Abstract
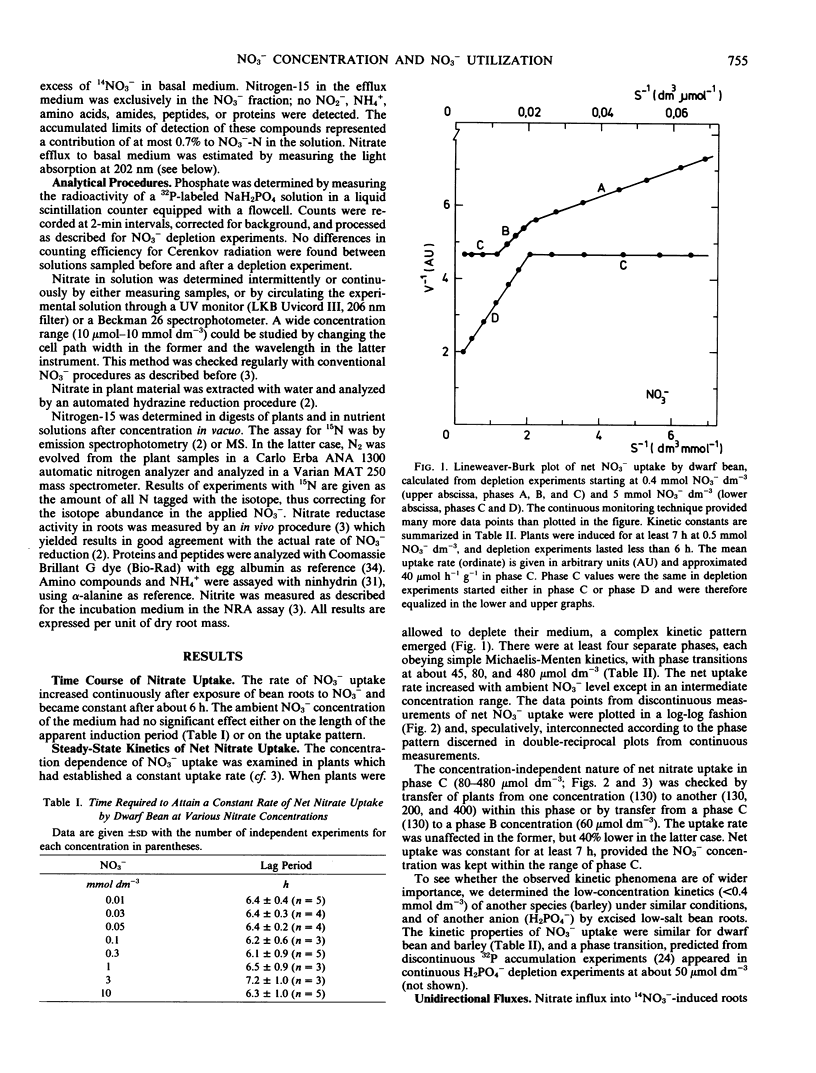
The effect of the exogenous and endogenous NO3− concentration on net uptake, influx, and efflux of NO3− and on nitrate reductase activity (NRA) in roots was studied in Phaseolus vulgaris L. cv. Witte Krombek. After exposure to NO3−, an apparent induction period of about 6 hours occurred regardless of the exogenous NO3− level. A double reciprocal plot of the net uptake rate of induced plants versus exogenous NO3− concentration yielded four distinct phases, each with simple Michaelis-Menten kinetics, and separated by sharp breaks at about 45, 80, and 480 micromoles per cubic decimeter.
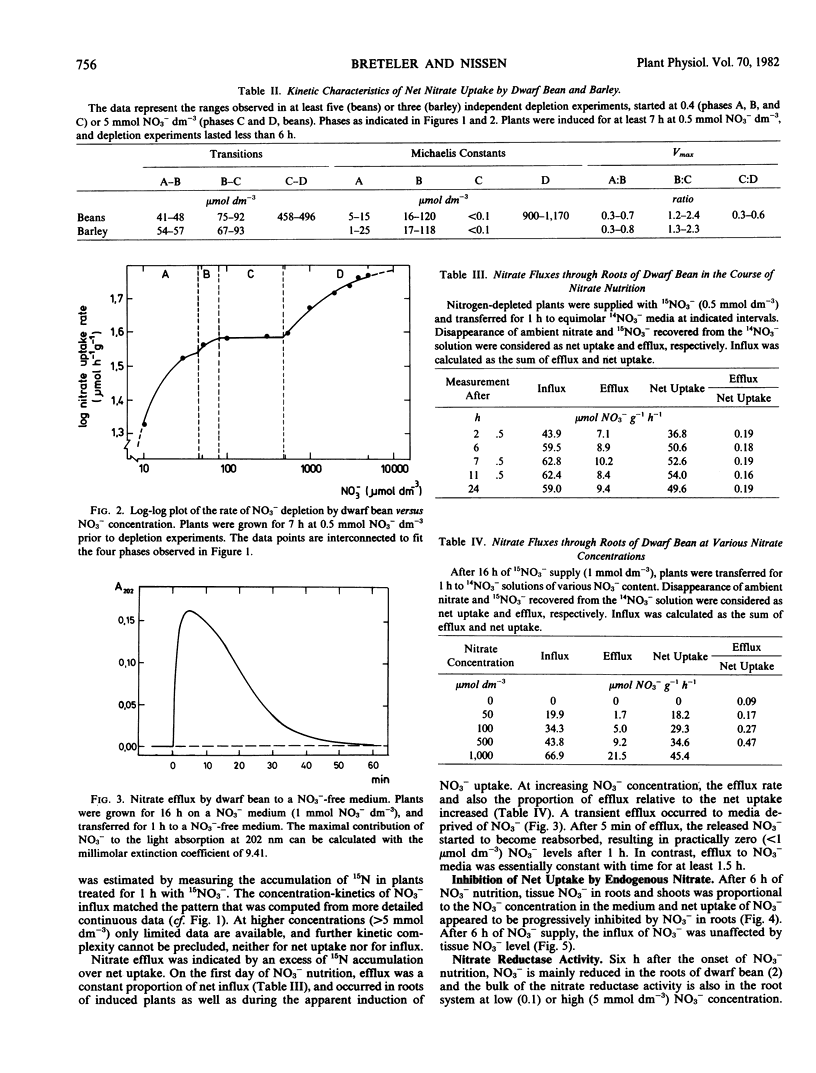
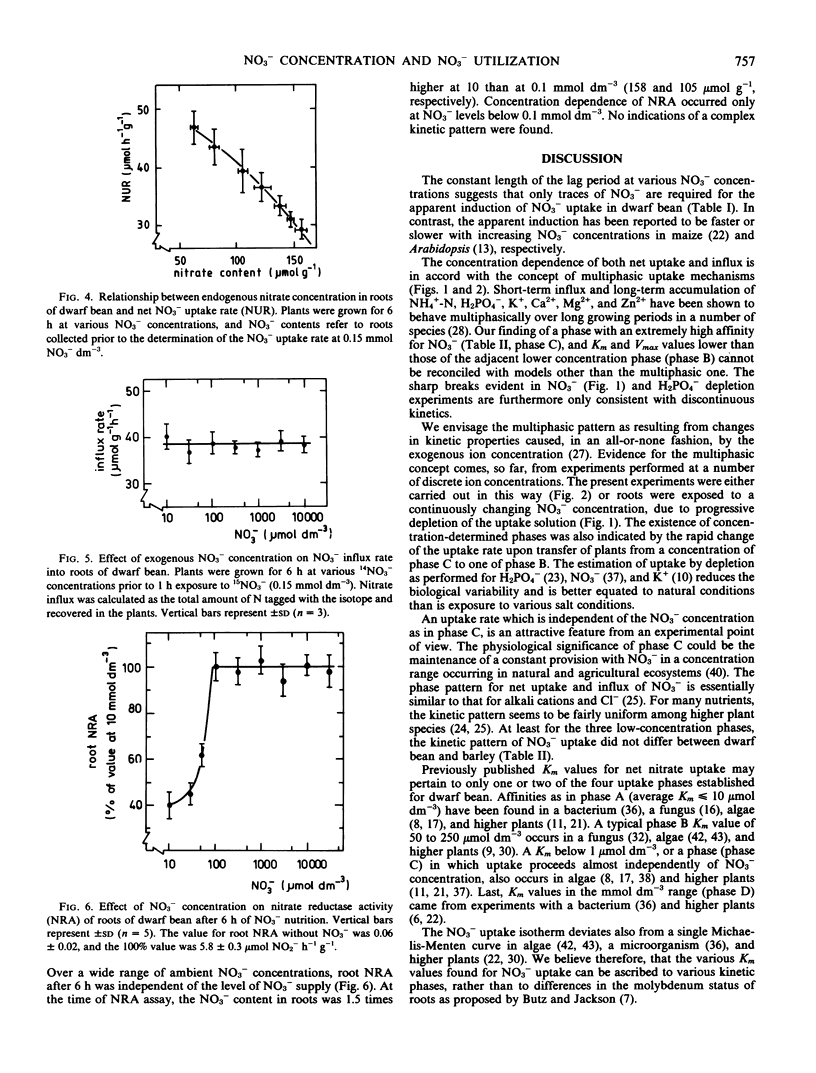
Influx was estimated as the accumulation of 15N after 1 hour exposure to 15NO3−. The isotherms for influx and net uptake were similar and corresponded to those for alkali cations and Cl−. Efflux of NO3− was a constant proportion of net uptake during initial NO3− supply and increased with exogenous NO3− concentration. No efflux occurred to a NO3−-free medium.
The net uptake rate was negatively correlated with the NO3− content of roots. Nitrate efflux, but not influx, was influenced by endogenous NO3−. Variations between experiments, e.g. in NO3− status, affected the values of Km and Vmax in the various concentration phases. The concentrations at which phase transitions occurred, however, were constant both for influx and net uptake. The findings corroborate the contention that separate sites are responsible for uptake and transitions between phases.
Beyond 100 micromoles per cubic decimeter, root NRA was not affected by exogenous NO3− indicating that NO3− uptake was not coupled to root NRA, at least not at high concentrations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chantarotwong W., Huffaker R. C., Miller B. L., Granstedt R. C. In vivo nitrate reduction in relation to nitrate uptake, nitrate content, and in vitro nitrate reductase activity in intact barley seedlings. Plant Physiol. 1976 Apr;57(4):519–522. doi: 10.1104/pp.57.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass A. D. Regulation of potassium absorption in barley roots: an allosteric model. Plant Physiol. 1976 Jul;58(1):33–37. doi: 10.1104/pp.58.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith J., Livoni J. P., Norberg C. L., Segel I. H. Regulation of Nitrate Uptake in Penicillium chrysogenum by Ammonium Ion. Plant Physiol. 1973 Oct;52(4):362–367. doi: 10.1104/pp.52.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyra C. A., Hageman R. H. Nitrate uptake and induction of nitrate reductase in excised corn roots. Plant Physiol. 1975 Nov;56(5):692–695. doi: 10.1104/pp.56.5.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Rao K. P., Rains D. W. Nitrate absorption by barley: I. Kinetics and energetics. Plant Physiol. 1976 Jan;57(1):55–58. doi: 10.1104/pp.57.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloemen R. H., Garrett R. H. Nitrate transport system in Neurospora crassa. J Bacteriol. 1974 Apr;118(1):259–269. doi: 10.1128/jb.118.1.259-269.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F. W., Thompson J. F. Regulation of nitrate reductase in excised barley roots. Plant Physiol. 1971 Aug;48(2):219–223. doi: 10.1104/pp.48.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Wallace W. The distribution and characteristics of nitrate reductase and glutamate dehydrogenase in the maize seedling. Plant Physiol. 1973 Sep;52(3):191–196. doi: 10.1104/pp.52.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]


