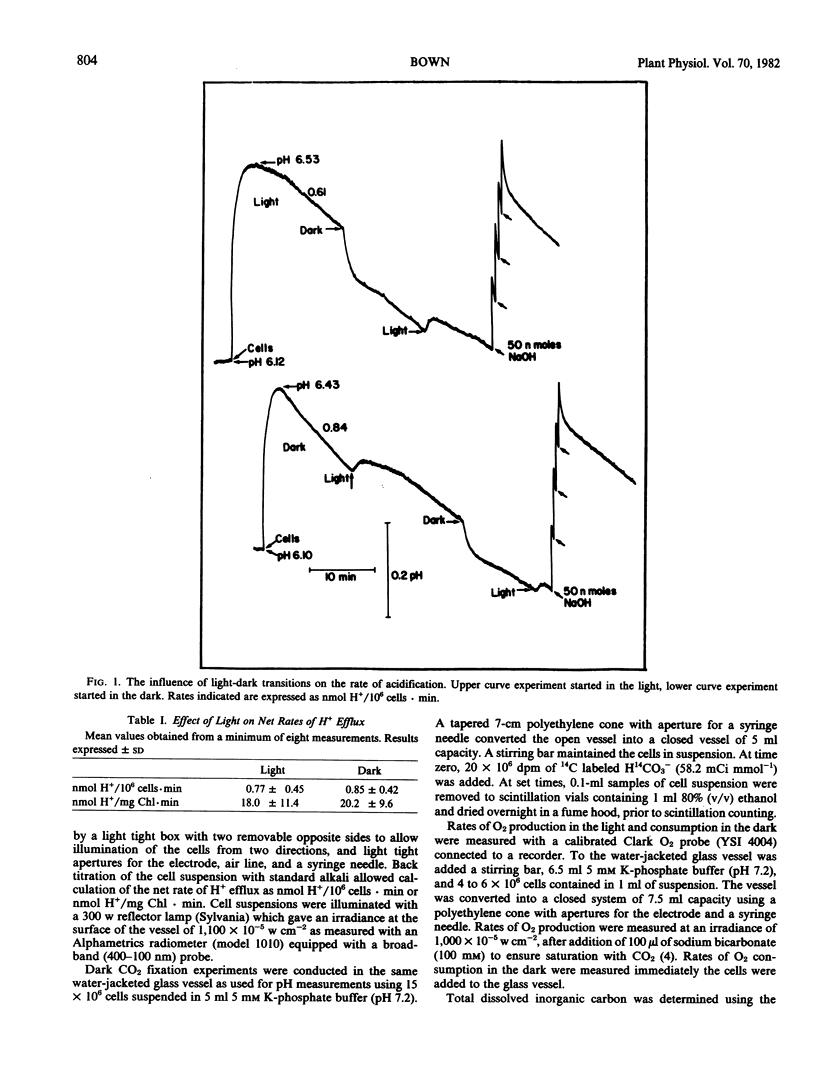
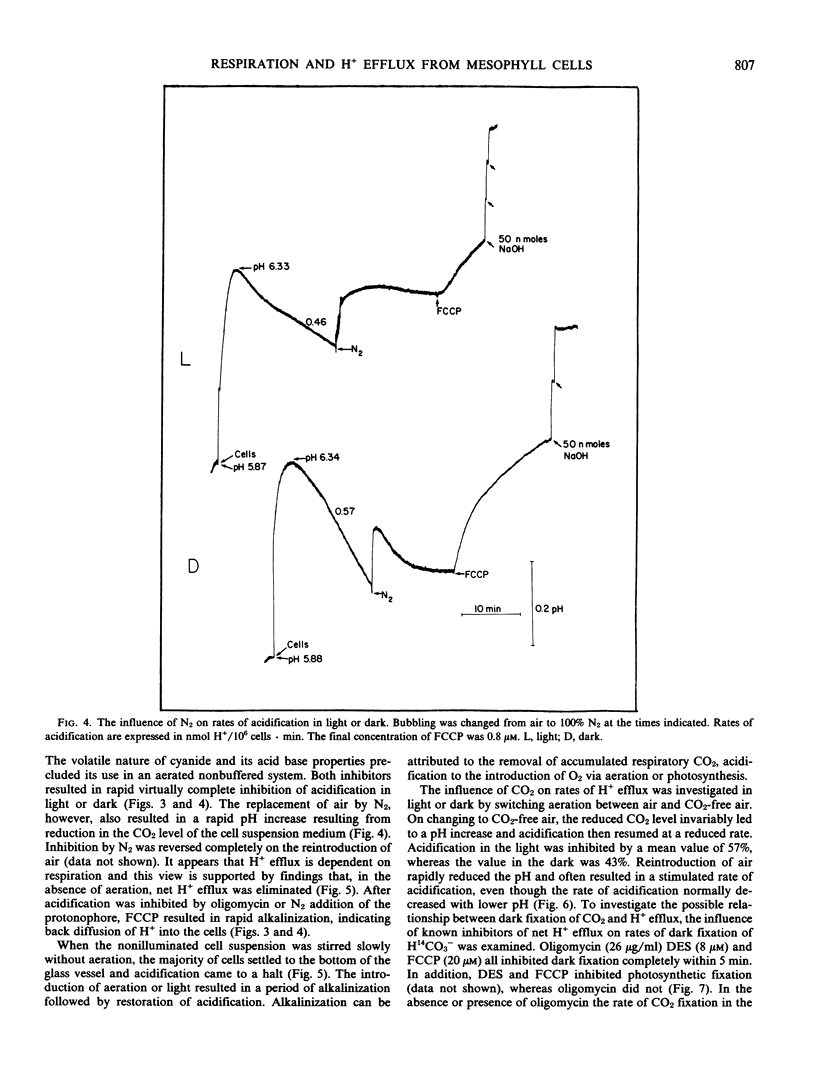
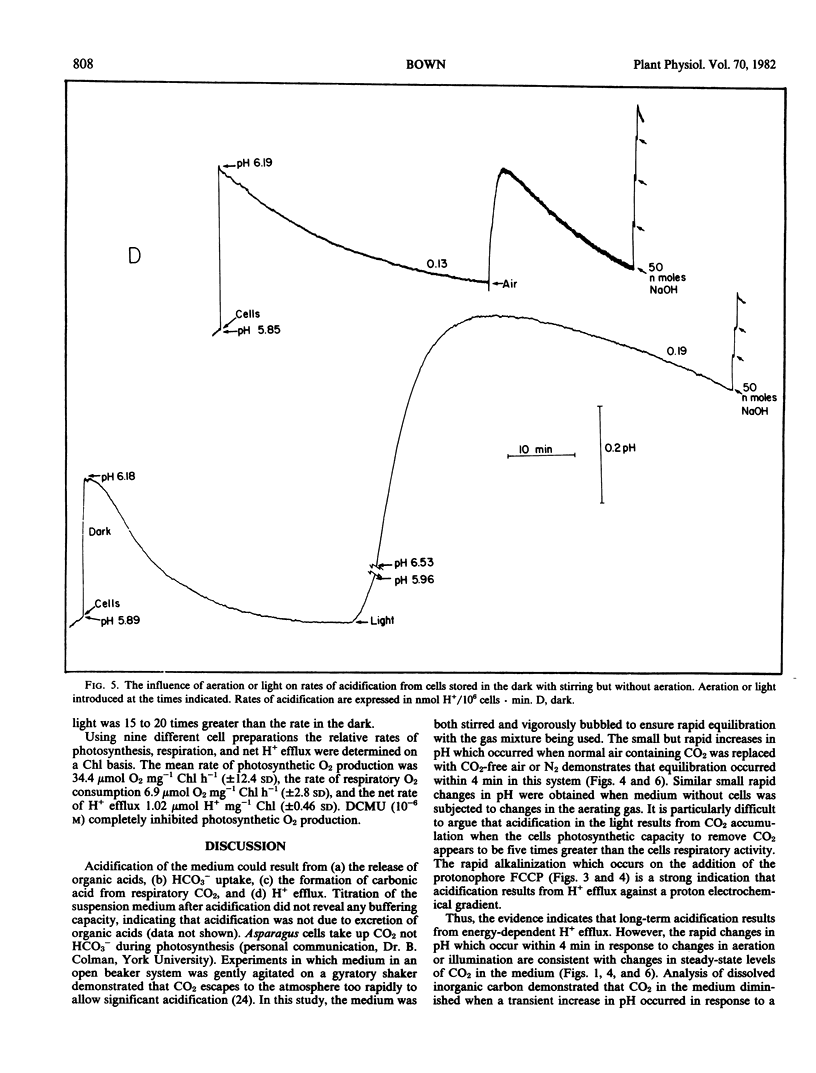
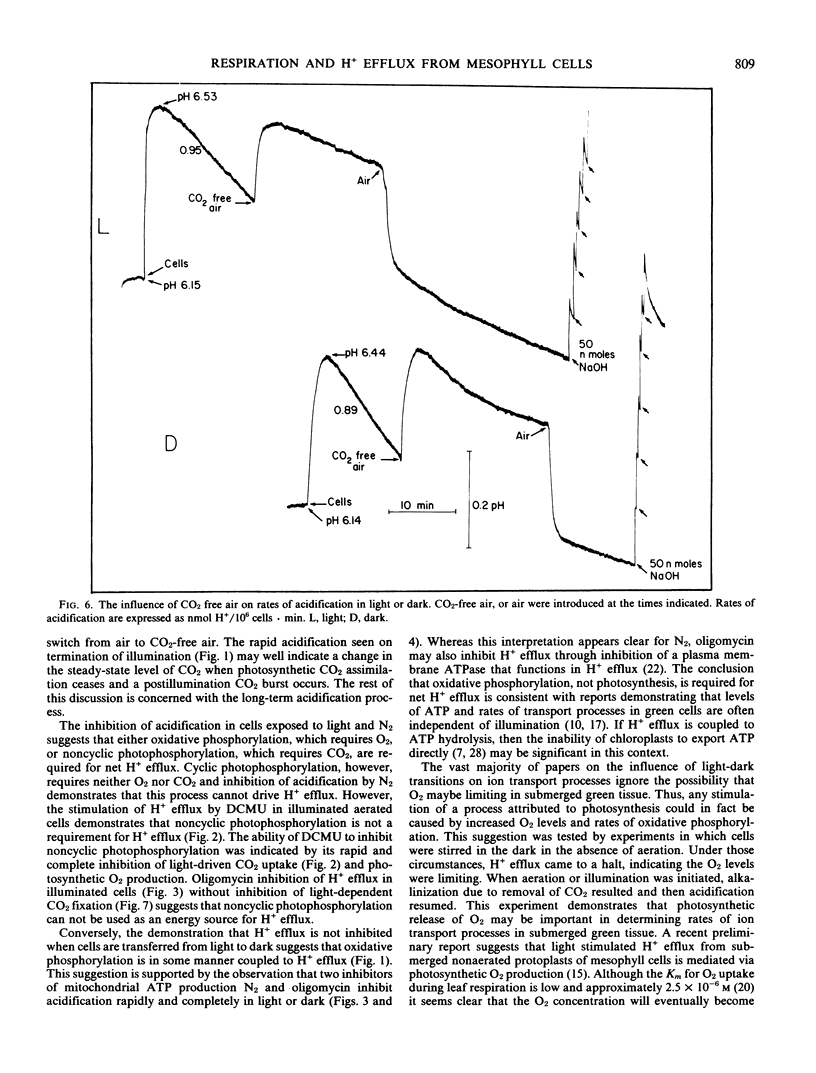
Abstract
Aerated and stirred suspensions of mechanically isolated Asparagus sprengeri Regel mesophyll cells were used to investigate the roles of respiration and photosynthesis in net H+ efflux. Rates varied between 0.12 and 1.99 nanomoles H+ per 106 cells per minute or 3 and 40 nanomoles H+ per milligram chlorophyll per minute. The mean rate of H+ efflux was 10% greater in the dark. 3-(3,4-Dichlorophenyl)-l,l-dimethylurea, an inhibitor of noncyclic photophosphorylation, did not inhibit H+ efflux from illuminated cells. Bubbling with N2 or addition of oligomycin, an inhibitor of mitochondrial ATP production, resulted in rapid and virtually complete inhibition of H+ efflux in light or dark. In the absence of aeration, H+ efflux came to a halt but resumed with aeration or illumination. When aeration was switched to CO2-free air, rates of H+ efflux were reduced 43% in the dark and 57% in the light. Oligomycin eliminated dark CO2 fixation but not photosynthetic CO2 fixation. It is suggested that H+ efflux is dependent on respiration and dark CO2 fixation, but independent of photosynthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B., Rubinstein B. Regulation of Ethylene Evolution and Leaf Abscission by Auxin. Plant Physiol. 1964 Nov;39(6):963–969. doi: 10.1104/pp.39.6.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. E., Lieberman M., Anderson J. D. Inhibition of ethylene production in fruit slices by a rhizobitoxine analog and free radical scavengers. Plant Physiol. 1978 Jun;61(6):886–888. doi: 10.1104/pp.61.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham B. C., Colman B. Measurement of carbon dioxide compensation points of freshwater algae. Plant Physiol. 1979 Nov;64(5):892–895. doi: 10.1104/pp.64.5.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Ethylene formation in pea seedlings; its relation to the inhibition of bud growth caused by indole-3-acetic Acid. Plant Physiol. 1968 Jul;43(7):1069–1074. doi: 10.1104/pp.43.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. The interaction between auxin and ethylene and its role in plant growth. Proc Natl Acad Sci U S A. 1966 Feb;55(2):262–269. doi: 10.1073/pnas.55.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp P. J., Wickliff J. L. Light or ethylene treatments induce transverse cell enlargement in etiolated maize mesocotyls. Plant Physiol. 1981 Jan;67(1):125–128. doi: 10.1104/pp.67.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y., Lieberman M. Effects of Kinetin, IAA, and Gibberellin on Ethylene Production, and Their Interactions in Growth of Seedlings. Plant Physiol. 1968 Dec;43(12):2029–2036. doi: 10.1104/pp.43.12.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeschl J. D., Rappaport L., Pratt H. K. Ethylene as a factor regulating the growth of pea epicotyls subjected to physical stress. Plant Physiol. 1966 May;41(5):877–884. doi: 10.1104/pp.41.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M. A., Kaufman P. B. Hormonal Regulation of Lateral Bud (Tiller) Release in Oats (Avena sativa L.). Plant Physiol. 1980 Dec;66(6):1123–1127. doi: 10.1104/pp.66.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haschke H. P., Lüttge U. Stoichiometric Correlation of Malate Accumulation with Auxin-dependent K-H Exchange and Growth in Avena Coleoptile Segments. Plant Physiol. 1975 Nov;56(5):696–698. doi: 10.1104/pp.56.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Rayle D. L. Enhancement of CO(2) Uptake in Avena Coleoptiles by Fusicoccin. Plant Physiol. 1976 May;57(5):806–811. doi: 10.1104/pp.57.5.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Purification of enzymatically isolated mesophyll protoplasts from c(3), c(4), and crassulacean Acid metabolism plants using an aqueous dextran-polyethylene glycol two-phase system. Plant Physiol. 1973 Nov;52(5):484–490. doi: 10.1104/pp.52.5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B. G., Newcomb W., Burg S. P. Mechanism of Auxin-induced Ethylene Production. Plant Physiol. 1971 Apr;47(4):504–509. doi: 10.1104/pp.47.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer D. W., Spanswick R. M. Correlation of Adenosine Triphosphate Levels in Chara corallina with the Activity of the Electrogenic Pump. Plant Physiol. 1979 Aug;64(2):165–168. doi: 10.1104/pp.64.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald I. R. Effect of vacuum infiltration on photosynthetic gas exchange in leaf tissue. Plant Physiol. 1975 Jul;56(1):109–112. doi: 10.1104/pp.56.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald I. R., Macklon A. E. Light-enhanced Chloride Uptake by Wheat Laminae: A Comparison of Chopped and Vacuum-infiltrated Tissue. Plant Physiol. 1975 Jul;56(1):105–108. doi: 10.1104/pp.56.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone C. P., Burke J. J., Hanson J. B. Histochemical Evidence for the Occurrence of Oligomycin-sensitive Plasmalemma ATPase in Corn Roots. Plant Physiol. 1977 Dec;60(6):916–922. doi: 10.1104/pp.60.6.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Anderson J. D., Chalutz E., Lieberman M. Influence of enol ether amino acids, inhibitors of ethylene biosynthesis, on aminoacyl transfer RNA synthetases and protein synthesis. Plant Physiol. 1979 Aug;64(2):289–292. doi: 10.1104/pp.64.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlasson W. B., Pratt H. K. Effects of Wounding on Respiration and Ethylene Production by Cantaloupe Fruit Tissue. Plant Physiol. 1964 Jan;39(1):128–132. doi: 10.1104/pp.39.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocking C. R., Larson S. A chloroplast cytoplasmic shuttle and the reduction of extraplastid NAD. Biochem Biophys Res Commun. 1969 Oct 8;37(2):278–282. doi: 10.1016/0006-291x(69)90731-1. [DOI] [PubMed] [Google Scholar]
- Zimmerman R. H., Lieberman M., Broome O. C. Inhibitory effect of a rhizobitoxine analog on bud growth after release from dormancy. Plant Physiol. 1977 Feb;59(2):158–160. doi: 10.1104/pp.59.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]


