Abstract
Objective
The relationship between serum selenium levels and papillary thyroid cancer (PTC), especially the pathological features, still remains controversial. We conducted this study to investigate the relationship between serum selenium levels and PTC in a Chinese population.
Methods
Cross-sectional data of 284 patients with PTC were collected from the First Affiliated Hospital of Shandong First Medical University. The general clinical characteristics, serum selenium levels, and tumor pathological features were described in PTC. The association between serum selenium levels and pathological features in PTC was analyzed using SPSS 26.0 statistical software.
Results
Our results showed that the median serum selenium level was 79.15 μg/L (IQR: 71.00 - 86.98 μg/L) in PTC patients. Serum selenium levels were lower in females than males (p = 0.035). Serum selenium levels were negatively correlated with the number of lymph node metastases (p = 0.048). High serum selenium (OR = 0.397, 95%CI: 0.217 - 0.725) and diastolic blood pressure (OR = 1.028, 95%CI: 1.005 - 1.051) were related factors for the incidence of bilateral tumors. High serum selenium (OR = 0.320, 95%CI: 0.166 - 0.617) and diastolic blood pressure (OR = 1.066, 95%CI: 1.031 - 1.103) were related factors for tumor multifocal incidence.
Conclusions
The serum selenium levels of PTC patients in females were lower than males. High serum selenium levels might be a protective factor in PTC patients. Further research is necessary to better understand the influence of selenium on PTC progression.
Keywords: papillary thyroid cancer, pathological features, nutrition, selenium, serum
Introduction
Nowadays cancer is the second leading cause of death, severely affecting people’s quality of life and longevity (1). Thyroid cancer is the most prevalent malignant tumor of the endocrine system, and its incidence has been on the rise in recent years (2, 3). Notably, papillary thyroid cancer (PTC) accounts for a vast majority of cases (4). Despite a favorable prognosis for most patients following systematic treatment, a subset experiences relapse and unfavorable outcomes (5). Previous research has identified large tumor diameters, extrathyroidal extension, and distant lymph node metastases as significant predictors of thyroid cancer recurrence and mortality (6, 7). While some risk factors for thyroid cancer have been predicted, most are uncontrollable, including advanced age, gender, race and family history. Therefore, the exploration of controllable risk factors becomes crucial for the prevention and treatment of thyroid cancer.
Selenium, an essential trace element, plays a pivotal role in maintaining homeostasis. Selenium manifests its anti-inflammatory, antioxidant, antiviral, and anticancer characteristics through its involvement in the synthesis of diverse selenoproteins (8–11). At present, a minimum of 25 selenoproteins with diverse biological functions have been identified (12). Maintaining proper selenium status is crucial for human health, as deficiency is associated with increased risks of infertility, autoimmune imbalance, inflammation, and cancer. Alternatively, excessive serum selenium levels may lead to hyperlipidemia, hyperinsulinemia, type 2 diabetes mellitus, and atherosclerosis (13).
Recently, the relationship between selenium and thyroid cancer has garnered attention. The thyroid has a significantly higher concentration of selenium than other organs (14). As oxidative stress may lead to cellular mutation and gene variation, selenoproteins have been considered to prevent the development of cancer through their antioxidant effects (15). However, studies on selenium and tumor risk have yielded inconsistent results. Most studies have found that low selenium appears to be associated with the incidence of thyroid cancer (16–19). In the study by Baltaci et al., patients with thyroid cancer demonstrated lower serum selenium levels compared to those without thyroid cancer (16). Kucharzewski et al. found that blood selenium concentrations in patients with thyroid cancer were lower than those in controls (19). In a case-control trial, serum selenium levels were inversely associated with the risk of thyroid cancer (17). Additionally, a meta-analysis involving 1291 subjects showed that blood selenium levels in thyroid cancer were lower than those in controls (18). However, a Polish study found no significant differences in serum selenium levels between patients with thyroid cancer and non-thyroid cancer patients (20). Moreover, no association between selenium intake and thyroid cancer was observed in a large prospective cohort study in the United States (14). In the study by Jonklaas et al., it was noted that serum selenium levels in patients with thyroid cancer were not significantly reduced, but they were negatively correlated with the disease stage (21).
Currently, studies focus on the relationship between serum selenium levels and the incidence of thyroid cancer, yet they lack further analysis of pathological features. Furthermore, most studies included other pathological types of thyroid cancer and did not study PTC separately. Therefore, we investigated serum selenium levels and pathological features in PTC patients to study the relationship between serum selenium levels and pathological features.
Materials and methods
Trial design and participants
This cross-sectional study was extracted from a larger prospective study. Patients with PTC who were treated at the First Affiliated Hospital of Shandong First Medical University (Qianfoshan Hospital of Shandong Province) from January 1, 2021 to December 31, 2022 were included in this study. All patients have completed the surgical pathological details, and serum selenium levels were detected during hospitalization.
Inclusion criteria
(1) Research period: January 1, 2021 to December 31, 2022.
(2) Surgical method: total thyroidectomy with or without lymph node dissection.
(3) Histopathological results: PTC was clearly diagnosed, and complete pathological details were recorded.
(4) Complete the detection of serum selenium index.
Exclusion criteria
(1) It is known that other primary tumors have metastasized to the thyroid gland.
(2) Those who have taken selenium preparations or selenium-enriched drugs in the past year.
(3) Patients who are pregnant or breastfeeding.
(4) Acute complications of diabetes (diabetic ketoacidosis, hyperglycemia and hyperosmolar state).
(5) Significant liver function abnormalities, defined as aspartate aminotransferase > 2 × ULN (upper limit of normal range) and/or alanine aminotransferase > 2 × ULN, and/or total bilirubin > 34umol/L or previous infectious hepatitis seropositive evidence.
(6) Patients with renal insufficiency (eGFR < 60ml/min/1.73m²).
(7) Patients with severe autoimmune diseases (rheumatoid, systemic lupus erythematosus and other diseases).
Data collection
(1) General clinical data, including name, age, gender, height, weight, waist, systolic blood pressure (SBP), diastolic blood pressure (DBP) and thyroid related indicators.
(2) Serum selenium levels: Inductively coupled plasma mass spectrometry (ICP-MS), collected by the Laboratory Department of the First Affiliated Hospital of Shandong First Medical University (Qianfoshan Hospital in Shandong Province), tested and provided by Guangzhou Jinyu Medical Laboratory Institute Report.
(3) Details of thyroid surgery (total thyroidectomy, with or without lymph node dissection), histopathological results (pathological type, tumor size, number of metastatic lymph nodes, whether the lesion is unilateral or bilateral, whether the lesion is multifocal, or whether the lesion is invasive) capsule, and presence or absence of metastasis), and TNM (tumor, node, metastasis) staging based on pathological details (American Joint Committee on Cancer, AJCC 8th ed.).
Statistical methods
All data were statistically analyzed using SPSS 26.0 statistical software. Describe patient demographics, clinical characteristics, and blood parameters using simple summary statistics. The Kolmogorov-Smirnov test was performed on the enumeration data, and the data that conformed to the normal distribution were expressed as mean ± standard deviation, and the data that did not conform to the normal distribution were expressed as the median (interquartile range). Univariate analysis was performed by t test or Wilcoxon signed rank test, and Logistic regression analysis was used for further analysis of variables with statistical differences. The independent samples Kruskal-Wallis rank sum test was used for comparison among multiple groups. Spearman or Pearson correlation analysis was used for bivariate analysis. All tests were two-sided, and p < 0.05 indicated a statistically significant difference.
Results
Patient characteristics
A total of 284 PTC patients were included in the study. The demographic and clinical data were shown in Table 1 . Among the 284 patients, 85 were males (85/284, 29.93%) and 199 were females (199/284, 70.07%). The median age was 44 years (IQR: 35.00 - 52.00 years). The median BMI was 25.71 kg/m2 (23.14 - 28.46 kg/m2). According to the standards established by the China Obesity Working Group Data Summary and Analysis Collaborative Group, 1 patient (1/284, 0.35%) was underweight (<18.5 kg/m2), 91 patients (91/284, 32.04%) were normal weight, and 113 patients (113/284, 39.79%) were overweight (24-27.9 kg/m2), and 79 patients (79/284, 27.82%) were obese (≥28 kg/m2). The median height was 163.00 cm (IQR: 160.00 - 170.00 cm), and the median weight was 69.5 kg (IQR: 60.25 - 80.00 kg). Waist was recorded in 282 patients, with an average of 90.90 cm. The median SBP and DBP in patients were 123.00 mmHg (IQR: 114.00 - 135.00 mmHg) and 80.48 mmHg (IQR: 73.00 - 88.75 mmHg), respectively. The clinical data of PTC patients with different genders were shown in Table S1 . Females exhibited higher levels of thyroid antibodies compared to males, while males demonstrated a higher BMI and blood pressure than females.
Table 1.
Demographic and clinical data observed in the cross-section study.
| Subjects | Data |
|---|---|
| Onset (Year) | 44.00 (35.00 - 52.00) |
| Gender (N, %) | |
| Male | 85 (29.93%) |
| Female | 199 (70.07%) |
| Waist (cm) | 90.90 ± 10.90 |
| BMI (kg/m2) | 25.71 (23.14 - 28.46) |
| Underweight (N, %) | 1 (0.35%) |
| Normal weight (N, %) | 91 (32.04%) |
| Overweight (N, %) | 113 (39.79%) |
| Obese (N, %) | 79 (27.82%) |
| SBP (mmHg) | 123.00 (114.00 - 135.00) |
| DBP (mmHg) | 80.48 (73.00 - 88.75) |
| FT3 (pmol/L) | 4.94 (4.62 - 5.41) |
| FT4 (pmol/L) | 17.73 (16.50 - 19.84) |
| TSH (uIU/mL) | 1.85 (1.16 - 2.64) |
| TPOAB (IU/mL) | 13.08 (10.04 - 20.13) |
| TGAB (IU/mL) | 20.24 (15.72 - 49.35) |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid stimulating hormone; TPOAB, thyroidperoxidase antibodies; TGAB, thyroglobulin antibody.
Characteristics of serum selenium levels in PTC patients
The distribution characteristics of serum selenium levels were shown in Figure 1 . The median serum selenium level was 79.15 μg/L (IQR: 71.00 - 86.98 μg/L). The serum selenium level of 232 patients (232/284, 81.69%) was below 90 μg/L. There were 199 female patients and 85 male patients. The serum selenium level was 78.30 μg/L (IQR: 68.80 - 86.30 μg/L) in female patients and 82.20 μg/L (IQR: 74.80 - 88.65 μg/L) in male patients. As shown in Figure 2A , the serum selenium level in female patients was lower than that in male patients (p = 0.035). Age categories for patients were classified according to the WHO criteria: 144 patients in the young group (<45 years old), 116 cases in the middle-aged group (45-59 years old), and 24 cases in the old group (≥60 years old). The serum selenium level was 79.05 μg/L (IQR: 69.65 - 86.60 μg/L) in the young group, 79.35 μg/L (IQR: 72.85 - 88.43 μg/L) in the middle-aged group, and 77.70 μg/L (IQR: 70.83 - 86.43 μg/L) in the elderly group. There was no significant difference among three groups in serum selenium levels (p = 0.944) ( Figure 2B ). BMI categories for patients were classified according to the China Obesity Working Group Data Summary and Analysis Collaborative Group: 91 patients were normal weight (18.5-23.9 kg/m2), 1 patient was underweight (<18.5 kg/m2), 113 patients were overweight (25-27.9 kg/m2), and 79 patients were obesity (≥28 kg/m2). Since only one patient was underweight, it was not included in the comparison. The serum selenium level in normal weight patients was 78.30 μg/L (IQR: 69.60 - 85.20 μg/L), the serum selenium level in overweight patients was 82.60 μg/L (IQR: 75.15 - 91.15 μg/L) and obesity patients was 76.10 μg/L (IQR: 68.50 - 86.20 μg/L). Serum selenium levels in overweight patients were significantly higher than those in normal weight groups (p < 0.01) ( Figure 2C ). The correlation between serum selenium levels and thyroid-related indicators was presented in Table S2 . The results showed that there was no significant correlation between selenium and thyroid related indicators.
Figure 1.
Overall characteristics of serum selenium levels in PTC patients.
Figure 2.
Serum selenium levels in PTC patients with different sexes, ages, and BMIs. (A) Serum selenium levels in PTC patients with different genders. (B) Serum selenium levels of PTC patients with different ages. (C) Serum selenium levels in PTC patients with different BMIs. ns, not significant; *, p < 0.05; **, p < 0.01.
Pathological features in PTC patients
The detailed pathological characteristics of PTC patients were shown in Table 2 . There were 142 patients (142/284, 50.00%) with unilateral tumors and 137 patients with bilateral lesions (137/284, 48.24%). There were 168 patients (168/284, 59.15%) with multifocal tumors, and 102 patients (102/284, 35.92%) with single tumor tumors. There were 159 (159/284, 55.99%) patients with thyroid capsule invasion. The median tumor size was 1.10 cm (IQR: 0.70 - 1.60 cm). The median number of positive lymph node metastases was 4 (IQR: 1.00 - 8.00). The results of T staging showed that most of the patients were in stage I, including 127 patients in stage Ia (127/284, 44.72%) and 79 patients in stage Ib (79/284, 27.82%). N staging results showed that more than half of the patients were stage Ia.
Table 2.
Pathological features of enrolled patients.
| Subjects | Data |
|---|---|
| Bilateral | |
| Unilateral lesion | 142 (50.00%) |
| Bilateral lesion | 137 (48.24%) |
| Not mentioned | 5 (1.76%) |
| Multifocality | |
| Yes | 168 (59.15%) |
| No | 102 (35.92%) |
| Not mentioned | 14 (4.93%) |
| Capsule invasion | |
| Yes | 159 (55.99%) |
| No | 30 (10.56%) |
| Not mentioned | 95 (33.45%) |
| Tumor size (cm) | 1.10 (0.70 - 1.60) |
| Number of metastatic lymph nodes | 4.00 (1.00 - 8.00) |
| TNM staging (AJCC 8th edition) | |
| T | |
| Ia | 127 (44.72%) |
| Ib | 79 (27.82%) |
| II | 28 (9.86%) |
| IIIa | 3 (1.06%) |
| IIIb | 11 (3.87%) |
| Iva | 9 (3.17%) |
| IVb | 4 (1.41%) |
| X | 23 (8.10%) |
| N | |
| 0 | 51 (17.96%) |
| Ia | 159 (55.99%) |
| Ib | 71 (25.00%) |
| NA | 3 (1.06%) |
NA, not available.
Serum selenium levels and pathological characteristics in PTC patients
The association between serum selenium levels and pathological features in PTC patients was analyzed by Spearman correlation analysis ( Table 3 ). Serum selenium levels were negatively correlated with the number of lymph node metastasis (r = -0.121, p = 0.048). There was no significant correlation with tumor T stage (p = 0.436), N stage (p = 0.811) and tumor maximum diameter (p = 0.795).
Table 3.
Spearman correlation analysis of serum selenium levels and pathological features in PTC patients.
| Subjects | Serum selenium | |
|---|---|---|
| r | p | |
| TNM staging-T | -0.048 | 0.436 |
| TNM staging-N | -0.015 | 0.811 |
| Tumor size (cm) | 0.016 | 0.795 |
| Metastatic lymph nodes | -0.121 | 0.048* |
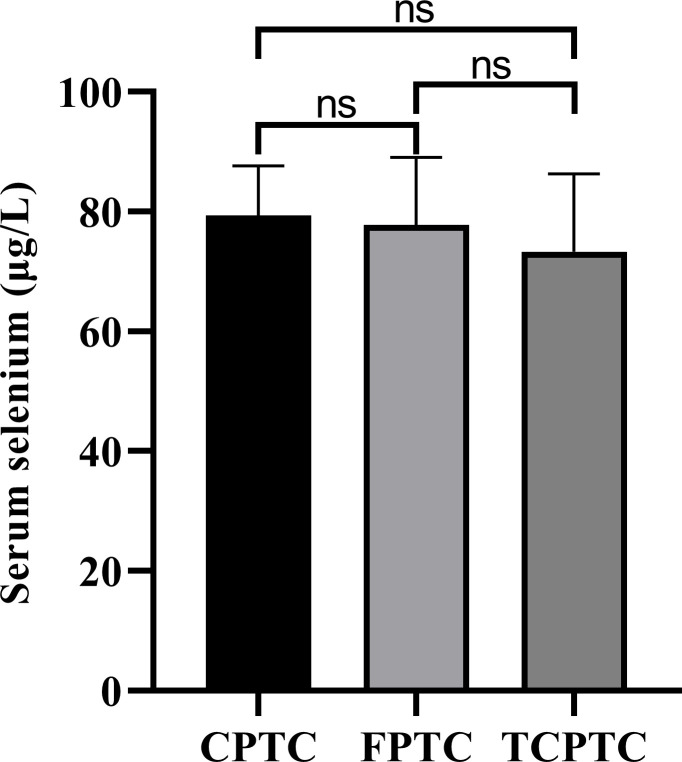
The serum selenium levels of different pathological subtypes in PTC patients were shown in Figure 3 . There were 270 patients with classical subtype PTC, 7 patients with follicular subtype PTC, and 7 patients with hypercellular subtype PTC. There was no significant difference in serum selenium levels among patients with different pathological subtypes of PTC (p = 0.860).
Figure 3.
Serum selenium levels of different pathological subtypes in PTC patients. CPTC, common papillary thyroid cancer; FPTC, follicular papillary thyroid cancer; TCPTC, tall cell papillary thyroid cancer. ns, not significant.
The comparison of serum selenium levels in PTC patients with different pathological features was shown in Figure 4 . Serum selenium levels in patients with bilateral tumors were significantly lower than those in patients with unilateral tumors (Z = -2.871, p = 0.004). Serum selenium levels in patients with multifocal tumors were significantly lower than those in patients with unifocal tumors (Z = -4.120, p < 0.001). There was no statistical difference in serum selenium levels of patients regardless of membrane invasion (t = -0.172, p = 0.864) and lymph node metastasis (t = 0.472, p = 0.637). The comparison of serum selenium levels in common papillary thyroid cancer patients with different pathological features was shown in Figure S1 .
Figure 4.
Serum selenium levels among different pathological features in PTC patients. CI, capsule invasion; NCI, no capsule invasion; ULT, unilateral tumor; BLT, bilateral tumor; UFT, unifocal tumor; MFT, multifocal tumor; M, metastasis; NM, no metastasis. ns, not significant; **, p < 0.01.
Factors related to unilateral and bilateral tumors in PTC patients
The status of unilateral or bilateral tumors in PTC patients was presented in Table 4 . Univariate analysis showed that there were statistical differences in serum selenium level (p = 0.004), BMI (p = 0.033) and DBP (p = 0.018), while age (p = 0.647), height (p = 0.971), Waist (p = 0.051) and SBP (p = 0.188) were not statistically different.
Table 4.
Comparison of data between unilateral tumor group and bilateral tumor group in PTC patients.
| Subject | Unilateral tumor | Bilateral tumors | t/Z | p |
|---|---|---|---|---|
| Number (%) | 142 (50.90) | 137 (49.10) | - | - |
| Age (years) | 45.00 (35.00 - 52.00) | 43.00 (35.00 - 52.50) | -0.458 | 0.647 |
| SSL (μg/L) | 82.70 (72.55 - 89.43) | 76.90 (70.05 - 85.05) | -2.871 | 0.004 |
| BMI (kg/m2) | 25.52 ± 3.91 | 26.66 ± 3.90 | -2.436 | 0.015 |
| Height (cm) | 163.00 (160.00 - 170.00) | 164.00 (160.00 - 170.00) | -0.036 | 0.971 |
| Weight (kg) | 66.50 (59.00 - 78.50) | 70.00 (62.00 - 81.00) | -2.129 | 0.033 |
| Waist (cm) | 88.00 (83.00 - 97.00) | 92.00 (84.25 - 98.75) | -1.949 | 0.051 |
| SBP (mmHg) | 122.00 (112.75 - 132.25) | 124.00 (114.50 - 138.00) | -1.316 | 0.188 |
| DBP (mmHg) | 78.00 (71.75 - 86.00) | 80.00 (74.00 - 92.00) | -2.364 | 0.018 |
| FT3 (pmol/L) | 4.92 (4.56 - 5.19) | 5.07 (4.69 - 5.87) | -0.107 | 0.915 |
| FT4 (pmol/L) | 17.59 (15.40 - 20.12) | 18.06 (16.97 - 19.65) | -0.721 | 0.471 |
| TSH (uIU/mL) | 2.04 (1.14 - 3.00) | 1.49 (1.26 - 2.42) | -1.477 | 0.140 |
| TPOAB (IU/mL) | 13.08 (10.01 - 20.00) | 12.67 (10.07 - 20.49) | -0.398 | 0.690 |
| TGAB (IU/mL) | 19.79 (15.06 - 44.08) | 21.30 (16.03 - 55.02) | -0.820 | 0.412 |
SSL, serum selenium level; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid stimulating hormone; TPOAB, thyroidperoxidase antibodies; TGAB, thyroglobulin antibody.
Statistically significant variables in univariate analysis (serum selenium level, BMI, DBP) were included in multivariate analysis ( Table 5 ). High serum selenium (OR = 0.387, 95%CI: 0.212 - 0.706) and DBP (OR = 1.029, 95%CI: 1.006 - 1.053) were the relevant factors for the incidence of bilateral tumors.
Table 5.
Multivariate logistic retrospective analysis of factors related to unilateral and bilateral tumor incidence in PTC patients.
| Subject | B | S.E. | Wald | p | OR | 95%CI |
|---|---|---|---|---|---|---|
| SSL (μg/L) | ||||||
| Low | - | - | - | - | 1 | - |
| Middle | -0.205 | 0.304 | 0.455 | 0.500 | 0.815 | 0.449 - 1.478 |
| High | -0.924 | 0.307 | 9.027 | 0.003 | 0.397 | 0.217 - 0.725 |
| BMI (kg/m2) | 0.048 | 0.034 | 2.042 | 0.153 | 1.049 | 0.982 - 1.121 |
| DBP (mmHg) | 0.028 | 0.012 | 5.690 | 0.017 | 1.028 | 1.005 - 1.051 |
Serum selenium levels were categorized into three grades: Low: 51.3 - 74.4 μg/L (n = 93), Middle: 74.5 - 85.3 μg/L (n = 93), and High : 85.4 - 175.3 μg/L (n = 93); BMI, body mass index; DBP, diastolic blood pressure. SSL, serum selenium level.
Factors related to single and multifocal tumors in PTC patients
The status of single or multifocal tumors in PTC patients was presented in Table 6 . Univariate analysis showed that there were statistical differences in serum selenium level (p = 0.004), BMI (p = 0.033) and DBP (p = 0.018), while age (p = 0.647), height (p = 0.971), Waist circumference (p = 0.051) and SBP (p = 0.188) were not statistically different.
Table 6.
Comparison of data between single-focal tumor group and multi-focal tumor group in PTC patients.
| Subject | Unilateral tumor | Bilateral tumors | t/Z | p |
|---|---|---|---|---|
| Number (%) | 102 (37.78) | 168 (62.22) | - | - |
| Age (years) | 43.00 (33.75 - 52.00) | 45.00 (36.00 - 53.00) | -1.120 | 0.263 |
| SSL (μg/L) | 85.05 (74.88 - 92.15) | 76.90 (69.43 - 85.25) | -4.120 | <0.001 |
| BMI (kg/m2) | 25.43 ± 4.10 | 26.44 ± 3.89 | -2.017 | 0.045 |
| Height (cm) | 163.00 (159.75 - 170.00) | 164.00 (160.00 - 170.00) | -0.256 | 0.798 |
| Weight (kg) | 66.00 (57.38 - 79.63) | 70.00 (62.00 - 80.00) | -1.940 | 0.052 |
| Waist (cm) | 90.19 ± 11.41 | 91.43 ± 10.58 | -0.906 | 0.366 |
| SBP (mmHg) | 122.16 ± 15.85 | 126.64 ± 17.43 | -2.120 | 0.035 |
| DBP (mmHg) | 77.00 (69.00 - 84.25) | 80.50 (75.00 - 91.75) | -3.985 | <0.001 |
| FT3 (pmol/L) | 4.96 (4.63 - 5.19) | 4.94 (4.61 - 5.46) | -1.368 | 0.171 |
| FT4 (pmol/L) | 17.43 (15.96 - 19.19) | 17.80 (16.50 - 19.90) | -0.681 | 0.496 |
| TSH (uIU/mL) | 2.28 (1.45 - 2.81) | 1.54 (1.10 - 2.64) | -1.007 | 0.314 |
| TPOAB (IU/mL) | 14.44 (10.30 - 19.37) | 12.55 (9.99 - 20.57) | -0.673 | 0.501 |
| TGAB (IU/mL) | 19.62 (15.81 - 56.31) | 20.76 (15.42 - 48.62) | -0.256 | 0.798 |
SSL, serum selenium level; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure yronine; FT4, free thyroxine; TSH, thyroid stimulating hormone; TPOAB, thyroidperoxidase antibodies; TGAB, thyroglobulin antibody.
Statistically significant variables in univariate analysis (serum selenium level, BMI, SBP and DBP) were included in multivariate analysis ( Table 7 ). High serum selenium (OR = 0.320, 95%CI: 0.166 - 0.617) and DBP (OR = 1.066, 95%CI: 1.031 - 1.103) were related factors for the onset of multifocal tumors.
Table 7.
Multivariate logistic retrospective analysis of factors related to unilateral and bilateral tumor incidence in PTC patients.
| Subject | B | S.E. | Wald | p | OR | 95%CI |
|---|---|---|---|---|---|---|
| SSL (μg/L) | ||||||
| Low | - | - | - | - | 1 | - |
| Middle | -0.283 | 0.343 | 0.680 | 0.409 | 0.754 | 0.385 - 1.476 |
| High | -1.139 | 0.335 | 11.586 | 0.001 | 0.320 | 0.166 - 0.617 |
| BMI (kg/m2) | 0.027 | 0.037 | 0.542 | 0.461 | 1.028 | 0.956 - 1.105 |
| SBP (mmHg) | -0.011 | 0.011 | 0.905 | 0.341 | 0.989 | 0.967 - 1.012 |
| DBP (mmHg) | 0.064 | 0.017 | 13.689 | <0.001 | 1.066 | 1.031 - 1.103 |
Serum selenium levels were categorized into three grades: Low: 51.3 - 74.5 μg/L (n = 90), Middle: 74.5 - 85.3 μg/L (n = 90), and High : 85.3 - 175.3 μg/L (n = 90). SSL, serum selenium level; BMI, body mass index; DBP, diastolic blood pressure.
Discussion
In this study, the relationship between serum selenium levels and the pathological features of PTC was analyzed. The results showed: (1) Serum selenium levels of female PTC patients were lower than those of male PTC patients. (2) Serum selenium levels of PTC patients were negatively correlated with the number of lymph node metastasis, but there was no significant correlation between serum selenium levels and the stage and pathological subtype of PTC. (3) High serum selenium levels might be a protective factor in PTC patients.
Serum selenium levels were negatively correlated with the positive number of lymph node metastasis. First, the effects of selenium on cancer cells are highly concentration-dependent (22). Cancer cell growth was promoted by low to moderate serum selenium levels, while high serum selenium levels were cytotoxic and inhibited cancer cell growth (23). Second, as a crucial component of deiodinase, selenium plays a pivotal role in the conversion of T4 to T3 (24, 25). When there is a deficiency of selenium, it may lead to a reduction in the synthesis of thyroid hormones, potentially resulting in an elevation of thyroid stimulating hormone (TSH) levels (25). Thyroid cell growth can be promoted by TSH, and long-term high levels of TSH might induce and promote the occurrence and development of thyroid cancer. Therefore, low selenium might indirectly promote the increase of TSH and lead to the occurrence and development of tumors. However, the effect of selenium on TSH in humans has not been fully established. Rostami et al. observed a significant negative correlation (p < 0.001) between serum selenium levels and TSH in a low selenium population (26). Similarly, Tong et al. reported a significant negative correlation (p < 0.001) between serum selenium levels and TSH in healthy individuals (27). Our study did not find the association between serum selenium levels and TSH. Similarly, studies based on the NHANSE database in the United States have not found any significant association between serum selenium levels and TSH (28). Third, glutathione peroxidase 3 (GPX3), a selenoprotein, suppressed the metastasis of thyroid cancer by inhibiting the Wnt/β-catenin signaling pathway (29). Insufficient selenium levels might contribute to a reduction in GPX3 levels, thereby facilitating the metastasis of thyroid cancer. Notably, 81.69% of patients in our study demonstrated serum selenium levels below 90 ng/L. When the serum selenium concentration exceeds 90 ng/L, selenoproteins, including selenoprotein P and GPX, could be optimized; whereas, levels below this threshold are insufficient to achieve the optimal states of selenium proteins (30, 31).
High serum selenium levels might be a protective factor in PTC patients. At high selenium levels, serum selenium levels were negatively correlated to bilateral and multifocal tumors. First, hypermetabolism in tumor cells leads to increased reactive oxygen species (ROS), and these cells appear to be susceptible to oxidative stress (8, 9). The supplementation of selenium may exert an anti-tumor effect by inhibiting the ROS-mediated Akt/mTOR pathway (11). Furthermore, selenium induces cell death and apoptosis through superoxide generation in mitochondria and activation of the mitochondrial apoptotic pathway (32). Selenium could also affect tumor proliferation by affecting gene expression. Selenium inhibits tumor cell proliferation through DNA repair by p53-dependent effectors (13). In drug intervention experiments, it was shown that sodium selenite can significantly reduce cell viability and induce thyroid cancer cell G0/G1 cell cycle arrest and apoptosis in a dose-dependent manner (11). At the same time, the activities of T lymphocytes and NK cells were significantly enhanced after Se supplementation, and tumor proliferation could be suppressed (33). Notably, tumor cells have a higher metabolism than resting cells compared to healthy cells (34). In order to maintain the balance of the oxidation-antioxidation system, more antioxidant molecules such as selenoproteins need to be consumed. Therefore, the reduction of serum selenium levels might also be caused by the depletion process of tumorigenesis and development.
There was no significant association between serum selenium levels and PTC stage. This notion was also supported by a study of Korean women with thyroid cancer (35). However, the study by Jonklaas et al. showed that serum selenium levels might be negatively correlated with thyroid cancer stage (21). This discrepancy might be due to a variety of factors. First, the study population was limited to patients with PTC in our study, whereas in Jonklaas et al.’s study it was patients with differentiated thyroid cancer. Results might have been influenced by incorporation of other types of thyroid cancer. Second, despite serum selenium levels might be associated with thyroid cancer risk, randomized controlled trials on the effect of selenium supplementation on cancer have yielded inconsistent results (36). Serum selenium levels might be associated with some features of PTC, but not with tumor stage.
There was no significant difference in serum selenium levels among patients with different pathological subtypes of PTC. This is a preliminary exploration of serum selenium levels in patients with different subtypes of PTC. Similarly, no correlation was found between selenium and the tumor pathological subtype in the study of breast cancer (31). However, care needs to be taken that, due to the rarity of some subtypes, a sufficiently large sample size could not be obtained to improve the reliability of the evidence. Therefore, future studies with larger sample sizes need to be implemented.
The serum selenium levels of PTC patients were lower in females than males. Previous study found that there was no gender difference in serum selenium levels in Austrian (37). Similarly, the study in Western Romanesia revealed slightly lower selenium levels in young females compared to young males (38). However, some studies have found that females have lower serum selenium levels than males (39, 40). Notably, previous studies paid little attention to the differences in serum selenium levels between genders in PTC populations. The multi-pathway anti-tumor effects of selenium suggest that low selenium levels might serve as a susceptibility factor for PTC in female patients. In the future, studies on the relationship between diet and in vivo selenium metabolism need to be conducted in the PTC population, and gender differences should be taken into account.
This study has several limitations. First, as a cross-sectional study, a causal relationship between serum selenium levels and pathological features in PTC patients could not be established. Second, this study did not include a healthy control group, and there might be an insufficient incorporation of covariates. Third, patients positive for thyroid antibodies were not excluded during the inclusion process. Fourth, this study might have selective bias, because female PTC patients account for a relatively high proportion. Fifth, given the rarity of some subtypes of PTC, future studies with larger sample sizes are necessary to validate the reliability of the conclusions. Sixth, the relationship between selenium and pathological features in PTC patients has only been investigated at the serum level, thus further exploration of the protein molecular mechanism is warranted for a more comprehensive understanding.
Conclusions
In conclusion, the serum selenium levels of PTC patients in females were lower than males. High serum selenium levels might be a protective factor in PTC patients. Therefore, further research is necessary in this aspect to better understand the influence of selenium on PTC progression.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the First Affiliated Hospital of Shandong First Medical University (Qianfoshan Hospital in Shandong Province) under the number YXLL-KY-2019031.
Author contributions
SG: Document Retrieval, Data Extraction, Data analysis, Essay writing, and Paper submission. JZ, JY, HF and YT: Data analysis. YS, MS and JF: Data Extraction. JD and LL: Article innovation and Paper submission. All authors contributed to the article and approved the submitted version.
Funding Statement
This work was funded by the National Natural Science Foundation of China (NO. 82303031, 82170847); Projects of Medicine and Health Science Technology Development Program in Shandong Province (NO. 2016WS0499); Shandong Provincial Natural Science Foundation of China Grants (NO. ZR2019PH025, ZR2020KH004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1242250/full#supplementary-material
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in globocan 2012. Int J Cancer (2015) 136(5):E359–86. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 3. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet (London England) (2016) 388(10061):2783–95. doi: 10.1016/s0140-6736(16)30172-6 [DOI] [PubMed] [Google Scholar]
- 4. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. Jama (2017) 317(13):1338–48. doi: 10.1001/jama.2017.2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tam AA, Ozdemir D, Aydın C, Bestepe N, Ulusoy S, Sungu N, et al. Association between preoperative thyrotrophin and clinicopathological and aggressive features of papillary thyroid cancer. Endocrine (2018) 59(3):565–72. doi: 10.1007/s12020-018-1523-6 [DOI] [PubMed] [Google Scholar]
- 6. Lango M, Flieder D, Arrangoiz R, Veloski C, Yu JQ, Li T, et al. Extranodal extension of metastatic papillary thyroid carcinoma: correlation with biochemical endpoints, nodal persistence, and systemic disease progression. Thyroid Off J Am Thyroid Assoc (2013) 23(9):1099–105. doi: 10.1089/thy.2013.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Niemann AC, Reid AT, Smith J, Hammond J, DeBolle SA, Wei I, et al. Association of patient age with high-risk pathologic features in papillary thyroid cancer. J Surg Res (2017) 211:228–32. doi: 10.1016/j.jss.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 8. Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, ME LL. Oxidative stress and cancer: an overview. Ageing Res Rev (2013) 12(1):376–90. doi: 10.1016/j.arr.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 9. Panieri E, Santoro MM. Ros homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis (2016) 7(6):e2253. doi: 10.1038/cddis.2016.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi WR, Roh JL, Gong G, Cho KJ, Choi SH, Nam SY, et al. Multifocality of papillary thyroid carcinoma as a risk factor for disease recurrence. Oral Oncol (2019) 94:106–10. doi: 10.1016/j.oraloncology.2019.05.023 [DOI] [PubMed] [Google Scholar]
- 11. Cheng Z, Yu S, He W, Li J, Xu T, Xue J, et al. Selenite induces cell cycle arrest and apoptosis via reactive oxygen species-dependent inhibition of the Akt/Mtor pathway in thyroid cancer. Front Oncol (2021) 11:668424. doi: 10.3389/fonc.2021.668424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuršvietienė L, Mongirdienė A, Bernatonienė J, Šulinskienė J, Stanevičienė I. Selenium anticancer properties and impact on cellular redox status. Antioxidants (Basel Switzerland) (2020) 9(1):134–5. doi: 10.3390/antiox9010080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fischer JL, Mihelc EM, Pollok KE, Smith ML. Chemotherapeutic selectivity conferred by selenium: A role for P53-dependent DNA repair. Mol Cancer Ther (2007) 6(1):355–61. doi: 10.1158/1535-7163.Mct-06-0472 [DOI] [PubMed] [Google Scholar]
- 14. O'Grady TJ, Kitahara CM, DiRienzo AG, Gates MA. The association between selenium and other micronutrients and thyroid cancer incidence in the Nih-Aarp diet and health study. PloS One (2014) 9(10):e110886. doi: 10.1371/journal.pone.0110886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Short SP, Williams CS. Selenoproteins in tumorigenesis and cancer progression. Adv Cancer Res (2017) 136:49–83. doi: 10.1016/bs.acr.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baltaci AK, Dundar TK, Aksoy F, Mogulkoc R. Changes in the Serum Levels of Trace Elements before and after the Operation in Thyroid Cancer Patients. Biol Trace Element Res (2017) 175(1):57–64. doi: 10.1007/s12011-016-0768-2 [DOI] [PubMed] [Google Scholar]
- 17. Glattre E, Thomassen Y, Thoresen SO, Haldorsen T, Lund-Larsen PG, Theodorsen L, et al. Prediagnostic serum selenium in a case-control study of thyroid cancer. Int J Epidemiol (1989) 18(1):45–9. doi: 10.1093/ije/18.1.45 [DOI] [PubMed] [Google Scholar]
- 18. Shen F, Cai WS, Li JL, Feng Z, Cao J, Xu B. The association between serum levels of selenium, copper, and magnesium with thyroid cancer: A meta-analysis. Biol Trace Element Res (2015) 167(2):225–35. doi: 10.1007/s12011-015-0304-9 [DOI] [PubMed] [Google Scholar]
- 19. Kucharzewski M, Braziewicz J, Majewska U, Góźdź S. Concentration of selenium in the whole blood and the thyroid tissue of patients with various thyroid diseases. Biol Trace Element Res (2002) 88(1):25–30. doi: 10.1385/bter:88:1:25 [DOI] [PubMed] [Google Scholar]
- 20. Przybylik-Mazurek E, Zagrodzki P, Kuźniarz-Rymarz S, Hubalewska-Dydejczyk A. Thyroid disorders-assessments of trace elements, clinical, and laboratory parameters. Biol Trace Element Res (2011) 141(1-3):65–75. doi: 10.1007/s12011-010-8719-9 [DOI] [PubMed] [Google Scholar]
- 21. Jonklaas J, Danielsen M, Wang H. A pilot study of serum selenium, vitamin D, and thyrotropin concentrations in patients with thyroid cancer. Thyroid (2013) 23(9):1079–86. doi: 10.1089/thy.2012.0548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gheorghiu ML, Badiu C. Selenium involvement in mitochondrial function in thyroid disorders. Hormones (Athens Greece) (2020) 19(1):25–30. doi: 10.1007/s42000-020-00173-2 [DOI] [PubMed] [Google Scholar]
- 23. Collery P. Strategies for the development of selenium-based anticancer drugs. J Trace elements Med Biol Organ Soc Minerals Trace Elements (GMS) (2018) 50:498–507. doi: 10.1016/j.jtemb.2018.02.024 [DOI] [PubMed] [Google Scholar]
- 24. Bates JM, Spate VL, Morris JS, St Germain DL, Galton VA. Effects of selenium deficiency on tissue selenium content, deiodinase activity, and thyroid hormone economy in the rat during development. Endocrinology (2000) 141(7):2490–500. doi: 10.1210/endo.141.7.7571 [DOI] [PubMed] [Google Scholar]
- 25. Gorini F, Vassalle C. Selenium and selenoproteins at the intersection of type 2 diabetes and thyroid pathophysiology. Antioxidants (Basel Switzerland) (2022) 11(6):589–620. doi: 10.3390/antiox11061188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rostami R, Nourooz-Zadeh S, Mohammadi A, Khalkhali HR, Ferns G, Nourooz-Zadeh J. Serum selenium status and its interrelationship with serum biomarkers of thyroid function and antioxidant defense in Hashimoto's thyroiditis. Antioxidants (Basel Switzerland) (2020) 9(11):626–8. doi: 10.3390/antiox9111070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tong YJ, Teng WP, Jin Y, Li YS, Guan HX, Wang WB, et al. [an epidemiological study on the relationship between selenium and thyroid function in areas with different iodine intake]. Zhonghua Yi Xue Za Zhi (2003) 83(23):2036–9. [PubMed] [Google Scholar]
- 28. Jain RB. Thyroid function and serum copper, selenium, and zinc in general U.S. Population. Biol Trace Element Res (2014) 159(1-3):87–98. doi: 10.1007/s12011-014-9992-9 [DOI] [PubMed] [Google Scholar]
- 29. Zhao H, Li J, Li X, Han C, Zhang Y, Zheng L, et al. Silencing Gpx3 expression promotes tumor metastasis in human thyroid cancer. Curr Protein Pept Sci (2015) 16(4):316–21. doi: 10.2174/138920371604150429154840 [DOI] [PubMed] [Google Scholar]
- 30. Xia Y, Hill KE, Li P, Xu J, Zhou D, Motley AK, et al. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: A placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am J Clin Nutr (2010) 92(3):525–31. doi: 10.3945/ajcn.2010.29642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sandsveden M, Nilsson E, Borgquist S, Rosendahl AH, Manjer J. Prediagnostic serum selenium levels in relation to breast cancer survival and tumor characteristics. Int J Cancer (2020) 147(9):2424–36. doi: 10.1002/ijc.33031 [DOI] [PubMed] [Google Scholar]
- 32. Xiang N, Zhao R, Zhong W. Sodium selenite induces apoptosis by generation of superoxide via the mitochondrial-dependent pathway in human prostate cancer cells. Cancer Chemotherapy Pharmacol (2009) 63(2):351–62. doi: 10.1007/s00280-008-0745-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roy M, Kiremidjian-Schumacher L, Wishe HI, Cohen MW, Stotzky G. Supplementation with selenium and human immune cell functions. I. Effect on lymphocyte proliferation and interleukin 2 receptor expression. Biol Trace element Res (1994) 41(1-2):103–14. doi: 10.1007/bf02917221 [DOI] [PubMed] [Google Scholar]
- 34. Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ros-mediated mechanisms: A radical therapeutic approach? Nat Rev Drug Discov (2009) 8(7):579–91. doi: 10.1038/nrd2803 [DOI] [PubMed] [Google Scholar]
- 35. Chung HK, Nam JS, Ahn CW, Lee YS, Kim KR. Some elements in thyroid tissue are associated with more advanced stage of thyroid cancer in Korean women. Biol Trace Element Res (2016) 171(1):54–62. doi: 10.1007/s12011-015-0502-5 [DOI] [PubMed] [Google Scholar]
- 36. Vinceti M, Dennert G, Crespi CM, Zwahlen M, Brinkman M, Zeegers MP, et al. Selenium for preventing cancer. Cochrane Database Systematic Rev (2014) 2014(3):Cd005195. doi: 10.1002/14651858.CD005195.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gundacker C, Komarnicki G, Zödl B, Forster C, Schuster E, Wittmann K. Whole blood mercury and selenium concentrations in a selected Austrian population: does gender matter? Sci Total Environ (2006) 372(1):76–86. doi: 10.1016/j.scitotenv.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 38. Bizerea-Moga TO, Pitulice L, Bizerea-Spiridon O, Moga TV. Evaluation of serum selenium status by age and gender: A retrospective observational cohort study in Western Romania. Nutrients (2021) 13(5):760, 897. doi: 10.3390/nu13051497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kafai MR, Ganji V. Sex, age, geographical location, smoking, and alcohol consumption influence serum selenium concentrations in the USA: third national health and nutrition examination survey, 1988-1994. J Trace elements Med Biol Organ Soc Minerals Trace Elements (GMS) (2003) 17(1):13–8. doi: 10.1016/s0946-672x(03)80040-8 [DOI] [PubMed] [Google Scholar]
- 40. Wasowicz W, Zachara BA. Selenium concentrations in the blood and urine of a healthy polish sub-population. J Clin Chem Clin Biochem Z fur klinische Chemie und klinische Biochemie (1987) 25(7):409–12. doi: 10.1515/cclm.1987.25.7.409 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.