Abstract
This Cerebellar Classic highlights the landmark discovery of the innervation of the cerebellar cortex and cerebellar nuclei by noradrenergic and serotoninergic axons emanating, respectively, from the locus coeruleus and the raphé nuclei. Since then, modulation of the activity of cerebellar neurons by the monoamine systems has been studied extensively, as well as their reorganization and modifications during development, plasticity, and disease. The discovery of noradrenergic and serotoninergic innervation of the cerebellum has been a crucial step in understanding the neurochemical relationships between brainstem nuclei and the cerebellum, and the attempts to treat cerebellar ataxias pharmacologically. The large neurochemical repertoire of the cerebellum represents one of the complexities and challenges in the modern appraisal of cerebellar disorders.
Keywords: Cerebellar afferents, Monoamines, Norepinephrine, Serotonin, Developmental plasticity
Divergent or “global” neural systems imply situations, whereby a relatively small number of neurons innervate a much larger number of terminal domains, in contrast to so-called “point-to-point” systems, where each neuron only contacts a few target nerve cells [1]. Since the time of Ramón y Cajal, the cerebellum had classically been considered a point-to-point system. With the discovery in the 1960s of the cerebellar monoaminergic innervation by neurons of the locus coeruleus and the raphé nuclei, the cerebellum has become a structure where “point-to-point” and “global” neural circuits converge. Moreover, monoamines may exert a widespread effect on neurons besides those receiving physical synaptic appositions—that is, they may subserve a paracrine function [2].
In the human brain, the locus coeruleus contains an average of 50,000 noradrenergic neurons [3, 4], while in rodents it contains about 3,000 cells [5]. The dorsal raphé nuclei of the human brain contain around 130,000–200,000 serotoninergic neurons [6], while that number in rodents is about 8,000–9,000 cells [7, 8]. Thus, the thousands of neurons in both these anatomical systems influence the physiological activity of extensively divergent domains that comprise several billion neurons, from the telencephalon to the spinal cord.
The Cerebellar Classic [9] by the pioneer Swedish neuroscientists Nils-Erik Andén, Kjell Fuxe, and Urban Ungerstedt revisited here has broadened the sources of afferent input to the cerebellum beyond the “traditional” climbing and mossy fibers. It has also paved the way for studies on the fate and reorganization of cerebellar monoamine systems in human diseases [10] and in experimental models of cerebellar degeneration [11–21], as well the elucidation of phylogenetic [22, 23], ontogenetic [24–26], developmental plasticity [27–29], and reinnervation issues [30].
Andén and colleagues [9] studied central monoamine neurons and their unmyelinated axons by means of fluorescence histochemistry after removing the cerebral cortex and cerebellum by suction with a fine glass cannula. In biochemical measurements, they found the mean concentration of norepinephrine in the normal rat cerebellum to be 0.18 μg/g, representing approximately 8% of the total brain amount; the mean concentration of serotonin was 0.07 μg/g or about 2.5% of the total brain amount. The authors concluded that most, if not all, norepinephrine nerve terminals in the cerebral cortex and the cerebellum belonged to axons originating from noradrenergic cell bodies primarily located in the reticular formation of the medulla oblongata and the pons. They further articulated the idea that the same noradrenergic neuron may innervate both the cerebral cortex and the cerebellum. That last organizing principle of the anatomical projections of coerulear noradrenergic neurons was subsequently confirmed with the identification of collateral axons in the cerebellar cortex, the cerebellar nuclei, and other areas of the central nervous system, including the cerebral cortex, the diencephalon, and the spinal cord [31, 32].
The monoaminergic innervation of the cerebellar cortex comprises norepinephrine- and serotonin-containing axons (Fig. 1) [9, 31]. The origin of the noradrenergic projection lies in neurons of the dorsal part of the locus coeruleus [33–35], the nucleus subcoeruleus, and fields A5/A7 [22, 36, 37]. Furthermore, horseradish peroxidase (HRP) tracing experiments in rats showed heavy innervation of the locus coeruleus by all raphé nuclei, in addition to many extra-raphé brainstem sources [38], suggesting close interactions between the two main brainstem monoaminergic nodes that target the cerebellar circuitry. Using fluorescence histochemistry, researchers have found that the noradrenergic innervation of the cerebellar cortex is more pronounced than its serotoninergic innervation [31]. Electron microscopic studies have shown that, in the rodent cerebellum, norepinephrine-containing axons are apposed to Purkinje cell dendrites [39, 40].
Fig. 1.
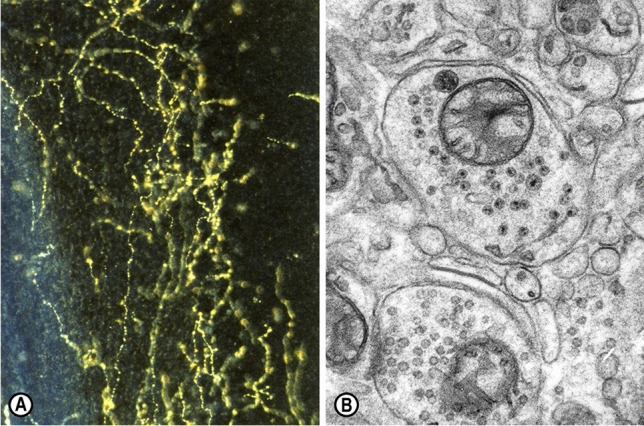
(A) Serotonin-immunoreactive fibers in the mouse cerebellum, displaying their typical axonal varicosities. Sternberger peroxidase-antiperoxidase (PAP) method, dark-field illumination, × 40 [18]. (B) Electron micrograph of a monoaminergic varicosity or bouton en passant in the molecular layer of the cerebellum of a “Purkinje cell degeneration” (Agtpbp1pcd/Agtpbp1.pcd) mutant mouse, containing small granular vesicles (40–60 nm in diameter) and a large granular vesicle (90–110 nm in diameter). Potassium permanganate (KMnO4) fixation method, ultrathin section stained with uranyl acetate and lead citrate, × 24,000 [16]
Physiological experiments have indicated a neuromodulatory role for norepinephrine [41, 42] and serotonin [43], both adjusting the activity of other synaptic inputs to the Purkinje cells rather than exerting a strict excitatory or inhibitory effect. The presence of α and β adrenergic receptors on Purkinje cells suggests the existence of bidirectional mechanisms of regulation that allow noradrenergic afferents to refine the signals arriving at Purkinje cells, including the parallel fiber input, under specific arousal states or during motor skill learning [44]. Cerebellar catecholamines, especially in the lateral cerebellar nucleus, might modulate certain aspects of cognitive and affective behavior, such as sensorimotor integration, associative fear learning, response inhibition, and working memory [45].
Serotonin-containing axons originate in neurons of the dorsal raphé nuclei of the pons and of the medullary and pontine reticular formation [46–48], and are distributed throughout the cerebellar cortex of the rat [9, 31, 48, 49] and the mouse [18]. A small contingent of serotonin terminals belong to typical mossy fibers; these are confined to the granule cell layer and establish synapses on dendrites of granule cells [24, 50]. The vast majority of serotonin nerve terminals belong to finer beaded axons of the so-called “diffuse system” and are distributed to all cerebellar cortical layers [50]. Serotonin axon terminals innervate the dendrites of Purkinje and granule cells; the parallel fibers; as well as basket, stellate, and Golgi cells and neurons of the cerebellar nuclei [24, 46, 48, 50]. Iontophoretic application of serotonin and electrophysiological stimulation of the raphé nuclei modulate the firing of Purkinje cells [43, 51–53]. Moreover, serotonin modulates the glutamate-induced excitation and the γ-aminobutyric acid (GABA)-elicited inhibition of Purkinje cells [54, 55].
With regard to the “third monoamine,” dopamine (3,4-dihydroxyphenethylamine), the cerebellum had not been considered an elective dopaminergic region, and the very small amounts of dopamine detected in it were thought to represent an intermediary product in the metabolism of norepinephrine [56]. Later studies have suggested the presence of a small dopaminergic contingent in the cerebella of rodents and primates [57–59], as well as the expression of dopamine D1–D5 receptors and dopamine transporters [21]. Still, the density of dopamine D2 receptors in the cerebellum represents about 1% of their density in the striatum [60]. Although unequivocal evidence on the functional role of a cerebellar dopaminergic system is still lacking, its involvement in associative and projective circuits has been discussed [61].
This Cerebellar Classic highlights a milestone in the elucidation of the neurochemistry of the cerebellum, whose main transmitters and neuromodulators also include glutamate, GABA, acetylcholine, nitric oxide, endocannabinoids, and neuropeptides. This large neurochemical arsenal is one the features of the cerebellum; they are involved in the numerous motor/non-motor functions of the cerebellum and have variable impacts on cerebellar ataxias.
Appendix
Author Contribution
Concept, writing, and approval of the final version: LCT and MM.
Funding
Open access funding provided by HEAL-Link Greece
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sotelo C, Alvarado-Mallart RM. Growth and differentiation of cerebellar suspensions transplanted into the adult cerebellum of mice with heredodegenerative ataxia. Proc Natl Acad Sci USA. 1986;83:1135–1139. doi: 10.1073/pnas.83.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mobley P, Greengard P. Evidence for widespread effects of noradrenaline on axon terminals in the rat frontal cortex. Proc Natl Acad Sci USA. 1985;82:945–947. doi: 10.1073/pnas.82.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mouton PR, Pakkenberg B, Gundersen HJ, Price DL. Absolute number and size of pigmented locus coeruleus neurons in young and aged individuals. J Chem Neuroanat. 1994;7:185–190. doi: 10.1016/0891-0618(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 4.Sharma Y, Xu T, Graf WM, Fobbs A, Sherwood CC, Hof PR, Allman JM, Manaye KF. Comparative anatomy of the locus coeruleus in humans and nonhuman primates. J Comp Neurol. 2010;518:963–971. doi: 10.1002/cne.22249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swanson LW. The locus coeruleus: A cytoarchitectonic, Golgi and immunohistochemical study in the albino rat. Brain Res. 1976;110:39–56. doi: 10.1016/0006-8993(76)90207-9. [DOI] [PubMed] [Google Scholar]
- 6.Baker KG, Halliday GM, Hornung JP, Geffen LB, Cotton RG, Törk I. Distribution, morphology and number of monoamine-synthesizing and substance P-containing neurons in the human dorsal raphé nucleus. Neuroscience. 1991;42:757–775. doi: 10.1016/0306-4522(91)90043-n. [DOI] [PubMed] [Google Scholar]
- 7.Ishimura K, Takeuchi Y, Fujiwara K, Tominaga M, Yoshioka H, Sawada T. Quantitative analysis of the distribution of serotonin-immunoreactive cell bodies in the mouse brain. Neurosci Lett. 1988;91:265–270. doi: 10.1016/0304-3940(88)90691-x. [DOI] [PubMed] [Google Scholar]
- 8.Aldahmash A. Cell numbers in the dorsal and median raphé nuclei of AS and AS/AGU rats. Biomed Res. 2010;21:15–22. https://www.alliedacademies.org/articles/cell-numbers-in-the-dorsal-and-median-raphe-nuclei-of-as-andasagu-rats.html. Accessed 16 June 2022.
- 9.Andén NE, Fuxe K, Ungerstedt U. Monoamine pathways to the cerebellum and cerebral cortex. Experientia. 1967;23:838–839. doi: 10.1007/BF02146876. [DOI] [PubMed] [Google Scholar]
- 10.Kish SJ, Shannak KS, Hornykiewicz O. Reduction of noradrenaline in cerebellum of patients with olivopontocerebellar atrophy. J Neurochem. 1984;42:1476–1478. doi: 10.1111/j.1471-4159.1984.tb02813.x. [DOI] [PubMed] [Google Scholar]
- 11.Landis SC, Bloom FE. Ultrastructural identification of noradrenergic boutons in mutant and normal mouse cerebellar cortex. Brain Res. 1975;96:299–305. doi: 10.1016/0006-8993(75)90738-6. [DOI] [PubMed] [Google Scholar]
- 12.Landis SC, Shoemaker WJ, Schlumpf M, Bloom FE. Catecholamines in mutant mouse cerebellum: fluorescence microscopic and chemical studies. Brain Res. 1975;93:253–266. doi: 10.1016/0006-8993(75)90349-2. [DOI] [PubMed] [Google Scholar]
- 13.Ghetti B, Fuller RW, Sawyer BD, Hemrick-Luecke SK, Schmidt MJ. Purkinje cell loss and the noradrenergic system in the cerebellum of pcd mutant mice. Brain Res Bull. 1981;7:711–714. doi: 10.1016/0361-9230(81)90123-4. [DOI] [PubMed] [Google Scholar]
- 14.Roffler-Tarlov S, Landis SC, Zigmond MJ. Effects of Purkinje cell degeneration on the noradrenergic projection to mouse cerebellar cortex. Brain Res. 1984;298:303–311. doi: 10.1016/0006-8993(84)91429-x. [DOI] [PubMed] [Google Scholar]
- 15.Ohsugi K, Adachi K, Ando K. Serotonin metabolism in the CNS in cerebellar ataxic mice. Experientia. 1986;42:1245–1247. doi: 10.1007/BF01946406. [DOI] [PubMed] [Google Scholar]
- 16.Triarhou LC, Ghetti B. Monoaminergic nerve terminals in the cerebellar cortex of Purkinje cell degeneration mutant mice: fine structural integrity and modification of cellular environs following loss of Purkinje and granule cells. Neuroscience. 1986;18:795–807. doi: 10.1016/0306-4522(86)90100-4. [DOI] [PubMed] [Google Scholar]
- 17.Ghetti B, Perry KW, Fuller RW. Serotonin concentration and turnover in cerebellum and other brain regions of pcd mutant mice. Brain Res. 1988;458:367–371. doi: 10.1016/0006-8993(88)90480-5. [DOI] [PubMed] [Google Scholar]
- 18.Triarhou LC, Ghetti B. Serotonin-immunoreactivity in the cerebellum of two neurological mutant mice and the corresponding wild-type genetic stocks. J Chem Neuroanat. 1991;4:421–428. doi: 10.1016/0891-0618(91)90022-5. [DOI] [PubMed] [Google Scholar]
- 19.Ghetti B, Triarhou LC, Fuller RW. Cerebellar monoamines in the “Purkinje cell degeneration” mutant mouse. In: Trouillas A, Fuxe K, editors. Serotonin, the cerebellum and ataxia. New York: Raven Press; 1993. pp. 297–306. [Google Scholar]
- 20.Abbott LC, Sotelo C. Ultrastructural analysis of catecholaminergic innervation in weaver and normal mouse cerebellar cortices. J Comp Neurol. 2000;426:316–329. doi: 10.1002/1096-9861(20001016)426:2<316::AID-CNE11>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Giompres P, Delis F. Dopamine transporters in the cerebellum of mutant mice. Cerebellum. 2005;4:105–111. doi: 10.1080/14734220510007851. [DOI] [PubMed] [Google Scholar]
- 22.Tohyama M. Comparative anatomy of cerebellar catecholamine innervations from teleosts to mammals. J Hirnforsch. 1976;17:43–60. [PubMed] [Google Scholar]
- 23.Nelson TE, King JS, Bishop GA. Distribution of tyrosine hydroxylase-immunoreactive afferents to the cerebellum differs between species. J Comp Neurol. 1997;379:443–454. doi: 10.1002/(sici)1096-9861(19970317)379:3<443::aid-cne9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Sotelo C, Beaudet A. Influence of experimentally induced agranularity on the synaptogenesis of serotonin nerve terminals in rat cerebellar cortex. Proc R Soc Lond B Biol Sci. 1979;206:133–138. doi: 10.1098/rspb.1979.0096. [DOI] [PubMed] [Google Scholar]
- 25.Yeh HH, Woodward DJ. Noradrenergic action in the developing rat cerebellum: interaction between norepinephrine and synaptically-evoked responses of immature Purkinje cells. Brain Res. 1983;313:207–218. doi: 10.1016/0165-3806(83)90218-3. [DOI] [PubMed] [Google Scholar]
- 26.Dopico AM, Zieher LM. Neurochemical characterization of the alterations in the noradrenergic afferents to the cerebellum of adult rats exposed to X-irradiation at birth. J Neurochem. 1993;61:481–489. doi: 10.1111/j.1471-4159.1993.tb02149.x. [DOI] [PubMed] [Google Scholar]
- 27.Kostrzewa RM, Harston CT, Fukushima H, Brus R. Noradrenergic fiber sprouting in the cerebellum. Brain Res Bull. 1982;9:509–517. doi: 10.1016/0361-9230(82)90159-9. [DOI] [PubMed] [Google Scholar]
- 28.Robain O, Lanfumey L, Adrien J, Farkas E. Developmental changes in the cerebellar cortex after locus coeruleus lesion with 6-hydroxydopamine in the rat. Exp Neurol. 1985;88:150–164. doi: 10.1016/0014-4886(85)90120-7. [DOI] [PubMed] [Google Scholar]
- 29.Sievers J, Mangold U, Berry M. 6-OHDA-induced ectopia of external granule cells in the subarachnoid space covering the cerebellum. III. Morphology and synaptic organization of ectopic cerebellar neurons: a scanning and transmission electron microscopic study. J Comp Neurol. 1985;232:319–30. doi: 10.1002/cne.902320305. [DOI] [PubMed] [Google Scholar]
- 30.Triarhou LC, Low WC, Ghetti B. Serotonin fiber innervation of cerebellar cell suspensions intraparenchymally grafted to the cerebellum of pcd mutant mice. Neurochem Res. 1992;17:475–482. doi: 10.1007/BF00969895. [DOI] [PubMed] [Google Scholar]
- 31.Hökfelt T, Fuxe K. Cerebellar monoamine nerve terminals, a new type of afferent fibers to the cortex cerebelli. Exp Brain Res. 1969;9:63–72. doi: 10.1007/BF00235452. [DOI] [PubMed] [Google Scholar]
- 32.Steindler DA. Locus coeruleus neurons have axons that branch to the forebrain and cerebellum. Brain Res. 1981;223:367–373. doi: 10.1016/0006-8993(81)91149-5. [DOI] [PubMed] [Google Scholar]
- 33.Olson L, Fuxe K. On the projections from the locus coeruleus noradrenaline neurons: the cerebellar innervation. Brain Res. 1971;28:165–171. doi: 10.1016/0006-8993(71)90533-6. [DOI] [PubMed] [Google Scholar]
- 34.Siggins GR, Hoffer BJ, Oliver AP, Bloom FE. Activation of a central noradrenergic projection to cerebellum. Nature. 1971;233:481–483. doi: 10.1038/233481a0. [DOI] [PubMed] [Google Scholar]
- 35.Hoffer BJ, Siggins GR, Oliver AP, Bloom FE. Activation of the pathway from locus coeruleus to rat cerebellar Purkinje neurons: pharmacological evidence of noradrenergic central inhibition. J Pharmacol Exp Ther. 1973;184:553–569. [PubMed] [Google Scholar]
- 36.Kimoto Y, Satoh K, Sakumoto T, Tohyama M, Shimizu N. Afferent fiber connections from the lower brain stem to the rat cerebellum by the horseradish peroxidase method combined with MAO staining, with special reference to noradrenergic neurons. J Hirnforsch. 1978;19:85–100. [PubMed] [Google Scholar]
- 37.Pasquier DA, Gold MA, Jacobowitz DM. Noradrenergic perikarya (A5–A7, subcoeruleus) projections to the rat cerebellum. Brain Res. 1980;196:270–275. doi: 10.1016/0006-8993(80)90737-4. [DOI] [PubMed] [Google Scholar]
- 38.Morgane PJ, Jacobs MS. Raphé projections to the locus coeruleus in the rat. Brain Res Bull. 1979;4:519–534. doi: 10.1016/0361-9230(79)90037-6. [DOI] [PubMed] [Google Scholar]
- 39.Bloom FE, Hoffer BJ, Siggins GR. Studies on norepinephrine-containing afferents to Purkinje cells of rat cerebellum. I. Localization of the fibers and their synapses. Brain Res. 1971;25:501–21. doi: 10.1016/0006-8993(71)90457-4. [DOI] [PubMed] [Google Scholar]
- 40.Kimoto Y, Toyama M, Satoh K, Sakumoto T, Takahashi Y, Shimizu N. Fine structure of rat cerebellar noradrenaline terminals as visualized by potassium permanganate ‘in situ perfusion’ fixation method. Neuroscience. 1981;6:47–58. doi: 10.1016/0306-4522(81)90242-6. [DOI] [PubMed] [Google Scholar]
- 41.Freedman R, Hoffer BJ, Puro D, Woodward DJ. Noradrenaline modulation of the responses of the cerebellar Purkinje cell to afferent synaptic activity. Br J Pharmacol. 1976;57:603–605. doi: 10.1111/j.1476-5381.1976.tb10391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodward DJ, Moises HC, Waterhouse BD, Yeh HH, Cheun JE. The cerebellar norepinephrine system: inhibition, modulation, and gating. Prog Brain Res. 1991;88:331–341. doi: 10.1016/s0079-6123(08)63820-0. [DOI] [PubMed] [Google Scholar]
- 43.Strahlendorf JC, Lee M, Strahlendorf HK. Effects of serotonin on cerebellar Purkinje cells are dependent on the baseline firing rate. Exp Brain Res. 1984;56:50–58. doi: 10.1007/BF00237441. [DOI] [PubMed] [Google Scholar]
- 44.Lippiello P, Hoxha E, Volpicelli F, Lo Duca G, Tempia F, Miniaci MC. Noradrenergic modulation of the parallel fiber-Purkinje cell synapse in mouse cerebellum. Neuropharmacology. 2015;89:33–42. doi: 10.1016/j.neuropharm.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 45.Carlson ES, Hunker AC, Sandberg SG, Locke TM, Geller JM, Schindler AG, Thomas SA, Darvas M, Phillips PEM, Zweifel LS. Catecholaminergic innervation of the lateral nucleus of the cerebellum modulates cognitive behaviors. J Neurosci. 2021;41:3512–3530. doi: 10.1523/JNEUROSCI.2406-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan-Palay V. Fine structure of labelled axons in the cerebellar cortex and nuclei of rodents and primates after intraventricular infusions with tritiated serotonin. Anat Embryol (Berl) 1975;148:235–265. doi: 10.1007/BF00319846. [DOI] [PubMed] [Google Scholar]
- 47.Taber Pierce E, Hoddevik GH, Walberg F. The cerebellar projection from the raphé nuclei in the cat as studied with the method of retrograde transport of horseradish peroxidase. Anat Embryol (Berl) 1977;152:73–87. doi: 10.1007/BF00341436. [DOI] [PubMed] [Google Scholar]
- 48.Bishop GA, Ho RH. The distribution and origin of serotonin immunoreactivity in the rat cerebellum. Brain Res. 1985;331:195–207. doi: 10.1016/0006-8993(85)91545-8. [DOI] [PubMed] [Google Scholar]
- 49.Takeuchi Y, Kimura H, Sano Y. Immunohistochemical demonstration of serotonin-containing nerve fibers in the cerebellum. Cell Tissue Res. 1982;226:1–12. doi: 10.1007/BF00217077. [DOI] [PubMed] [Google Scholar]
- 50.Beaudet A, Sotelo C. Synaptic remodelling of serotonin axon terminals in rat agranular cerebellum. Brain Res. 1981;206:305–329. doi: 10.1016/0006-8993(81)90534-5. [DOI] [PubMed] [Google Scholar]
- 51.Bloom FE, Hoffer BJ, Siggins GR, Barker JL, Nicoli RA. Effects of serotonin on central neurons: microiontophoretic application. Fed Proc. 1972;31:97–106. [PubMed] [Google Scholar]
- 52.Weiss M, Pellet J. Raphé-cerebellum interactions. II. Effects of midbrain raphé stimulation and harmaline administration on single unit activity of cerebellar cortical cells in the rat. Exp Brain Res. 1982;48:171–6. doi: 10.1007/BF00237212. [DOI] [PubMed] [Google Scholar]
- 53.Strahlendorf JC, Strahlendorf HK, Lee M. Enhancement of cerebellar Purkinje cell complex discharge activity by microiontophoretic serotonin. Exp Brain Res. 1986;61:614–624. doi: 10.1007/BF00237588. [DOI] [PubMed] [Google Scholar]
- 54.Lee M, Strahlendorf JC, Strahlendorf HK. Modulatory action of serotonin on glutamate-induced excitation of cerebellar Purkinje cells. Brain Res. 1986;361:107–113. doi: 10.1016/0006-8993(85)91280-6. [DOI] [PubMed] [Google Scholar]
- 55.Strahlendorf JC, Lee M, Strahlendorf HK. Modulatory role of serotonin on GABA-elicited inhibition of cerebellar Purkinje cells. Neuroscience. 1989;30:117–126. doi: 10.1016/0306-4522(89)90358-8. [DOI] [PubMed] [Google Scholar]
- 56.Björklund A, Lindvall O. Dopamine-containing systems in the C.N.S. In: Björklund A, Hökfelt T, editors. Classical transmitters in the C.N.S., part I (Handbook of chemical neuroanatomy, vol. 2) Amsterdam: Elsevier; 1984. pp. 55–122. [Google Scholar]
- 57.Panagopoulos NT, Papadopoulos GC, Matsokis NA. Dopaminergic innervation and binding in the rat cerebellum. Neurosci Lett. 1991;130:208–212. doi: 10.1016/0304-3940(91)90398-d. [DOI] [PubMed] [Google Scholar]
- 58.Ikai Y, Takada M, Shinonaga Y, Mizuno N. Dopaminergic and non-dopaminergic neurons in the ventral tegmental area of the rat project, respectively, to the cerebellar cortex and deep cerebellar nuclei. Neuroscience. 1992;51:719–728. doi: 10.1016/0306-4522(92)90310-x. [DOI] [PubMed] [Google Scholar]
- 59.Melchitzky DS, Lewis DA. Tyrosine hydroxylase- and dopamine transporter-immunoreactive axons in the primate cerebellum: evidence for a lobular- and laminar-specific dopamine innervation. Neuropsychopharmacology. 2000;22:466–472. doi: 10.1016/S0893-133X(99)00139-6. [DOI] [PubMed] [Google Scholar]
- 60.Martres MP, Sales N, Bouthenet ML, Schwartz JC. Localisation and pharmacological characterisation of D2 dopamine receptors in rat cerebral neocortex and cerebellum using [125I]iodosulpride. Eur J Pharmacol. 1985;118:211–219. doi: 10.1016/0014-2999(85)90131-1. [DOI] [PubMed] [Google Scholar]
- 61.Flace P, Livrea P, Basile GA, Galletta D, Bizzoca A, Gennarini G, Bertino S, Branca JJV, Gulisano M, Bianconi S, Bramanti A, Anastasi G. The cerebellar dopaminergic system. Front Syst Neurosci. 2021;15:650614 . doi: 10.3389/fnsys.2021.650614. [DOI] [PMC free article] [PubMed] [Google Scholar]




