Significance
Animals can attend to conspecifics’ fear expressions and thus be warned of potential threats. Social animals interact not only with conspecifics but also with individuals of other species. Yet, it is unknown whether the between-species transmission of threat information activates similar neural circuits as the within-species one. Here, we document that rats’ amygdala responds to the emotional arousal of caregivers who had recently undergone fear conditioning procedure. The basolateral and centromedial amygdalar nuclei are crucial for integrating sensory cues indicating threats and orchestrating defensive responses, respectively. We found that both parts are involved during human–rat and human–human interactions, which suggests a similar brain circuit involved in cross- and within-species responses to aversive arousal induced in the interaction partner.
Keywords: social transmission of threat information, basolateral amygdala, centromedial amygdala, cross-species, interspecies
Abstract
Reading danger signals may save an animal’s life, and learning about threats from others allows avoiding first-hand aversive and often fatal experiences. Fear expressed by other individuals, including those belonging to other species, may indicate the presence of a threat in the environment and is an important social cue. Humans and other animals respond to conspecifics’ fear with increased activity of the amygdala, the brain structure crucial for detecting threats and mounting an appropriate response to them. It is unclear, however, whether the cross-species transmission of threat information involves similar mechanisms, e.g., whether animals respond to the aversively induced emotional arousal of humans with activation of fear-processing circuits in the brain. Here, we report that when rats interact with a human caregiver who had recently undergone fear conditioning, they show risk assessment behavior and enhanced amygdala activation. The amygdala response involves its two major parts, the basolateral and central, which detect a threat and orchestrate defensive responses. Further, we show that humans who learn about a threat by observing another aversively aroused human, similar to rats, activate the basolateral and centromedial parts of the amygdala. Our results demonstrate that rats detect the emotional arousal of recently aversively stimulated caregivers and suggest that cross-species social transmission of threat information may involve similar neural circuits in the amygdala as the within-species transmission.
Many animals take advantage of social cues to guide their behavior. Such cues allow them to detect threats and find food or mates safely and efficiently (1). One critical social cue essential for the animal’s survival is negative emotion expressed by others that signals threat (2–4). By sensing such cues from conspecifics, animals can detect threats before encountering them (5). Humans are no exception, they robustly respond to the fear of other people (6, 7).
Animals can also recognize the emotional expressions of other species. Such “emotional eavesdropping” often appears in interactions between humans and other animals, especially domesticated species. For example, dogs respond with a heightened cortisol level to exposure to human infant cries (8). They are more attentive to human emotional vocalizations than to nonemotional ones (9). Cats and dogs can also differentiate between negative and positive emotions in humans (10, 11) and are sensitive to chemical signals related to human emotional states (12). Also, goats (13) and horses (14) are sensitive to human emotional cues. However, it is still to be determined what the neural mechanisms of the between-species emotional eavesdropping are. Such studies require an animal model in which simultaneous recording of behavior and neural circuits activity is possible. Here, we asked whether rats, commonly used in research laboratories, respond to emotional arousal of recently fear-conditioned human caregivers, and, if so, whether the response involves the amygdala, the core of the evolutionarily conserved brain system processing fear (15).
We hypothesized that detecting negative emotional arousal in other species engages well-defined preexisting neural circuits responsible for detecting these emotional states in conspecifics. Previous research in rats determined that observing the emotional states of conspecifics activates the basolateral and central amygdala (2, 16), which are also necessary for threat recognition and expression of defensive responses during first-hand threat experience (15). The amygdala has also been reported to be involved in humans when witnessing others receiving aversive stimuli (17, 18), and heterogeneity of its nuclei has been shown in human fMRI studies (19–21). However, particular amygdala subparts have not been investigated during fear transmission in humans. Here, we expected to find similar patterns of amygdala activation in between-species (human to rat) and within-species (human to human) threat detection. Verifying this hypothesis is a powerful argument for evolutionarily conserved neural circuitry responsible for detecting the emotional states of other individuals.
To address this hypothesis, we tested whether laboratory rats respond differently when interacting with human caregivers who had recently undergone fear conditioning, and whether the basolateral and centromedial amygdala are engaged in this process. Next, to test whether within-human fear transmission involves a similar brain circuit, we investigated activation of the basolateral and centromedial amygdala when a human observer watched another individual in a fearful situation.
Results
Human–Rat Interaction.
After initial habituation (Fig. 1A), rats interacted with human caregivers who underwent either aversive stimulation (Fig. 1C) or a sham procedure (Fig. 1B). Using skin conductance response, we confirmed that fear conditioning, compared to the sham procedure, induced a stronger physiological response in the caregivers [a repeated measures ANOVA with Group and Stimulus factors: Group × Stimulus interaction (F(1,8) = 32.47, P < 0.001, ηg2 = 0.15); pairwise comparisons with FDR correction: US-EXP > US-CTRL (P < 0.001) and EXP-US > EXP-noUS (P < 0.001)]. The post hoc sensitivity analysis is provided in supplement. Skin conductance responses to the conditioned stimuli (CS+ and CS−) were more robust in participants who underwent the experimental task, compared to those from the control group [a repeated measures ANOVA with Group and Stimulus factors: main effect of Group (F(1,8) = 17.29, P < 0.01, ηg2 = 0.18)], but no Group x Stimulus interaction was observed (F(1,8) = 0.02, P = 0.88).
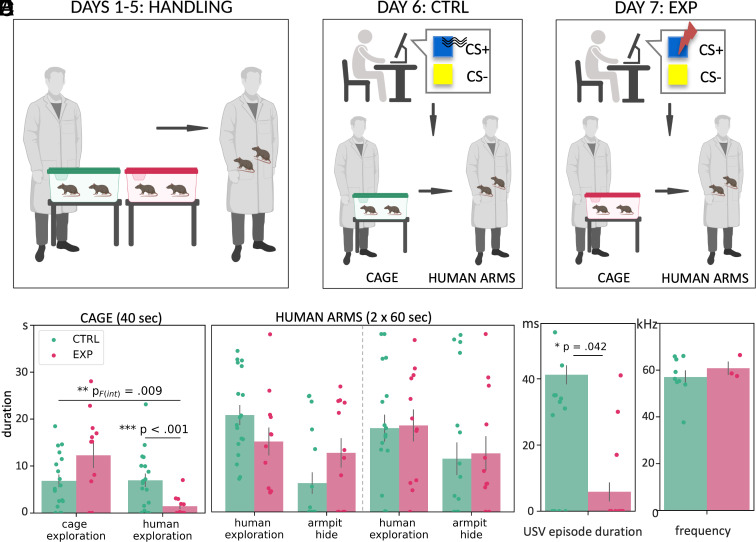
Fig. 1.
Experimental design and rat behavioral results. (A) During the first 5 d, every human caregiver handled four rats (2 sessions involving 2 rats): First, they kept their hands on the edge of the cage for 40 s and then took the rats into the arms and handled them for 2 min, both at the same time. (B) On day 6, mild vibrations were applied to the caregiver during the computer task, and only rats from the control (marked in green) cage were handled. (C) On day 7, uncomfortable electric shocks were applied to the caregiver during the computer task, and only rats from the experimental (marked in pink) cage were handled. (D) Rats’ behavior during 40 s in the cage with the caregivers holding their hands on the edge of the cage (phase 1) followed by the period during which the humans handled the two rats (phase 2, divided into two 60-s blocks). Colors mark the rats interacting with the caregivers on day 6 (green) and 7 (pink). Rats exposed to aversively aroused caregivers interacted less with human hands and spent more time exploring the cage. The dashed line divides the arms phase into two 60-s periods. Error bars indicate SEM; nexp = 18; nctrl = 18. (E) Mean durations of a single USV episode (nexp = 15, nctrl = 15; USV from five cages measured in three time points; one extreme outlier excluded from the graph for visualization purposes) and the USV frequency indicative of appetitive signaling (nexp = 3, nctrl = 9). USV data were recorded in pairs of rats at three time points. Error bars indicate SEM.
Next, we analyzed the rats’ behavior during the interaction with caregivers: during 40 s in the home cage (when the caregivers held their hands on the edge of the cage, phase 1) and the subsequent 120 s in the arms of the caregivers (phase 2). In phase 1, we measured how much time the rats explored the cage and stayed close to the caregivers’ hands. In phase 2, we compared how much time the rats spent on walking on the caregiver’s arms and sniffing his body (human exploration), and how much time they tried to hide in the caregiver’s armpit. We found that rats tested with the caregivers who underwent fear conditioning explored the human’s hands significantly less and instead spent more time exploring the cage [a mixed model ANOVA (within-subject factor: Behavior, between-subject factors: Group, Caregiver) for phase 1: Group × Behavior interaction (F(1,12) = 9.54, P < 0.01, ηg2 = 0.38); the effect of group on the duration of the human exploration (F(1,22) = 14.6, P < 0.002), pairwise comparisons with FDR correction; Fig. 1D]. The post hoc sensitivity analysis is provided in supplement.
When transferred to the caregivers’ arms, the rats explored the human handler and sought shelter in the armpit. No statistically significant effects were observed [a mixed model ANOVA (within-subject factors: Behavior, Time, between-subject factor: Group) for phase 2: nonsignificant interactions: Behavior × Time × Group (F(1,10) = 1.63, P = 0.23, ηg2 = 0.05), Behavior × Time (F(1,10) = 0.30, P = 0.60, ηg2 = 0.01), Time × Group (F(1,10) = 0.80, P = 0.39, ηg2 = 0.002), Behavior × Group (F(1,10) = 0.75, P = 0.41, ηg2 = 0.04)], a trend-level main effect of Behavior (F(1,10) = 4.76+, P = 0.05, ηg2 = 0.21).
Additionally, the analysis of ultrasonic vocalizations (USV) in a subgroup of rats indicated that during the human–rat interaction, the mean USV episode duration was shorter in animals interacting with a caregiver who underwent fear conditioning (U = 546.5, P = 0.042, nexp = 30, nctrl = 30; Fig. 1E). The number of USV episodes also tended to be lower in these rats (number of episodes: U = 538, statistical trend P = .06, nexp = 30, nctrl = 30; total duration of the USV episodes: U = 541.5, statistical trend P = 0.05, nexp = 30, nctrl = 30). The rats in both groups vocalized in similar frequencies c.a. 50 to 60 kHz (the experimental group: M = 60.80 kHz, the control group: M = 57.05 kHz, Fig. 1E), which often accompanies social interactions in rats. No 22-kHz alarm calls were detected.
Taken together, the changes in the behavior of rats interacting with caregivers that underwent fear conditioning and a decrease in ultrasonic communication indicate increased anxiety. They also suggest that rats tried to reduce contact with the aversively aroused caregivers.
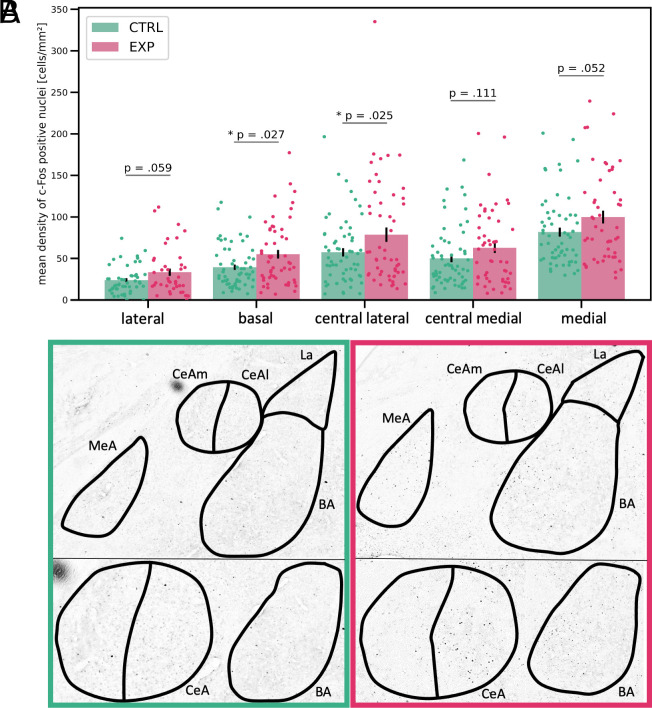
Then, we investigated the activation of the rat amygdala following interaction with the caregivers recently subjected to fear conditioning or the sham procedure. We found increased expression of c-Fos, a marker of neuronal activation, in the amygdala of rats handled by the aversively aroused caregivers when compared to those handled by the caregivers who experienced the sham procedure [the mixed model ANOVA with two between- (Group, Caregiver) and one within-subject (Nucleus) factors: main effect of group (F(1,13) = 11.63, P = 0.005)].
Further, we investigated the activation of the separate nuclei of the amygdala. We found that the basal nucleus and the lateral division of the central nucleus were more active in rats interacting with the caregiver who underwent fear conditioning than with the caregiver subjected to the sham procedure [the repeated measures ANOVA with one Category factor combining the information about both the group and the nucleus: main effect of the category (F(9, 72) = 20.19, P < 0.001, ηg2 = 0.35), pairwise comparisons with FDR correction: P = 0.03 for both basal (d = 0.63) and central lateral (d = 0.47) nuclei; Fig. 2 A and B]. The post hoc sensitivity analysis is provided in supplement. We found a similar trend in the medial (P = 0.05, d = 0.63) and lateral (P = 0.06, d = 0.66) nuclei of the amygdala. The difference in the medial division of the central nucleus was not significant (P = 0.11).
Fig. 2.
(A) Rat amygdala activations following interaction with human caregivers. Error bars indicate SEM; nLa-ctrl = 49, nLa-exp = 41, nBA-ctrl = 61, nBA-exp = 55, nCeAl-ctrl = 60, nCeAl-exp = 52, nCeAm-ctrl = 61, nCeAm-exp = 52, nMeA-ctrl = 59, nMeA-exp = 50; n indicates the number of brain sections analyzed in 18 rats in each group. (B) The sample brain sections showing c-Fos expression in the rats interacting with caregivers subjected to the sham procedure (Left) and with aversively aroused caregivers (Right). The upper part shows five main amygdalar nuclei: La: lateral, BA: basal, CeAl: central, lateral division, CeAm: central, medial division, MeA: medial. In the lower part, the central (central lateral + central medial, CeA) and basal (BA) parts are zoomed in.
Human–Human Interaction.
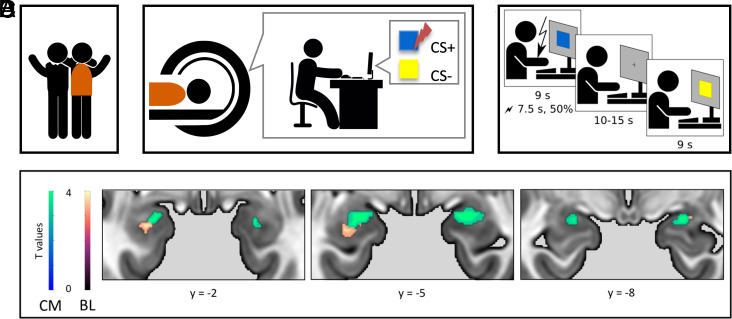
To investigate whether human–human fear contagion results in a similar pattern of amygdala activation as the one we found in the human–rat pairs, we used the observational fear learning procedure (17, 22). It involved an interaction of a pair of friends, one of whom (the observer) was watching the other (the demonstrator) undergo the classical fear conditioning (Fig. 3 A–C; see also the Materials and Methods section).
Fig. 3.
Fear transfer between humans. (A) In the neuroimaging experiment, pairs of friends were invited to the lab. (B) The observer was lying in the functional MRI (fMRI) scanner and watching his friend (the demonstrator) performing the fear conditioning task. (C) The task consisted of two squares presented one by one, one of which was repeatedly paired with an uncomfortable electric shock applied to the forearm. (D) Centromedial (CM, green) and basolateral (BL, orange) amygdala were activated in the observers; US > no US contrast, small volume corrected within the CM and BL masks, N = 48.
In this procedure, demonstrators’ performance modulated physiological arousal and resulted in conditioned fear responses in the observers (17). Here, the demonstrators’ performance was rated by the observers as expressive (ME = 6.0, IQR = 2.0), natural (ME = 7.0, IQR = 4.25), and showing discomfort (ME = 6.0, IQR = 2.25; all measured on the 0–9 scale). The observers identified with their friends (ME = 7.0, IQR = 2.0), felt empathy toward them (ME = 5.5, IQR = 3.25; both measured on the 0–9 scale), and perceived the shocks as very unpleasant (ME = 1.5, IQR = 1.0, 1–5 scale). All the medians were significantly greater from 0 (P < 0.001 in all of the cases).
We compared activation of the amygdala when the subjects observed the friend receiving an electric stimulation (unconditional stimulus, US) to the periods without stimulation (no US). Using the small volume correction approach, we found significant clusters within the bilateral amygdalae mask (44 voxels’ cluster on the left side and 36 voxels’ cluster on the right side). Further, we found significant activations in the two main subparts of the amygdala, the basolateral and centromedial divisions (Fig. 3D and Table 1.).
Table 1.
Results of direct comparison of the US > no US contrast
| Region | Extent | t-value | X | Y | Z |
|---|---|---|---|---|---|
| US > no US | |||||
| Left amygdala | 44 | 5.65 | −22 | −2 | −20 |
| Right amygdala | 36 | 5.61 | 22 | −6 | −14 |
| Left centromedial amygdala | 30 | 5.65 | −22 | −2 | −20 |
| Right centromedial amygdala | 32 1 |
5.61 3.44 |
22 26 |
−6 −0 |
−14 −24 |
| Left basolateral amygdala | 14 | 4.53 | −24 | −2 | −22 |
| Right basolateral amygdala | 2 1 |
3.76 3.66 |
24 24 |
−0 −8 |
−24 14 |
P- and t-values presented were obtained using FWE correction at a voxel (peak) level P < 0.05 within small volume correction within bilateral amygdala, bilateral centromedial amygdala, and bilateral basolateral amygdala masks. Each row corresponds to a significant cluster of voxels.
A Shared Perspective.
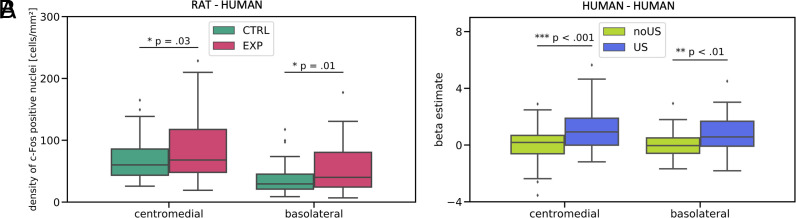
We investigated the homologous parts of the amygdala, the centromedial and basolateral nuclei, in rats and humans. Rats’ data were aggregated: central lateral, central medial, and medial nuclei were treated together as the centromedial division, while basal and lateral nuclei were counted as the basolateral division. We found that both parts of the amygdala were activated in the rats interacting with aversively aroused caregivers [the centromedial amygdala: t(111) = −2.20, P = 0.03, the basolateral amygdala: t(114) = −2.57, P = 0.01] (Fig. 4A). Similarly, during the human–human interaction, the reaction to the US applied to the partner was significantly greater compared to the no US (control) condition in both the centromedial [t(46) = 4.24, P < 0.001] and basolateral [t(46) = 2.79, P = 0.008] amygdala (Fig. 4B). The post hoc sensitivity analysis is provided in supplement.
Fig. 4.
Aversively aroused partner activated the centromedial and basolateral amygdala in rats (human–rat interaction) and humans (human–human interaction). (A) The mean c-Fos expression was higher in the rats interacting with caregivers who had undergone fear conditioning (EXP) compared to the rats interacting with caregivers subjected to the sham procedure (CTRL), nCM-ctrl = 61, nCM-exp = 52, nBL-ctrl = 61, nBL-exp = 55, n indicates the number of brain sections analyzed in 18 rats in each group. (B) In humans, the BOLD signal was increased in response to the US applied to the interaction partner compared to the no US condition. Error bars extend to data points placed no further than 1.5*IQR (interquartile range) beyond the 1st quartile and above the 3rd quartile, N = 48.
Discussion
Our findings demonstrate that rats respond differently to human caregivers who underwent fear conditioning and to the caregivers subjected to the sham procedure. Interaction with a human caregiver who had recently undergone fear conditioning changed rats’ behavior and activated their amygdala, the brain structure crucial for threat detection and orchestrating defensive responses. In addition, comparing the pattern of amygdala responses in human–rat and human–human experiments, we found that the basolateral and centromedial amygdala is activated in both rats and humans receiving social cues about a threat, indicating the involvement of homologous areas in the rat’s and human’s brain. Cross-species social transmission of threat information might therefore involve similar neural circuits as the within-species transmission.
Our previous research demonstrated that interaction with a recently fear-conditioned conspecific activates most of the rat amygdala nuclei, including the lateral, basal, medial, as well as lateral and medial divisions of the central amygdala (2, 23). The current study shows that interaction with an aversively stimulated caregiver activates mainly the basal nucleus and lateral division of the central nucleus of the amygdala in rats. These patterns of activations suggest that the rat–rat interaction activates more neuronal circuits within the amygdala than the human–rat interaction. The more robust activation of the medial amygdala in the rat–rat interaction may stem from the significant role of the olfactory social communication between the rats, as it strongly involves this part of the amygdala (24, 25). On the other hand, activation of the basal nucleus and lateral division of the central nucleus of the amygdala may reflect the major involvement of the core fear-related circuits during human–rat interaction (15).
We also found that interaction with a caregiver who recently experienced fear conditioning significantly decreased rats’ human-oriented exploratory behaviors. Instead, the animals focused on exploring their cage. This behavioral pattern resembles the changes observed when rats interact with a conspecific that was fear-conditioned immediately before the interaction; our earlier studies showed that the rat–rat emotional transfer results in a risk assessment behavior, which, in a home cage, is expressed as increased cage exploration (2, 3). Risk assessment is an information-gathering behavior displayed in potentially threatening aversive situations (26). In line, the aversiveness of the situation for rats interacting with caregivers who underwent aversive stimulation is evidenced by reduced 50-kHz calling, which typically occurs in the presence of aversive stimuli (27–29).
While the human amygdala has been reported to be involved when witnessing others receiving aversive stimuli (17, 18), the involvement of its different nuclei has not been investigated in this context. Here, we demonstrate that both the basolateral and centromedial amygdala engage in human observational fear. Animal studies showed that the basolateral amygdala processes sensory information about the threat, while the central amygdala orchestrates defensive responses (7, 25, 30, 31). The human and rat findings presented here suggest a common brain circuit activating when humans and rats interact with humans subjected to fear conditioning. The results of human–rat and human–human studies are consistent with the previous reports showing similarities between the rodents’ and humans’ neural processing of the aversive emotional content (32–34) and the underlying molecular mechanisms (35).
Another interesting question is whether the pattern of amygdala activation in rats and humans mirrors the pattern of amygdala activation in the fear-conditioned individuals. In our previous rat–rat studies, we observed similar amygdala activations in the directly fear-conditioned animals and in the rats interacting with them (2, 23). Concerning humans, here we did not test the amygdala activations in the aversively stimulated caregivers, or in the demonstrators in the human–human study. However, previous research on human fear conditioning showed that direct and observational fear conditioning largely share their neural underpinnings (36). In particular, the basolateral and centromedial amygdala are activated during fear conditioning (19, 20). Thus, we can infer that the homologous parts of the amygdala are activated in rats and humans, both when they experience fear directly, and when they observe fearful others. However, it is plausible that there are some quantitative or nuanced anatomical differences between the amygdala activations in the demonstrators and observers that we could not detect because of methodological limitations. Taken together, our results suggest that within-species and cross-species sharing of the other individuals’ negative emotions uses already existing neural fear circuits. Such neural reuse has been reported earlier in different species for both innate and learned behaviors (37–39). The ability to receive information about potential threat from others, particularly the between-species emotional eavesdropping, probably evolved because it increases animals’ chances of survival (40). Our data show that rats are responsive to human emotional arousal evoked by recent aversive stimulation. However, whether this ability is universal across species or limited to domesticated species is unclear.
Laboratory rats are a domesticated form of wild rats with a relatively long history of coexistence with humans. Domesticated animals interact more with humans (41, 42) and, as recent studies show, can detect and respond to human emotions (8, 10, 11). This ability to “sense” human emotions can be either innate, i.e., explained by evolutionary changes in their brains, or learned as the effect of the lifetime experience with humans. Studies comparing wolves and dogs have shown that wolves raised by humans can communicate with humans using gaze (43), suggesting the role of experience. On the other hand, when dogs and wolves with similar rearing experiences were compared, dogs had a stronger tendency to be in proximity with a human partner (44), suggesting that domestication also influenced the response of dogs to humans. The present results cannot answer the question about the role of domestication in detecting human emotions; more research investigating interactions between different species is needed.
When comparing the human–rat and human–human experimental paradigms, it is worth noting that there are differences in the communication modalities. Previous research has shown that visual cues are sufficient for social fear learning in humans (17, 18, 22). Thus in the current human–human paradigm, the participants observed their partners’ responses on the screen while lying in the scanner. In contrast, in the human–rat experimental paradigm, the rats interacted with caregivers directly, which allowed the rats to use both visual and olfactory cues, as both are instrumental for social communication in rodents (45, 46). The caregivers underwent fear conditioning immediately before the interaction. Similar procedure was also used in our previous rat–rat studies (2, 23). We chose the paradigms that seemed the most ecologically valid, but further studies are needed to understand the modalities needed to detect human fear in rats and humans.
Together, these results concur with what has previously been shown in rat studies on responding to conspecifics with aversively induced emotional arousal and suggest that behavioral and neural response to threat communicated by both the conspecifics and humans is similar. Further works on rats interacting with humans who experienced fear-inducing stimuli could shed more light on the specifics of emotional transmission between species, strengthening the interface between neuroscience, ethology, and psychology and laying the groundwork for translational research on empathy disorders.
Materials and Methods
Experiment 1: Human–Rat Interaction.
Human caregivers and rat subjects.
Nine human caregivers participated. Based on previous reports showing that the experimenter’s sex affects the rodents’ stress responses (47, 48), we employed only male caregivers and compared the responses of rats to the same caregivers in two conditions. All caregivers were scientists and had valid permissions to work with animals. During the recruitment process, they were interviewed regarding the length of experience in working with rodents (M = 11.9 y) and their proficiency in handling procedures (ME = 4.0 on a 1–5 scale, self-report). Caregivers were informed that the electrical stimulation was a part of the experiment, and thus, they were interviewed in terms of any health issues that could disallow their participation (e.g., heart diseases or metal elements inside the forearm). They received a financial remuneration of 350 PLN (~85 EUR) for participation.
We used 36 male Wistar rats weighing 180–200 g at the start of the experiment. The rats were provided by the Center of Experimental Medicine in Bialystok, Poland. They were randomly paired and housed in cages measuring 56 × 37 × 20 cm, following a 12/12 light–dark cycle. The rats had unlimited access to food and water.
To ensure ethical treatment, we adhered to the guidelines of the Polish Act on Animal Welfare. Prior to conducting the experiment, we obtained specific permission from the First Warsaw Ethical Committee on Animal Research. The protocol involving human participants was approved by the Ethics Committee of the Faculty of Psychology at the University of Warsaw (decision made on November 28, 2017). Our procedure was based on the Ethical Principles of Psychologists and Code of Conduct established by both the Polish and the American Psychological Associations.
Procedure.
Each cage housed two rats that were randomly assigned to the same caregiver and the same condition (either experimental or control). The symbols enabling their identification were placed on the cages. Each caregiver had four rats assigned (two rats in the “experimental” cage and two in the “control” cage). The tail of one of the rats in each cage was marked with a permanent marker so that the rats could be distinguished and the order of handling controlled. Before the beginning of the experiment, rats were prehandled by one of the experimenters to get them used to human contact.
At the very beginning of the experiment, all caregivers filled in the safety screening form and signed the informed consent. The first 5 d of the experiment were devoted to the handling procedure (Fig. 1A), which resulted with the rats’ habituation. Each caregiver came to the laboratory individually, every day at the same time. The cage with the dedicated rats was placed on the experimental table and opened by the experimenter. The human placed his hands on the edge of the open cage for 40 s and then took both rats into his arms and handled them for two more minutes. Afterward, the rats from another cage were handled in the same manner. The phases of the interaction followed in the same order for all animals. Keeping hands on the cage’s edge was always followed by taking rats in the arms. Within each cage, the order of the rats being taken in the arms changed every day. Caregivers were not given any other specific instructions on how handling should be performed.
Days 6 and 7 were the control and experimental days, respectively. Such an order aimed at avoiding the transfer of strong negative emotions induced in the caregivers in the experimental task to the control condition. On day 6 (Fig. 1B), caregivers were informed that they would do a short computer task, in which squares of two colors (blue and yellow) would be presented. One of the colors was said to be repeatedly paired with mild vibrations that the caregivers would feel on the forearm thanks to the small device attached to this place. The vibrations were explained as not painful and similar to the ones emitted by a cell phone. The task was analogous on day 7 (Fig. 1C), but instead of mild vibrations, uncomfortable electrictical stimulation was applied to the caregivers’ forearms. The stimulation used in the experiment involved five unipolar pulses lasting 1 ms each, with intervals of 200 ms between them. Before starting the task, the experimenter gradually increased the intensity of the stimulation. Caregivers were asked to rate the intensity on a scale ranging from 1 (barely noticeable) to 8 (painful). The target level of stimulation, which was considered very unpleasant but not painful, was chosen individually for each caregiver and set at a rating of 6. On both days, caregivers were informed that at the end of the day, they would do the same computer task once again. The aim of such repetition was to maintain the induced emotional state in caregivers throughout the whole control/experimental day. On both days, two galvanic skin response (GSR) electrodes were attached to the caregivers’ fingers besides the vibration or stimulation device.
On days 6 and 7, caregivers performed the computer task: 12 squares (conditioned stimuli, CS) were presented on the screen one by one, each lasting for 9 s. The caregiver observed a total of 6 squares of one color (referred to as CS+) and 6 squares of another color (referred to as CS−) on a screen. The squares were displayed in a pseudorandom order, ensuring that a particular square was repeated at most twice consecutively. The assignment of colors to CS+ and CS− was balanced across different caregivers. Four out of six CS+ (first, second, fourth, and sixth) were reinforced with the unconditioned stimulus (US; mild haptic vibrations on day 6 and uncomfortable electric shock on day 7), which appeared 8 s after the CS+ onset. CS− were never associated with the US. Between the CS presentations, a fixation cross appeared on the screen with a jittered duration (10 to 15 s). The caregivers were asked to simply watch the squares. Previous studies employing this discriminatory fear conditioning procedure have shown robust responses to the US, indicating fear acquisition (17, 22).
The computer task lasted around 5 min and immediately afterward the electrodes were detached. The caregivers were asked to put on the lab coat and gloves, and enter the animal room. They took the appropriate cage, placed it on the dedicated place on the table, and opened it. Then, the handling followed exactly in the same manner as during the preexperimental phase. The experimenter informed the caregivers when to move from the “hands on the edge” (40 s) to the “rats in the arms” (2 min) phase. When the interaction was over, the cage was closed and placed back on the shelf. On day 6, caregivers had recording electrodes attached once again, and they underwent the computer task once more (because we wanted them to believe that on the next day, they would also be asked to do the task once again after the interaction). However, when the interaction phase was finished on day 7, there was no need to repeat the task. Caregivers were debriefed and received a remuneration for their participation.
Skin conductance recordings.
During the computer tasks, we registered skin conductance responses of the human participants. It was recorded using a Biopac EDA100C amplifier, sampled at 250 Hz.
USV recordings.
We used an UltraSoundGate Condenser Microphone CM16 from Avisoft Bioacoustics in Berlin, Germany, to record rat calls. The microphone was positioned about 25 to 30 cm above the floor of the cage. It was capable of capturing frequencies between 15 and 180 kHz, with a flat frequency response (±6 dB) between 25 and 140 kHz. We connected the microphone to an UltraSoundGate 416H device (Avisoft Bioacoustics, Berlin, Germany), which was then connected to a computer. For recording the data, we used Avisoft-RECORDER software. The recorded signals were processed using custom-made RAT-REC PRO 7.0 software. We applied Fast Fourier Transformation (FFT; 1024, Hamming window) to analyze the signals. The results were displayed as color spectrograms, which provided visual representations of the sound frequencies. We manually marked each signal with the corresponding section label for automated parameter measurement. Several parameters were automatically determined, including the number of ultrasonic vocalization (USV) calls, the total calling time (s), the average call length (s), the frequency bandwidth (kHz), the number of gaps, the average gap length (s), and the average peak frequency (kHz). We analyzed the FFT spectrograms across the entire recorded frequency spectrum (10 to 130 kHz) to evaluate the presence of both 50-kHz (appetitive) and 22-kHz (aversive) calls. However, our detailed analysis of the spectrograms indicated the absence of 22-kHz alarm calls in the recordings.
Perfusion.
Two hours after the interaction, the rats were administered a lethal dose of morbital (133.3 mg/mL sodium pentobarbital and 26.7 mg/mL pentobarbital). Then, the rats were perfused transcardially by injecting ice-cold 0.1 M PBS (pH 7.4, Sigma) and a 4% paraformaldehyde (wt/vol, POCh) in PBS (pH 7.4). The brains were then carefully removed and kept in the same fixative solution for 24 h at 4 °C. Afterward, they were immersed in a 30% sucrose solution (wt/vol) at 4 °C. The brains were then gradually frozen and sliced into 40-μm sections using a cryostat. Specifically, we collected coronal brain sections that contained the amygdala.
c-Fos immunohistochemistry.
The immunohistochemical staining process was conducted on coronal brain sections that were free-floating. The sections were initially incubated in PBS (pH 7.45, Gibco #18912014) overnight at 4 °C and then washed three times with PBS. Next, the sections were exposed to a solution of 0.3% hydrogen peroxide in PBS for 10 min, followed by two washes with PBS. The sections were then incubated with a primary antibody (anti-c-Fos, 1:1000, Abcam #ab190289) in a solution containing PBST (PBS with 0.02% Triton X-100, Chempur #498418109) and 3% NGS (Normal Goat Serum, Vector Laboratories, #S-1000-20) for 48 h at 4 °C. After incubation, the brain slices were washed three times with PBST and incubated with a biotinylated secondary antibody (goat anti-rabbit, 1:1000, Vector Laboratories #BA-1000) in PBST for 2 h at room temperature. Following another three washes with PBST, the sections were incubated with an avidin–biotin complex (1:1000 in PBST, Vector Laboratories 494 ABC kit #PK-6100) for 1 h and then washed three times with PBS. To develop the immunostaining reaction, the sections were incubated in a mixture of diaminobenzidine, urea hydrogen peroxide (Sigmafast #D4293-50SET), and 0.5M nickel chloride dissolved in distilled water for approximately 5 min. The reaction was stopped by rinsing the sections three times in PBS. The c-Fos-positive nuclei appeared dark brown in color. After staining, the sections were mounted onto slides, air-dried, dehydrated in xylene, and covered with Entellan™ new (Sigma #107961) for preservation.
Data analysis.
We used the PsPM 4.3.0 software to analyze skin conductance data (SCR, https://bachlab.github.io/PsPM/). We ran this software on MATLAB 2020a (MathWorks, Natick, MA, USA). Our analysis focused on event-related SCR using a nonlinear model.
Before conducting the analysis, we visually inspected the data to identify any artifacts or irregularities and manually marked any missing parts of the data. For preprocessing, we used the default settings. The signals were filtered using bidirectional 1st-order Butterworth filters with 5 Hz low-pass and 0.0159 Hz high-pass cutoff frequencies, and resampled to 10 Hz. We did not perform any response normalization. To calculate the average values, we considered the four US and two no US responses for each caregiver in both the experimental and control conditions. For statistical analysis, we used the Scipy package version 1.9.3, specifically running a mixed model ANOVA which included one between-subject factor (Group) and one within-subject factor (Stimulus).
Due to technical difficulties, we were only able to collect USV recordings from rats handled by five caregivers. In the statistical analysis, we used data recorded within three time windows: 0–1 min, 1–2 min, and 2–3 min. Since the data were collected from pairs of rats and not individual rats, each group included 15 samples (5 caregivers × 3 time points). While examining the mean call length, we observed one data point that was significantly longer than the others. However, due to the small sample size and the non-normal distribution of the data, we decided not to exclude this observation from the analysis. To compare the mean call length and mean peak frequency between groups, we used Mann–Whitney U tests in the Scipy package version 1.9.3. When calculating the mean frequency, any values of zero were omitted from the analysis.
The analysis of the rats’ behavior was done using BehaView software (http://www.pmbogusz.net/?a=behaview). The following behaviors were coded and analyzed: approach, avoidance, exploration, human exploration, hiding under the human’s armpit, responding to the human’s activity, freezing, hiding in the other rat’s body, interaction with the other rat, and waiting. Only the behaviors that were observed in all animals, including cage exploration, human exploration, and hiding under the human’s armpit, were used in the analysis. A mixed model ANOVA was done separately for the 40-s period in the cage and the 120-s period in the arms. For the “cage” phase, the between factors were caregiver and group, and the within factor was behavior. For the “arms” phase, the between factor was group, and the within factors were behavior and time point (initial 60 s vs. final 60 s).
The c-Fos-positive nuclei were counted using ImageJ (NIH) software. As two slices were chosen from each rat brain and we did not take lateralization into account, four amygdalae were analyzed in each rat brain. Out of 144 amygdalae that we initially planned to analyze (4 amygdalae × 2 rats × 2 groups × 9 caregivers), 21 were assessed as damaged and were excluded from the analysis. In the remaining dataset, five main amygdala nuclei (basal, lateral, central medial, central lateral, and medial) were distinguished on each slice. Due to the imperfect condition of the slices, it was not possible to analyze all five nuclei in each and every slice but it was done for the vast majority. The amygdala nuclei were delineated manually (Fig. 3B) based on the corresponding sections from the rat brain atlas (49). Pixels with a value in a 40 to 255 range were identified as c-Fos-positive nuclei. The area (in mm2) and the number of the c-Fos-positive nuclei within each nucleus were exported, and the mean density was calculated for each nucleus (number of cells divided by the area, the conventional unit: cells/mm2).
To calculate the mean c-Fos expression in the five amygdala nuclei, ten categories involving information about the nucleus and the group were created (e.g., basal-exp, basal-ctrl, lateral-exp etc.). The observations that entered the analysis were the values of mean density for every nucleus in every single amygdala. A repeated measures ANOVA was run and FDR correction was applied for the pairwise comparisons. The order of taking rats into the arms was found to have no impact on the results. To calculate the mean c-Fos expression in the centromedial and basolateral nuclei, the areas of nuclei and the number of c-Fos-positive cells were summed within the two complexes (basolateral = basal + lateral, centromedial = central medial + central lateral + medial). Based on such sums, mean density was calculated in the two main amygdala divisions. For each division, the difference between the experimental and control group was calculated using independent samples t test in the Scipy package v. 1.9.3.
Experiment 2: Human–Human Interaction.
We gathered data during a study on observational fear learning, in which we modified the method used by Haaker et al. (50) to allow us to directly observe the interaction between the individuals demonstrating fear and those observing it (22). The experiment involved two groups of participants who, during the first phase, observed either their friends or strangers undergoing the classical fear conditioning task. Then, during the second phase, the effects of fear learning were measured (17). Here, we present only the data collected from the observers watching their friends being conditioned.
The Ethics Committee at the Faculty of Psychology, University of Warsaw, reviewed and approved the protocol we used (decision from 28 November 2017). The procedure we followed adhered to the ethical guidelines outlined by both the Polish and American Psychological Associations, which include the Ethical Principles of Psychologists and Code of Conduct.
Participants.
The sample size was estimated for the original between-group study (17), where the friends group consisted of 48 pairs of friends (Fig. 3A). One participant in a pair was assigned the role of a demonstrator while another was given the role of an observer, and only the observers underwent fMRI. We recruited heterosexual males between the ages of 18 and 30 who were right-handed and native or fluent Polish speakers. Only heterosexual participants were chosen (self-declared) to ensure that the relationships examined were nonromantic male friendships, thus reducing variability in the sample. Handedness was assessed through self-report in both the recruitment and fMRI safety-screening processes. Certain individuals were excluded from participation, including students and graduates of psychology or cognitive science programs, those with neurological disorders or medical conditions that would interfere with MRI scanning or electrical stimulation, and individuals using psychoactive drugs. Moreover, the participants needed to have known each other for at least three years and obtain a minimum score of 30 out of 60 on the McGill Friendship Questionnaire–Respondent's Affection (51). In the analyzed group, the observers’ average age was 22.4 years (SD = 2.8), the average length of friendship between observers and demonstrators was 8.6 years (SD = 4.9), and the average score of observers on the McGill Friendship Questionnaire was 50.7 (SD = 9.1). Participants’ anxiety levels remained similar before and after the experiment, as indicated by the STAI score (32 points before and 33 points after; t(47) = −0.10, P = 0.92). All participants were compensated with a financial remuneration of 100 PLN (approximately 23 EUR) for their involvement in the study.
Experimental setup, task, and stimuli.
Inside the MRI scanner, the observer viewed a live video stream (without sound) on a monitor compatible with MRI through a mirror box placed on the head coil. Meanwhile, the demonstrator, located in a separate room adjacent to the MRI room, was recorded by a GoPro Hero7 camera, and the live video feed was transmitted to the observer via the monitor (Fig. 3B). To minimize distractions, the walls of the room were covered with gray acoustic foam.
The stimuli used in the task were similar to those used in Experiment 1 and involved colored squares and cutaneous electrical stimulation. However, in this experiment, the number and timing of the stimuli were different. The demonstrator watched a total of 24 CS+ and 24 CS− in a semirandom order. It was ensured that a particular CS was repeated at most twice consecutively. Each CS was displayed on the screen for 9 s, with half of the CS+ being followed by the presentation of the US. The US was presented for the first and last CS+ trials, starting 7.5 s after the CS onset to ensure the demonstrator’s reaction aligned with the CS. The CS− trials were never reinforced with the US. Between trials, there were random intertrial intervals (ITIs) lasting between 10 and 15 s, during which a fixation symbol (+) was displayed at the center of the screen (Fig. 3C). After completing this task, the observers themselves underwent a similar task, which is not relevant to the current manuscript.
The stimulating electrodes were placed above the flexor carpi radialis muscle, causing visible muscle flexion in response to even low-intensity stimulation. The demonstrators individually adjusted the intensity of the electrical shocks to be unpleasant but not painful, as described in the details of Experiment 1.
Procedure.
When the participants arrived at the laboratory, they received a brief explanation of the experiment, which included the possibility of receiving unpleasant electrical stimulation. They then provided written consent and completed safety forms. Subsequently, they were randomly assigned the roles of either a demonstrator or an observer by receiving color-coded envelopes. The demonstrators were escorted to a room adjacent to the MR room, while the observers were informed that they would witness their friends engaging in a task that involved the presentation of colored symbols and the administration of unpleasant electrical stimulation. They were also informed that they would later perform the same task themselves. The observers had skin conductance and sham stimulation electrodes attached. Inside the scanner, the observers had sham leads connected to the stimulation electrodes to enhance the realism and believability of receiving electrical stimulation, despite no actual electrical stimulation being administered.
Meanwhile, the demonstrators were informed that they would carry out a task involving the presentation of colored symbols and the administration of unpleasant, but not painful, electrical stimulation. They were instructed to respond naturally but noticeably to the stimulation in order to convey the notion that one of the colored symbols was associated with discomfort (they were informed of which color to associate with discomfort). The demonstrators watched a recording demonstrating a model’s reaction to the stimulation. They also had stimulation and sham skin conductance electrodes attached. They adjusted the intensity of the stimulation as described earlier.
Following that, the experimenter ensured that the camera was appropriately positioned to capture the demonstrator’s face, hand, and computer screen. Video transmission was activated to allow the observer to see the demonstrator during the task. A brief reminder was given to the observer, stating that they would observe their friend performing a task, after which the MRI scanning commenced. Upon completion of the observational stage, the observer undertook a task where their responses to the colored symbols were evaluated, but no electrical stimulation was administered to them. The results of this task are not elaborated upon in the manuscript. Finally, all participants received a debriefing at the conclusion of the experiment.
Behavioral measures.
We collected the data using McGill Friendship Questionnaire and State Anxiety Inventory. McGill Friendship Questionnaire - Respondent’s Affection (51), translated by A. Kaźmierowska, P. Pączek, and A. Schudy): This questionnaire was used to assess the respondents’ feelings toward their friend and their satisfaction with the friendship. It contained 16 positive statements about the friend, and the respondents rated their agreement or disagreement with each statement on a scale from −4 (strongly disagree) to 4 (strongly agree). One question was accidentally left out of the questionnaire. A score of 30 points or higher was considered as the minimum requirement for participants to be included in the study. State Anxiety Inventory (STAI, ref. 52): This inventory was used to measure participants’ anxiety levels. It consisted of 20 self-report items that participants completed twice, both at the beginning and the end of the experiment. The scale helped the researchers control for the influence of anxiety on their findings. The Polish adaptation of the State Anxiety Inventory was used in this study (53).
Evaluation of the demonstrator’s expression (the observational US).
We used a set of questions to assess how the observers perceived the behavior of the demonstrators in the study, as suggested by Haaker et al. (50). At the end of the experiments, we asked the observers to rate the following aspects: Discomfort of the Demonstrator: The observers rated how much discomfort they thought the demonstrator experienced when receiving electrical stimulation; Expressiveness of the Demonstrator: The observers rated the level of expressiveness displayed by the demonstrator during the experiment; Naturalness of the Demonstrator’s Reactions: The observers rated how natural the demonstrator’s reactions seemed to them; Empathy toward the Demonstrator: The observers rated the amount of empathy they felt toward the demonstrator; Unpleasantness Attribution: The observers rated the degree of unpleasantness they attributed to the demonstrators. Additionally, the observers were asked to score the extent to which they identified with their friends. The ratings used a 10-point Likert scale, ranging from 0 (not at all) to 9 (very much), except for the unpleasantness rating, which used a 5-point Likert scale ranging from 1 (very unpleasant) to 5 (very pleasant). The medians of the ratings were tested against 0 using the one-sample Wilcoxon signed-rank test in R.
Neuroimaging data acquisition.
We acquired MRI data using a 3T Siemens Magnetom Trio scanner equipped with a 12-channel head coil. The session began by obtaining a T1-weighted anatomical image through an MPRAGE sequence. The image had a resolution of 1 × 1 × 1 mm and was captured with specific settings: an inversion time (TI) of 1,100 ms, GRAPPA parallel imaging with an acceleration factor (PE) of 2, and an acquisition time (TA) of 6 min and 3 s. Following the anatomical scans, two functional imaging runs were conducted: observational and direct tasks. The initial run comprised 362 volumes, with each volume containing 47 axial slices. These slices had a thickness of 2.3 mm, an in-plane resolution of 2.3 × 2.3 mm, and a 30% distance factor. The acquisition of the slices employed a T2*-sensitive gradient echo-planar imaging (EPI) sequence, with the following parameters: a repetition time (TR) of 2870 ms, an echo time (TE) of 30 ms, a flip angle (FA) of 90 degrees, a field of view (FoV) of 212 mm, a matrix size of 92 × 92, interleaved acquisition order, and a GRAPPA acceleration factor (PA) of 2.
Neuroimaging data preprocessing.
We performed preprocessing on the fMRI data using fMRIPrep 1.4.0 software (54, 55), which is based on Nipype 1.2.0 (56, 57) and Nilearn 0.5.2 (58). The preprocessing pipeline started by applying intensity nonuniformity correction to the anatomical images using N4BiasFieldCorrection (59) from ANTs 2.2.0 (60). These corrected images were used as the anatomical reference. Additionally, we conducted skull-stripping on the anatomical reference using antsBrainExtraction from ANTs and segmentation using fast from FSL 5.0.9 (61). Finally, we normalized the anatomical images to MNI space through nonlinear registration with antsRegistration (ANTs 2.2.0), using the ICBM 152 Nonlinear Asymmetrical template version 2009c as the reference (62).
Regarding the functional images, we followed these preprocessing steps: First, we generated a reference volume using a customized methodology in fMRIPrep. This reference volume was then coregistered to the anatomical reference using flirt from FSL 5.0.9 (63), employing the boundary-based registration cost-function (64). Prior to any spatiotemporal filtering, we estimated head-motion parameters, including transformation matrices and corresponding rotation and translation parameters, using mcflirt from FSL 5.0.9 (65). Slice-time correction was performed on the functional scanning runs using 3dTshift from AFNI 20160207 (66). Subsequently, the functional images were resampled into MNI space with a voxel size of 2 × 2 × 2 mm postnormalization. To accomplish this, we conducted all resampling operations in a single interpolation step by combining head-motion transform matrices and coregistrations to anatomical and output spaces through antsApplyTransforms (ANTs). Finally, we applied a 6 mm FWHM 3D Gaussian kernel smoothing to the functional images using spm_smooth (SPM 12 v7487, Wellcome Centre for Human Neuroimaging, London, UK). Framewise displacement (FD) and the derivative of rms variance over voxels (DVARS) were calculated for each functional scan using fMRIPrep. The implementations in Nipype were used, following the definitions provided by ref. 67. Frames exceeding a threshold of 0.5 mm FD or 1.5 standardized DVARS were identified as motion outliers.
Neuroimaging data analyses.
For data analysis, we employed a mass univariate approach based on a general linear model, utilizing SPM 12 software running on MATLAB 2020a (MathWorks, Natick, MA, USA). Our analysis focused on the observational learning stage, and we constructed first-level models that included four types of events: CS+, CS−, US, and no US (lack of US during 50% of CS+ trials). The observational CS events were treated as instantaneous events, capturing the onset of the CS, while the observational US/no US events were modeled as lasting 1.5 s (from the onset of the US to the offset of the CS). Alongside the event regressors, we included six motion parameters (translation and rotation) as regressors of no interest. Additionally, we incorporated delta regressors for each volume identified as a motion outlier by fMRIPrep. For the purposes of this manuscript, we specifically analyzed the observational stage, with the primary contrast of interest being US > no US. We estimated beta values at the first-level and used them for the second-level analysis with a paired t test design. The resulting statistical maps at the second level were thresholded using small volume correction, separately for each mask, with a significance threshold of P = 0.05.
The anatomical ventrolateral and dorsomedial amygdala masks (68) were combined and treated as a single ROI. We also investigated each of the amygdala divisions separately. Since it was reported that the functional connectivity patterns between the ventrolateral and basolateral amygdala as well as between the dorsomedial and centromedial amygdala were found to be similar, we decided to use general labels commonly used in animal research (69).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
US: unconditioned stimulus; no US: no unconditioned stimulus; EXP: experimental condition; CTRL: control condition; USV: ultrasonic vocalizations; SEM: standard error of the mean; FDR: false discovery rate; La: lateral; BA: basal; CeAl: central, lateral division; CeAm: central, medial division; MeA: medial; CeA: central; fMRI: functional MRI; MR: magnetic resonance; CM: centromedial; BL: basolateral; BOLD: blood oxygen level dependent; IQR: interquartile range; GSR: galvanic skin response; CS: conditioned stimulus; SCR: skin conductance response; ROI: region of interest. Data collection and analysis were sponsored by the National Science Centre grant 2015/19/B/HS6/02209. Ewelina Knapska was supported by the European Research Council Starting Grant (H 415148). We thank Sven Muller for his helpful comments on the final version of the manuscript.
Author contributions
A.M.K., M.K., M.S., J.M.M., M.W., A.M., and E.K. designed research; A.M.K., M.K., and M.S. performed research; A.M.K., M.K., M.S., T.N., and A.H. analyzed data; and A.M.K., M.K., M.S., J.M.M., M.W., A.M., and E.K. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. J.H. is a guest editor invited by the Editorial Board.
Data, Materials, and Software Availability
Raw data and code for the analysis data have been deposited in OSF and GitHub (https://osf.io/gx3jv/; https://github.com/nencki-lobi/emocon-humans-rats) (70, 71).
Supporting Information
References
- 1.Dall S. R. X., Giraldeau L.-A., Olsson O., McNamara J. M., Stephens D. W., Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 20, 187–193 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Knapska E., et al. , Between-subject transfer of emotional information evokes specific pattern of amygdala activation. Proc. Natl. Acad. Sci. U.S.A. 103, 3858–3862 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andraka K., et al. , Distinct circuits in rat central amygdala for defensive behaviors evoked by socially signaled imminent versus remote danger. Curr. Biol. 31, 2347–2358.e6 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Jeon D., et al. , Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat. Neurosci. 13, 482–488 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puścian A., et al. , Ability to share emotions of others as a foundation of social learning. Neurosci. Biobehav. Rev. 132, 23–36 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Olsson A., Phelps E. A., Social learning of fear. Nat. Neurosci. 10, 1095–1102 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Olsson A., Knapska E., Lindström B., The neural and computational systems of social learning. Nat. Rev. Neurosci. 21, 197–212 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Yong M. H., Ruffman T., Emotional contagion: Dogs and humans show a similar physiological response to human infant crying. Behav. Processes 108, 155–165 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Huber A., Barber A. L. A., Faragó T., Müller C. A., Huber L., Investigating emotional contagion in dogs (Canis familiaris) to emotional sounds of humans and conspecifics. Anim. Cogn. 20, 703–715 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albuquerque N., et al. , Dogs recognize dog and human emotions. Biol. Lett. 12, 20150883 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quaranta A., d’Ingeo S., Amoruso R., Siniscalchi M., Emotion recognition in cats. Animals (Basel) 10, 1107 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Aniello B., Semin G. R., Alterisio A., Aria M., Scandurra A., Interspecies transmission of emotional information via chemosignals: From humans to dogs (Canis lupus familiaris). Anim. Cogn. 21, 67–78 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Nawroth C., Albuquerque N., Savalli C., Single M.-S., McElligott A. G., Goats prefer positive human emotional facial expressions. R. Soc. Open Sci. 5, 180491 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baba C., Kawai M., Takimoto-Inose A., Are horses (Equus caballus) sensitive to human emotional cues? Animals (Basel) 9, 630 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeDoux J., The amygdala. Curr. Biol. 17, R868–R874 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Debiec J., Sullivan R. M., Intergenerational transmission of emotional trauma through amygdala-dependent mother-to-infant transfer of specific fear. Proc. Natl. Acad. Sci. U.S.A. 111, 12222–12227 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaźmierowska A. M., et al. , Learning about threat from friends and strangers is equally effective: An fMRI study on observational fear conditioning. Neuroimage 263, 119648 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Olsson A., Nearing K. I., Phelps E. A., Learning fears by observing others: The neural systems of social fear transmission. Soc. Cogn. Affect. Neurosci. 2, 3–11 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balderston N. L., Schultz D. H., Hopkins L., Helmstetter F. J., Functionally distinct amygdala subregions identified using DTI and high-resolution fMRI. Soc. Cogn. Affective Neurosci. 10, 1615–1622 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michely J., Rigoli F., Rutledge R. B., Hauser T. U., Dolan R. J., Distinct processing of aversive experience in amygdala subregions. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 5, 291–300 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawada M., et al. , Mapping effective connectivity of human amygdala subdivisions with intracranial stimulation. Nat. Commun. 13, 4909 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szczepanik M., et al. , Observational learning of fear in real time procedure. Sci. Rep. 10, 16960 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mikosz M., Nowak A., Werka T., Knapska E., Sex differences in social modulation of learning in rats. Sci. Rep. 5, 18114 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keshavarzi S., Power J. M., Albers E. H. H., Sullivan R. K. S., Sah P., Dendritic organization of olfactory inputs to medial amygdala neurons. J. Neurosci. 35, 13020–13028 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janak P. H., Tye K. M., From circuits to behaviour in the amygdala. Nature 517, 284–292 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanchard D. C., Griebel G., Pobbe R., Blanchard R. J., Risk assessment as an evolved threat detection and analysis process. Neurosci. Biobehav. Rev. 35, 991–998 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Knutson B., Burgdorf J., Panksepp J., Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J. Comp. Psychol. 112, 65–73 (1998). [DOI] [PubMed] [Google Scholar]
- 28.Burgdorf J., Knutson B., Panksepp J., Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behav. Neurosci. 114, 320–327 (2000). [PubMed] [Google Scholar]
- 29.Knutson B., Burgdorf J., Panksepp J., Ultrasonic vocalizations as indices of affective states in rats. Psychol. Bull. 128, 961–977 (2002). [DOI] [PubMed] [Google Scholar]
- 30.LeDoux J. E., Iwata J., Cicchetti P., Reis D. J., Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 8, 2517–2529 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeDoux J. E., Cicchetti P., Xagoraris A., Romanski L. M., The lateral amygdaloid nucleus: Sensory interface of the amygdala in fear conditioning. J. Neurosci. 10, 1062–1069 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pattwell S. S., et al. , Altered fear learning across development in both mouse and human. Proc. Natl. Acad. Sci. U.S.A. 109, 16318–16323 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andero R., et al. , Amygdala-dependent fear is regulated by Oprl1 in mice and humans with PTSD. Sci. Transl. Med. 5, 188ra73 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayo L. M., et al. , Protective effects of elevated anandamide on stress and fear-related behaviors: Translational evidence from humans and mice. Mol. Psychiatry 25, 993–1005 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Soliman F., et al. , A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science 327, 863–866 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindström B., Haaker J., Olsson A., A common neural network differentially mediates direct and social fear learning. Neuroimage 167, 121–129 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Anderson M. L., Penner-Wilger M., Neural reuse in the evolution and development of the brain: Evidence for developmental homology? Dev. Psychobiol. 55, 42–51 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Newcomb J. M., Katz P. S., Homologues of serotonergic central pattern generator neurons in related nudibranch molluscs with divergent behaviors. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 193, 425–443 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Dehaene S., Cohen L., The unique role of the visual word form area in reading. Trends Cogn. Sci. 15, 254–262 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Keysers C., Knapska E., Moita M. A., Gazzola V., Emotional contagion and prosocial behavior in rodents. Trends Cogn. Sci. 26, 688–706 (2022). [DOI] [PubMed] [Google Scholar]
- 41.Albert F. W., et al. , Phenotypic differences in behavior, physiology and neurochemistry between rats selected for tameness and for defensive aggression towards humans. Horm. Behav. 53, 413–421 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Albert F. W., et al. , A comparison of brain gene expression levels in domesticated and wild animals. PLoS Genet. 8, e1002962 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heberlein M. T. E., Turner D. C., Range F., Virányi Z., A comparison between wolves, Canis lupus, and dogs, Canis familiaris, in showing behaviour towards humans. Anim. Behav. 122, 59–66 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lazzaroni M., et al. , The effect of domestication and experience on the social interaction of dogs and wolves with a human companion. Front. Psychol. 11, 785 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monfils M. H., Agee L. A., Insights from social transmission of information in rodents. Genes Brain Behav. 18, e12534 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Sterley T.-L., Bains J. S., Social communication of affective states. Curr. Opin. Neurobiol. 68, 44–51 (2021). [DOI] [PubMed] [Google Scholar]
- 47.Sorge R. E., et al. , Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat. Methods 11, 629–632 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Faraji J., et al. , Sex-specific stress and behavioural responses to human experimenters in rats. bioRxiv [Preprint] (2021). 10.1101/2021.12.01.470834 (Accessed 28 June 2023). [DOI] [Google Scholar]
- 49.Paxinos G., Watson C., The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition (Academic Press, 2013). [Google Scholar]
- 50.Haaker J., Golkar A., Selbing I., Olsson A., Assessment of social transmission of threats in humans using observational fear conditioning. Nat. Protoc. 12, 1378–1386 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Mendelson M. J., Aboud F. E., Measuring friendship quality in late adolescents and young adults: McGill Friendship Questionnaires. Can. J. Behav. Sci. 31, 130–132 (1999). [Google Scholar]
- 52.Spielberger C. D., Gorsuch R. L., Lushene R., Vagg P. R., Jacobs G. A., Manual for the State-Trait Anxiety Inventory (Consulting Psychologists Press, 1983). [Google Scholar]
- 53.Spielberger C. D., Strelau J., Tysarczyk M., Wrześniewski K., STAI–Inwentarz Stanu I Cechy Lęku (Pracownia Testów Psychologicznych Polskiego Towarzystwa Psychologicznego, 2012). [Google Scholar]
- 54.Esteban O., et al. , fMRIPrep: A robust preprocessing pipeline for functional MRI (2019). 10.5281/zenodo.2851559 (Accessed 28 June 2023). [DOI] [PMC free article] [PubMed]
- 55.Esteban O., et al. , fMRIPrep: A robust preprocessing pipeline for functional MRI. Nat. Methods 16, 111–116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gorgolewski K., et al. , Nipype: A flexible, lightweight and extensible neuroimaging data processing framework in python. Front. Neuroinform. 5, 13 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gorgolewski K. J., et al. , nipy/nipype (2019). 10.5281/zenodo.2685428 (Accessed 28 June 2023). [DOI]
- 58.Abraham A., et al. , Machine learning for neuroimaging with scikit-learn. Front. Neuroinform. 8, 14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tustison N. J., et al. , N4ITK: Improved N3 bias correction. IEEE Trans. Med. Imaging 29, 1310–1320 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Avants B. B., Epstein C. L., Grossman M., Gee J. C., Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 12, 26–41 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y., Brady M., Smith S., Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 20, 45–57 (2001). [DOI] [PubMed] [Google Scholar]
- 62.Fonov V. S., Evans A. C., McKinstry R. C., Almli C. R., Collins D. L., Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage 47 (suppl. 1), S102 (2009). [Google Scholar]
- 63.Jenkinson M., Smith S., A global optimisation method for robust affine registration of brain images. Med. Image Anal. 5, 143–156 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Greve D. N., Fischl B., Accurate and robust brain image alignment using boundary-based registration. Neuroimage 48, 63–72 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jenkinson M., Bannister P., Brady M., Smith S., Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841 (2002). [DOI] [PubMed] [Google Scholar]
- 66.Cox R. W., Hyde J. S., Software tools for analysis and visualization of fMRI data. NMR Biomed. 10, 171–178 (1997). [DOI] [PubMed] [Google Scholar]
- 67.Power J. D., et al. , Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 84, 320–341 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bielski K., Adamus S., Kolada E., Rączaszek-Leonardi J., Szatkowska I., Parcellation of the human amygdala using recurrence quantification analysis. Neuroimage 227, 117644 (2021). [DOI] [PubMed] [Google Scholar]
- 69.McDonald A. J., Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 55, 257–332 (1998). [DOI] [PubMed] [Google Scholar]
- 70.Kaźmierowska A. M., Fear transmission: human-human and human-ratstudies. OSF. https://osf.io/gx3jv/. Deposited 28 June 2023.
- 71.Kaźmierowska A. M., Szczepanik M., Analysis code from the fear contagion studies: an fMRI human -> human experiment and an inter-species human -> rat study. GitHub. https://github.com/nencki-lobi/emocon-humans-rats. Deposited 28 June 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Raw data and code for the analysis data have been deposited in OSF and GitHub (https://osf.io/gx3jv/; https://github.com/nencki-lobi/emocon-humans-rats) (70, 71).