Abstract
Background
Previously reported post hoc multivariable analyses exploring predictors of confirmed virologic failure (CVF) with cabotegravir + rilpivirine long-acting (CAB + RPV LA) were expanded to include data beyond week 48, additional covariates, and additional participants.
Methods
Pooled data from 1651 participants were used to explore dosing regimen (every 4 or every 8 weeks), demographic, viral, and pharmacokinetic covariates as potential predictors of CVF. Prior dosing regimen experience was accounted for using 2 populations. Two models were conducted in each population—baseline factor analyses exploring factors known at baseline and multivariable analyses exploring baseline factors plus postbaseline model-predicted CAB/RPV trough concentrations (4 and 44 weeks postinjection). Retained factors were evaluated to understand their contribution to CVF (alone or in combination).
Results
Overall, 1.4% (n = 23/1651) of participants had CVF through 152 weeks. The presence of RPV resistance-associated mutations, human immunodeficiency virus-1 subtype A6/A1, and body mass index ≥30 kg/m2 were associated with an increased risk of CVF (P < .05 adjusted incidence rate ratio), with participants with ≥2 of these baseline factors having a higher risk of CVF. Lower model-predicted CAB/RPV troughs were additional factors retained for multivariable analyses.
Conclusions
The presence of ≥2 baseline factors (RPV resistance-associated mutations, A6/A1 subtype, and/or body mass index ≥30 kg/m2) was associated with increased CVF risk, consistent with prior analyses. Inclusion of initial model-predicted CAB/RPV trough concentrations (≤first quartile) did not improve the prediction of CVF beyond the presence of a combination of ≥2 baseline factors, reinforcing the clinical utility of the baseline factors in the appropriate use of CAB + RPV LA.
Keywords: long-acting antiretroviral therapy, cabotegravir, rilpivirine, virologic response, multivariable analysis
Confirmed virologic failure occurred in 1.4% of long-acting cabotegravir + rilpivirine participants up to 3 years on study. Having ≥2 baseline factors (rilpivirine resistance-associated mutations, human immunodeficiency virus-1 subtype A6/A1, and/or body mass index ≥30 kg/m2) was associated with increased failure risk, consistent with prior analyses.
Cabotegravir (CAB), an integrase strand transfer inhibitor (INSTI), plus rilpivirine (RPV), a nonnucleoside reverse transcriptase inhibitor (NNRTI), is the first complete long-acting (LA), injectable antiretroviral therapy (ART) regimen approved and recommended by treatment guidelines [1, 2]. CAB + RPV LA is administered intramuscularly monthly or every 2 months by a healthcare professional for the maintenance of human immunodeficiency virus-1 (HIV-1) virologic suppression.
CAB + RPV LA demonstrated noninferior efficacy and was well tolerated across phase 3/3b trials (FLAIR; ATLAS; ATLAS-2M) [3–10]. Confirmed virologic failure (CVF; 2 consecutive plasma HIV-1 RNA measurements ≥200 copies/mL) occurred in ∼1% (n = 19/1651) of participants in phase 3/3b clinical trials through week 48 of CAB + RPV therapy, with only 4 additional cases after week 48 [3–10]. Successful implementation and high rates of virologic suppression were also demonstrated in clinic-based implementation studies (CARISEL and CUSTOMIZE) [11–14]. Notably, CVF rates in these studies were numerically lower [11, 12, 15].
In FLAIR and ATLAS, 5/6 participants with CVF on LA therapy through week 48 were from Russia, had their HIV-1 subtype previously reported as A or A1, and had the integrase polymorphism L74I detected at baseline [16]. Further investigation based on the updated Los Alamos National Laboratory library [17] highlighted the prevalence of L74I and identified these viruses as A6 [18, 19]. L74I is a natural polymorphism present at low rates in different subtypes, except for A6, in which it is very common [18, 19]. Although detected on rare occasions in laboratory or clinical isolates exposed to integrase inhibitors, including CAB [20–22], L74I is not clearly associated with resistance and alone does not impact INSTI susceptibility [23].
A post hoc multivariable analysis (MVA) using pooled data from participants on CAB + RPV LA in FLAIR, ATLAS, and ATLAS-2M explored drug, viral, and participant factors potentially predictive of virologic failure through week 48 [24]. Preexisting RPV resistance-associated mutations (RAMs), HIV-1 subtype A6/A1, higher body mass index (BMI), and lower week 8 RPV trough concentrations (4 weeks after first injection) were significantly (P < .05) associated with increased odds of CVF. An additional analysis found CVF to be a multifactorial event whereby the presence of ≥2 baseline factors (RPV RAMs, HIV-1 subtype A6/A1, and/or BMI ≥30 kg/m2) was associated with increased odds of CVF [24]. Consideration of these baseline factors can guide clinicians in patient selection and minimize CVF risk.
Since the original analysis, data beyond week 48 are available, including a population with different lengths of exposure, participants who switched from every 4 weeks (Q4W) to every 8 weeks (Q8W) dosing, as well as additional participants who switched to CAB + RPV LA during the extension phases of the studies. We now report expanded analyses exploring predictors of CVF beyond the first year of CAB + RPV LA in these studies based on data from the most recent reporting period for each study, including additional factors and participants.
METHODS
Study Population
Data from participants who received CAB + RPV LA dosed Q4W and/or Q8W in FLAIR, ATLAS, or ATLAS-2M were pooled in post hoc analyses. Data cutoffs were through week 124 for FLAIR, week 96 for ATLAS, and week 152 for ATLAS-2M [4, 7, 10]. This analysis includes participants initially randomized to CAB + RPV LA and participants who switched to CAB + RPV LA during extension phases of the studies.
FLAIR, ATLAS, and ATLAS-2M are randomized, multicenter, parallel-group, open-label, phase 3/3b studies. FLAIR and ATLAS evaluated CAB + RPV LA dosed Q4W versus continuing daily oral therapy and ATLAS-2M evaluated CAB + RPV LA dosed Q8W versus Q4W. The full study designs and eligibility criteria have been published elsewhere [5, 8, 9]. Participants were ≥18 years of age and virologically suppressed (plasma HIV-1 RNA <50 copies/mL) at randomization. Historical genotypic evidence of any major INSTI or NNRTI RAMs, excluding K103N in plasma, was exclusionary per the 2015 (FLAIR and ATLAS) and 2019 (ATLAS-2M) International Antiviral Society-USA (IAS-USA) guidelines (Supplementary Table 1) [25, 26]. Both FLAIR and ATLAS had an extension phase in which participants randomized to the daily oral therapy comparator arm could switch to CAB + RPV LA Q4W or, for ATLAS only, could transition to either Q4W or Q8W dosing in ATLAS-2M. Thus, in ATLAS-2M, approximately half of participants rolled over from the daily oral therapy or CAB + RPV LA Q4W arms of the ATLAS study. Baseline characteristics were broadly similar across the studies [5, 8, 9].
All studies were conducted in accordance with the Declaration of Helsinki [27]. All participants provided written informed consent, and the study protocols were approved by an investigational review board.
Factors Explored for Association With Virologic Failure
Sex at birth, baseline BMI (continuous, linear term), HIV-1 subtype A6/A1, L74I polymorphism (including L74/L/I, but not any L74I mixtures containing M), CAB RAMs, other INSTI (non-CAB-specific) RAMs, RPV RAMs, other (non-RPV-specific) NNRTI RAMs, and Q4W or Q8W dosing regimen were explored as potential predictors of CVF in baseline factor analyses (BFAs).
Additional MVAs evaluated the same covariates but also included the postbaseline factors: population pharmacokinetic (PK) model-predicted CAB and RPV trough plasma concentrations [28, 29]. Time points for model-predicted plasma concentrations were after first injections (week 4 postinjection) and at week 44 postinjection (at the end of 6 Q8W injections or 11 Q4W injections). For participants who withdrew before week 44 postinjection, week 44 values were predicted based on available time points.
Factors identified as significant predictors in the final selected models were also used to evaluate CVF risk when present alone or in combination in the overall population. Results were further evaluated by geographical region (North America, Europe, and “other” regions) and by country.
Full details of the methodology of genotypic and phenotypic analyses have been published (Supplementary page 1) [24]. IAS-USA 2019 HIV-1 drug resistance mutation guidelines were used to identify RAMs (Supplementary Table 2) [25].
Statistical Analysis
To explore potential factors associated with CVF, comprehensive statistical modeling was performed with and without PK covariates: these are referred to as MVAs (including PK covariates) and BFAs (no PK covariates). Kaplan–Meier curves of time to CVF were produced to summarize CVF according to dosing regimen.
In addition to calculating the unadjusted CVF incidence rate per 100 participant-years, multivariable Poisson modeling with backwards variable elimination was used to account for the complexities of the expanded analysis population (Supplementary Figure 1). Dosing regimen experience was accounted for using 2 distinct populations: “single-regimen” analyses and “all-regimen” analyses, with an MVA and BFA in each population. The single-regimen population comprised all intention-to-treat exposed participants who received only 1 regimen—either Q4W or Q8W. For the MVA and BFA in this population, a zero-inflated Poisson model was used. The all-regimens model comprised all intention-to-treat exposed participants. In this analysis, participants who received both Q4W and Q8W regimens were included twice in the model, once for each regimen, contributing twice to the complete records count and only once to individual participant count. For the MVA and BFA in this population, a repeated measures quasi-Poisson model, including a sandwich covariance estimator, was used. Model-predicted troughs after 44 weeks of injections were not included in the all-regimens MVA model because of the complexity of the repeated data structure for participants receiving multiple regimens.
A conventional backwards elimination variable selection algorithm was used in the models. Covariates were deemed to be statistically significant at a level of P < .05. Using significant predictors in the final selected models, the risk of CVF was then examined in the overall population to understand their contribution to CVF (when present alone or in combination). Positive predictive value (PPV), negative predictive value (NPV), sensitivity, and specificity were calculated for each BFA outcome, along with subsets of MVA model variables, to ascertain which combination of factors is optimal in predicting CVF.
The primary outcome of interest was the occurrence of CVF. The incidence of model-selected factors in participants with HIV-1 RNA <50 copies/mL (per the Food and Drug Administration Snapshot algorithm) was also summarized. The time points for HIV-1 RNA <50 copies/mL Snapshot analyses were week 124 for FLAIR, week 48 for ATLAS (no Snapshot analysis performed at week 96 [4]), and week 152 for ATLAS-2M.
RESULTS
Participants
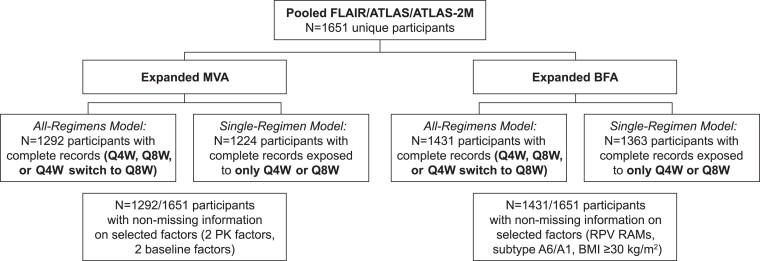
Across 3 studies, 1651 unique participants were included, 23/1651 (1.4%) of whom had CVF. The number of unique participants with complete records for the covariates in each analysis is shown in Figure 1.
Figure 1.
Data collation. BFA, baseline factor analysis; BMI, body mass index; CAB, cabotegravir; MVA, multivariable analysis; PK, pharmacokinetics; Q4W, every 4 weeks; Q8W, every 8 weeks; RAM, resistance-associated mutation; RPV, rilpivirine.
Time to CVF by Regimen
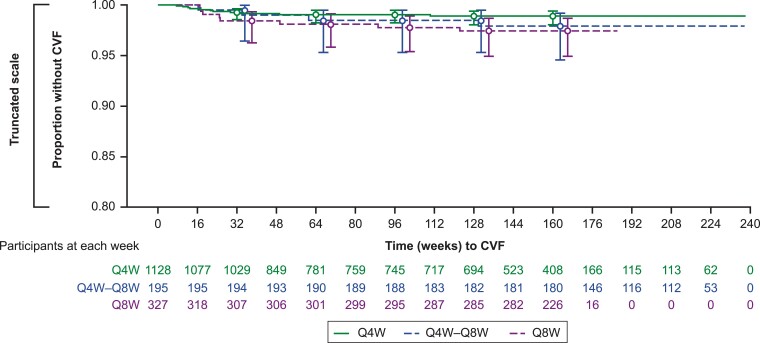
The overall unadjusted incidence rate (95% confidence interval [CI]) of CVF per 100 person-years was 0.54 (0.34–0.80). The unadjusted incidence rate (95% CI) by dosing regimen was 0.42 (0.21–0.75) for Q4W dosing, 0.85 (0.37–1.68) for Q8W dosing, and 0.54 (0.15–1.40) for participants who switched from Q4W to Q8W (Supplementary Table 3). Figure 2 shows time to CVF for the 3 regimen groups; CVF occurred infrequently, with time to CVF similar by regimen. Overall, median (interquartile range) time to suspected virologic failure (first of 2 consecutive measurements of HIV-1 RNA ≥200 copies/mL) was 24.9 (16.9–49.3) weeks.
Figure 2.
Time to CVF by regimen. Confidence intervals around point estimates are shown for each of the 3 groups. CVF, confirmed virologic failure; Q4W, every 4 weeks; Q8W, every 8 weeks.
Predictors of Virologic Failure
Multivariable and Baseline Analyses
Tables 1 and 2 show the results of the MVAs and BFAs for the single-regimen and all-regimens populations. Supplementary Figure 2 shows CVF outcome in relation to the individual covariates included in the models. Of the 199 participants with HIV-1 subtype A6/A1, 11/180 (6%) with subtype A6 had CVF and 2/19 (11%) with subtype A1 had CVF.
Table 1.
Baseline Factor Analyses of CVFa (Single- and All-Regimens Models)
| Covariate | Single-Regimen Adjusted Incidence Rate Ratio (95% CI) [P Value], n = 1363 |
All-Regimens Adjusted Incidence Rate Ratio (95% CI) [P Value], n = 1431b |
||
|---|---|---|---|---|
| Full Model | Final Model | Full Model | Final Model | |
| RPV RAMs: yes/no | 16.9 (3.74–76.1) [.0002] | 21.7 (5.80–80.8) [<.0001] | 11.3 (4.83–26.5) [<.0001] | 10.4 (3.88–27.9) [<.0001] |
| HIV-1 subtype A6/A1: yes/no | 24.5 (3.47–173) [.0013] | 12.9 (4.42–37.5) [<.0001] | 9.17 (0.984–85.3) [.0516] | 9.15 (3.79–22.1) [<.0001] |
| Baseline BMI: kg/m2 | 1.09 (0.994–1.19) [.0671] | 1.09 (1.00–1.19) [.0447] | 1.09 (1.01–1.17) [.0205] | 1.10 (1.02–1.18) [.0145] |
| Regimen: Q8W/Q4W | 1.89 (0.536–6.67) [.3221] | d | 1.90 (0.756–4.79) [.1719] | d |
| Integrase L74I:c yes/no | 0.480 (0.068–3.40) [.4629] | d | 1.23 (0.117–12.9) [.8642] | d |
| Sex at birth: female/male | 0.796 (0.222–2.85) [.7254] | d | 0.827 (0.329–2.08) [.6858] | d |
| Other NNRTI RAMs: yes/no | 1.87 (0.465–7.51) [.3787] | d | 2.65 (1.12–6.31) [.0273] | 2.78 (1.15–6.76) [.0237] |
| CAB RAMs: yes/no | 2.01 (0.115–35.0) [.6332] | d | 1.66 (0.28–9.79) [.5742] | d |
| Other INSTI RAMs: yes/no | 0 [.9998] | d | 0.401 (0.022–7.28) [.5368] | d |
Abbreviations: BMI, body mass index; CAB, cabotegravir; CI, confidence interval; CVF, confirmed virologic failure; IAS-USA, International Antiviral Society-USA; INSTI, integrase strand transfer inhibitor; LA, long-acting; NNRTI, nonnucleoside reverse transcriptase inhibitor; Q4W, every 4 weeks; Q8W, every 8 weeks; RAM, resistance-associated mutation; RPV, rilpivirine.
Bolded values represent statistically significant predictors (P < .05).
aThrough week 124 for FLAIR, week 96 for ATLAS, and week 152 for ATLAS-2M.
bParticipants who received both Q4W and Q8W CAB + RPV LA were included twice in the model, once for each regimen. A total of 1600 complete records was included.
cL74I (including L74/L/I, but not any L74I mixtures containing M).
dCovariates eliminated from the selected models. RAMs were determined per 2019 IAS-USA guidelines [25].
Table 2.
Multivariable Analyses of CVFa (Single- and All-Regimens Models)
| Covariate | Single-Regimen Adjusted Incidence Rate Ratio (95% CI) [P Value], n = 1224 |
All-Regimens Adjusted Incidence Rate Ratio (95% CI) [P Value], n = 1292b |
||
|---|---|---|---|---|
| Full Model | Final Model | Full Model | Final Model | |
| RPV RAMs: yes/no | 31.2 (7.54–129) [<.0001] | 25.7 (7.17–92.2) [<.0001] | 14.0 (4.85–40.7) [<.0001] | 12.1 (4.66–31.2) [<.0001] |
| HIV-1 subtype A6/A1: yes/no | 19.5 (2.22–172) [.0074] | 15.5 (4.69–50.9) [<.0001] | 2.93 (0.461–18.7) [.2542] | e |
| Baseline BMI: kg/m2 | 1.02 (0.910–1.15) [.7151] | e | 1.03 (0.947–1.13) [.4661] | e |
| Regimen: Q8W/Q4W | 1.84 (0.306–11.1) [.5050] | e | 2.56 (0.97–6.80) [.0589] | 2.39 (0.96–5.96) [.0612] |
| Integrase L74I:c yes/no | 0.630 (0.079–5.03) [.6631] | e | 3.07 (0.482–19.6) [.2351] | 5.96 (2.13–16.70) [.0007] |
| Sex: female/male | 1.05 (0.244–4.50) [.9489] | e | 0.552 (0.186–1.64) [.2847] | e |
| Other NNRTI RAMs: yes/no | 3.11 (0.804–12.01) [.1001] | 3.03 (0.93–9.93) [.0667] | 2.36 (0.914–6.08) [.0762] | 2.13 (0.87–5.17) [.0963] |
| CAB RAMs: yes/no | 3.00 (0.192–47.0) [.4330] | e | 1.50 (0.248–9.11) [.6573] | e |
| Other INSTI RAMs: yes/no | 0 (0–0) [.9998] | e | 0.320 (0.015–6.90) [.4668] | e |
| Model-predicted log2 week 44 CAB Ctrough (μg/mL)d | 7.65 (2.05–28.5) [.0025] | 5.99 (1.94–18.5) [.0019] | NAf | NAf |
| Model-predicted log2 week 44 RPV Ctrough (ng/mL)d | 1.37 (0.170–11.0 [.7684] | 4.16 (1.04–16.7) [.0441] | NAf | NAf |
| Model-predicted log2 week 4 CAB Ctrough (μg/mL)d | 1.55 (0.57–4.22) [.3893] | 2.20 (1.21–4.00) [.0100] | 2.54 (1.18–5.48) [.0174] | 2.68 (1.29–5.57) [.0081] |
| Model-predicted log2 week 4 RPV Ctrough (ng/mL)d | 3.46 (0.62–19.4) [.1588] | e | 1.72 (0.707–4.17) [.2326] | 1.71 (0.78–3.74) [.1785] |
Abbreviations: BMI, body mass index; CAB, cabotegravir; CI, confidence interval; Ctrough, trough concentration; CVF, confirmed virologic failure; INSTI, integrase strand transfer inhibitor; LA, long-acting; MVA, multivariable analysis; NA, not applicable; NNRTI, nonnucleoside reverse transcriptase inhibitor; Q4W, every 4 weeks; Q8W, every 8 weeks; RAM, resistance-associated mutation; RPV, rilpivirine.
Bolded values represent statistically significant predictors (P < .05).
aThrough week 152 for ATLAS-2M, week 124 for FLAIR, and week 96 for ATLAS.
bParticipants who received both Q4W and Q8W CAB + RPV LA were included twice in the model, once for each regimen. A total of 1462 complete records was included.
cL74I (including L74/L/I, but not any L74I mixtures containing M).
dIncidence rate ratios correspond to a 1 log2 unit decrease.
eCovariates eliminated from the selected models.
fWeek 44 CAB and RPV trough concentrations were not included in the all-regimens MVA because of the complexity of the repeated data structure for participants receiving multiple regimens.
The BFAs included 1363 and 1431 unique participants with complete records in the single-regimen and all-regimens models, respectively. Three baseline factors were retained and significantly associated with increased risk of CVF in both models: RPV RAMs, HIV-1 subtype A6/A1, and higher BMI (Table 1). Additionally, the other NNRTI RAMs covariate was retained and significantly associated with increased risk of CVF in the all-regimens model. All other factors were not significant in the final models (including Q8W dosing regimen and L74I).
The MVA single-regimen model included 1224 participants with complete records and included all baseline factors plus model-predicted plasma CAB and RPV troughs. Five factors were significantly associated with increased risk of CVF: RPV RAMs, HIV-1 subtype A6/A1 (associated with integrase L74I polymorphism [18, 19]), model-predicted CAB trough concentration 4 weeks following initial injection dose (early CAB plasma concentrations are inversely correlated with high BMI [29, 30]), and model-predicted CAB and RPV trough concentrations at week 44 postinjection (Table 2). The other NNRTI RAMs covariate was retained in the final selected model but did not reach statistical significance (P = .0667). Notably, in a sensitivity analysis in which only CAB trough concentrations were excluded, RPV troughs were retained.
In the all-regimens MVA model, which included 1292 unique participants with complete records, RPV RAMs, integrase L74I, and model-predicted CAB trough concentration 4 weeks after initial injection were statistically associated with increased risk of CVF. Dosing regimen, other NNRTI RAMs, and model-predicted RPV trough concentration 4 weeks following initial injection dose were retained in the final selected model but did not reach statistical significance (P = .0612, P = .0963, and P = .1785, respectively).
Risk of CVF According to Combinations of Baseline Factors
CVF risk according to combinations of the baseline factors identified as significant predictors in both BFAs (RPV RAMs, HIV-1 subtype A6/A1, and BMI ≥30 kg/m2) were examined in 1431 participants with complete records for these factors.
CVF risk was higher in the presence of ≥2 baseline factors; 19.3% (n = 11/57) of participants in this category met the CVF criterion through 3 years on study (Table 3). Time to CVF by the presence of none, 1, or ≥2 baseline factors is shown in Supplementary Figure 3. Notably, the presence of ≥2 baseline factors occurred in 4.0% (n = 57/1431) of the overall population. The proportion of participants who had CVF with no factors (0.4%, n = 4/970) or any 1 factor (2.0%, n = 8/404) was similar to the overall population rate of 1.6% (n = 23/1431). CVF among participants with a sole baseline factor was driven by RPV RAMs (3.2%, n = 1/31 with CVF) and HIV-1 subtype A6/A1 (3.8%, n = 6/157 with CVF); CVF occurred in 0.5% (n = 1/216) of participants with BMI ≥30 kg/m2 as their only baseline factor. The model sensitivity and specificity of having ≥2 contributing baseline factors was considered optimal given the 47.8% sensitivity and 96.7% specificity, with a PPV of 19.3% and an NPV of 99.1% (Supplementary Table 4). Supplementary Tables 5–7 show outcomes by regimen, region, and country. Overall, 11 participants with CVF were from Russia, all of whom had HIV-1 subtype A6/A1 (Supplementary Table 7A). Because the other NNRTI RAMs covariate was significant in 1 model, including this as a “fourth factor” was explored further. Inclusion of other NNRTI RAMs had minimal impact, identifying 1 additional participant with CVF, and did not improve the overall accuracy of the diagnostic measures. When outcomes were assessed by K103N specifically, CVF only occurred when preexisting RPV RAMs were also present (Supplementary Table 8).
Table 3.
Virologic Outcomes by the Presence of Key Baseline and Postbaseline Factors
|
Three Baseline Factors:
RPV RAMs, Subtype A6/A1, and BMI ≥30 kg/m2 |
Two Baseline Factors + CAB and RPV PK:
a
RPV RAMs, Subtype A6/A1, Low Initial Model-Predicted CAB Trough,a and Low Initial Model-Predicted RPV Trougha |
||||
|---|---|---|---|---|---|
| Baseline Factors (Number) | Virologic Suppression, n (%)b |
CVF, n (%)c |
Factors (No.) | Virologic Suppression, n (%)b | CVF, n (%)c |
| 0 | 844/970 (87.0) | 4/970 (0.4)d | 0 | 584/664 (88.0) | 0/664 (0)g |
| 1 | 343/404 (84.9) | 8/404 (2.0)e | 1 | 339/396 (85.6) | 5/396 (1.3)h |
| ≥2 | 44/57 (77.2) | 11/57 (19.3)f | ≥2 | 190/232 (81.9) | 17/232 (7.3) |
| ≥3 | 28/39 (71.8) | 8/39 (20.5)i | |||
| TOTAL | 1231/1431 (86.0) | 23/1431 (1.6) | TOTAL | 1113/1292 (86.1) | 22/1292 (1.7) |
| (95% CI) | (84.1–87.8) | (1.0–2.4) 18/1224 (1.47)j |
(95% CI) | (84.1–88.0) | (1.1–2.6) |
Reproduced/adapted from C. Orkin, et al. J Int AIDS Soc. 2022; 25(suppl 6):e26009. doi: 10.1002/jia2.26009 under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) license (https://creativecommons.org/licenses/by/4.0/).
Abbreviations: BMI, body mass index; CAB, cabotegravir; CI, confidence interval; CVF, confirmed virologic failure; FDA, US Food and Drug Administration; NPV, negative predictive value; PK, pharmacokinetics; PPV, positive predictive value; RAM, resistance-associated mutation; RPV, rilpivirine.
aBelow first quartile.
bBased on the FDA Snapshot algorithm of HIV-1 RNA <50 copies/mL at week 48 for ATLAS, week 124 for FLAIR, and week 152 for ATLAS-2M.
cDefined as 2 consecutive measurements of HIV-1 RNA ≥200 copies/mL.
dPPV 0.4%; NPV 95.9%; sensitivity 17.4%; specificity 31.4%.
ePPV 2.0%; NPV 98.5%; sensitivity 34.8%; specificity 71.9%.
fPPV 19.3%; NPV 99.1%; sensitivity 47.8%; specificity 96.7%.
gPPV 0%; NPV 96.5%; sensitivity 0%; specificity 47.7%.
hPPV 1.3%; NPV 98.1%; sensitivity 22.7%; specificity 69.2%.
iPPV 20.5%; NPV 98.9%; sensitivity 36.4%; specificity 97.6%.
jAnalysis dataset for the multivariable modeling.
Risk of CVF According to Combinations of Baseline and Postbaseline Factors
Virologic outcomes were summarized according to combinations of those factors found to be important predictors in the MVA to see how these findings could be applied clinically to assess CVF risk. Given the correlation between HIV-1 subtype A6/A1 and the integrase L74I polymorphism [18, 19], only HIV-1 subtype A6/A1 was included. Model-predicted first troughs were included versus troughs after 44 weeks of injections, given their proximity to baseline.
A total of 1292 participants with complete records were available for this analysis (Figure 1). Of participants with ≥3 baseline and postbaseline factors present (3% [n = 39/1292]), 20.5% (n = 8/39) had CVF with a 36.4% sensitivity and 97.6% specificity, and a PPV and NPV of 20.5% and 98.9%, respectively (Table 3 and Supplementary Table 4). As the number of factors decreased, so did the proportion of participants with CVF (≥2 factors, 7.3% [n = 17/232]; 1 factor, 1.3% [n = 5/396]; 0 factors, 0% [n = 0/664]). This same pattern was observed when including BMI ≥30 kg/m2 as a fifth factor (≥2 factors, 5.7% [n = 18/318]; 1 factor, 1.0% [n = 4/391]; 0 factors, 0% [n = 0/583]). Among participants with initial CAB or RPV troughs ≤first quartile as their only factor, 0.6% (n = 1/160) and 0.7% (n = 1/137) had CVF, respectively; when both CAB and RPV troughs were ≤first quartile, this rate was 2.7% (n = 3/113).
Pharmacokinetics in Relation to Virologic Outcome
Of the 22 MVA participants who received CAB + RPV LA and had CVF, 18/22 (82%) had model-predicted CAB and/or RPV trough concentrations within the first quartile 4 weeks after initial injection, including 10/22 (45%) with concentrations for both drugs in the lower quartiles (Supplementary Figures 4A–D).
DISCUSSION
In this expanded analysis, CAB + RPV LA demonstrated high rates of virologic suppression, with CVF occurring in 1.4% of participants. Of note, numerically lower CVF rates have been reported (0%-0.5%) in the CARLOS, CARISEL, SOLAR, and CUSTOMIZE implementation studies [11, 12, 15, 31]. The presence of a combination of ≥2 baseline factors (preexisting RPV RAMs, A6/A1 subtype, and/or BMI ≥30 kg/m2) increased the risk of CVF, consistent with prior analyses exploring potential predictors of CVF within the first year of CAB + RPV LA [24].
The absolute difference in CVF incidence between the Q4W and Q8W regimens equates to ∼1 extra participant with CVF on Q8W over 200 person-years. Consistent with the previous MVA and BFA [24], the Q8W regimen was not identified as a statistically significant predictor in any of the 4 models. This finding aligns with the noninferior efficacy of Q8W versus Q4W dosing demonstrated by the phase 3b ATLAS-2M study [6, 9, 10]. The presence of a combination of ≥2 of the significant baseline factors increased the proportion of participants with CVF 10- to 12-fold compared with a single factor across both regimens.
In the BFAs, preexisting RPV RAMs, HIV-1 subtype A6/A1, and baseline BMI were significant in both the single-regimen and all-regimens models. The other NNRTI RAMs covariate was also found to be significant in the all-regimens model, but when considered as an additional factor did not improve the diagnostic measures. In the MVAs, which included postbaseline PK, RPV RAMs and HIV-1 subtype A6/A1 (single-regimen model)/L74I (all-regimens model) were retained as significant factors. Model-predicted CAB trough concentration 4 weeks following initial injection was significant in the single- and all-regimens models, with model-predicted CAB and RPV trough concentrations at week 44 postinjection being significant factors in the single-regimen model; however, most participants with CAB and/or RPV concentrations in the first quartile did not have CVF (Supplementary Figure 4A).
In contrast to the BFAs, BMI was not retained in the MVAs, potentially in lieu of CAB concentrations because of the known inverse relationship (Supplementary Figure 4B) [29, 30]. Given this correlation, and that trough concentrations of LA therapy cannot be known before treatment initiation, a patient's BMI may be more useful to clinicians. Using longer (2-inch) needles results in higher CAB troughs early in treatment for individuals with higher BMIs; however, most participants with a BMI ≥30 kg/m2 in the phase 3/3b studies used standard needles [32]. The integrase polymorphism L74I was retained in the all-regimens MVA, with HIV-1 subtype A6/A1 not retained; this contrasts with the other 3 models and is likely because of the high correlation with HIV-1 subtype A6/A1 [18, 19].
When exploring combinations of the 3 significant baseline factors, participants with ≥2 predictive baseline factors (RPV RAMs, HIV-1 subtype A6/A1, BMI ≥30 kg/m2) had an increased risk of CVF (19.3% [n = 11/57]). Notably, the presence of ≥2 baseline factors was uncommon (4%, n = 57/1431). The absence of any baseline factors was associated with a low incidence of CVF (0.4% [n = 4/970]). No single predictor had a CVF incidence of >4%; notably, participants with BMI ≥30 kg/m2 as their sole factor had a CVF rate of 0.5% (n = 1/216). This is aligned with a previous post hoc analysis demonstrating similar outcomes, regardless of BMI category (BMI <30 or ≥30 kg/m2), for pooled participants across FLAIR, ATLAS, and ATLAS-2M through week 48 [32]. For participants with preexisting RPV RAMs as their sole factor, only 3.2% (n = 1/31) had CVF supporting the multifactorial model. Notably, RPV RAMs for ATLAS and ATLAS-2M participants were identified by a retrospective proviral DNA analysis. Reflecting clinical practice, virologically suppressed participants were not screened for archived resistance as part of CAB + RPV LA clinical trials. When considering CAB + RPV LA, understanding the patient's treatment history is important, including a review of RNA-based resistance tests, to exclude any history of treatment failure. Patient treatment history should be considered in combination with the presence of subtype A6/A1 and a BMI ≥30 kg/m2 to inform treatment decisions; screening for archived resistance is not a requirement for initiating CAB + RPV LA.
When including model-predicted initial CAB and RPV trough concentrations as additional factors, the presence of ≥3 baseline and postbaseline factors was associated with an increased risk of CVF, but only marginally improved prediction of CVF beyond the presence of a combination of ≥2 baseline factors. This suggests that clinicians can apply the ≥2 baseline factors to inform patient selection. Although consideration of PK concentrations as additional factors may lead to a small incremental reduction in risk, given the complexity of measuring drug levels, the clinical utility of therapeutic dose monitoring is considered to be low.
Limitations
The relative clinical weight that can be placed on these findings requires additional context, most importantly that the majority of participants with any of the individual factors significantly associated with an increased risk of CVF continued to maintain suppression; thus, these findings should not be overgeneralized to each subgroup. Although the findings presented are important in guiding appropriate use of CAB + RPV LA, the results would benefit from validation in additional patient cohorts. The number of participants with CVF was low in these analyses (∼1% of total population); because we did not measure PK concentrations at each time for every participant, we used model-predicted values at weeks 4 and 44 in lieu of observed concentrations. Given the low frequency and multifactorial nature of CVF, PK cutoffs associated with virologic nonresponse have not been established for CAB + RPV LA.
CONCLUSIONS
Overall, CVF occurred in 1.4% of participants up to 3 years on study with an unadjusted CVF incidence rate of approximately 1 per 200 person-years among 4291 person-years. The CVF rate was ≤0.5% for participants with no baseline factors, or with BMI ≥30 kg/m2 as the only factor. A combination of ≥2 baseline factors (preexisting RPV RAMs, HIV-1 subtype A6/A1, and/or BMI ≥30 kg/m2) retains potential clinical utility to inform CVF risk, which helps guide appropriate use of this novel LA treatment option.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Chloe Orkin, SHARE Collaborative, Department of Immunobiology, Queen Mary University of London, London, United Kingdom.
Jonathan M Schapiro, National Hemophilia Center, Sheba Medical Center, Ramat Gan, Israel.
Carlo F Perno, IRCCS Bambino Gesù Pediatric Hospital, Rome, Italy.
Daniel R Kuritzkes, Division of Infectious Diseases, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Parul Patel, ViiV Healthcare, Durham, North Carolina, USA.
Rebecca DeMoor, GSK, Collegeville, Pennsylvania, USA.
David Dorey, GSK, Mississauga, Canada.
Yongwei Wang, ViiV Healthcare, Durham, North Carolina, USA.
Kelong Han, GSK, Collegeville, Pennsylvania, USA.
Veerle Van Eygen, Janssen Research & Development, Beerse, Belgium.
Herta Crauwels, Janssen Research & Development, Beerse, Belgium.
Susan L Ford, GSK, Durham, North Carolina, USA.
Christine L Latham, ViiV Healthcare, Durham, North Carolina, USA.
Marty St. Clair, ViiV Healthcare, Durham, North Carolina, USA.
Joseph W Polli, ViiV Healthcare, Durham, North Carolina, USA.
Simon Vanveggel, Janssen Research & Development, Beerse, Belgium.
Kati Vandermeulen, Janssen Research & Development, Beerse, Belgium.
Ronald D’Amico, ViiV Healthcare, Durham, North Carolina, USA.
Harmony P Garges, ViiV Healthcare, Durham, North Carolina, USA.
Andrew Zolopa, ViiV Healthcare, Durham, North Carolina, USA.
William R Spreen, ViiV Healthcare, Durham, North Carolina, USA.
Jean van Wyk, ViiV Healthcare, Brentford, United Kingdom.
Amy G Cutrell, ViiV Healthcare, Durham, North Carolina, USA.
Notes
Author contributions. P. P., R. D., D. D., Y. W., K. H., V. V. E., H. C., S. L. F., C. L. L., M. S. C., J. W. P., S. V., K. V., R. D’A., H. P. G., A. Z., W. R. S., J. v. W., and A. G. C. participated in the research design. C. O., J. M. S., C. F. P., D. R. K., K. H., V. V. E., H. C., S. L. F., C. L. L., M. S. C., and R. D’A. conducted experiments for the study analyses. R. D., D. D., Y. W., and A. G. C. performed statistical analyses. All authors interpreted the findings, were involved in the drafting and review of the manuscript, and approved the final version.
Acknowledgments. The authors thank everyone who has contributed to the FLAIR, ATLAS, and ATLAS-2M studies: all study participants and their families, and the clinical investigators and their staff. Professional medical writing and editorial assistance was provided by Daniel Williams, MSc, at Scimentum (Nucleus Global) and funded by ViiV Healthcare.
Financial support. FLAIR, ATLAS, and ATLAS-2M were funded by ViiV Healthcare and Janssen Pharmaceuticals. This analysis was funded by ViiV Healthcare. The funders participated in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. All authors vouch for the accuracy and completeness of the data, data analyses, and interpretation, and fidelity to the protocol.
Data sharing. Data sharing requests will be considered by the management group on written request to the corresponding author. Deidentified participant data or other prespecified data will be available subject to a written proposal and a signed data sharing agreement.
References
- 1. U.S. Department of Health and Human Services . Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Available at:https://clinicalinfo.hiv.gov/en/guidelines. Accessed 29 June 2023.
- 2. Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA panel. JAMA 2020; 324:1651–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Orkin C, Bernal Morell E, Tan DHS, et al. Initiation of long-acting cabotegravir plus rilpivirine as direct-to-injection or with an oral lead-in in adults with HIV-1 infection: week 124 results of the open-label phase 3 FLAIR study. Lancet HIV 2021; 8:e668–e78. [DOI] [PubMed] [Google Scholar]
- 4. Swindells S, Lutz T, Van Zyl L, et al. Week 96 extension results of a phase 3 study evaluating long-acting cabotegravir with rilpivirine for HIV-1 treatment. AIDS 2022; 36:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swindells S, Andrade-Villanueva JF, Richmond GJ, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med 2020; 382:1112–23. [DOI] [PubMed] [Google Scholar]
- 6. Jaeger H, Overton ET, Richmond G, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 96-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet HIV 2021; 8:e679–e89. [DOI] [PubMed] [Google Scholar]
- 7. Orkin C, Oka S, Philibert P, et al. Long-acting cabotegravir plus rilpivirine for treatment in adults with HIV-1 infection: 96-week results of the randomised, open-label, phase 3 FLAIR study. Lancet HIV 2021; 8:e185–e96. [DOI] [PubMed] [Google Scholar]
- 8. Orkin C, Arasteh K, Górgolas Hernández-Mora M, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med 2020; 382:1124–35. [DOI] [PubMed] [Google Scholar]
- 9. Overton ET, Richmond G, Rizzardini G, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 48-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet 2021; 396:1994–2005. [DOI] [PubMed] [Google Scholar]
- 10. Overton E, Richmond G, Rizzardini G, Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with human immunodeficiency virus 1 type 1 (HIV-1) infection: 152-week results from ATLAS-2M, a randomized, open-label, phase 3b, noninferiority study. Clin Infect Dis 2023; 76:1646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sinclair G, Benson P, Mena L, et al. Perspectives of people living with HIV-1 on implementation of long-acting cabotegravir plus rilpivirine in US healthcare settings: results from the CUSTOMIZE hybrid III implementation-effectiveness study. J Int AIDS Soc 2022; 25:e26006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jonsson-Oldenbüttel C, Ghosn J, van der valk M, et al. Safety and effectiveness from the CARISEL study: phase 3b hybrid-III implementation study integrating cabotegravir + rilpivirine long-acting into European clinical settings. J Int AIDS Soc 2022; 25(Suppl 3):e25935. Abstract EPLBB05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Welzen BJ, Vandekerckhove L, Jonsson-Oldenbüttel C, et al. Implementation of cabotegravir and rilpivirine long-acting (CAB + RPV LA): primary results from the CAB + RPV implementation study in European locations (CARISEL). Poster presented at HIV Glasgow 2022. Poster P069.
- 14. Czarnogorski M, Garris CP, Dalessandro M, et al. Perspectives of healthcare providers on implementation of long-acting cabotegravir plus rilpivirine in US healthcare settings from a hybrid III implementation-effectiveness study (CUSTOMIZE). J Int AIDS Soc 2022; 25:e26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borch J, Scherzer J, Jonsson-Oldenbüttel C, et al. 6-month outcomes of every 2 months long-acting cabotegravir and rilpivirine in a real-world setting—effectiveness, adherence to injections, and patient-reported outcomes of people living with HIV in the German CARLOS cohort presented at HIV Drug Therapy Glasgow 2022; virtual and Glasgow, Scotland. Presentation 043.
- 16. Rizzardini G, Overton ET, Orkin C, et al. Long-acting injectable cabotegravir + rilpivirine for HIV maintenance therapy: week 48 pooled analysis of phase 3 ATLAS and FLAIR trials. J Acquir Immune Defic Syndr 2020; 85:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Los Alamos National Laboratory . HIV sequence database. Available at:http://www.hiv.lanl.gov/. Accessed 29 June 2023.
- 18. Lapovok I, Laga V, Kazennova E, Bobkova M. HIV type 1 integrase natural polymorphisms in viral variants circulating in FSU countries. Curr HIV Res 2017; 15:318–26. [DOI] [PubMed] [Google Scholar]
- 19. Kirichenko A, Lapovok I, Baryshev P, et al. Genetic features of HIV-1 integrase sub-subtype A6 predominant in Russia and predicted susceptibility to INSTIs. Viruses 2020; 12:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eron JJ, Clotet B, Durant J, et al. Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING study. J Infect Dis 2013; 207:740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oliveira M, Ibanescu RI, Anstett K, et al. Selective resistance profiles emerging in patient-derived clinical isolates with cabotegravir, bictegravir, dolutegravir, and elvitegravir. Retrovirology 2018; 15:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boyce CL, Brummel S, Ziemba L, et al. HIV drug resistance in women randomized to DTG vs EFV or TDF vs TAF in pregnancy [CROI abstract 509]. Abstracts from CROI 2022 Conference on Retroviruses and Opportunistic Infections. CROI 2022 Abstract eBook. 2022; 483.
- 23. Jeffrey JL, St Clair M, Wang P, et al. Impact of integrase sequences from HIV-1 subtypes A6/A1 on the in vitro potency of cabotegravir or rilpivirine. Antimicrob Agents Chemother 2022; 66:e0170221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cutrell AG, Schapiro JM, Perno CF, et al. Exploring predictors of HIV-1 virologic failure to long-acting cabotegravir and rilpivirine: a multivariable analysis. AIDS 2021; 35:1333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wensing AM, Calvez V, Ceccherini-Silberstein F, et al. 2019 update of the drug resistance mutations in HIV-1. Top Antivir Med 2019; 27:111–21. [PMC free article] [PubMed] [Google Scholar]
- 26. Wensing AM, Calvez V, Gunthard HF, et al. 2015 update of the drug resistance mutations in HIV-1. Top Antivir Med 2015; 23:132–41. [PMC free article] [PubMed] [Google Scholar]
- 27. World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310:2191–4. [DOI] [PubMed] [Google Scholar]
- 28. Neyens M, Crauwels HM, Perez-Ruixo JJ, Rossenu S. Population pharmacokinetics of the rilpivirine long-acting formulation after intramuscular dosing in healthy subjects and people living with HIV. J Antimicrob Chemother 2021; 76:3255–62. [DOI] [PubMed] [Google Scholar]
- 29. Han K, Baker M, Lovern M, et al. Population pharmacokinetics of cabotegravir following administration of oral tablet and long-acting intramuscular injection in adult HIV-1-infected and uninfected subjects. Br J Clin Pharmacol 2022; 88:4607–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patel P, Ford S, Crauwels H, et al. Pharmacokinetics of cabotegravir and rilpivirine long-acting injectables in HIV-infected individuals through 48 weeks in the FLAIR and ATLAS phase 3 studies. Open Forum Infect Dis 2019; 6(Suppl 2):S865–6. Abstract 2495. [Google Scholar]
- 31. Ramgopal MN, Castagna A, Cazanave, et al. Efficacy, safety, and tolerability of switching to long-acting cabotegravir + rilpivirine versus continuing bictegravir/emtricitabine/tenofovir alafenamide in virologically suppressed adults with HIV, 12-month results (SOLAR): A randomised, open-label, phase 3b non-inferiority trial. Lancet 2023. In press. [DOI] [PubMed] [Google Scholar]
- 32. Elliot E, Polli JW, Patel P, et al. Efficacy and safety outcomes by BMI category over 48 weeks in phase 3/3b cabotegravir and rilpivirine long-acting trials. Presented at the European AIDS conference 2021; October 27–30, 2021; virtual and London, UK. Presentation BPD1/8. Available from: https://www.natap.org/2021/EACS/EACS_23.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.