Abstract
Older organs provide a substantial unrealized potential with the capacity to close the gap between demand and supply in organ transplantation.
The potential of senolytics in improving age-related conditions has been shown in various experimental studies and early clinical trials. Those encouraging data may also be of relevance for transplantation.
As age-differences between donor and recipients are not uncommon, aging may be accelerated in recipients when transplanting older organs; young organs may, at least in theory, have the potential to ‘rejuvenate’ old recipients.
Here, we review the relevance of senescent cells and the effects of senolytics on organ quality, alloimmune responses and outcomes in solid organ transplantation.
Keywords: Senolytics, Transplantation, Senescent cells, immunosenescence, SASP, old donors, Aging
Senescent cells accumulate in aging and impact organ quality and -function
Senescent cells are characterized by the expression of p21CIP1 and/or p16INK4a, DNA segments with chromatin alterations reinforcing senescence (Rodier et al., 2011), senescence-associated heterochromatin foci (Narita et al., 2003), senescence-associated β-galactosidase (Lee et al., 2006), short telomeres (Bernadotte et al., 2016) and the production of a senescence-associated secretory phenotype (SASP). From a physiological perspective, senescence represents a cell-autonomous mechanism, preventing the transformation into malignant cells (Campisi, 2001). Indeed, approaches aimed to inhibit cellular senescence by targeting p16INK4a, Rb, p53 or p21CIP1 have been shown to promote cancer development (Larsen, 2004; Takeuchi et al., 2010).
Characteristically, senescent cells accumulate in various tissues with aging (Dimri et al., 1995; Korolchuk et al., 2017) and have a compromised mitochondrial capacity with a limited tissue regeneration (Jurk et al., 2014). Senescent cells also have the ability to induce senescence in surrounding, non-senescent cells through the production of SASP consisting of pro-inflammatory cytokines, chemokines, proteases, and other factors, (Coppe et al., 2008; Xu et al., 2015b). SASP constitutes a fundamental feature of senescent cells disrupting tissue homeostasis and impeding neighboring cellular functions resulting into age-related tissue dysfunction, chronic age-related diseases, and biological aging (Kirkland and Tchkonia, 2017). Consequently, senescent cell accumulation promotes a variety of age-related diseases. For instance, senescent cells accumulate in adipose tissue in individuals with diabetes, contributing to age-related metabolic dysfunction (Minamino et al., 2009; Tchkonia et al., 2010; Xu et al., 2015a), in the aorta during atherosclerosis (Roos et al., 2016), and in the lungs as observed in idiopathic pulmonary fibrosis (Schafer et al., 2017).
Multimorbidity, defined asthe coexistence of multiple chronic diseases a clinical condition that is particularly common in the elderly. Metabolic syndrome and cardiovascular disease both display established causes of aging. These conditions are associated with changes that are also observed in aging, such as increased intensity of intracellular processes that generate free radicals and cause chronic low-grade inflammation, a phenomenon also called ‘Inflamm-aging’ (Edrey and Salmon, 2014; Franceschi et al., 2007). Inflamm-aging itself is a risk factor not only for cardiovascular disease, cancer and chronic kidney disease, but also an indicator of poor health status lined to multimorbidity, sarcopenia, frailty and premature death (Ferrucci and Fabbri, 2018). Based on these findings, an interactive concept is suggested: while the accumulation of senescent cells is the cause of age-related diseases, the presence of multiple chronic diseases may, in turn, accelerate aging. (Hodes et al., 2016) Since, both transplant recipients and donors have frequently an extensive past-medical history it is also possible that accelerated biological aging due to senescence derived multimorbidity may affect transplant organ function and outcome.
Senescent cell accumulation and Immunogenicity
Senescent cells secrete various inflammatory proteins most of which are transcriptionally regulated by nuclear factor κB and CCAAT/Enhancer-binding transcribed by protein β (Kuilman et al., 2008). Epigenetic factors such as G9a/GLP, SIRT1, MLL1, BRD4, and HMGB2 have been shown to drive the expression of SASP factors including IL-6, IFN-γ, and TNF-α (Freund et al., 2010; Loo et al., 2020; Takahashi et al., 2012) that contribute to the sterile, low grade, chronic inflammation in aging (Franceschi et al., 2000; Tchkonia et al., 2013).
Ischemia-reperfusion injury (IRI) is an inevitable component of transplantation occurring during organ procurement, transport and, finally, while reconstructing blood flow. Oxygen demand increases rapidly in ischemic tissues after organ reperfusion. IRI induces oxidative stress, mitochondrial damage, electrolyte imbalance resulting into local inflammation, the release of ROS (Fisher et al., 1991), pro-inflammatory cytokines, especially TNF-α, IL-1, IL-6, IL-8 (Krishnadasan et al., 2003; Naidu et al., 2003) in addition to various proteases (Yano et al., 2001). All of those factors have been implicated in accelerated senescence via SASP activation, mitochondrial dysfunction, and activation of the p53 and RAS pathways. Recent studies in experimental models confirm that IRI induces senescence in both cardiomyocytes and interstitial cells, both within and around the infarcted left ventricular myocardium (Dookun et al., 2020). Senomorphic drugs that inhibit SASP including ATM kinase inhibitors and Janus kinase inhibitors may ameliorate the consequences of SASP factors released upon IRI in transplanted aged organs (Birch and Gil, 2020).
IRI also activates the recipient’s innate immunity through the release of damage associated molecular patterns (DAMPs) consisting of intracellular contents (e.g., mt-DNA, HMGB1) or membrane components (e.g., hyaluron, syndecan). Donor and recipient derived antigen presenting cells recognize and activated DAMPs through pattern recognition receptors (PRR) that initiate adaptive immune responses targeting the allograft through MHC-class-II mediated antigen-presentation (Yi et al., 2012).
Old donor organs contain augmented frequencies of senescent cells as a key source of cell-free-mt-DNA. IRI, in turn, leads to an increased release of cf-mt-DNA, particularly in old organs promoting an amplified dendritic cell mediated Th1/Th17 alloimmune response leading to an inferior graft survival of older organs (Iske et al., 2020; Oberhuber et al., 2015). Clinically, increased levels of mt-DNA have also been linked to higher rejection rates and delayed graft function following kidney transplantation (Kim et al., 2019).
These findings are consistent with preclinical and clinical studies showing that donor age poses a significant risk for adverse outcomes, an augmented immunogenicity and more frequent acute rejections (Oberhuber et al., 2015; Tullius and Milford, 2011).
Improving outcomes of older organs for transplantation
Although organ transplantation represents the treatment of choice for end-stage organ failure, the approach cannot achieve its potential as there is a dramatic gap between demand and supply. While the number of organ transplants has increased over the last years, more than 100,000 patients are currently waiting for an organ transplant in the United States (Hart et al., 2021). In contrast, it is estimated that approximately 24.000 organs from older donors are currently not utilized or discarded (Klassen et al., 2016). The age of organ donors is the most prominent reason for organ discard. (Messina et al., 2017) Utilizing available organs for transplantation successfully has therefore the potential to substantially reduce wait-times, morbidity and mortality for patients on transplant waitlists.
Senolytics have the capacity to selectively clear senescent cells. In preclinical models, senolytics delay, prevent or alleviate age-related dysfunction and diseases in multiple organs. Several clinical studies testing the potential of senolytics are currently underway (Kirkland and Tchkonia, 2020). As senolytics effectively reduce the burden of senescent cells and alleviate age-related dysfunction and inflammation, these agents may ultimately be effective when utilizing organs from older donors. We have been able to show that Dasatinib plus Quercetin (D & Q) applied prior to experimental IRI and transplantation significantly reduced the burden of senescent cells while alleviating systemic inflammation and decreasing levels of cf-mt-DNA, Th17 and IFNγ+ T cells. Notably, pretreatment of old donor mice with senolytics prolonged the survival of older to that of young organs in a mouse cardiac transplant model (Iske et al., 2020).
In the heart, for example, senescence accumulate with aging, resulting in augmented amounts of senescent cardiomyocytes, endothelial cells, smooth muscle cells, cardiac fibroblasts and cardiac progenitor cells (Anderson et al., 2019; Walaszczyk et al., 2019). Preclinical models of biological aging have demonstrated that senescence contributes to the pathophysiology of age-related remodeling and cardiac dysfunction indicating a role for senolytics (Chimenti et al., 2003). Navitoclax has been shown to reduce cardiomyocyte senescence and SASP of cardiomyocytes attenuating cardiac hypertrophy reducing the size of the left ventricle (Anderson et al., 2019; Owens et al., 2021; Walaszczyk et al., 2019).
During and following the transplant procedure, several factors including ischemia reperfusion injury, early allo-immune responses in addition to immunosuppressive treatment mediate graft damage and impede tissue regeneration. Moreover, tissue senescence with aberrant p21 expression has been identified to further compromise tissue generation following partial hepatectomy (Ritschka et al., 2020). Accumulating senescent cells have also been shown to impair tissue regeneration following cardiac infarction (Lewis-McDougall et al., 2019). Notably, ABT-737, a senolytic drug inhibiting Bcl-2 and Bcl-x, has been shown to disrupt aberrant p21 expression, thus restoring liver generation in mice (Ritschka et al., 2020) while global senescent cell elimination in the INK-ATTAK system increased the number of proliferating cardiomyocytes in old hearts (Lewis-McDougall et al., 2019).
There are several time points during which an organ for transplant could be treated with senolytics: Starting prior to transplantation with a treatment of the donor (Fig. 1A), during organ preservation with a treatment of the graft itself (Fig. 1 B), and finally, following transplantation with a treatment of the recipient (Fig. 1C).
Figure 1. Potential Opportunities to Apply Senolytics in Organ Transplantation.
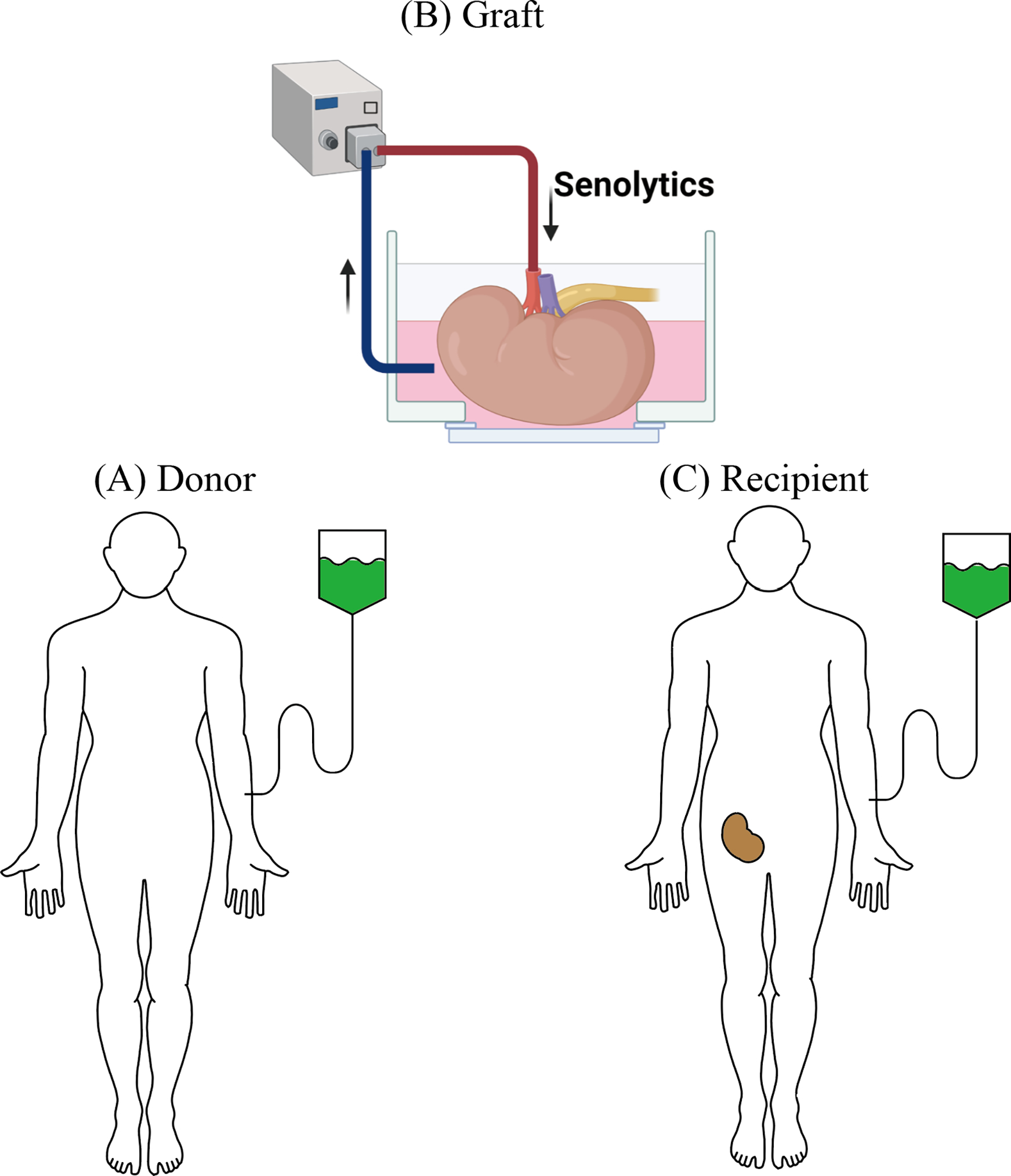
(A) Donor: Senolytics may be administered prior to organ donation. When applied in living donors, a regimen with minimal to no side effects is desirable.
(B) Graft: Treatment of the graft after organ procurement and prior to transplantation represents an attractive therapeutic intervention. Ex-vivo perfusion systems allowing for prolonged organ evaluation may not only support the treatment with senolytics but may also aide in monitoring treatment effects.
(C) Recipient: Recipients can be treated with senolytics at the time of transplantation or in the immediate post-transplant period; interaction between senolytics and immunosuppressants need to be considered.
Administering senolytics to the donor may ameliorate the consequences of IRI in older organs. Senolytics that target only a single senescent cell anti-apoptotic pathways (SCAP), such as navitoclax, are likely to have significant off-target apoptotic effects on non-aging cell types including platelets and immune cells while eliminating a limited range of senescent cells (Zhu et al., 2016). Moreover, some senescent cells have a beneficial role in tissue repair. (Demaria et al., 2014) Intermittent administration, combined with the short elimination half-life of senolytics may thus be most effective, minimizing drug toxicity and off-target effects (Christopher et al., 2008; Graefe et al., 2001). Notably, only a brief exposure to senolytics is required for senescent cells to initiate apoptosis thus avoiding the risks of long-term exposure and side-effects (Palmer et al., 2021). Indeed, the tolerability of dasatinib in patients with chronic myeloid leukemia has improved with intermittent dosing schedules (La Rosee et al., 2013).
Treatment of the allograft itself following organ procurement and prior to transplantation, may provide another opportunity for intervention. Adding senolytics to organ preservation solutions represents a unique opportunity for targeted delivery. New technologies including ex-vivo hypo- or normothermic organ perfusion do not only facilitate the assessment of organ function prior to transplantation but may also provide a platform for an isolated treatment of donor organs (Chandak et al., 2019; Nasralla et al., 2018; Reddy et al., 2009; Van Raemdonck et al., 2015). Overall, we submit that that senolytics may help to significantly expand the donor pool and improve outcomes of transplants derived from older donors. With an increased utilization of older organs, organ repair ‘centers’, an increased utilization of ex vivo organ perfusion systems and an adjusted allocation may be become necessary. The cost of machine perfusion can be an obstacle to their wider use. However, the cost of performing normothermic machine perfusion on grafts that have already been discarded was estimated to be only slightly more than the estimated monthly Medicare expenditure for the care of a MELD 30 patient, in addition to preventing deaths on the waiting list (Raigani et al., 2020). Moreover, economic analysis of kidney transplantation demonstrated that transplantation of low-quality kidneys is more cost-effective that waiting and receiving dialysis (Senanayake et al., 2021).
Biological age affecting transplant outcome
While the effects of donor age on transplant outcomes are clearly recognized (Tullius and Milford, 2011), it has become clear that biological rather than chronological donor age determines organ quality and long-term function after transplantation. Indeed, cyclin-dependent kinase inhibitor 2A (CDKN2A) expression in zero-hour biopsies in kidney transplantation was associated with donor age and graft function. In addition, linear regression analysis showed that CDKN2A was the best single predictor, followed by donor age and telomere length (Koppelstaetter et al., 2008). The concept that biological organ age impacting outcomes after transplantation is also supported by findings that delayed graft function, acute rejection, and chronic allograft dysfunction, are associated with telomere shortening (Domanski et al., 2015). At the same time, improved graft survival of renal transplants from p16 INK4a−/− mice compared to wild-type mice (Braun et al., 2012) underscore the concept of a cellular based biological age affecting graft outcomes. Thus, assessing chronological donor age utilizing markers of biological organ age, may provide additional valuable information.
Interference of senolytics with immunosuppressants
When administering senolytics to transplant recipient both, the pharmacological interaction as well as the mechanistic interaction between senolytics and immunosuppressive drugs require consideration. Notably, a broad range of senolytics has been shown to exert immunosuppressive effects on various immune cells which may augment the effects of established immunosuppressants.
Dasatinib is a Tyrosine kinase inhibitor (TKI) targeting the Src family (O’Hare et al., 2005) that also interferes with T cell receptor signaling (Smith-Garvin et al., 2009). Phosphorylation of Lck and Fyn, both members of the Src family, for instance, represent an early step within the T cell receptor activation cascade. Indeed, depleting Lcy and Fyn simultaneously has been shown to impair T cell function in mice (Denny et al., 2000; Lovatt et al., 2006; Tsun et al., 2011). SRC family proteins also display relevant targets of glucocorticoids mediated T cell suppression (Giese et al., 2004; Moes et al., 2014). Co-administration of Dasatinib and glucocorticoids may therefore affect similar pathways with potential additive or synergistic effects on T-cell suppression. Indeed, Dasatinib has been shown to exert synergistic effects with glucocorticoids in a murine model of acute T-cell leukemia (Serafin et al., 2017).
Calcineurin inhibitors (CNI) including tacrolimus (FK506) and cyclosporine (CsA) are widely administered as maintenance immunosuppressants in solid organ transplantation (Matas et al., 2015). Calcineurin dephosphorylates the cytoplasmic nuclear factor of activated T cells (NFAT), facilitating the migration into the nucleus, thus preventing the transcription of critical cytokines including IL-2 (Giese et al., 2004; Moes et al., 2014). Notably, the histone deacetylase inhibitor and senolytic drug Panobinostat have also been shown to induce calcineurin degradation through inhibiting the chaperone function of heat shock protein 90 (Imai et al., 2016) suggesting synergistic effects with tacrolimus or CsA.
Further evidence for synergistic effects of senolytics drugs with immunosuppressants derives from clinical studies assessing the impact of combinatorial treatment on hematopoietic tumors. Of relevance, synergistic effects of the bcl-family inhibitor navitoclax and rituximab treating B cell lymphoma have been reported (Ackler et al., 2012).
Senolytics may also interfere with the immunosuppressive effects of mTOR inhibitors such as rapamycin. In addition to their senolytic effects, the flavonoids quercetin and fisetin have been identified as inhibitors of the mTOR pathway which may result in synergistic effects on inhibiting T cell alloimmunity. In support, quercetin has been shown to inhibit the induction and effector function of cytotoxic T cells in mixed leucocyte reactions (Schwartz et al., 1982) while fisetin compromised Th1 and Th2 cytokine production, and proliferation of murine T cells in vitro (Song et al., 2013).
Interestingly, rapamycin reduces cellular senescence and systemic inflammation in the elderly while improving physical performance (Kim and Guan, 2015; Singh et al., 2016) suggesting a potential interference of immunosuppressants with senolytics. Mechanistically, mTOR inhibition has been shown to impede on protein translation and augmentation of autophagy expanded lifespan in flies and worms (Bjedov et al., 2010; Hansen et al., 2008; Hansen et al., 2007; Toth et al., 2008) (Figure 2).
Figure 2. Interaction of Senolytic and Immunosuppressive drugs.
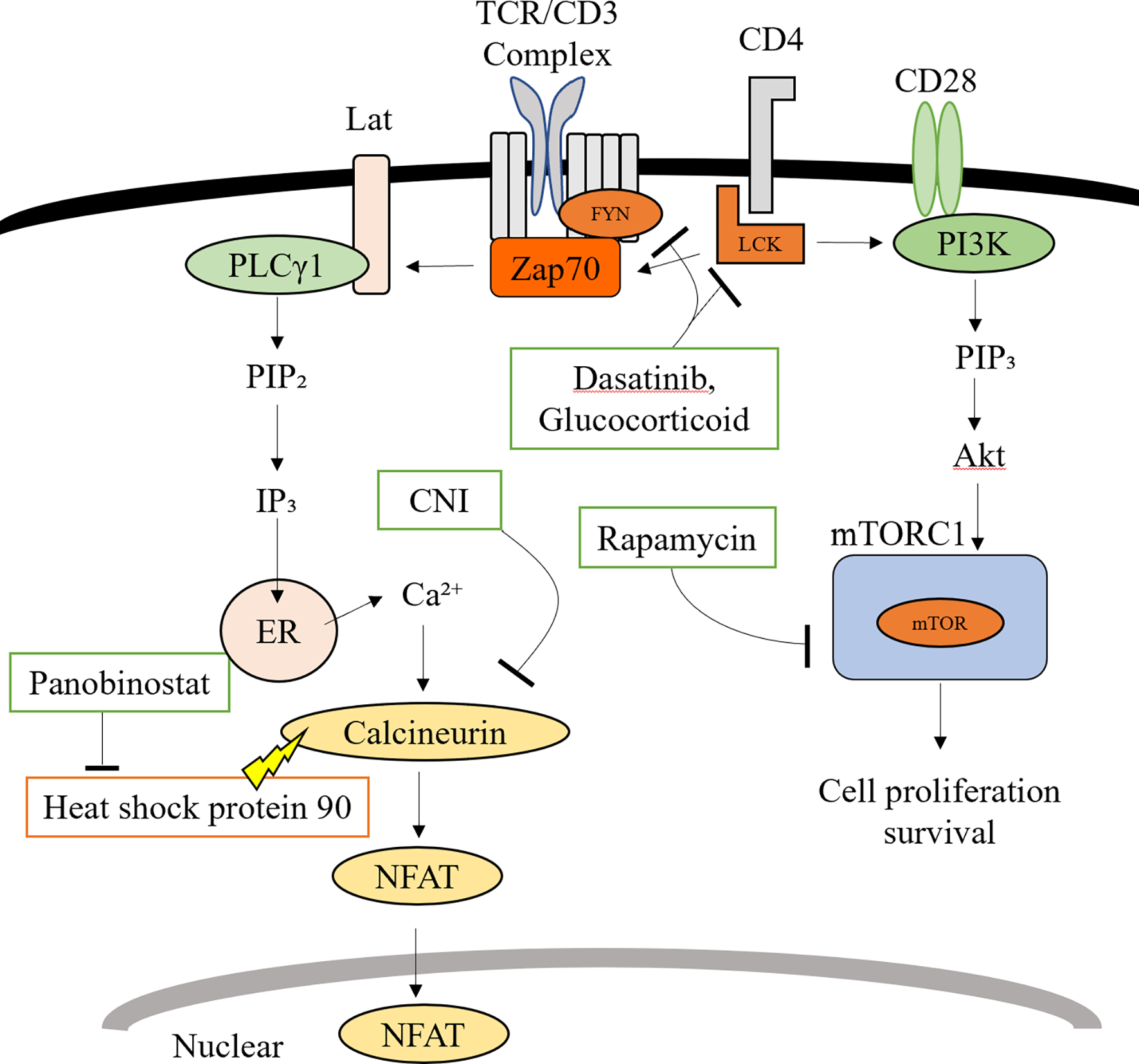
Members of the Src family including Lck and Fyn are involved in early TCR signal transduction. Both, Dasatinib and Glucocorticoids target Src family proteins.
IP3 is the trigger for Ca2+ release from the endoplasmic reticulum (ER) promoting the entry of extracellular Ca2+ into the cell via calcium release-activated Ca2+ channels. Calmodulin bound to calcium activates calcineurin while promoting the transcription of IL-2 through the NFAT transcription factor. CNI inhibits calcineurin, thus suppressing dephosphorylations of NFAT. Panobinostat induces calcineurin degradation through inhibiting the chaperone function of heat shock protein 90.
CD28 co-stimulation of the TCR phosphorylates and activates PI3K, thereby activating the PI3K/mTOR pathway. Rapamycin inhibits T cell proliferation by inhibiting the formation of mTORC1.
Transfer of senescent cells with organ transplantation: A theoretical concept with relevance for senolytics
Old organs represent an underutilized source for transplantation (Saidi and Hejazii Kenari, 2014; Tullius and Rabb, 2018). Transplanting older organs, in turn, may lead to the transfer of senescent cells that may accumulate in recipients, potentially accelerating aging.
Intraperitoneal transplantation of relatively small numbers of senescent cells transferred into young mice accelerated aging of visceral adipose tissue and has been accompanied by a decline in physical performance (Xu et al., 2018). In addition, autologous transplantation of senescent cells into healthy knee joints of young mice promoted the development of an osteoarthritis-like condition (Xu et al., 2017). Moreover, transplanting senescent cells into the skeletal muscle of immunocompromised NOD SCID gamma mice revealed an increase in the number of senescent cells with an enhanced expression of SASP markers including IL-1α, IL-1β, IL-6, and TNF-α (da Silva et al., 2019). Most recently it has been shown that the transfer of splenocytes augmented SASP proteins systemically in recipient mice resulting into accelerated aging (Yousefzadeh et al., 2021). These observations are consistent with our preliminary findings showing that young mice transplanted with old hearts accumulated senescent cells in various tissues with an inferior physical performance that improved if grafts had been treated with senolytics.
Thus, removing senescent cells from donor organs may not only reduce immunogenicity and improve organ function but also inhibit the transfer of senescent cells in organ transplantation. The finding that D&Q slowed aging and the associated decline in physical function in mice injected intraperitoneally with senescent cells supports this concept (Xu et al., 2018).
At least in theory, it is also possible that young donor organs could exert a rejuvenating effect when transplanted into older recipients. Experiments with parabiosis models have shown the potential to rejuvenate brain, heart muscle, pancreas, bone, and skeletal muscle of old mice. (Baht et al., 2015; Conboy et al., 2005; Katsimpardi et al., 2014; Villeda et al., 2011; Villeda et al., 2014). It has also been shown that the transfer of plasma from young into old mice augmented the plasticity of hippocampal neurons and improved cognitive function (Villeda et al., 2014). Subsequent studies have indicated that several blood soluble factors may mediate rejuvenation. Moreover, extracellular vesicles derived from young mesenchymal stromal cells rejuvenated old endothelial progenitor cells in vitro (Kang and Yang, 2020; Rybtsova et al., 2020; Wang et al., 2020). Although representing a theoretical possibility, the rejuvenation potential when transplanting a young organ remains speculative currently.
Conclusion
Reducing the burden of senescent cells to improve organ quality while ameliorating alloimmune responses represents a novel concept. Diminishing the amounts of senescent cells in old organs with senolytics may thus have the potential of increasing organ availability and improving transplant outcomes. Confirmatory clinical trials will be necessary.
With many open questions remaining, optimal treatment time points, regimens and detailed mechanisms including an interfere with immunosuppressants will require more detailed investigations.
Transferring aging with the transplantation of older organs will be of clinical relevance if confirmed. Mechanisms linking the transplantation of old organs with an acceleration of senescence in recipients need to be delineated in detail.
Acknowledgements
This work was supported by the Osaka Medical College Foundation (to TM), NIH grant R01AG064165 (to SGT) and a grant by the Pepper Foundation (to HZ).
Glossary & Abbreviations
- Akt
Protein kinase B
- ATM
ataxia telangiectasia mutated
- Bcl-2 /
Bcl-x B-cell lymphoma 2 / B-cell lymphoma x
- BRD4
Bromodomain-containing protein 4
- CaA
cyclosporine
- CCAAT
cytosine-cytosine-adenosine-adenosine-thymidine
- CDKN2A
cyclin-dependent kinase inhibitor 2A
- CNI
Calcineurin inhibitors
- DAMPs
damage associated molecular patterns
- D & Q
Dasatinib plus Quercetin
- ER
endoplasmic reticulum
- FK506
Tacrolimus
- GLP
G9a-like-protein
- HMGB2
high-mobility group box 2
- IFN
Interferon
- IL
interleukin
- INK-ATTAK-mouse
INK-linked apoptosis through targeted activation of caspase), which selectively expresses a FK506-binding protein (FKBP)–CASP8 fusion protein in p16INK4A-postive senescent cells and triggers apoptosis following administration of AP20187, a molecule that dimerizes the FKBP–CASP8 fusion protein
- IP3
inositol trisphosphate
- IRI
Ischemia-reperfusion injury
- Lat
linker for activation of T cells
- Lck
lymphocyte protein tyrosine kinase
- MLL1
Mixed lineage leukemia protein-1
- mTOR
mammalian/mechanistic target of rapamycin
- mTORC1
mTOR complex 1
- NFAT
nuclear factor of activated T cells
- NMP
normothermic mechanical perfusion
- NOD
nucleotide-binding oligomerization domain-containing protein
- PI3K
Phosphoinositide 3-kinase
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PIP3
Phosphatidylinositol 3,4,5-trisphosphate
- PLCγ1
Phosphorylation of phospholipase C γ1
- PRR
pattern recognition receptors
- RAS
Rat sarcoma
- ROS
reactive oxygen species
- SASP
senescence-associated secretory phenotype
- SCAP
single senescent cell anti-apoptotic pathways
- SCID
severe combined immunodeficiency
- SIRT1
stimulator of interferon genes 1
- TCR
T cell antigen receptor
- TKI
Tyrosine kinase inhibitor
- TNF
tumor necrosis factor
- Zap70
Zeta-chain associated protein kinase 70
References:
- Ackler S, Mitten MJ, Chen J, Clarin J, Foster K, Jin S, Phillips DC, Schlessinger S, Wang B, Leverson JD, Boghaert ER, 2012. Navitoclax (ABT-263) and bendamustine +/− rituximab induce enhanced killing of non-Hodgkin’s lymphoma tumours in vivo. Br J Pharmacol 167, 881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Lagnado A, Maggiorani D, Walaszczyk A, Dookun E, Chapman J, Birch J, Salmonowicz H, Ogrodnik M, Jurk D, Proctor C, Correia-Melo C, Victorelli S, Fielder E, Berlinguer-Palmini R, Owens A, Greaves LC, Kolsky KL, Parini A, Douin-Echinard V, LeBrasseur NK, Arthur HM, Tual-Chalot S, Schafer MJ, Roos CM, Miller JD, Robertson N, Mann J, Adams PD, Tchkonia T, Kirkland JL, Mialet-Perez J, Richardson GD, Passos JF, 2019. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baht GS, Silkstone D, Vi L, Nadesan P, Amani Y, Whetstone H, Wei Q, Alman BA, 2015. Exposure to a youthful circulaton rejuvenates bone repair through modulation of beta-catenin. Nat Commun 6, 7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernadotte A, Mikhelson VM, Spivak IM, 2016. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY) 8, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch J, Gil J, 2020. Senescence and the SASP: many therapeutic avenues. Genes Dev 34, 1565–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L, 2010. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab 11, 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun H, Schmidt BM, Raiss M, Baisantry A, Mircea-Constantin D, Wang S, Gross ML, Serrano M, Schmitt R, Melk A, 2012. Cellular senescence limits regenerative capacity and allograft survival. J Am Soc Nephrol 23, 1467–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, 2001. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol 11, S27–31. [DOI] [PubMed] [Google Scholar]
- Chandak P, Phillips BL, Uwechue R, Thompson E, Bates L, Ibrahim I, Sewpaul A, Figueiredo R, Olsburgh J, Hosgood S, Nicholson ML, Wilson C, Callaghan CJ, 2019. Dissemination of a novel organ perfusion technique: ex vivo normothermic perfusion of deceased donor kidneys. Artif Organs 43, E308–E319. [DOI] [PubMed] [Google Scholar]
- Chimenti C, Kajstura J, Torella D, Urbanek K, Heleniak H, Colussi C, Di Meglio F, Nadal-Ginard B, Frustaci A, Leri A, Maseri A, Anversa P, 2003. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res 93, 604–613. [DOI] [PubMed] [Google Scholar]
- Christopher LJ, Cui D, Wu C, Luo R, Manning JA, Bonacorsi SJ, Lago M, Allentoff A, Lee FY, McCann B, Galbraith S, Reitberg DP, He K, Barros A Jr., Blackwood-Chirchir A, Humphreys WG, Iyer RA, 2008. Metabolism and disposition of dasatinib after oral administration to humans. Drug Metab Dispos 36, 1357–1364. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA, 2005. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J, 2008. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6, 2853–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva PFL, Ogrodnik M, Kucheryavenko O, Glibert J, Miwa S, Cameron K, Ishaq A, Saretzki G, Nagaraja-Grellscheid S, Nelson G, von Zglinicki T, 2019. The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell 18, e12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dolle ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J, 2014. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31, 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny MF, Patai B, Straus DB, 2000. Differential T-cell antigen receptor signaling mediated by the Src family kinases Lck and Fyn. Mol Cell Biol 20, 1426–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. , 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 92, 9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanski L, Kloda K, Kwiatkowska E, Borowiecka E, Safranow K, Drozd A, Ciechanowicz A, Ciechanowski K, 2015. Effect of delayed graft function, acute rejection and chronic allograft dysfunction on kidney allograft telomere length in patients after transplantation: a prospective cohort study. BMC Nephrol 16, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dookun E, Walaszczyk A, Redgrave R, Palmowski P, Tual-Chalot S, Suwana A, Chapman J, Jirkovsky E, Donastorg Sosa L, Gill E, Yausep OE, Santin Y, Mialet-Perez J, Andrew Owens W, Grieve D, Spyridopoulos I, Taggart M, Arthur HM, Passos JF, Richardson GD, 2020. Clearance of senescent cells during cardiac ischemia-reperfusion injury improves recovery. Aging Cell 19, e13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edrey YH, Salmon AB, 2014. Revisiting an age-old question regarding oxidative stress. Free Radic Biol Med 71, 368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Fabbri E, 2018. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 15, 505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AB, Dodia C, Tan ZT, Ayene I, Eckenhoff RG, 1991. Oxygen-dependent lipid peroxidation during lung ischemia. J Clin Invest 88, 674–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G, 2000. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908, 244–254. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S, 2007. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128, 92–105. [DOI] [PubMed] [Google Scholar]
- Freund A, Orjalo AV, Desprez PY, Campisi J, 2010. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med 16, 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese T, Zeier M, Schemmer P, Uhl W, Schoels M, Dengler T, Buechler M, Meuer S, 2004. Monitoring of NFAT-regulated gene expression in the peripheral blood of allograft recipients: a novel perspective toward individually optimized drug doses of cyclosporine A. Transplantation 77, 339–344. [DOI] [PubMed] [Google Scholar]
- Graefe EU, Wittig J, Mueller S, Riethling AK, Uehleke B, Drewelow B, Pforte H, Jacobasch G, Derendorf H, Veit M, 2001. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J Clin Pharmacol 41, 492–499. [DOI] [PubMed] [Google Scholar]
- Hansen M, Chandra A, Mitic LL, Onken B, Driscoll M, Kenyon C, 2008. A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet 4, e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C, 2007. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell 6, 95–110. [DOI] [PubMed] [Google Scholar]
- Hart A, Lentine KL, Smith JM, Miller JM, Skeans MA, Prentice M, Robinson A, Foutz J, Booker SE, Israni AK, Hirose R, Snyder JJ, 2021. OPTN/SRTR 2019 Annual Data Report: Kidney. Am J Transplant 21 Suppl 2, 21–137. [DOI] [PubMed] [Google Scholar]
- Hodes RJ, Sierra F, Austad SN, Epel E, Neigh GN, Erlandson KM, Schafer MJ, LeBrasseur NK, Wiley C, Campisi J, Sehl ME, Scalia R, Eguchi S, Kasinath BS, Halter JB, Cohen HJ, Demark-Wahnefried W, Ahles TA, Barzilai N, Hurria A, Hunt PW, 2016. Disease drivers of aging. Ann N Y Acad Sci 1386, 45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Ohta E, Takeda S, Sunamura S, Ishibashi M, Tamura H, Wang YH, Deguchi A, Tanaka J, Maru Y, Motoji T, 2016. Histone deacetylase inhibitor panobinostat induces calcineurin degradation in multiple myeloma. JCI Insight 1, e85061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iske J, Seyda M, Heinbokel T, Maenosono R, Minami K, Nian Y, Quante M, Falk CS, Azuma H, Martin F, Passos JF, Niemann CU, Tchkonia T, Kirkland JL, Elkhal A, Tullius SG, 2020. Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation. Nat Commun 11, 4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, Saretzki G, Fox C, Lawless C, Anderson R, Hewitt G, Pender SL, Fullard N, Nelson G, Mann J, van de Sluis B, Mann DA, von Zglinicki T, 2014. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun 2, 4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Yang YR, 2020. Circulating plasma factors involved in rejuvenation. Aging (Albany NY) 12, 23394–23408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL, 2014. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Moon H, Lee YH, Seo JW, Kim YG, Moon JY, Kim JS, Jeong KH, Lee TW, Ihm CG, Lee SH, 2019. Clinical relevance of cell-free mitochondrial DNA during the early postoperative period in kidney transplant recipients. Sci Rep 9, 18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Guan KL, 2015. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest 125, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T, 2017. Cellular Senescence: A Translational Perspective. EBioMedicine 21, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T, 2020. Senolytic drugs: from discovery to translation. J Intern Med 288, 518–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen DK, Edwards LB, Stewart DE, Glazier AK, Orlowski JP, Berg CL, 2016. The OPTN Deceased Donor Potential Study: Implications for Policy and Practice. Am J Transplant 16, 1707–1714. [DOI] [PubMed] [Google Scholar]
- Koppelstaetter C, Schratzberger G, Perco P, Hofer J, Mark W, Ollinger R, Oberbauer R, Schwarz C, Mitterbauer C, Kainz A, Karkoszka H, Wiecek A, Mayer B, Mayer G, 2008. Markers of cellular senescence in zero hour biopsies predict outcome in renal transplantation. Aging Cell 7, 491–497. [DOI] [PubMed] [Google Scholar]
- Korolchuk VI, Miwa S, Carroll B, von Zglinicki T, 2017. Mitochondria in Cell Senescence: Is Mitophagy the Weakest Link? EBioMedicine 21, 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnadasan B, Naidu BV, Byrne K, Fraga C, Verrier ED, Mulligan MS, 2003. The role of proinflammatory cytokines in lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg 125, 261–272. [DOI] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS, 2008. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031. [DOI] [PubMed] [Google Scholar]
- La Rosee P, Martiat P, Leitner A, Klag T, Muller MC, Erben P, Schenk T, Saussele S, Hochhaus A, 2013. Improved tolerability by a modified intermittent treatment schedule of dasatinib for patients with chronic myeloid leukemia resistant or intolerant to imatinib. Ann Hematol 92, 1345–1350. [DOI] [PubMed] [Google Scholar]
- Larsen CJ, 2004. [pRB, p53, p16INK4a, senescence and malignant transformation]. Bull Cancer 91, 399–402. [PubMed] [Google Scholar]
- Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, Kleijer WJ, DiMaio D, Hwang ES, 2006. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell 5, 187–195. [DOI] [PubMed] [Google Scholar]
- Lewis-McDougall FC, Ruchaya PJ, Domenjo-Vila E, Shin Teoh T, Prata L, Cottle BJ, Clark JE, Punjabi PP, Awad W, Torella D, Tchkonia T, Kirkland JL, Ellison-Hughes GM, 2019. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell 18, e12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo TM, Miyata K, Tanaka Y, Takahashi A, 2020. Cellular senescence and senescence-associated secretory phenotype via the cGAS-STING signaling pathway in cancer. Cancer Sci 111, 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovatt M, Filby A, Parravicini V, Werlen G, Palmer E, Zamoyska R, 2006. Lck regulates the threshold of activation in primary T cells, while both Lck and Fyn contribute to the magnitude of the extracellular signal-related kinase response. Mol Cell Biol 26, 8655–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Stewart DE, Cherikh WS, Wainright JL, Boyle G, Snyder JJ, Israni AK, Kasiske BL, 2015. OPTN/SRTR 2013 Annual Data Report: kidney. Am J Transplant 15 Suppl 2, 1–34. [DOI] [PubMed] [Google Scholar]
- Messina M, Diena D, Dellepiane S, Guzzo G, Lo Sardo L, Fop F, Segoloni GP, Amoroso A, Magistroni P, Biancone L, 2017. Long-Term Outcomes and Discard Rate of Kidneys by Decade of Extended Criteria Donor Age. Clin J Am Soc Nephrol 12, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, Ishikawa F, Komuro I, 2009. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med 15, 1082–1087. [DOI] [PubMed] [Google Scholar]
- Moes AD, Hesselink DA, Zietse R, van Schaik RH, van Gelder T, Hoorn EJ, 2014. Calcineurin inhibitors and hypertension: a role for pharmacogenetics? Pharmacogenomics 15, 1243–1251. [DOI] [PubMed] [Google Scholar]
- Naidu BV, Krishnadasan B, Farivar AS, Woolley SM, Thomas R, Van Rooijen N, Verrier ED, Mulligan MS, 2003. Early activation of the alveolar macrophage is critical to the development of lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg 126, 200–207. [DOI] [PubMed] [Google Scholar]
- Narita M, Nunez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW, 2003. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113, 703–716. [DOI] [PubMed] [Google Scholar]
- Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, Chiocchia V, Dutton SJ, Garcia-Valdecasas JC, Heaton N, Imber C, Jassem W, Jochmans I, Karani J, Knight SR, Kocabayoglu P, Malago M, Mirza D, Morris PJ, Pallan A, Paul A, Pavel M, Perera M, Pirenne J, Ravikumar R, Russell L, Upponi S, Watson CJE, Weissenbacher A, Ploeg RJ, Friend PJ, Consortium for Organ Preservation in E, 2018. A randomized trial of normothermic preservation in liver transplantation. Nature 557, 50–56. [DOI] [PubMed] [Google Scholar]
- O’Hare T, Walters DK, Stoffregen EP, Jia T, Manley PW, Mestan J, Cowan-Jacob SW, Lee FY, Heinrich MC, Deininger MW, Druker BJ, 2005. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res 65, 4500–4505. [DOI] [PubMed] [Google Scholar]
- Oberhuber R, Heinbokel T, Cetina Biefer HR, Boenisch O, Hock K, Bronson RT, Wilhelm MJ, Iwakura Y, Edtinger K, Uehara H, Quante M, Voskuil F, Krenzien F, Slegtenhorst B, Abdi R, Pratschke J, Elkhal A, Tullius SG, 2015. CD11c+ Dendritic Cells Accelerate the Rejection of Older Cardiac Transplants via Interleukin-17 A. Circulation 132, 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens WA, Walaszczyk A, Spyridopoulos I, Dookun E, Richardson GD, 2021. Senescence and senolytics in cardiovascular disease: Promise and potential pitfalls. Mech Ageing Dev 198, 111540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AK, Tchkonia T, Kirkland JL, 2021. Senolytics: Potential for Alleviating Diabetes and Its Complications. Endocrinology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raigani S, De Vries RJ, Carroll C, Chen YW, Chang DC, Shroff SG, Uygun K, Yeh H, 2020. Viability testing of discarded livers with normothermic machine perfusion: Alleviating the organ shortage outweighs the cost. Clin Transplant 34, e14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SP, Brockmann J, Friend PJ, 2009. Normothermic perfusion: a mini-review. Transplantation 87, 631–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritschka B, Knauer-Meyer T, Goncalves DS, Mas A, Plassat JL, Durik M, Jacobs H, Pedone E, Di Vicino U, Cosma MP, Keyes WM, 2020. The senotherapeutic drug ABT-737 disrupts aberrant p21 expression to restore liver regeneration in adult mice. Genes Dev 34, 489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Munoz DP, Teachenor R, Chu V, Le O, Bhaumik D, Coppe JP, Campeau E, Beausejour CM, Kim SH, Davalos AR, Campisi J, 2011. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci 124, 68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD, 2016. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15, 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybtsova N, Berezina T, Kagansky A, Rybtsov S, 2020. Can Blood-Circulating Factors Unveil and Delay Your Biological Aging? Biomedicines 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidi RF, Hejazii Kenari SK, 2014. Challenges of organ shortage for transplantation: solutions and opportunities. Int J Organ Transplant Med 5, 87–96. [PMC free article] [PubMed] [Google Scholar]
- Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y, Mazula DL, Brooks RW, Fuhrmann-Stroissnigg H, Pirtskhalava T, Prakash YS, Tchkonia T, Robbins PD, Aubry MC, Passos JF, Kirkland JL, Tschumperlin DJ, Kita H, LeBrasseur NK, 2017. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 8, 14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A, Sutton SL, Middleton E Jr., 1982. Quercetin inhibition of the induction and function of cytotoxic T lymphocytes. Immunopharmacology 4, 125–138. [DOI] [PubMed] [Google Scholar]
- Senanayake S, Graves N, Healy H, Baboolal K, Barnett A, Kularatna S, 2021. Time-to-event analysis in economic evaluations: a comparison of modelling methods to assess the cost-effectiveness of transplanting a marginal quality kidney. Health Econ Rev 11, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafin V, Capuzzo G, Milani G, Minuzzo SA, Pinazza M, Bortolozzi R, Bresolin S, Porcu E, Frasson C, Indraccolo S, Basso G, Accordi B, 2017. Glucocorticoid resistance is reverted by LCK inhibition in pediatric T-cell acute lymphoblastic leukemia. Blood 130, 2750–2761. [DOI] [PubMed] [Google Scholar]
- Singh M, Jensen MD, Lerman A, Kushwaha S, Rihal CS, Gersh BJ, Behfar A, Tchkonia T, Thomas RJ, Lennon RJ, Keenan LR, Moore AG, Kirkland JL, 2016. Effect of Low-Dose Rapamycin on Senescence Markers and Physical Functioning in Older Adults with Coronary Artery Disease: Results of a Pilot Study. J Frailty Aging 5, 204–207. [DOI] [PubMed] [Google Scholar]
- Smith-Garvin JE, Koretzky GA, Jordan MS, 2009. T cell activation. Annu Rev Immunol 27, 591–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Guan S, Lu J, Chen Z, Huang G, Li G, Xiong Y, Zhang S, Yue Z, Deng X, 2013. Suppressive effects of fisetin on mice T lymphocytes in vitro and in vivo. J Surg Res 185, 399–409. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Imai Y, Yamakoshi K, Kuninaka S, Ohtani N, Yoshimoto S, Hori S, Tachibana M, Anderton E, Takeuchi T, Shinkai Y, Peters G, Saya H, Hara E, 2012. DNA damage signaling triggers degradation of histone methyltransferases through APC/C(Cdh1) in senescent cells. Mol Cell 45, 123–131. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Takahashi A, Motoi N, Yoshimoto S, Tajima T, Yamakoshi K, Hirao A, Yanagi S, Fukami K, Ishikawa Y, Sone S, Hara E, Ohtani N, 2010. Intrinsic cooperation between p16INK4a and p21Waf1/Cip1 in the onset of cellular senescence and tumor suppression in vivo. Cancer Res 70, 9381–9390. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL, 2010. Fat tissue, aging, and cellular senescence. Aging Cell 9, 667–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL, 2013. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 123, 966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth ML, Sigmond T, Borsos E, Barna J, Erdelyi P, Takacs-Vellai K, Orosz L, Kovacs AL, Csikos G, Sass M, Vellai T, 2008. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy 4, 330–338. [DOI] [PubMed] [Google Scholar]
- Tsun A, Qureshi I, Stinchcombe JC, Jenkins MR, de la Roche M, Kleczkowska J, Zamoyska R, Griffiths GM, 2011. Centrosome docking at the immunological synapse is controlled by Lck signaling. J Cell Biol 192, 663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullius SG, Milford E, 2011. Kidney allocation and the aging immune response. N Engl J Med 364, 1369–1370. [DOI] [PubMed] [Google Scholar]
- Tullius SG, Rabb H, 2018. Improving the Supply and Quality of Deceased-Donor Organs for Transplantation. N Engl J Med 378, 1920–1929. [DOI] [PubMed] [Google Scholar]
- Van Raemdonck D, Neyrinck A, Cypel M, Keshavjee S, 2015. Ex-vivo lung perfusion. Transpl Int 28, 643–656. [DOI] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Despres S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T, 2011. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, Wabl R, Udeochu J, Wheatley EG, Zou B, Simmons DA, Xie XS, Longo FM, Wyss-Coray T, 2014. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med 20, 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaszczyk A, Dookun E, Redgrave R, Tual-Chalot S, Victorelli S, Spyridopoulos I, Owens A, Arthur HM, Passos JF, Richardson GD, 2019. Pharmacological clearance of senescent cells improves survival and recovery in aged mice following acute myocardial infarction. Aging Cell 18, e12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wei J, Da Fonseca Ferreira A, Wang H, Zhang L, Zhang Q, Bellio MA, Chu XM, Khan A, Jayaweera D, Hare JM, Dong C, 2020. Rejuvenation of Senescent Endothelial Progenitor Cells by Extracellular Vesicles Derived From Mesenchymal Stromal Cells. JACC Basic Transl Sci 5, 1127–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Bradley EW, Weivoda MM, Hwang SM, Pirtskhalava T, Decklever T, Curran GL, Ogrodnik M, Jurk D, Johnson KO, Lowe V, Tchkonia T, Westendorf JJ, Kirkland JL, 2017. Transplanted Senescent Cells Induce an Osteoarthritis-Like Condition in Mice. J Gerontol A Biol Sci Med Sci 72, 780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N, Jensen MD, LeBrasseur NK, Tchkonia T, Kirkland JL, 2015a. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife 4, e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL, 2018. Senolytics improve physical function and increase lifespan in old age. Nat Med 24, 1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera V, Giorgadze N, Jensen MD, LeBrasseur NK, Kirkland JL, 2015b. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A 112, E6301–6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Omoto Y, Yamakawa Y, Nakashima Y, Kiriyama M, Saito Y, Fujii Y, 2001. Increased matrix metalloproteinase 9 activity and mRNA expression in lung ischemia-reperfusion injury. J Heart Lung Transplant 20, 679–686. [DOI] [PubMed] [Google Scholar]
- Yi T, Fogal B, Hao Z, Tobiasova Z, Wang C, Rao DA, Al-Lamki RS, Kirkiles-Smith NC, Kulkarni S, Bradley JR, Bothwell AL, Sessa WC, Tellides G, Pober JS, 2012. Reperfusion injury intensifies the adaptive human T cell alloresponse in a human-mouse chimeric artery model. Arterioscler Thromb Vasc Biol 32, 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadeh MJ, Flores RR, Zhu Y, Schmiechen ZC, Brooks RW, Trussoni CE, Cui Y, Angelini L, Lee KA, McGowan SJ, Burrack AL, Wang D, Dong Q, Lu A, Sano T, O’Kelly RD, McGuckian CA, Kato JI, Bank MP, Wade EA, Pillai SPS, Klug J, Ladiges WC, Burd CE, Lewis SE, LaRusso NF, Vo NV, Wang Y, Kelley EE, Huard J, Stromnes IM, Robbins PD, Niedernhofer LJ, 2021. An aged immune system drives senescence and ageing of solid organs. Nature 594, 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD, Kirkland JL, 2016. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]


