Abstract
Ferroptosis is a type of cell death characterized by the accumulation of intracellular iron and an increase in hazardous lipid peroxides. Ferroptosis and autophagy are closely related. Ionizing radiation is a frequently used cancer therapy to kill malignancies. We found that ionizing radiation induces both ferroptosis and autophagy and that there is a form of mutualism between the two processes. Ionizing radiation also causes lipid droplets to form in proximity to damaged mitochondria, which, through the action of mitophagy, results in the degradation of the peridroplet mitochondria by lysosomes and the consequent release of free fatty acids and a significant increase in lipid peroxidation, thus promoting ferroptosis. Ionizing radiation has a stronger, fatal effect on cells with a high level of mitophagy, and this observation suggests a novel strategy for tumor treatment.
Subject terms: Macroautophagy, Lipids
Introduction
Ferroptosis is a unique type of programmed cell death that is brought about by the accumulation of iron-dependent lipid peroxides [1, 2]. Transferrin is implicated in iron endocytosis, and blocking the expression of SLC39A14, which governs the ingress of non-transferrin bound iron (NTBI) into a cell, greatly inhibits ferroptosis, which demonstrates that the protein encoded by SLC39A14 is essential to ferroptosis [3, 4]. Intracellular free iron (Fe2+) increases the production of ROS via the Fenton reaction, which in turn causes the enzymatic oxidation of lipid membrane polyunsaturated fatty acids and increases lipid peroxidation [5, 6]. The overexpression of lipid metabolism-related proteins (e.g., COX2, and acyl-CoA synthase long-chain family member 4 (ACSL4)) also significantly contributes to ferroptosis [7, 8]. In addition, overactive glycolysis and glutamine metabolism are also required for ferroptosis to occur. Inhibition of glycolysis and glutamine metabolism can greatly reduce the level of lipid peroxidation generated by erastin (a ferroptosis inducer) in tumors, which demonstrates that ferroptosis is primarily linked to metabolism [9, 10]. Glucose enters tumor cells through the GLUT3 (glucose transporter 3) transporter to generate pyruvate through glycolysis and is converted into acetyl-CoA [11]. Glutamine enters tumor cells through ASCT2/SLC38A1 transporters and is converted to glutamate by glutaminase (GLS). Glutamate is then converted to α-ketoglutaric acid (α-KG) by glutamate dehydrogenase (GLUD) [12, 13]. Glutamate can also be converted into glutamine by glutamine synthetase (GS) to maintain intracellular glutamine metabolism [14, 15]. Finally, acetyl-CoA and α-ketoglutaric acid enter the tricarboxylic acid (TCA) cycle in order to provide cells with energy. However, a high nutrient supply flux results in excessive accumulation of mitochondrial ROS. If the continual accumulation of ROS is not eliminated in a timely manner, the phospholipid-related polyunsaturated fatty acid side chains, nucleic acids, and other macromolecules will undergo a peroxidation reaction, leading to ferroptosis [16, 17]. The proteins encoded by SLC7A11 and SLC3A2 are crucial and constitute the system xc−, a cystine–glutamate antiporter, which mediates glutathione (GSH) production via translocation of intracellular glutamate out of the cell and simultaneous translocation of cystine into the cell [18–20]. Glutathione peroxidase 4 (GPX4) is an important antioxidant protein that selectively eliminates phospholipid hydroperoxides. If GPX4 is deactivated, lipid peroxides accumulate, eventually leading to ferroptosis [21, 22].
The morphological characteristics of ferroptosis include mitochondrial shrinkage and cristae reduction [1]. Mitochondrial membrane potential hyperpolarization and accumulation of lipid peroxides were found in cystine deprivation-induced ferroptosis, and the loss of fumarate hydratase confers resistance to cystine deprivation-induced ferroptosis [23]. The mitochondrial antioxidant MitoTEMPO inhibits ferroptosis [24]. Ferrostatin-1 is also an inhibitor of ferroptosis and protects mitochondrial function [25]. Mitophagy is a selective autophagic mechanism for scavenging damaged mitochondria and is mainly mediated by PINK1/Parkin and BNIP3 pathways [26]. PINK1 accumulates on the outer mitochondrial membrane and recruits and activates Parkin. VDAC1 and MFN1/2 proteins are ubiquitinated by Parkin to induce mitophagy [27, 28]. BNIP3 is an external mitochondria-associated atypical BH3 member of the Bcl-2 family. BNIP3 is a mitophagy receptor with an LC3B interaction region (LIR) motif that helps target damaged mitochondrial bodies for lysosomal degradation [29]. Studies have found that cardiac ischemia-reperfusion injury can cause the upregulation of Parkin and BNIP3, deplete mitochondrial mass, lead to mitochondrial metabolic disorders, and eventually lead to mitophagy-mediated cell death [30]. Mitophagy inhibitor Mdivi-1 blocks doxycycline (DOX) - activated Parkin upregulation, thus decreasing DOX-induced ROS generation [31]. Lipid droplets (LDs) are essential organelles that store lipids within cells. LDs form neutral lipids between the phospholipid bilayers of the endoplasmic reticulum (ER), which accumulate and finally split into mature LDs. The enzyme DGAT1 is primarily responsible for fat synthesis in this process, and DGAT2 maintains LDs equilibrium [32, 33]. Studies have shown that LDs anchoring protein perilipin5 (PLIN5) promotes LDs association with damaged mitochondria [34]. The protein TPD52 is critical in LDs expansion [35], and CPT supplies fatty acids to damaged mitochondria for β-oxidation to supply energy during stress [36]. However, as a stress response, mitophagy of peridroplet mitochondria results in an increase of free fatty acids and the production of lipid peroxides after ROS oxidation, which leads to ferroptosis [37].
Radiotherapy is a widely used cancer therapy that causes various types of cell death. Ionizing radiation directly induces DNA strand breakage, but it can also cause cellular production of large quantities of ROS, resulting in oxidative stress damage. Some studies have found that ionizing radiation induces ferroptosis by causing iron aggregation and lipid peroxidation [38–40]. In the process of ferroptosis, the knockdown of ACSL4 greatly reduces ferroptosis activity and reduces the sensitivity of malignancies to radiation [38]. Ionizing radiation also induces autophagy, which provides nutrients to alleviate metabolic load and maintain cell viability. Many investigations have indicated that autophagy is essential for ferroptosis [41–43]. However, the specific mechanism linking ionizing radiation-induced ferroptosis with autophagy and the relationship between mitophagy and ionizing radiation-induced ferroptosis are unknown. Nevertheless, these processes are strongly associated with the development of ferroptosis, and the aim of this study is to explain these relationships in terms of suborganelle autophagy to propose a novel theory as a foundation for the enhancement of radiotherapy efficacy.
Materials and methods
Cell culture
PANC-1 and SW1990 human pancreatic cancer cell lines were cultured in Dulbecco’s Modified Eagle medium (DMEM). The human non-small cell lung cancer cell line A549 was grown in DMEM/F-12 Ham’s (1:1) medium. B16 and S91 mouse melanoma cell lines were grown in Roswell Park Memorial Institute (RPMI) medium. All media were supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cell lines were obtained from the cell bank of the Chinese Academy of Sciences and were maintained in a humidified incubator at 37 °C with 5% CO2 and were confirmed to be free of microbial contamination.
Cell line construction
We used siRNA and shRNA plasmids from Santa Cruz Biotechnology (Dallas, TX, USA) to knockdown or knockout Parkin or BNIP3 genes in cells. Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) was used as the transfection reagent. The procedures were executed following manufacturer guidelines. Additional experiments were performed on knockdown cells at 48 h after transfection. shRNA transfected cells were cultured in a complete cell culture medium containing puromycin (final concentration 2 μg/mL) to identify stable knockout subclones. Lipofectamine 3000 was also used to transfect cells with the overexpression plasmid pEX-1 (pGVMV/MCS/EGFP/Neo), and G-418 disulfate (1000 μg/ml) and EGFP-fluorescence were used to screen stable subclones. Finally, all the constructed cell lines were validated using western blotting. Supplementary Table S1 contains reagent and antibody information. Supplementary Table S2 contains the nucleotide sequence information.
Experimental mouse model
The Lanzhou Veterinary Research Institute provided C57BL/6 male mice aged 6–8 weeks. The mice were housed in the animal facility at the Institute of Modern Physics and kept in specified conditions: pathogen-free; constant temperature 26 °C; humidity 50%; noise levels <85 dB; air changed every 12 h; 12 h light–12 h dark cycles; and an unlimited supply of clean water and food. Each mouse was subcutaneously implanted with 500,000 melanoma cells to create a tumor-bearing model.
Radiotherapy
X-rays were produced by a PXI Precision X-Rad 225 generator (Minneapolis, MN, USA) with energy 225 kV/13.3 mA. The carbon ion beam (12C6+) was supplied by the medical department of the Heavy Ion Research Facility in Lanzhou (energy: 80 MeV/u, peak LET: 50 KeV/μm, SOBP). Tumor cells were irradiated during the logarithmic growth phase in culture dishes. When tumor volume reached about 50 mm3, the melanoma-bearing mice were anesthetized with pentobarbital sodium (50 mg/kg IP) and placed on the platform. The tumor site was irradiated with a 4 Gy X-ray dose; the mice were protected with a tailored lead device. All animal experiments were approved by the Institute of Modern Physics Ethical Committee and conducted in accordance with EU Directive 2010/63/EU guidelines.
Clone formation
The cells were exposed to X-ray radiation or a carbon ion beam. The supernatant was then removed and washed with phosphate-buffered saline (PBS). Trypsin digestion was performed, and the cells were resuspended in a complete cell culture medium for cell counting. The cells were transferred into 60 mm Petri dishes and maintained in an incubator for 14 d, with renewed medium every 3 d, until the majority of single clones had >50 cells. The clones were then washed with pre-cooled PBS, followed by 5 min of 75% ethanol fixation, 10 min of 0.5% crystal violet staining, rewashing, scanning, and being photographed. Survival scores were calculated using GraphPad Prism 8.0. Clone formation rate = number of effective clones/number of seeded cells expressed as a percentage.
Flow cytometry
The tumor cells were radiated with X-rays. After 48 h, the supernatant was removed and washed with PBS. The serum-free medium was mixed with acridine orange (AO; 15 μg/mL) dye to detect acid bodies; dihydrorhodamine 123 (DHR123; 2 μM) dye to detect ROS in mitochondria; BODIPY 581/591 C11 (1 μM) dye to detect lipid peroxidation; and Nile Red (50 nM) dye to detect intracellular lipid droplets. After 30 min incubation at 37 °C, in air containing 5% CO2, the supernatant was discarded, and the cells were again washed with PBS and digested with trypsin. The supernatant was removed after centrifugation, and the cells were resuspended in PBS. Each sample had 10,000 cells taken for examination, and the experiment was repeated three times using Merck-Millipore FlowSight flow cytometer. Analysis was performed using an IDEAS application program.
Transmission electron microscopy
The medium was removed 48 h after irradiation, and the cells were rapidly fixed with 2.5% glutaraldehyde (v/v) in 0.1 M phosphate buffer. The medium was scraped gently in one direction to collect the cells. The collected cells were centrifuged and fixed with a new 2.5% glutaraldehyde solution for 2 h at room temperature before being transferred to 4 °C storage for overnight fixation. The pellet was post-fixed with 1% osmium tetroxide (w/v) in 0.1 M phosphate buffer for 2 h. Cells were dehydrated for 20 min each time using a graded ethanol series (30%, 50%, 70%, 80%, 90%, and 95%) and then embedded in Epon 812 agent and polymerized for 48 h at 60 °C. The 60 nm ultrathin sections were stained with 2% uranyl acetate and 2.6% lead citrate. An HT7800 transmission electron microscope (Hitachi, Tokyo, Japan) was used for imaging.
Western blot
Tumor cells were washed with PBS and lysed with RIPA buffer (150 mM sodium chloride, 1.0% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, and a protease inhibitor cocktail) at 48 h after irradiation, then ultrasonicated and mixed with 5× loading buffer. The cell lysis mixture was then placed in a metal bath at 98 °C for 10 min and then transferred to an incubator and incubated at 4 °C for 30 min. NuPAGE Novex 4–12% Bis-Tris protein gels (Thermo Fisher Scientific, Waltham, MA, USA) were used with voltages of 80 V for protein concentration and 120 V for separation. The proteins were then electrotransferred onto the PVDF membrane (Merck-Millipore, Darmstadt, Germany) at 120 V and 0.2 A, with the transfer time being determined by the protein molecular weight. After blocking with 5% bovine serum albumin in 1× PBS containing 0.1% Tween-20 (1× PBST) for 2 h at 24 °C, the membrane was incubated with the corresponding primary antibody in 5% BSA overnight at 4 °C, washed 5 times with 1× PBST, and then incubated with the HRP-conjugated secondary antibody (Southern Biotech, Birmingham, AL, USA) in 5% BSA for 2 h at 24 °C. ECL western blotting detection reagent (Merck–Millipore) was used to evaluate peroxidase activity, and the bands were captured using the Alliance LD4 (UVItec, Cambridge, UK).
Co-immunoprecipitation
Tumor cells were washed with pre-cooled PBS before being incubated with pre-cooled 1× lysis/wash buffer for 5 min on ice. The lysed sample was centrifuged at 13 000 g for 10 min to separate the debris. The supernatant was collected and labeled as a cell lysis sample. A quantity of 20 μL (0.2 mg) of protein A/G MagPoly beads (ACE Biotechnology, Nanjing, China) was placed in a tube; 180 μL of 1× lysis/wash buffer was added to the magnetic beads, and the tube was gently centrifuged. When all the magnetic beads had been absorbed, the supernatant was discarded. Then the magnetic bead was incubated with 10 μg antibody for 6 h. After the magnetic bead adsorbed the antibody, the supernatant was discarded. The magnetic bead was then co-incubated with the protein lysate for 24 h. When all the magnetic beads had been absorbed, the supernatant was collected in a tube. The collected magnetic beads was placed in a metal bath and heated to 98 °C for 10 min after adding 50 μL 1× loading buffer. The magnetic beads were separated out using a magnetic separator, and the supernatant containing the target antigen was detected by a western blot assay.
Quantitative real-time PCR
Total RNA was extracted using TRIzol, and cDNA was obtained using an RT Master Mix for qPCR II kit (MedChemExpress, Princeton, NJ, USA) in accordance with the manufacturer’s instructions. The cDNA was diluted three times before being quantified using SYBR Green. The total volume was 20 μL, and 40 cycles were performed using a Bio-Rad CFX96 PCR system (Hercules, CA, USA). Each cycle was pre-denatured for 5 min at 95 °C, denaturated for 10 s at 95 °C, annealed at 60 °C for 35 s, followed by primer-template extension at 72 °C for 15 s, with a heating rate of 0.5 °C/s between 72 °C and 95 °C. The 2-∆CT method was used to examine the target fold gene expression. Supplementary Table S3 contains commercial primers and sequence information.
Immunofluorescence
Fixed image collection
The medium was removed 48 h after irradiation, and cells were washed three times with PBS before being fixed for 10 min in 4% paraformaldehyde. We used 0.5% Triton X-100 to permeabilize the cell membrane at 24 °C for 5 min; 2% goat serum in PBS was blocked for 2 h at 24 °C before the primary antibody (dilution 1:100) was incubated overnight at 4 °C. The cells were washed three times with PBS before being incubated with the fluorescent secondary antibody (dilution 1:500) for 1.5 h at 24 °C. We used 4′,6-diamidino-2-phenylindole (DAPI) to stain the nuclei. The cells were photographed with a Revolve RVL-100-G microscope (Discover Echo, San Diego, CA, USA) and analyzed using Fiji ImageJ software.
Live image collection
The dyes MitoTracker Green (150 nM), LysoTracker Red (50 nM), MitoTracker Red (100 nM), JC-1 (1 μg/mL), Dihydrorhodamine123 (2 μM), Nile Red (50 nM), BODIPY 493/503 (200 ng/mL), BODIPY 581/591 C11 (1 μM), and BODIPY 558/568 C12 (1 μM) were each diluted in serum-free medium. Cells were stained with the dye-containing medium in an incubator (37 °C with 5% CO2) for 30 min. Hoechst 33342 (1 μM) was then added to the medium for 10 min to label the nuclei. The cells were washed with PBS and then photographed using the Echo Revolve microscope.
Mito–keima model
The Mito–keima model was created using the Public Protein/Plasmid Library’s Mt-Kemia-Cox8 lentivirus (LV01230-2a). To generate single-cell suspension, cells were cultured to logarithmic growth stage, digested with trypsin, centrifuged (400 g for 5 min), and re-suspended in 1 mL serum-free medium. The cells were then infected with lentivirus (transduction enhancer 1) and grown at 37 °C for 60 min. The cells were then placed in 6-well plates (transduction enhancer 2) and incubated at 37 °C for 24 h, removed the supernatant and washed three times with PBS, and cultured in fresh medium for another 24 h. Purinycin was then utilized for screening. The Keima fluorescence (green) signals were taken at 440 nm wavelength in a neutral environment and the Keima fluorescence (red) signals were taken at 580 nm wavelength in an acidic environment.
Cytoskeleton analysis
The skeleton plugin (http://imagej.net/AnalyzeSkeleton) of Fiji ImageJ software was used to analyze extracted mitochondria skeletons. We derived line shape mitochondria skeleton structures. The number of branches, branch length, and average branch length were used to determine the extent of mitochondrial skeleton change.
Colocalization analysis
Immunofluorescence images were processed using MATLAB. The color fraction H in the HSI vector space was used to identify the colocalized area, and the brown–yellow color (positive area) was extracted by setting the angle range; the positive area could be extracted when the angle range was set to 0–30°. The average colocalized area was used to assess the degree of colocalization. The program was developed in our laboratory.
Immunohistochemistry
Tumor samples were embedded in paraffin after being fixed with 4% paraformaldehyde. The paraffin blocks were sectioned into 3 μm thicknesses and placed on positively charged slides. Dewaxed sections were placed in a 0.01 M citrate buffer for 10 min at 95 °C for antigen repair. We used 0.5% Triton X-100 to permeabilize the cell membrane, and endogenous peroxides were removed by 3% H2O2. The section was evenly coated with 5% BSA and blocked at 24 °C for 30 min. The sections were then incubated overnight in 5% BSA at 4 °C with the primary antibody (dilution 1:200). The slides were washed three times with 1× PBST. The sections were covered with secondary antibodies and incubated at 24 °C for 30 min. The Lab Vision UltraVision Quanto Detection System (Thermo Fisher Scientific) was then used with 3,3′-diaminobenzidine tetrahydrochloride (DAB) as the chromogen. Hematoxylin-eosin staining was in accordance with standard protocol. Slides were scanned and analyzed using Fiji ImageJ software.
Enzyme-linked immunosorbent assay
The concentration of intracellular factors was evaluated using ELISA (enzyme-linked immunosorbent assay). Tumour cells were exposed to X-ray or carbon ion radiation for 48 h and washed in PBS before being digested with trypsin. The cells were then centrifuged (1200 rpm for 5 min), resuspended in PBS and frozen with liquid nitrogen, and then thawed in a 37 °C water bath; this process was repeated three times. The supernatant was collected after centrifugation (3000 rpm for 20 min) and tested in accordance with the manufacturer’s instructions. Absorbance was determined using an i3 Paradigm multi-label reader (Molecular Devices, San Jose, CA, USA). Supplementary Table S1 contains commercial ELISA kit information.
Statistical analysis
All experiments were conducted in triplicate unless otherwise noted. Origin 9.0 software was used to analyze the data and generate the graphs. Significant differences were tested using Student’s t-test, one-way ANOVA with Dunnett’s post hoc test, and Kaplan–Meier analysis with a log-rank test, with *p < 0.05, **p < 0.01, and ***p < 0.001 levels of statistical significance.
Results
Ionizing radiation increases intracellular ROS
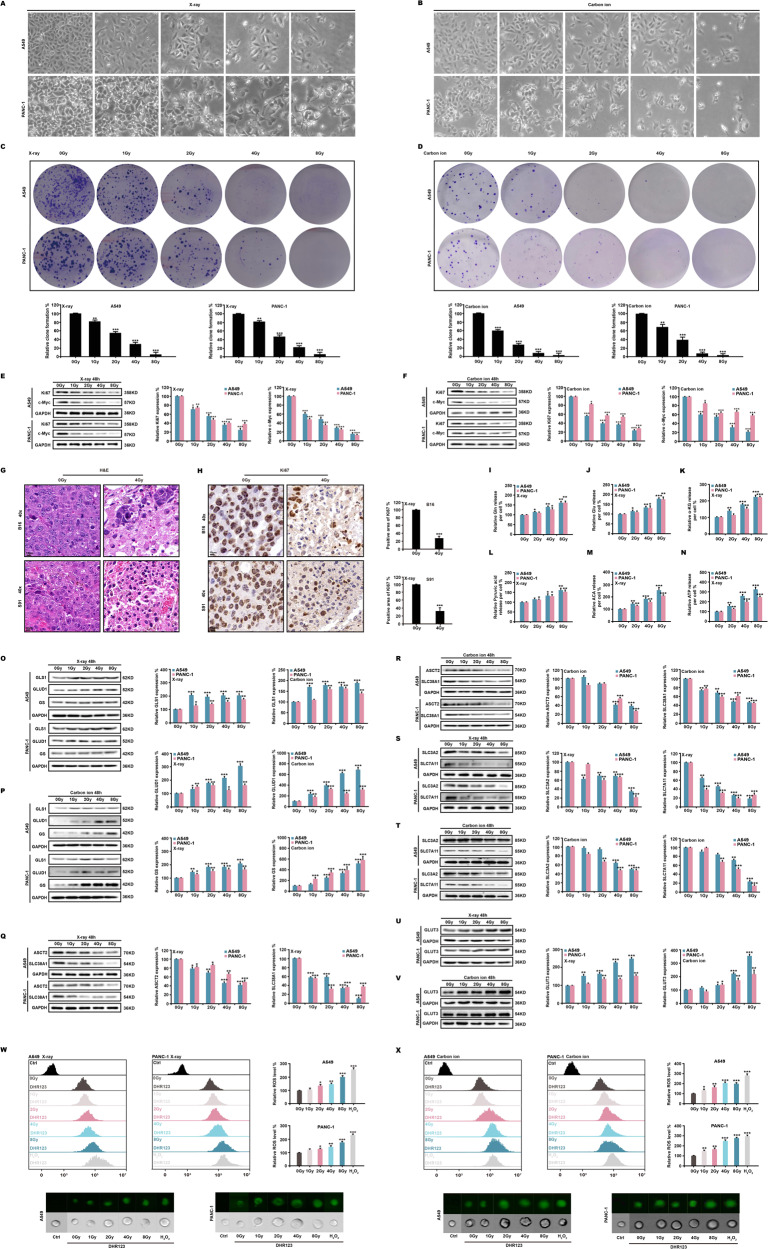
We used X-ray and carbon ion beam radiation to irradiate human non-small cell lung cancer cells from cell line A549 and human pancreatic cancer cells from cell line PANC-1. We found that as the radiation dose increased, the number of cells decreased, and the tumor cells swelled (Fig. 1A, B). Subsequent clone formation experiments confirmed that ionizing radiation dose-dependently inhibited cell proliferation (Fig. 1C, D). Western blotting showed that different dose of X-ray and carbon ion beam downregulated the expression of malignant proliferating proteins Ki67 and c-Myc (Fig. 1E, F). Similar results were found for mouse melanoma cell lines B16 and S91 (Fig. S1A–C). We discovered that ionizing radiation induced cell death in mouse melanoma (Fig. 1G) and significantly decreased Ki67 expression in B16 and S91 tumors (Fig. 1H). Since c-Myc is a major driver of glutamine utilization and is closely involved in glycolytic metabolic energy supply in cell proliferation [44–46], we used ELISA to detect the key products of these two metabolic pathways. At 48 h after X-ray radiation, the results showed that accumulations of intracellular glutamine (Fig. 1I), glutamate (Fig. 1J), and α-ketoglutarate (Fig. 1K) increased. The levels of pyruvate (Fig. 1L), acetyl-CoA (Fig. 1M), and ATP (Fig. 1N) significantly increased dose-dependently. Moreover, real-time PCR analysis revealed significant increases in the expression of glutaminase 1 (Fig. S1D), glutamate dehydrogenase 1 (Fig. S1E) and glutamine synthetase (Fig. S1F). Western blotting also demonstrated that the protein expression of glutaminase 1, glutamate dehydrogenase 1 and glutamine synthetase were upregulated (Fig. 1O, P). However, at a 4 Gy X-ray dose, administered at 24, 48 and 72 h, the expression of glutamine and glutamate decreased over time (Fig. S1G, H). The expression of ASCT2 and of SLC38A1, which are key proteins in the glutamine transport system (Fig. 1Q, R), and of SLC3A2/SLC7A11, which are key proteins in the cystine/glutamate antiporter system (xCT) (Fig. 1S, T), were all downregulated as radiation intensity increased. However, the expression of the glucose transporter GLUT3 (Fig. 1U, V) was significantly upregulated. Dihydrorhodamine123 (DHR123) staining revealed a significant increase in intracellular ROS (Fig. 1W, X) and a widespread distribution in tumor cells (Fig. S1I, J). Our explanation for this is that ionizing radiation inhibits cell proliferation, and since tumor cells must overproduce energy to counter the excessive levels of radiation stress, the tumor cells also produce a large quantity of ROS which accelerates cell death.
Fig. 1. Ionizing radiation increases intracellular ROS.
A549 and PANC-1 cells were irradiated with X-ray and carbon ion beams. At 48 h, the morphology (A, B), and clone formation rate (C, D) were evaluated. Ki67 and c-Myc were identified in A549 and PANC-1 cells (E, F); H&E staining and ki67 expression were also performed in B16 and S91 melanomas (G, H). ELISA was used to assess glutamine (I–K) and glycolytic (L–N) metabolites. Western blotting was used to indicate glutaminase 1, glutamate dehydrogenase 1, glutamine synthetase (O, P), glutamine (Q, R) and glutamate (S, T) transport proteins and glucose transport proteins (U, V). Intracellular ROS production (W, X) was measured by flow cytometry.
Ionizing radiation induces ferroptosis
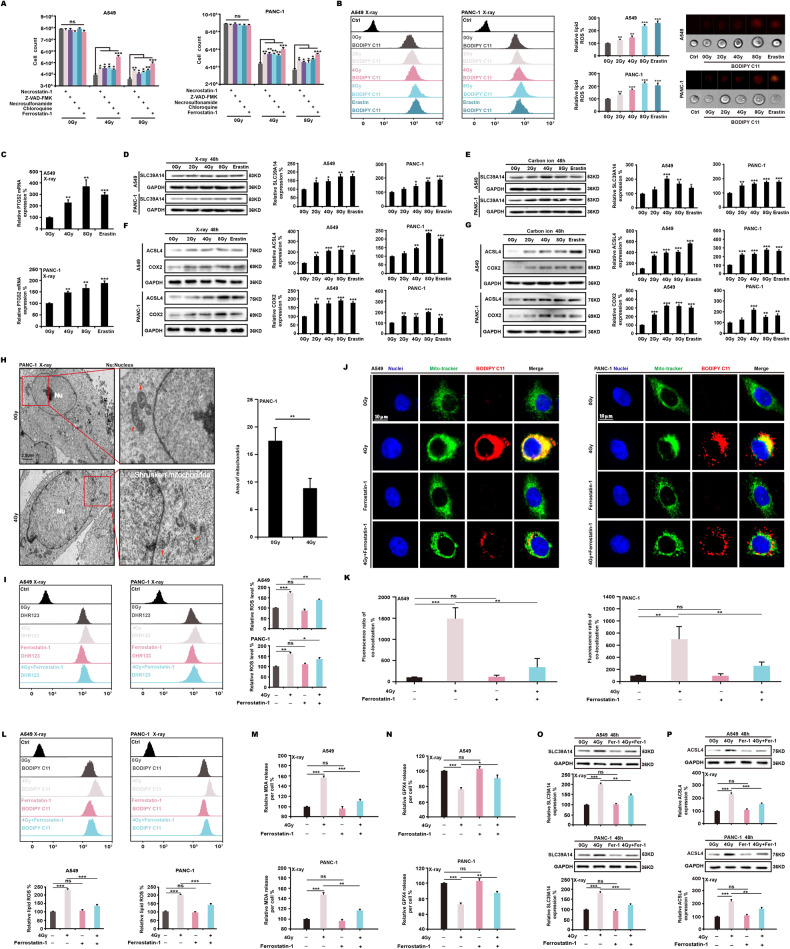
To investigate the specific mode of cell death, we used inhibitors of different forms of cell death. We found that ferrostatin-1, a ferroptosis inhibitor, significantly reduced cell death induced by ionizing radiation (Fig. 2A). Using BODIPY C11 staining, we found that X-ray radiation and ferroptosis activator erastin greatly increased intracellular lipid ROS levels (Fig. 2B). The expression of PTGS2 increased at the mRNA level as the radiation intensity increased (Fig. 2C). Expression of transferrin SLC39A14 (Fig. 2D, E) and ferroptosis signature proteins ACSL4 and COX2 (Fig. 2F, G) upregulated as X-ray and carbon ion radiation intensity increased. We also found that SLC39A14 and the lipid peroxide indicator MDA significantly increased in mouse melanomas after 4 Gy X-ray radiation (Fig. S2A, B). Transmission electron microscopy showed a decrease in X-ray-induced mitochondrial cristae, or even their disappearance, resulting in vacuolation (Fig. 2H). As the radiation dose increased, the branched or unbranched mitochondrial skeleton shrank (Fig. S2C, D). In addition, after labeling with JC-1 dye, X-rays reduced the mitochondrial membrane potential of A549 and PANC-1 cells (Fig. S2E, F). Using MitoTracker Green and BODIPY C11 labeling, we found that when A549 and PANC-1 cells were exposed to 4 Gy X-ray doses, the level of colocalization between the two dyes greatly increased over time (Fig. S2G–J). We then treated cells with a 4 Gy X-ray dose and used ferrostatin-1. The results demonstrated that ferrostatin-1 suppressed the production of intracellular ROS (Fig. 2I). Ferrostatin-1 also reduced the colocalization of MitoTracker and BODIPY C11 following ionizing radiation (Fig. 2J, K), decreased lipid peroxides (Fig. 2L, M), and raised GPX4 levels (Fig. 2N). Ferrostatin-1 following exposure to ionizing radiation also inhibited the expression of SLC39A14 (Fig. 2O) and ACSL4 (Fig. 2P). These results indicate that ionizing radiation can induce ferroptosis in tumor cells.
Fig. 2. Ionizing radiation induces ferroptosis.
A549 and PANC-1 cells were exposed to X-ray radiation and treated with various cell death inhibitor. At 48 h, it was found that ferrostatin-1 (2 μM) was effective in restoring cell survival (A). Using BODIPY C11 staining (1 μM), the amounts of lipid ROS generated by ionizing radiation were determined (B). Real-time PCR (C) and western blotting (D–G) were used to assess the expression of major ferroptosis markers. The morphological changes induced in mitochondria by ionizing radiation were observed by transmission electron microscopy (H). Using flow cytometry and immunofluorescence, the levels of ROS (I), lipid peroxides (J–M), and GPX4 (N) were determined. Eventually, the expression of critical ferroptosis proteins (O, P) was indicated.
Ferroptosis and autophagy are reciprocally influenced by ionizing radiation
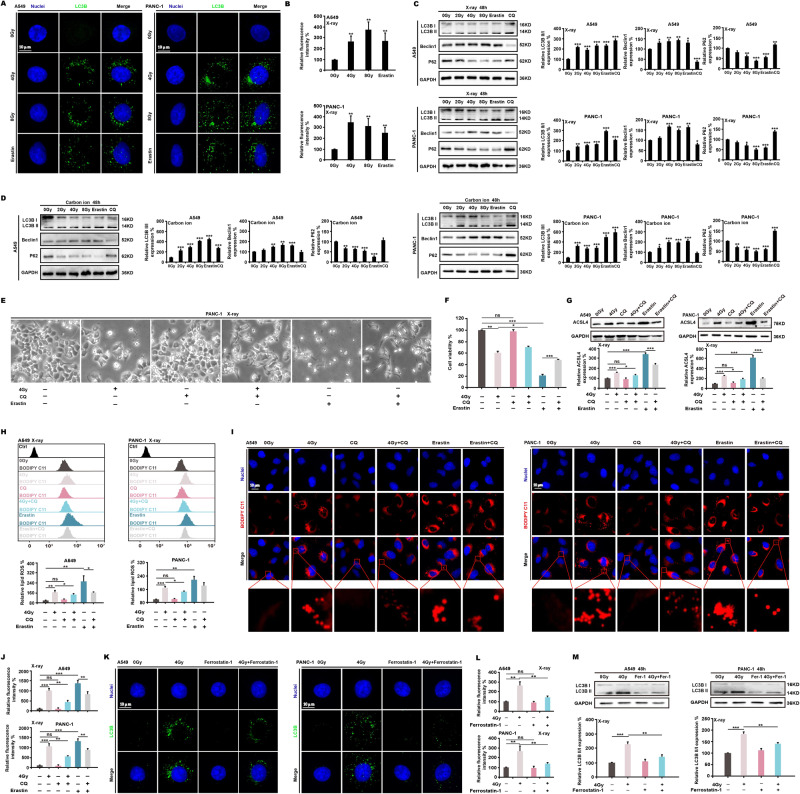
Previous studies have shown that ionizing radiation induces autophagy so we investigated the relationship between ionizing radiation-induced ferroptosis and autophagy. At 48 h, X-ray radiation and erastin caused the aggregation of LC3B dots in A549 and PANC-1 cells (Fig. 3A, B). The autophagy-related proteins Beclin1 and LC3B II/LC3B I ratio were highly upregulated after X-ray and carbon ion radiation, and the expression of p62 noticeably decreased (Fig. 3C, D). We then exposed PANC-1 cells to X-ray radiation, the autophagy inhibitor chloroquine (CQ), and erastin. We found that CQ partially reversed cell death due to ionizing radiation and prevented cell death induced by erastin (Fig. 3E, F). We also found that CQ significantly downregulated the expression of ACSL4 induced by X-ray radiation and erastin (Fig. 3G), as well as lipid ROS (Fig. 3H) and lipid peroxides (Fig. 3I, J). We infer that ionizing radiation-induced autophagy promotes ferroptosis. Next, we treated cells with X-ray radiation and ferrostatin-1 respectively. The results showed that ferrostatin-1 greatly inhibited the aggregation of ionizing radiation-induced LC3B dots (Fig. 3K, L) and LC3B II/LC3B I ratio (Fig. 3M). These results suggest that ionizing radiation induces a reciprocal action between autophagy and ferroptosis.
Fig. 3. Ferroptosis and autophagy are reciprocally influenced by ionizing radiation.
At 48 h of X-ray irradiation、erastin (5 μM) or CQ (2 μM) treatment, the expression of autophagy-related proteins in A549 and PANC-1 was assessed using immunofluorescence (A, B) and western blotting (C, D). The autophagy inhibitor CQ was used to treat the cells with X-ray radiation and erastin, and it was found that CQ restored cell survival (E, F). Through the measurement of ACSL4 (G), lipid ROS (H), and lipid peroxides (I, J), it was determined that CQ prevented ferroptosis. In addition, ferrostatin-1 (2 μM) combined with X-ray radiation inhibited the expression of the autophagy-essential protein LC3B (K–M).
Mitophagy induced by ionizing radiation promotes ferroptosis
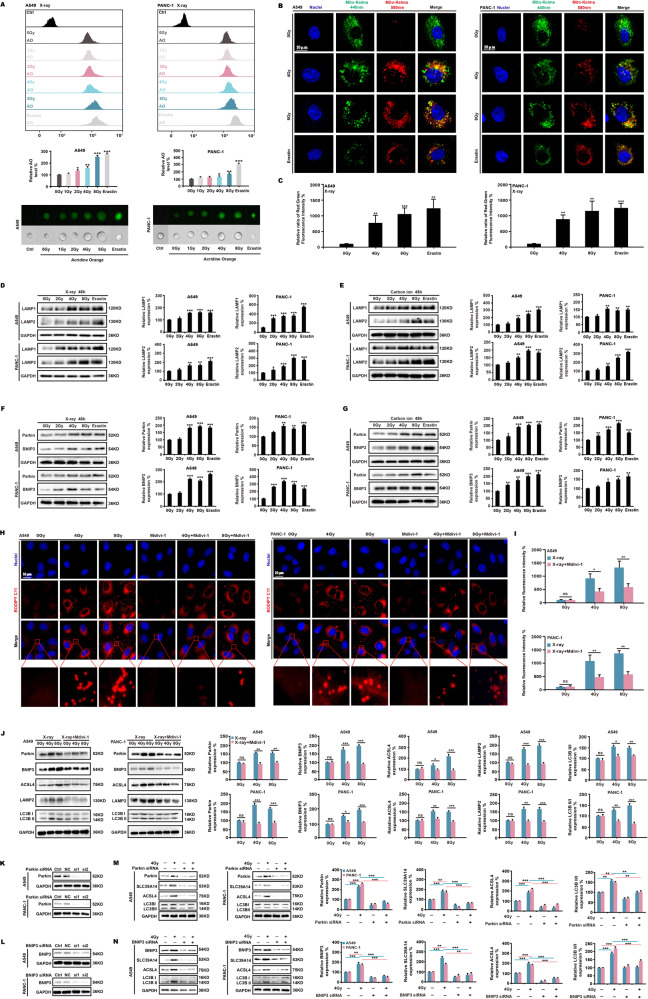
The preceding studies suggest that ionizing radiation damages mitochondria and induces autophagy. We continued to investigate the association between mitophagy and ferroptosis by treating A549 and PANC-1 cells with X-ray radiation and erastin. Staining with acridine orange (AO) showed there was a considerable increase in the acidities of intracellular bodies following the treatment (Fig. 4A). Using the Mito-Keima model, it was discovered that radiation and erastin can significantly increase the shift of green fluorescence to red fluorescence (Fig. 4B, C). MitoTracker Green and LysoTracker Red labeling showed that radiation and erastin increased the colocalization of mitochondria and lysosomes (Fig. S3A, B). We then found that the X-ray radiation and erastin treatment upregulated the expression of lysosomal marker proteins LAMP1 and LAMP2 (Fig. 4D, E) and greatly increased the expression of mitophagy marker proteins Parkin and BNIP3 (Fig. 4F, G). We searched the OncoLnc database (http://www.oncolnc.org/) and found an increased survival rate for lung adenocarcinoma (LUAD) and pancreatic adenocarcinoma (PAAD) in patients with a high Parkin expression level (Fig. S3C).
Fig. 4. Mitophagy induced by ionizing radiation promotes ferroptosis.
A549 and PANC-1 cells were exposed to X-ray radiation and erastin (5 μM). At 48 h, AO staining (15 μg/mL) was used to identify intracellular acidic bodies (A). Using the Mito-Keima model, the shift of fluorescence was detected (B, C). In addition, the expression of lysosomal proteins (D, E) and mitophagy proteins (F, G) was evaluated. Immunofluorescence was used to investigate the formation of lipid peroxides (H, I) and the expression of ferroptosis- and mitophagy-related proteins (J) following X-ray radiation of cells with Mdivi-1 (2 μM). We then used siRNA to inhibit the expression of the essential genes of mitophagy (K, L), and X-ray radiation was used to assess the expression of the key proteins of ferroptosis (M, N).
After treating both cell lines with X-ray radiation and mitophagy inhibitor Mdivi-1, we found that Mdivi-1 decreased the recruitment of Parkin to the mitochondria (Fig. S3D, E) and reduced lipid peroxidation induced by ionizing radiation (Fig. 4H, I). Mdivi-1 also downregulated the ionizing radiation-stimulated protein expression of Parkin, BNIP3, ACSL4, and LAMP2, and decreased the LC3B II/LC3BI ratio (Fig. 4J). In contrast, after treating the cells with X-ray radiation and mitophagy inducers carbonyl cyanide m-chlorophenylhydrazone (CCCP) (Fig. S3F–H) or valproic acid (VPA) (Fig. S3I–K), we observed an increased rate of cell death and reduced clone formation, as well as a considerable rise in lipid peroxide production in A549, PANC-1, B16, and S91 cell lines. Finally, we used siRNA to inhibit the expression of Parkin and BNIP3 (Fig. 4K, L) and determined that ionizing radiation did not increase the expression of SLC39A14 or ACSL4 and did not increase LC3B II/LC3BI ratio (Fig. 4M, N). These results suggest that ionizing radiation-induced mitophagy enhances ferroptosis.
Free fatty acids released by mitophagy boost lipid peroxidation
Taking into account the importance of lipids in ferroptosis, we investigated the effects of ionizing radiation on lipids. Using transmission electron microscopy (Fig. 5A) and immunofluorescence (Fig. 5B), we demonstrated that X-ray radiation and ferroptosis activator RSL3 initiated considerable LDs aggregation around mitochondria. As the radiation dose increased, LDs aggregation increased. Using Nile red dye, we found that following X-ray irradiation and RSL3 treatment of PANC-1 and SW1990 cell lines, intracellular LDs increased as the irradiation dose increased (Fig. S4A, B), and colocalization of LDs with mitochondria increased as treatment time increased (Fig. S4C). As the radiation dose increased, intracellular free fatty acids also increased (Fig. 5C). After irradiating PANC-1 and SW1990 cells with X-rays and carbon ions, we found that LDs formation proteins DGAT2, TPD52, PLIN5, and β-oxidation related protein CPT1A all increased as the radiation dose increased (Fig. 5D, E). We also observed that X-ray radiation and RSL3 significantly induced the colocalization of LAMP1 and PLIN5 (Fig. 5F, G). We then used Parkin siRNA to generate a PANC-1 knockdown cell line (ParkinKD). We irradiated PANC-1 wild-type cells and ParkinKD cells with X-ray doses and found no apparent production of lipid droplets in ParkinKD cells with or without irradiation (Fig. S4D). We then used siRNA to interfere with Parkin or BNIP3 genes and observed significant decreases in the expression of DGAT2, PLIN5, TPD52, and CPT1A (Fig. 5H, I). The expression of these proteins did not increase after X-ray radiation compared with wild-type cells (Fig. 5J, K). Identical outcomes were also observed in B16 and S91 ParkinKO and BNIP3KO cells (Fig. S4E–H). Additionally, treating PANC-1 cells with X-ray radiation combined with ferrostatin-1 showed that LAMP1 and PLIN5 colocalization was much less than that of X-ray radiation alone (Fig. 5L, M). Using BODIPY C12 to label-free fatty acids, we found that ionizing radiation-induced the release of abundant intracellular free fatty acids but that this phenomenon did not occur when ParkinKD cells were irradiated with the same X-ray dose (Fig. S4I, J). ELISA analysis of the free fatty acids yielded identical results (Fig. 5N). We then used a co-immunoprecipitation test to confirm that Parkin and BNIP3 interacted with DGAT2, PLNI5, and TPD52 (Fig. 5O). We hypothesized that ionizing radiation upregulates the expression of Parkin and BNIP3, which interact with LDs and target proteins to facilitate LDs production and proximity to mitochondria in order to provide fatty acids to damaged mitochondria. Therefore, we generated PANC-1 and SW1990 CPT1KD cells (Fig. S4K). And we found that fatty acids did not accumulate in mitochondria after 4 Gy X-ray irradiation (Fig. S4L–N). Mitophagy wraps peridroplet mitochondria in autophagosomes that are subsequently degraded into free fatty acids and dispersed in the cytoplasm, and then oxidized to create lipid peroxides that accelerate ferroptosis.
Fig. 5. Free fatty acids released by mitophagy boost lipid peroxidation.
At 48 h after irradiation and RSL3 (4 μM) treatment, transmission electron microscopy (A) and immunofluorescence (B) revealed the formation and aggregation of lipid droplets. The quantity of free fatty acids in PANC-1 and SW1990 cells was then determined using ELISA (C), and the expression of proteins involved in the production and localization of lipid droplets (D, E) was determined using western blotting. The colocalization of lipid droplets and lysosomes was subsequently examined (F, G). ParkinKD and BNIP3KD cells irradiated with X-ray radiation were used to assess the expression of lipid drop-related proteins (H–K), and immunofluorescence was used to examine the colocalization of lysosomes and lipid drops (L, M). We used siRNA to inhibit the mitophagy genes in order to detect the quantity of free fatty acids (N). Finally, the co-immunoprecipitation approach was used to confirm the relationship between mitophagy and proteins involved in lipid droplet production and localization (O).
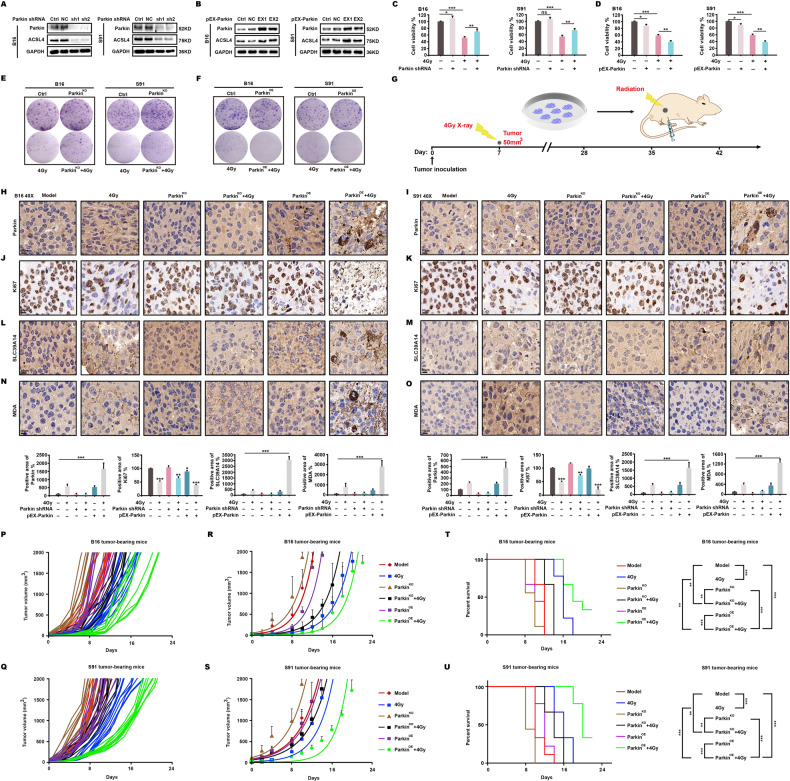
Ionizing radiation enhances killing effects on mitophagy-activated tumors through ferroptosis in mice
The conclusions described in the preceding section necessitated further investigation of the association between mitophagy and ferroptosis induced by ionizing radiation in vivo. We generated stable mouse melanoma B16 and S91 knockout (ParkinKO) and overexpression (ParkinOE) cell lines using Parkin shRNA and pEX-Parkin plasmids (Fig. 6A, B). After a 4 Gy dose of X-ray radiation, the survival rate of ParkinKO cells was significantly higher than that of wild-type cells (Fig. 6C), whereas the survival rate of ParkinOE cells was lower than that of wild-type cells (Fig. 6D). In the clone formation experiment, ParkinKO produced more clones following irradiation (Fig. 6E), whereas ParkinOE produced fewer clones (Fig. 6F). C57BL/6 mice were then subcutaneously implanted with wild-type, ParkinKO, or ParkinOE cells. When tumor volume measured 50 mm3, a 4 Gy X-ray dose was administered (Fig. 6G). We found that irradiation of ParkinOE B16 and S91 melanomas (Fig. 6H, I) significantly decreased the expression of Ki67 (Fig. 6J, K) and increased the expression of SLC39A14 (Fig. 6L, M) and MDA (Fig. 6N, O). After continuous measurement of tumor volume, we found that irradiation greatly slowed the growth of mitophagy-activated (ParkinOE) tumors (Fig. 6P–S) and significantly extended the lifespan of tumor-bearing mice (Fig. 6T, U).
Fig. 6. Ionizing radiation boosts killing in mitophagy-activated tumors through ferroptosis in mice.
Using shRNA and pEX-Parkin plasmids, stable B16 and S91ParkinKO cells (A) and ParkinOE cells (B) were created. At 48 h after irradiation, both cell survival (C, D) and clone formation (E, F) were identified. C57BL/6 mice were injected subcutaneously with B16 and S91 cells when tumor volume reached 50 mm3, then locally treated with a 4 Gy X-ray dose (G). After 48 h, tumors were harvested and assessed for signs of malignant proliferation and ferroptosis by immunohistochemistry (H–O). On the remaining animals, tumor size was evaluated every two days following irradiation, and growth (P–S) and survival (T, U) curves were plotted.
Discussion and conclusion
Ionizing radiation can generate ROS such as oxygen free radicals and hydrogen peroxide through radiolysis of cellular water and stimulation of oxidase, thereby damaging nucleic acids, proteins, and lipids and even triggering cell death [47, 48]. We found that X-ray and carbon ion beams induced a dose-dependent increase in ROS. The excessive activation of the glycolysis pathway overproduces pyruvate, which is converted to acetyl-CoA and lactate [49, 50]. Acetyl-CoA participates in various metabolic pathways, which is able to involve in the TCA cycle [51–53]. In addition, glutamine converted to α-KG and is also added to the TCA cycle [54]. These two reactions increased the energy supply for tumor cells under radiation stress. A substantial amount of ROS was also unavoidably produced. However, over time, the ability of ionizing radiation to activate the two pathways weakens until the cell dies.
We note with interest that intracellular glutamine concentration was maintained at a specific level even when glutamine uptake was restricted. We suspect that the glutamate and glutamine transport channels are close to shutting down under radiation stress, causing significant intracellular glutamate accumulation. Thus, there is an open metabolic pathway for glutamate dehydrogenase-generated α-KG, and also, through glutamine synthetase reverse, for glutamine [55–57]. As a result, it appears that intracellular glutamine content increases when glutamine transport capacity decreases. X-ray and carbon ion radiation suppress GPX4 expression by blocking xCT systems in tumor cells, thus preventing GSH production. We also discovered that ionizing radiation decreased the mitochondrial membrane potential, and, due to the absence of GSH, much ROS could not be removed, resulting in a dose-dependent increase in lipid peroxidation. In light of the findings of Guang et al. [38], we identified ACSL4 as a key protein to indicate ferroptosis and discovered that ionizing radiation greatly upregulated ACSL4 expression and induced ferroptosis. Previous research has shown that active glutaminolysis promotes the development of ferroptosis [9]. We found that although ionizing radiation inhibited the glutamine transporter ASCT2/SLC38A1, it stimulated GLS1, GLUD1, and GS, increased glutaminolysis, and still induced ferroptosis. A previous study has shown that SLC39A14 is a critical protein that regulates intracellular iron accumulation [4]. We also discovered that ionizing radiation increased the expression of SLC39A14, which indicates that SLC39A14 is also responsive to ionizing radiation-induced ferroptosis.
Ionizing radiation-induced cell death, including ferroptosis, apoptosis, necrosis, and autophagy, is not an isolated phenomenon, and there are many links between the various modes of death. Several investigations have demonstrated that autophagy is involved in ferroptosis [41–43, 58, 59]. Autophagy-related gene ATG5/ATG7 knockdown reduces iron accumulation and significantly inhibits erastin-induced ferroptosis [60]. We found that the autophagy inhibitor CQ significantly decreased ionizing radiation-induced ferroptosis. Ferroptosis inhibitor ferrostatin-1 was used for reverse verification, and the results showed that ferrostatin-1 also reduced autophagy under radiation stress. Ferroptosis is thought to be a lysosomal-dependent mode of cell death, and the extent of the dependency is determined by the lysosomal number. We found that ionizing radiation significantly increased LAMP1 and LAMP2 expression. All these findings suggest that ionizing radiation-induced ferroptosis and autophagy are mutually beneficial.
Additionally, ferroptosis is known to be strongly related to mitochondria. The fluorescent probe BODIPY C11 was found to be localized in mitochondria together with the hyperpolarization of mitochondrial membrane potential and the accumulation of lipid peroxides in erastin-induced ferroptosis, which indicates that mitochondria were involved in the process of ferroptosis. In investigating the relationship between ferroptosis and mitochondria under radiation stress, we found that MitoTracker Green and BODIPY C11 colocalization was dose- and time-dependent. Damaged mitochondria are removed via mitophagy.
The function of mitophagy is still debatable. For example, neural cells rely on mitophagy to remove damaged mitochondria, and deficiency of mitophagy marker protein Parkin will result in mitophagy defects, leading to Parkinson’s disease [61, 62]. The midbrain of Parkinson’s patients has high iron and lipid peroxide levels [63]. Mitophagy inhibitor Mdivi-1 prevents doxorubicin-induced Parkin upregulation, thereby maintaining mitochondrial biosynthesis [31]. Mdivi-1 also inhibits myoferlin-related ROS production, thereby maintaining cellular homeostasis [64]. Our study showed that ionizing radiation increased the expression of Parkin and BNIP3. Therefore, we used ionizing radiation in combination with Mdivi-1 to significantly inhibit the expression of ACSL4 in tumor cells. We irradiated ParkinKD and BNIP3KD tumor cell lines and found ACSL4 expression and iron transporter SLC39A14 expression greatly decreased. Interestingly, ferroptosis dramatically increased when ionizing radiation was combined with the mitophagy activators CCCP or VPA. We also discovered that the killing effect of ionizing radiation on ParkinKO tumors was not apparent in the animal model, and the expression of ferroptosis-related markers was not significant. On the contrary, the survival time of ParkinOE tumor-bearing mice was significantly prolonged after irradiation. All of these findings demonstrate that mitophagy promoted ferroptosis, which implies that ionizing radiation-induced ferroptosis depended on mitophagy activation.
Fatty acids are essential to maintain biological activity [65, 66]. Fatty acids that enter mitochondria provide energy by β-oxidation, but free fatty acids in the cytoplasm are easily oxidized and contribute to ferroptosis [67, 68]. Under stress, LDs accumulate near mitochondria and channel fatty acids to mitochondria [37, 69, 70]. We also found that ionizing radiation increased the proximity of LDs to damaged mitochondria, but due to mitophagy, lysosomes phagocytosed peridroplet mitochondria and released free fatty acids to the cytoplasm. This process was a powerful driving force of ionizing radiation-induced ferroptosis. Mitophagy inhibition significantly reduced LDs aggregation around mitochondria and free fatty acid content in cells under radiation stress. Interestingly, we also evaluated the expression of DGAT1 and DGAT2, key proteins in triglyceride synthesis, and discovered that DGAT1 remained highly expressed in ParkinKD and BNIP3KD cell lines after radiation, but DGAT2 did not. We believe this illustrates Nguyen’s discovery that DGAT1 selectively transports lysosome-released fatty acids to form new LDs clusters [71]. This process would also explain why so many LDs form after being exposed to ionizing radiation.
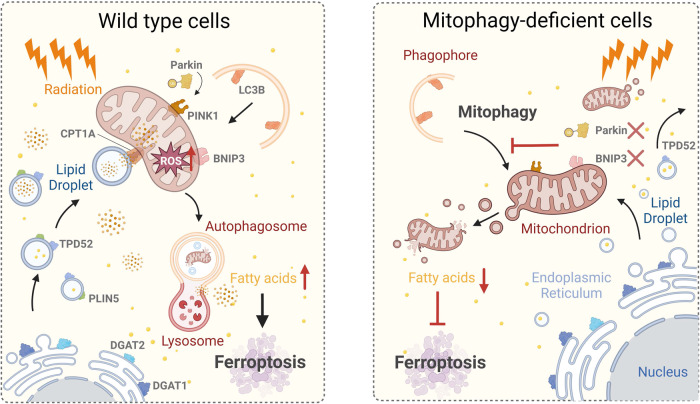
Finally, our findings suggest that ionizing radiation induces ferroptosis in tumor cells. Mitophagy is required for the incidence of ferroptosis under radiation stress. The release of free fatty acids into the cytoplasm during mitophagy is the cause of ionizing radiation-induced ferroptosis (Fig. 7). Our research investigated the action of mitophagy in ferroptosis under ionizing radiation stress in the hopes of providing a basis for future clinical treatment.
Fig. 7. Schematic of the proposed mechanism.
Ionizing radiation induces substantial ROS in mitochondria, resulting in mitochondrial damage. Rapidly formed lipid droplets, under radiation stress, concentrate around damaged mitochondria to transfer fatty acids there. Mitophagy is simultaneously induced. Damaged mitochondria and lipid droplets form autolysosomes by lysosomal phagocytosis. Autolysosomes release free fatty acids into the cytoplasm, and after peroxidation by ROS, ferroptosis is strongly enhanced. This behavior does not occur in mitophagy-deficient cells; thus ionizing radiation-induced mitophagy promotes ferroptosis by increasing intracellular free fatty acid release.
Supplementary information
Author contributions
HZ conceived and designed the experiment. PY, TZ, and YR performed the western blot and real-time PCR assays. PY and HL performed the flow cytometry assays. QZ performed the ELISA assays. PY, RL, and JH performed the immunofluorescence assays. TZ and YR performed the transmission electron microscopy assays. PY and JL performed the animal experiment. PY and RL performed the immunohistochemistry assays. JL, WW and TZ analyzed the data. HZ and PY wrote the original draft. JW modified and polished the manuscript.
Funding
This work was supported by the National Nature Science Foundation of China (NO.11905264, HZ; NO.12175289, JW), the Hundred-Talent Program of the Chinese Academy of Sciences (NO.29Y763050, HZ), and the Science and Technology Research Project of Gansu Province (NO.22YF7WA024, HZ; NO.22JR5RA128, PY).
Data availability
All data generated and analyzed during the study are included in this article. Each experiment was performed at least three times independently.
Competing interests
The authors declare no competing interests.
Ethics
All animal experiments were approved by the Institute of Modern Physics Ethical Committee and conducted in accordance with EU Directive 2010/63/EU guidelines.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jufang Wang, Email: jufangwang@impcas.ac.cn.
Heng Zhou, Email: hengzhouyz@163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41418-023-01230-0.
References
- 1.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang C, Zhang X, Yang M, Dong X. Recent Progress in Ferroptosis Inducers for Cancer Therapy. Adv Mater (Deerfield Beach, Fla) 2019;31:e1904197. doi: 10.1002/adma.201904197. [DOI] [PubMed] [Google Scholar]
- 3.Ding H, Chen S, Pan X, Dai X, Pan G, Li Z, et al. Transferrin receptor 1 ablation in satellite cells impedes skeletal muscle regeneration through activation of ferroptosis. J cachexia, Sarcopenia Muscle. 2021;12:746–68. doi: 10.1002/jcsm.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu Y, Jiang L, Wang H, Shen Z, Cheng Q, Zhang P, et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood. 2020;136:726–39. doi: 10.1182/blood.2019002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salnikow K. Role of iron in cancer. Semin Cancer Biol. 2021;76:189–94. doi: 10.1016/j.semcancer.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Henning Y, Blind US, Larafa S, Matschke J, Fandrey J. Hypoxia aggravates ferroptosis in RPE cells by promoting the Fenton reaction. Cell Death Dis. 2022;13:662. doi: 10.1038/s41419-022-05121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang HL, Hu BX, Li ZL, Du T, Shan JL, Ye ZP, et al. PKCβII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat Cell Biol. 2022;24:88–98. doi: 10.1038/s41556-021-00818-3. [DOI] [PubMed] [Google Scholar]
- 8.Zhu L, Chen D, Zhu Y, Pan T, Xia D, Cai T, et al. GPX4-Regulated Ferroptosis Mediates S100-Induced Experimental Autoimmune Hepatitis Associated with the Nrf2/HO-1 Signaling Pathway. Oxid Med Cell Longev. 2021;2021:6551069. doi: 10.1155/2021/6551069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C, Dong X, Du W, Shi X, Chen K, Zhang W, et al. LKB1-AMPK axis negatively regulates ferroptosis by inhibiting fatty acid synthesis. Signal Transduct Target Ther. 2020;5:187. doi: 10.1038/s41392-020-00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hochrein SM, Wu H, Eckstein M, Arrigoni L, Herman JS, Schumacher F, et al. The glucose transporter GLUT3 controls T helper 17 cell responses through glycolytic-epigenetic reprogramming. Cell Metab. 2022;34:516–532.e511. doi: 10.1016/j.cmet.2022.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Zheng Y, Miao YM, Yan WX, Geng YZ, Dai Y, et al. Bergenin, a PPARγ agonist, inhibits Th17 differentiation and subsequent neutrophilic asthma by preventing GLS1-dependent glutaminolysis. Acta Pharmacol Sin. 2022;43:963–76. doi: 10.1038/s41401-021-00717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zavorka Thomas ME, Lu X, Talebi Z, Jeon JY, Buelow DR, Gibson AA, et al. Gilteritinib Inhibits Glutamine Uptake and Utilization in FLT3-ITD-Positive AML. Mol Cancer Ther. 2021;20:2207–17. doi: 10.1158/1535-7163.MCT-21-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao JS, Shi S, Qu HY, Keckesova Z, Cao ZJ, Yang LX, et al. Glutamine synthetase licenses APC/C-mediated mitotic progression to drive cell growth. Nat Metab. 2022;4:239–53. doi: 10.1038/s42255-021-00524-2. [DOI] [PubMed] [Google Scholar]
- 15.Dai W, Shen J, Yan J, Bott AJ, Maimouni S, Daguplo HQ, et al. Glutamine synthetase limits b-catenin-mutated liver cancer growth by maintaining nitrogen homeostasis and suppressing mTORC1.J Clin Investig. 2022;132:e161408. [DOI] [PMC free article] [PubMed]
- 16.Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D, et al. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid Med Cell Longev. 2019;2019:5080843. doi: 10.1155/2019/5080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Li X, Ge C, Min J, Wang F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 2022;29:467–80. doi: 10.1038/s41418-022-00941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12:599–620. doi: 10.1007/s13238-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–31. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Z, Song J, Gao Y, Huang S, Dou R, Zhong P, et al. Hypoxia-induced HIF-1α/lncRNA-PMAN inhibits ferroptosis by promoting the cytoplasmic translocation of ELAVL1 in peritoneal dissemination from gastric cancer. Redox Biol. 2022;52:102312. doi: 10.1016/j.redox.2022.102312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao Y, Chen Y, Xue F, Liu K, Zhu B, Gao J, et al. Contribution of ferroptosis and GPX4’s dual functions to osteoarthritis progression. EBioMedicine. 2022;76:103847. doi: 10.1016/j.ebiom.2022.103847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou L, Chen J, Li R, Wei L, Xiong H, Wang C, et al. Metal-Polyphenol-Network Coated Prussian Blue Nanoparticles for Synergistic Ferroptosis and Apoptosis via Triggered GPX4 Inhibition and Concurrent In Situ Bleomycin Toxification. Small (Weinh der Bergstr, Ger) 2021;17:e2103919. doi: 10.1002/smll.202103919. [DOI] [PubMed] [Google Scholar]
- 23.Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, et al. Role of Mitochondria in Ferroptosis. Mol cell. 2019;73:354–363.e353. doi: 10.1016/j.molcel.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang X, Wang H, Han D, Xie E, Yang X, Wei J, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci. 2019;116:2672–80. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wenzel SE, Tyurina YY, Zhao J, St Croix CM, Dar HH, Mao G, et al. PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals. Cell. 2017;171:628–641.e626. doi: 10.1016/j.cell.2017.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang T, Liu Q, Gao W, Sehgal SA, Wu H. The multifaceted regulation of mitophagy by endogenous metabolites. Autophagy. 2022;18:1216–39. doi: 10.1080/15548627.2021.1975914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samuvel DJ, Li L, Krishnasamy Y, Gooz M, Takemoto K, Woster PM, et al. Mitochondrial depolarization after acute ethanol treatment drives mitophagy in living mice. Autophagy. 2022: 1–15:2671–85. [DOI] [PMC free article] [PubMed]
- 29.Springer MZ, Poole LP, Drake LE, Bock-Hughes A, Boland ML, Smith AG, et al. BNIP3-dependent mitophagy promotes cytosolic localization of LC3B and metabolic homeostasis in the liver. Autophagy. 2021;17:3530–46. doi: 10.1080/15548627.2021.1877469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin Q, Li R, Hu N, Xin T, Zhu P, Hu S, et al. DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol. 2018;14:576–87. doi: 10.1016/j.redox.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin J, Guo J, Zhang Q, Cui L, Zhang L, Zhang T, et al. Doxorubicin-induced mitophagy and mitochondrial damage is associated with dysregulation of the PINK1/parkin pathway. Toxicol Vitr. 2018;51:1–10. doi: 10.1016/j.tiv.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Li T, Jin Y, Shen J. Dgat2 reduces hepatocellular carcinoma malignancy via downregulation of cell cycle-related gene expression. Biomed. Pharmacother. 2019;115:108950. doi: 10.1016/j.biopha.2019.108950. [DOI] [PubMed] [Google Scholar]
- 33.Yenilmez B, Wetoska N, Kelly M, Echeverria D, Min K, Lifshitz L, et al. An RNAi therapeutic targeting hepatic DGAT2 in a genetically obese mouse model of nonalcoholic steatohepatitis. Mol Ther: J Am Soc Gene Ther. 2022;30:1329–42. doi: 10.1016/j.ymthe.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui L, Mirza AH, Zhang S, Liang B, Liu P. Lipid droplets and mitochondria are anchored during brown adipocyte differentiation. Protein Cell. 2019;10:921–6. doi: 10.1007/s13238-019-00661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan Y, Hou T, Gao Y, Dan W, Liu T, Liu B, et al. Acetylation-dependent regulation of TPD52 isoform 1 modulates chaperone-mediated autophagy in prostate cancer. Autophagy. 2021;17:4386–4400. doi: 10.1080/15548627.2021.1917130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu L, Surolia R, Larson-Casey JL, He C, Davis D, Kang J, et al. Targeting Cpt1a-Bcl-2 interaction modulates apoptosis resistance and fibrotic remodeling. Cell Death Differ. 2022;29:118–32. doi: 10.1038/s41418-021-00840-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rambold AS, Cohen S, Lippincott-Schwartz J. Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev Cell. 2015;32:678–92. doi: 10.1016/j.devcel.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lei G, Zhang Y, Koppula P, Liu X, Zhang J, Lin SH, et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020;30:146–62. doi: 10.1038/s41422-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye LF, Chaudhary KR, Zandkarimi F, Harken AD, Kinslow CJ, Upadhyayula PS, et al. Radiation-Induced Lipid Peroxidation Triggers Ferroptosis and Synergizes with Ferroptosis Inducers. ACS Chem Biol. 2020;15:469–84. doi: 10.1021/acschembio.9b00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou H, Zhou YL, Mao JA, Tang LF, Xu J, Wang ZX, et al. NCOA4-mediated ferritinophagy is involved in ionizing radiation-induced ferroptosis of intestinal epithelial cells. Redox Biol. 2022;55:102413. doi: 10.1016/j.redox.2022.102413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Kuang F, Kroemer G, Klionsky DJ, Kang R, Tang D. Autophagy-Dependent Ferroptosis: Machinery and Regulation. Cell Chem Biol. 2020;27:420–35. doi: 10.1016/j.chembiol.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou B, Liu J, Kang R, Klionsky DJ, Kroemer G, Tang D. Ferroptosis is a type of autophagy-dependent cell death. Semin Cancer Biol. 2020;66:89–100. doi: 10.1016/j.semcancer.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Liu L, Li L, Li M, Luo Z. Autophagy-Dependent Ferroptosis as a Therapeutic Target in Cancer. ChemMedChem. 2021;16:2942–50. doi: 10.1002/cmdc.202100334. [DOI] [PubMed] [Google Scholar]
- 44.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA. 2008;105:18782–7. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–5. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu X, Li J, Sun X, Guo Y, Chu D, Wei L, et al. Tumor suppressor NDRG2 inhibits glycolysis and glutaminolysis in colorectal cancer cells by repressing c-Myc expression. Oncotarget. 2015;6:26161–76. doi: 10.18632/oncotarget.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baidoo KE, Yong K, Brechbiel MW. Molecular pathways: targeted α-particle radiation therapy. Clin Cancer Res. 2013;19:530–7. doi: 10.1158/1078-0432.CCR-12-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grimes DR. Radiofrequency Radiation and Cancer: A Review. JAMA Oncol. 2022;8:456–61. doi: 10.1001/jamaoncol.2021.5964. [DOI] [PubMed] [Google Scholar]
- 49.DeBerardinis RJ, Chandel NS. We need to talk about the Warburg effect. Nat Metab. 2020;2:127–9. doi: 10.1038/s42255-020-0172-2. [DOI] [PubMed] [Google Scholar]
- 50.Stine ZE, Schug ZT, Salvino JM, Dang CV. Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discov. 2022;21:141–62. doi: 10.1038/s41573-021-00339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Röhrig F, Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. 2016;16:732–49. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- 52.Prendeville H, Lynch L. Diet, lipids, and antitumor immunity. Cell Mol Immunol. 2022;19:432–44. doi: 10.1038/s41423-021-00781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Padilla J, Lee J A Novel Therapeutic Target, BACH1, Regulates Cancer Metabolism. Cells 2021, 10:634. [DOI] [PMC free article] [PubMed]
- 54.Yin X, Peng J, Gu L, Liu Y, Li X, Wu J, et al. Targeting glutamine metabolism in hepatic stellate cells alleviates liver fibrosis. Cell Death Dis. 2022;13:955. doi: 10.1038/s41419-022-05409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan S, Wang Y, Zhang Z, Lu J, Wu Z, Shan Q, et al. High expression of glutamate-ammonia ligase is associated with unfavorable prognosis in patients with ovarian cancer. J Cell Biochem. 2018;119:6008–15. doi: 10.1002/jcb.26797. [DOI] [PubMed] [Google Scholar]
- 56.Frieg B, Görg B, Gohlke H, Häussinger D. Glutamine synthetase as a central element in hepatic glutamine and ammonia metabolism: novel aspects. Biol Chem. 2021;402:1063–72. doi: 10.1515/hsz-2021-0166. [DOI] [PubMed] [Google Scholar]
- 57.Eelen G, Dubois C, Cantelmo AR, Goveia J, Brüning U, DeRan M, et al. Role of glutamine synthetase in angiogenesis beyond glutamine synthesis. Nature. 2018;561:63–69. doi: 10.1038/s41586-018-0466-7. [DOI] [PubMed] [Google Scholar]
- 58.Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–8. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen M, Li Y, Wang Y, Shao J, Zhang F, Yin G, et al. N(6)-methyladenosine modification regulates ferroptosis through autophagy signaling pathway in hepatic stellate cells. Redox Biol. 2021;47:102151. doi: 10.1016/j.redox.2021.102151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao H, Bai Y, Jia Y, Zhao Y, Kang R, Tang D, et al. Ferroptosis is a lysosomal cell death process. Biochem Biophys Res Commun. 2018;503:1550–6. doi: 10.1016/j.bbrc.2018.07.078. [DOI] [PubMed] [Google Scholar]
- 61.Chen H, Chan DC. Mitochondrial dynamics-fusion, fission, movement, and mitophagy-in neurodegenerative diseases. Hum Mol Genet. 2009;18:R169–176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doric Z, Nakamura K. Mice with disrupted mitochondria used to model Parkinson’s disease. Nature. 2021;599:558–60. doi: 10.1038/d41586-021-02955-z. [DOI] [PubMed] [Google Scholar]
- 63.Devos D, Moreau C, Devedjian JC, Kluza J, Petrault M, Laloux C, et al. Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid Redox Signal. 2014;21:195–210. doi: 10.1089/ars.2013.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rademaker G, Boumahd Y, Peiffer R, Anania S, Wissocq T, Liégeois M, et al. Myoferlin targeting triggers mitophagy and primes ferroptosis in pancreatic cancer cells. Redox Biol. 2022;53:102324. doi: 10.1016/j.redox.2022.102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oteng AB, Kersten S. Mechanisms of Action of trans Fatty Acids. Adv Nutr (Bethesda, Md) 2020;11:697–708. doi: 10.1093/advances/nmz125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buchan GJ, Bonacci G, Fazzari M, Salvatore SR, Gelhaus Wendell S. Nitro-fatty acid formation and metabolism. Nitric Oxide. 2018;79:38–44. doi: 10.1016/j.niox.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee JY, Nam M, Son HY, Hyun K, Jang SY, Kim JW, et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc Natl Acad Sci USA. 2020;117:32433–42. doi: 10.1073/pnas.2006828117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoy AJ, Nagarajan SR, Butler LM. Tumour fatty acid metabolism in the context of therapy resistance and obesity. Nat Rev Cancer. 2021;21:753–66. doi: 10.1038/s41568-021-00388-4. [DOI] [PubMed] [Google Scholar]
- 69.Herms A, Bosch M, Reddy BJ, Schieber NL, Fajardo A, Rupérez C, et al. AMPK activation promotes lipid droplet dispersion on detyrosinated microtubules to increase mitochondrial fatty acid oxidation. Nat Commun. 2015;6:7176. doi: 10.1038/ncomms8176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tan Z, Xiao L, Tang M, Bai F, Li J, Li L, et al. Targeting CPT1A-mediated fatty acid oxidation sensitizes nasopharyngeal carcinoma to radiation therapy. Theranostics. 2018;8:2329–47. doi: 10.7150/thno.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nguyen TB, Louie SM, Daniele JR, Tran Q, Dillin A, Zoncu R, et al. DGAT1-Dependent Lipid Droplet Biogenesis Protects Mitochondrial Function during Starvation-Induced Autophagy. Dev Cell. 2017;42:9–21.e25. doi: 10.1016/j.devcel.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed during the study are included in this article. Each experiment was performed at least three times independently.