Abstract
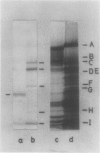
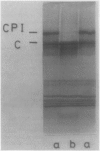
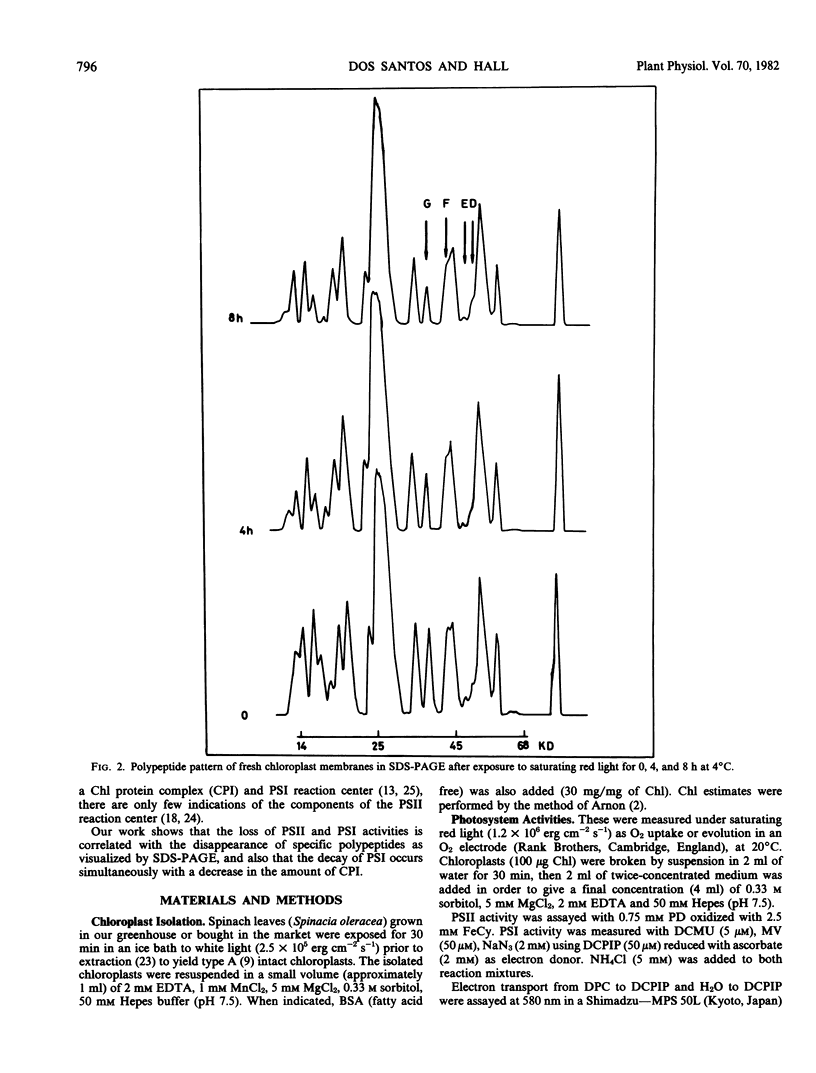
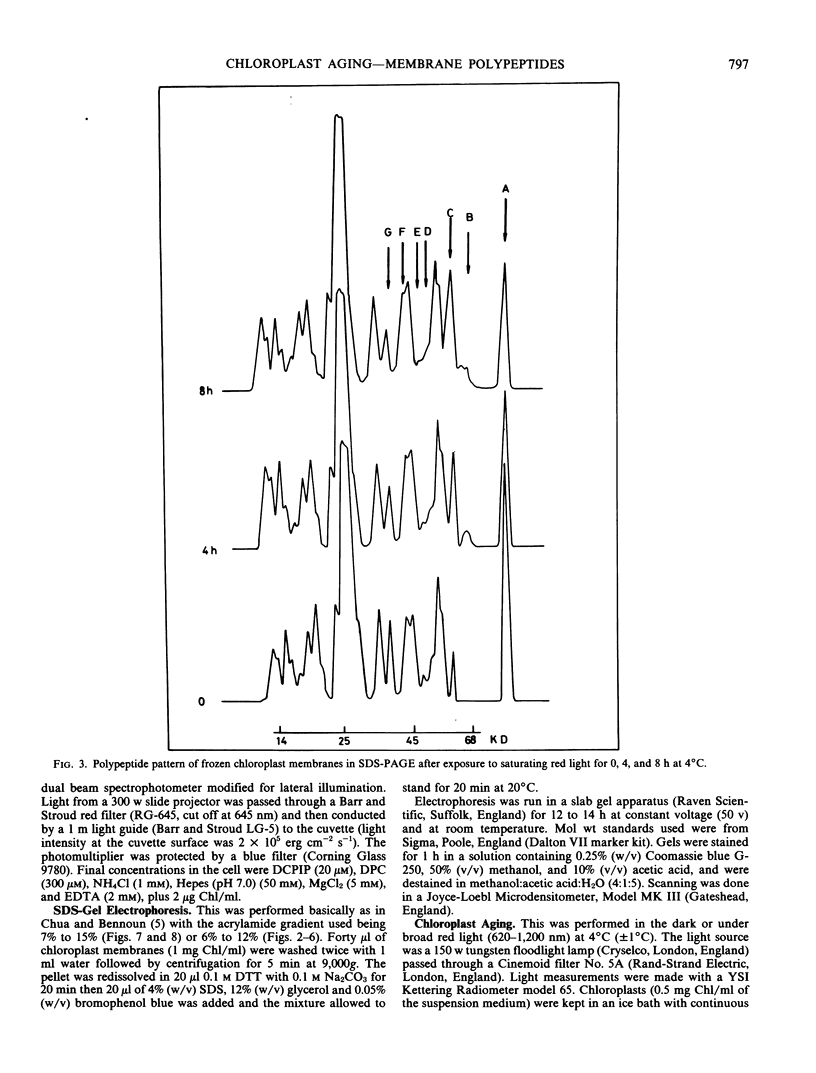
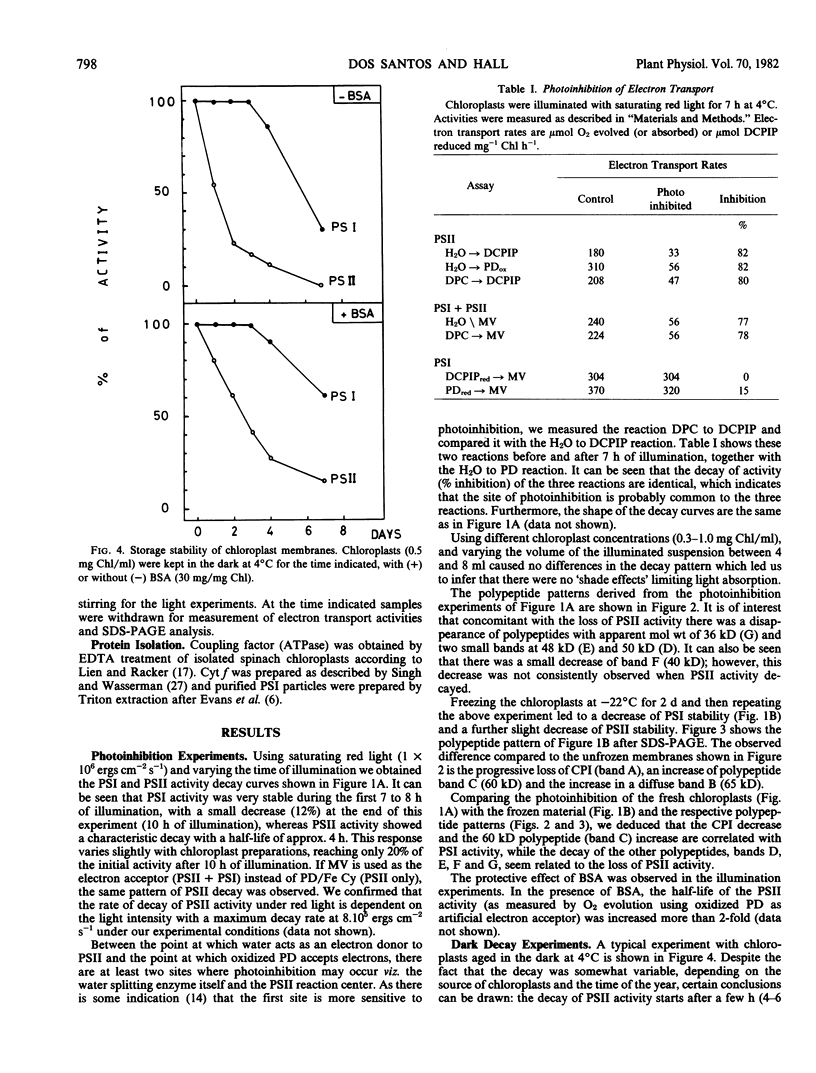
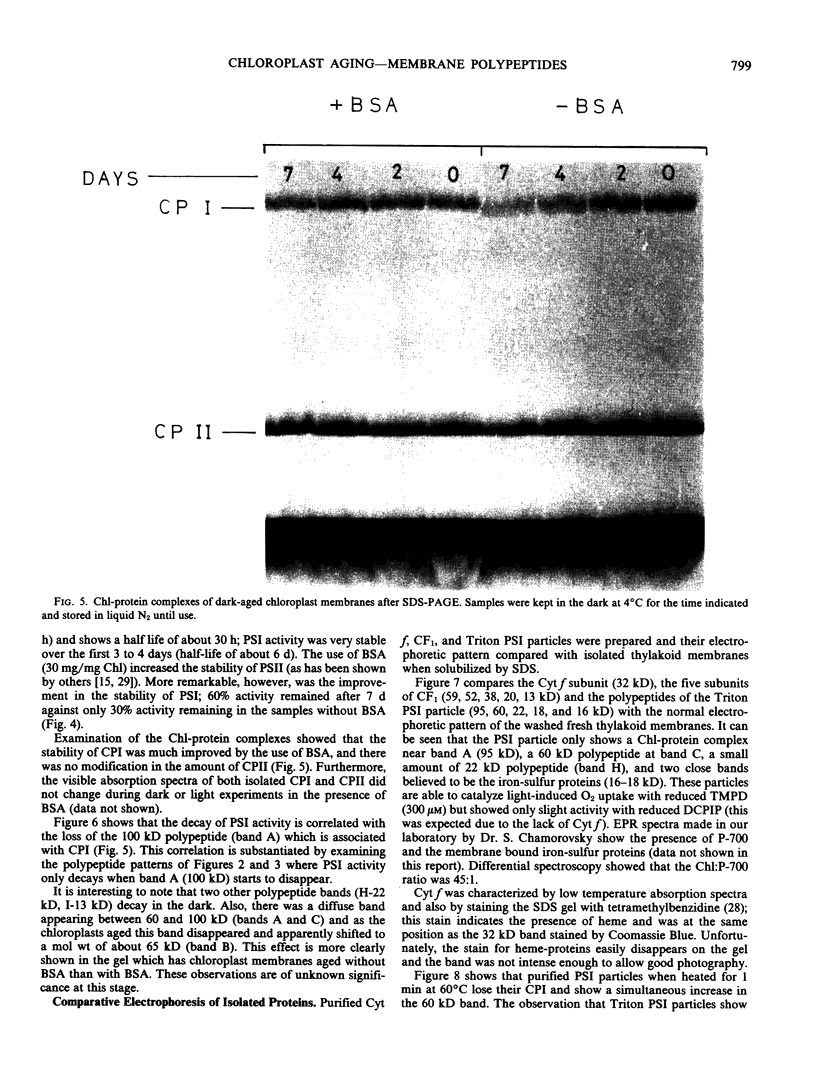
Spinach (Spinacia oleracea) chloroplasts were aged at 4°C under red light and in the dark. The electron transport activity was monitored together with the thylakoid polypeptide patterns in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The light-induced decay of photosystem II (PSII) activity (half-life, about 4 hours) was correlated with a decrease in polypeptides with apparent molecular weights of 36, 48, and 50 kilodaltons. There was very little decay of photosystem I (PSI) activity until after 8 hours illumination. Prior freezing of the chloroplasts enhanced the decrease in PSI activity which was correlated with chlorophyll-protein complex I (CPI) disappearance and an increase in a polypeptide with apparent molecular weight of 60 kilodalton. No variations were detected in the light-harvesting chlorophyll a/b protein. In the dark, the decay of PSII started at 4 to 6 hours and showed a half life of about 30 hours. PSI activity decay (half life about 6 days) occurred simultaneously with the disappearance of CPI. The use of bovine serum albumin (30 mg/mg of chlorophyll) in the light-induced decay experiments increased the stability of PSII more than 2-fold; in the dark experiments, the stability of both photosystems was also more than doubled and the stability of the CPI complex was considerably improved. Comparative electrophoresis of the purified proteins indicated no changes in the cytochrome f band or in the subunits of the ATPase coupling factor during the light-induced decay experiments. Heating of purified PSI particles prior to electrophoresis showed that the 60 kilodaltons polypeptide increased with the disappearance of CPI.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M. P-700 content and polypeptide profile of chlorophyll-protein complexes of spinach and barley thylakoids. Biochim Biophys Acta. 1980 Jun 10;591(1):113–126. doi: 10.1016/0005-2728(80)90225-x. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengis C., Nelson N. Subunit structure of chloroplast photosystem I reaction center. J Biol Chem. 1977 Jul 10;252(13):4564–4569. [PubMed] [Google Scholar]
- Chua N. H., Bennoun P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2175–2179. doi: 10.1073/pnas.72.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley C. Studies on the Mechanism of Photoinhibition in Higher Plants: I. EFFECTS OF HIGH LIGHT INTENSITY ON CHLOROPLAST ACTIVITIES IN CUCUMBER ADAPTED TO LOW LIGHT. Plant Physiol. 1981 Jun;67(6):1161–1165. doi: 10.1104/pp.67.6.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., Sihra C. K., Slabas A. R. The oxidation-reduction potential of the reaction-centre chlorophyll (P700) in Photosystem I. Evidence for multiple components in electron-paramagnetic-resonance signal 1 at low temperature. Biochem J. 1977 Jan 15;162(1):75–85. doi: 10.1042/bj1620075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbeck J. H., Martin I. F., Fowler C. F. Mechanism of Linolenic Acid-induced Inhibition of Photosynthetic Electron Transport. Plant Physiol. 1980 Apr;65(4):707–713. doi: 10.1104/pp.65.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Izawa S. Hydrogen ion buffers. Methods Enzymol. 1972;24:53–68. doi: 10.1016/0076-6879(72)24054-x. [DOI] [PubMed] [Google Scholar]
- Hall D. O. Nomenclature for isolated chloroplasts. Nat New Biol. 1972 Jan 26;235(56):125–126. doi: 10.1038/newbio235125a0. [DOI] [PubMed] [Google Scholar]
- Heath R. L., Packer L. Photoperoxidation in isolated chloroplasts. II. Role of electron transfer. Arch Biochem Biophys. 1968 Jun;125(3):850–857. doi: 10.1016/0003-9861(68)90523-7. [DOI] [PubMed] [Google Scholar]
- Kalberer P. P., Buchanan B. B., Arnon D. I. Rates of photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1542–1549. doi: 10.1073/pnas.57.6.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok B., Gassner E. B., Rurainski H. J. Photoinhibition of chloroplast reactions. Photochem Photobiol. 1966 Mar;4(2):215–227. doi: 10.1111/j.1751-1097.1965.tb05739.x. [DOI] [PubMed] [Google Scholar]
- Reeves S. G., Hall D. O. The stoichiometry (ATP-2e- ratio) of non-cyclic photophosphorylation in isolated spinach chloroplasts. Biochim Biophys Acta. 1973 Jul 26;314(1):66–78. doi: 10.1016/0005-2728(73)90064-9. [DOI] [PubMed] [Google Scholar]
- Satoh K. Polypeptide composition of the purified photosystem II pigment-protein complex from spinach. Biochim Biophys Acta. 1979 Apr 11;546(1):84–92. doi: 10.1016/0005-2728(79)90172-5. [DOI] [PubMed] [Google Scholar]
- Shiozawa J. A., Alberte R. S., Thornber J. P. The P700-chlorophyll a-protein. Isolation and some characteristics of the complex in higher plants. Arch Biochem Biophys. 1974 Nov;165(1):388–397. doi: 10.1016/0003-9861(74)90177-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. E., Ryan D., Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976 Sep;75(1):168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Wasserman A. R., Fleischer S. The stabilization of chloroplast function. Biochim Biophys Acta. 1968 Jan 15;153(1):154–169. doi: 10.1016/0005-2728(68)90156-4. [DOI] [PubMed] [Google Scholar]
- Wessels J. S., Borchert M. T. Polypeptide profiles of chlorophyll . protein complexes and thylakoid membranes of spinach chloroplasts. Biochim Biophys Acta. 1978 Jul 6;503(1):78–93. doi: 10.1016/0005-2728(78)90163-9. [DOI] [PubMed] [Google Scholar]