a real-world setting found that 5-year survival was 47.9% in resectable patients with stage III NSCLC (18). Studies from Spain (NADIM and NADIM II) also showed that the addition of neoadjuvant nivolumab to platinum-based therapy in patients with resectable stage IIIA NSCLC resulted in improved PFS and OS; both studies also included nivolumab as adjuvant monotherapy, which may have impacted PFS and OS (19,20). The OS at 24 months was 84.7% with nivolumab plus chemotherapy vs. 63.4% with chemotherapy [hazard ratio =0.40; 95% confidence interval (CI): 0.17–0.93; P=0.034] in NADIM II (20). Based on these findings, the authors hoped that the perception of locally advanced lung cancer could change from one that is potentially lethal to one that is curable (19).
The surgery rates and median OS observed in our study were generally consistent with those observed in other real-world settings, such as the KINDLE study—a retrospective study of 3,151 patients with stage III NSCLC from 19 countries (in Africa, Asia, Latin America, and the Middle East) in which the curative resection rate was 21.4%, median PFS was 12.5 months, and median OS was 34.9 months; concurrent chemoradiotherapy was also the most common treatment (29.4%) (21). These investigators found significant associations with sex, stage, histology, and ECOG performance status, similar to the findings from the factor analysis models conducted in our study, which emphasises the importance of taking these associations into account when devising treatment plans; this is in line with guideline recommendations (22). We also note the high rate of tobacco use, which is consistent with the smoking prevalence in Spain. This is a key area where lifestyle interventions are warranted.
With respect to PD-L1 expression, a predictor of response to some anti-PD-1/L1 monoclonal antibodies, we found that nearly 30% of patients were tested, and a positive PD-L1 status (≥1%) was observed in >50% of these patients. This large proportion of positive results suggests that it is being used prognostically and that a significant number of patients could benefit from treatment directed at overexpression of PD-L1. The increasing use of PD-1/L1 testing in patients with NSCLC is a key area for further research in the TTR study cohort.
It should be noted, however, that the findings reflect staging and treatment recommendations at the time this study was undertaken [2010–2019], and staging procedures had significantly changed during these years. During this time, concurrent chemoradiotherapy was treatment of choice in unresectable locally advanced stage IIIA or IIIB NSCLC and was usually based on platinum-doublet chemotherapy (7). The initial SACT used in this study was primarily platinum-doublet chemotherapy for stage IIIA [832 (99.4%)] and stage IIIB [587 (97.4%)] NSCLC, which is consistent with the recommendations available during the study period. Surgical resection remains treatment of choice for resectable locally advanced stage III NSCLC (7). For patients with stage IIIA NSCLC, we found lower than expected use of surgery (37.0%). It is possible that the resectable patients in this study only received chemoradiotherapy as thoracic surgery teams/departments are not available at all sites in Spain. This may partly explain the range of treatments observed in this study, and we can see the impact that this had on PFS and OS, which varied widely.
The NADIM study, which evaluated induction therapy in stage III NSCLC has reported promising results, with the addition of checkpoint inhibitors to platinum-based chemotherapy resulting in an unprecedented major pathological response rate of 50–83% and pathological complete response of 21–59% (16). However, newer treatments such as immunotherapies and tyrosine kinase inhibitors were not widely used as initial therapy at the time of the analysis [2010–2019]. Durvalumab has been available in Spain since January 2020 for patients with stage IIIA/IIIB NSCLC who receive and complete concurrent chemoradiotherapy, but its use is restricted to PD-L1-positive tumours (usually >1% PD-L1 expression). Durvalumab is already included in the Sociedad Española de Oncología Médica (SEOM) and ESMO clinical guidelines based on the findings from the PACIFIC trial (8,23-25). In this trial of 713 patients with unresectable stage III NSCLC, durvalumab demonstrated durable median PFS (16.8 vs. 5.6 months), median OS/time to distant metastasis (23.2 vs. 14.6 months) benefits, and estimated 5-year survival probability (95% CI) of 0.43 (0.38–0.47) vs. 0.33 (0.28–0.38) after chemoradiotherapy versus placebo (26). The 5-year OS rates seen in the PACIFIC trial are comparable with results presented here for patients receiving neoadjuvant SACT plus surgery, which range from 0.39 (95% CI: 0.15–1.00) in stage IIIB patients with non-squamous histology to 0.55 (95% CI: 0.39–0.77) in stage IIIA patients with squamous histology.
Since the release of the ESMO and SEOM guidelines, several new trials have reported data for treatment in stage III NSCLC. Osimertinib (EGFR targeted therapy) was associated with significantly longer disease-free survival in a study of 682 patients with EGFR-positive stage IB–IIIA NSCLC (27). At 3 years, 84% of patients (stage II–IIIA) were disease free compared with 34% who received placebo; OS data were immature at the time of publication (28). The phase II KEYNOTE-799 trial has also been published and found that immunotherapy (pembrolizumab) plus concurrent chemoradiotherapy in 112 patients with stage III NSCLC had promising antitumour activity based on objective response rate (>70%) (29). Furthermore, the US Food and Drug Administration (FDA) recently approved nivolumab with platinum-doublet chemotherapy for adults with resectable NSCLC in the neoadjuvant setting based on the findings of the CheckMate 816 trial in patients with resectable, histologically confirmed stage IB, II, or IIIA NSCLC (30). Atezolizumab was also recently approved by the FDA for adjuvant treatment following resection and platinum-based chemotherapy in patients with stage II–IIIA NSCLC whose tumours have PD-L1 expression ≥1% (31) and by the EMA in patients with stage II–IIIA NSCLC whose tumours have PD-L1 expression ≥50% (32). However, some of these findings have not yet resulted in updates to official European guidelines for the treatment of stage III NSCLC.
Strengths and limitations
Our data were taken from a large registry in Spain that has undergone rigorous protocol assessment by an independent GECP committee. This registry represents a nationwide cohort and Spain has a National Health System with universal coverage ensuring that all patients undergo the same diagnostic work-up (12). Another strength of this study was that data collected from the GECP would cover a sizeable population receiving secondary care in multiple hospital centres in Spain. There were minimal missing data for the variables assessed within this population. The study design was longitudinal, which allowed tracking of all patients over time. However, selection bias may have reduced the generalisability of the results, and a change in treatment guidelines over the period of this study may mean that the prognosis for patients differed depending on when the patients were treated. All contributing centres with <100 patients were excluded from the study due to poor data quality on vital status and they did not frequently review data in the TTR. In addition, hospitals in the GECP network have a special focus on research and inclusion in the registry is voluntary; therefore, it is likely that the largest medical centres with access to a wider range of treatment options were over-represented and that care received in these hospitals may not be representative of care received across the entire Spanish population. This study was descriptive by design and statistical testing was hampered by confounding, small patient numbers among certain sub-cohorts, and limited 5-year follow-up for outcome evaluation.
Conclusions
This analysis of the TTR study describes the clinical reality surrounding the initial management and survival outcomes for stage III NSCLC in Spain over a 9-year period. It provides insights into the diverse approaches adopted for the treatment of stage III NSCLC prior to the availability of immunotherapies and targeted treatments in this setting. Survival outcomes are comparable with other real-world evidence, but improvement is still warranted. This is also a pivotal time in the management of NSCLC as (neo)adjuvant immunotherapies increasingly become the standard of care—and further research exploring the impact of these novel treatment strategies, including various immunotherapy regimens, may provide hope for improved patient outcomes. It may be necessary to monitor how these new clinical trial data are being incorporated into clinical practice and how they could improve the lung cancer patient’s outcomes.
Supplementary
The article’s supplementary files as
Acknowledgments
Professional writing and editorial assistance were provided by Sam Phillips, PhD, DMS, from Parexel, funded by Bristol Myers Squibb.
Funding: This work was supported by Bristol Myers Squibb.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the GECP ethics committee (IRB Number PI 148.15). For prospective enrolment, informed consent was taken from all individual participants. For retrospective enrolment, individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-176/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-176/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-176/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-176/coif). MP served as an unpaid editorial board member of Translational Lung Cancer Research from October 2021 to September 2023. All authors report that this work was supported by Bristol Myers Squibb. MP reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events and support for attending meetings and/or travel from AstraZeneca, Bristol Myers Squibb, Janssen, MSD, Pfizer, Roche and Takeda. RLC reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Aristo, AstraZeneca, Bristol Myers Squibb, MSD, Novartis, Pierre-Fabre, Roche and Takeda; and support for attending meetings and/or travel from Novartis, Roche and Takeda. VC reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, MSD, Pfizer, Roche and Takeda. DRA reports consulting fees and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, MSD, Novartis and Roche. DRA also reports support for attending meetings and/or travel from MSD, Novartis and Roche. BM reports consulting fees from AstraZeneca, Roche and Sanofi. BM also reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, Boehringer Ingelheim, MSD, Roche and Takeda; and support for attending meetings and/or travel from AstraZeneca and MSD. JBB reports institutionally awarded grants and personal fees from Pfizer and Roche. JBB also reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, MSD, Roche and Sanofi; and support for attending meetings and/or travel from MSD, Roche and Takeda. ER is an employee of IQVIA. RC, Carlos Chaib, JP, LV and MJD are employees of Bristol Myers Squibb, the study sponsor. Carlos Chaib, JP and LV also report stock ownership in Bristol Myers Squibb. The authors have no other conflicts of interest to declare.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer, World Health Organization. GLOBOCAN 2020: Lung. [cited January 2023]. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf
- 3.International Agency for Research in Cancer, World Health Organization. GLOBOCAN 2020: Spain. 2020. [cited January 2023]. Available online: https://gco.iarc.fr/today/data/factsheets/populations/724-spain-fact-sheets.pdf
- 4.National Cancer Institute. Surveillance, Epidemiology, and End Results Program. SEER cancer statistics review (CSR) 1975-2014. Updated April 2, 2018. [cited January 2023]. Available online: https://seer.cancer.gov/archive/csr/1975_2014/index.html#contents
- 5.Ganti AK, Klein AB, Cotarla I, et al. Update of Incidence, Prevalence, Survival, and Initial Treatment in Patients With Non-Small Cell Lung Cancer in the US. JAMA Oncol 2021;7:1824-32. 10.1001/jamaoncol.2021.4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gildea TR, DaCosta Byfield S, Hogarth DK, et al. A retrospective analysis of delays in the diagnosis of lung cancer and associated costs. Clinicoecon Outcomes Res 2017;9:261-9. 10.2147/CEOR.S132259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-21. 10.1093/annonc/mdx222 [DOI] [PubMed] [Google Scholar]
- 8.Remon J, Soria JC, Peters S, et al. Early and locally advanced non-small-cell lung cancer: an update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol 2021;32:1637-42. 10.1016/j.annonc.2021.08.1994 [DOI] [PubMed] [Google Scholar]
- 9.IMFINZI® (durvalumab) [prescribing information]. Wilmington, DE, USA: AstraZeneca Pharmaceuticals LP; 2022. [Google Scholar]
- 10.IMFINZI® (durvalumab) [summary of product characteristics]. Södertälje, Sweden: AstraZeneca AB; 2022. [Google Scholar]
- 11.Ekman S, Griesinger F, Baas P, et al. I-O Optimise: a novel multinational real-world research platform in thoracic malignancies. Future Oncol 2019;15:1551-63. 10.2217/fon-2019-0025 [DOI] [PubMed] [Google Scholar]
- 12.Provencio M, Carcereny E, Rodríguez-Abreu D, et al. Lung cancer in Spain: information from the Thoracic Tumors Registry (TTR study). Transl Lung Cancer Res 2019;8:461-75. 10.21037/tlcr.2019.08.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Medical Association (WMA). WMA Declaration of Helsinki – ethical principles for medical research involving human subjects. 09 July 2018. [cited January 2023]. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
- 14.International Society for Pharmacoepidemiology. Guidelines for good pharmacoepidemiology practices (GPP), revision 3. 2015. [cited January 2023]. Available online: https://www.pharmacoepi.org/resources/policies/guidelines-08027/
- 15.Remon J, Reguart N, García-Campelo R, et al. Lung Cancer in Spain. J Thorac Oncol 2021;16:197-204. 10.1016/j.jtho.2020.09.026 [DOI] [PubMed] [Google Scholar]
- 16.Palmero R, Vilariño N, Navarro-Martín A, et al. Induction treatment in patients with stage III non-small cell lung cancer. Transl Lung Cancer Res 2021;10:539-54. 10.21037/tlcr-20-420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NSCLC Meta-analysis Collaborative Group . Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561-71. 10.1016/S0140-6736(13)62159-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peer M, Azzam S, Cyjon A, et al. Major pulmonary resection after neoadjuvant chemotherapy or chemoradiation in potentially resectable stage III non-small cell lung carcinoma. Sci Rep 2021;11:20232. 10.1038/s41598-021-99271-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:1413-22. 10.1016/S1470-2045(20)30453-8 [DOI] [PubMed] [Google Scholar]
- 20.Provencio M, Serna R, Nadel E, et al. Progression free survival and overall survival in NADIM II study. World Conference on Lung Cancer (WCLC 2022): Vienna, Austria. 6-9 August 2022. [Google Scholar]
- 21.Jazieh AR, Onal HC, Tan DSW, et al. Real-World Treatment Patterns and Clinical Outcomes in Patients With Stage III NSCLC: Results of KINDLE, a Multicountry Observational Study. J Thorac Oncol 2021;16:1733-44. 10.1016/j.jtho.2021.05.003 [DOI] [PubMed] [Google Scholar]
- 22.Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192-237. 10.1093/annonc/mdy275 [DOI] [PubMed] [Google Scholar]
- 23.Majem M, Juan O, Insa A, et al. SEOM clinical guidelines for the treatment of non-small cell lung cancer (2018). Clin Transl Oncol 2019;21:3-17. 10.1007/s12094-018-1978-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faivre-Finn C, Vicente D, Kurata T, et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial. J Thorac Oncol 2021;16:860-7. 10.1016/j.jtho.2020.12.015 [DOI] [PubMed] [Google Scholar]
- 25.Spigel DR, Faivre-Finn C, Gray JE, et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:1301-11. 10.1200/JCO.21.01308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 27.Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:1711-23. 10.1056/NEJMoa2027071 [DOI] [PubMed] [Google Scholar]
- 28.Tsuboi M, Wu Y, Grohe C, et al. LBA47 – Osimertinib as adjuvant therapy in patients (pts) with resected EGFR-mutated (EGFRm) stage IB-IIIA non-small cell lung cancer (NSCLC): updated results from ADAURA. Ann Oncol 2022;33:abstr S808-69.
- 29.Jabbour SK, Lee KH, Frost N, et al. Pembrolizumab Plus Concurrent Chemoradiation Therapy in Patients With Unresectable, Locally Advanced, Stage III Non-Small Cell Lung Cancer: The Phase 2 KEYNOTE-799 Nonrandomized Trial. JAMA Oncol 2021;7:1-9. 10.1001/jamaoncol.2021.2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.OPDIVO® (nivolumab) [prescribing information]. Princeton, NJ, USA: Bristol Myers Squibb; 2022. [Google Scholar]
- 31.TECENTRIQ® (atezolizumab) [prescribing information]. South San Francisco, CA, USA: Genentech, Inc.; 2022. [Google Scholar]
- 32.TECENTRIQ® (atezolizumab) [summary of product characteristics]. Grenzach-Wyhlen, Germany: Roche Pharma AG; 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as