Abstract
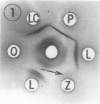
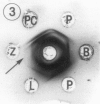
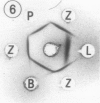
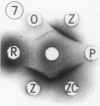
Preparation and characterization of antisera against lettuce (Lactuca sativa L., cv. Grand Rapids) and pea (Pisum sativum L., cv. Alaska) phytochrome is described. These antisera, together with previously obtained antisera against zucchini (Cucurbita pepo L., cv. Black Beauty) and oat (Avena sativa L., cv. Garry) phytochrome, were used to compare by Ouchterlony double immunodiffusion phytochrome isolated from etiolated lettuce, pea, bean (Phaseolus vulgaris L., cv. Taylor Horticultural Bush), zucchini, oat and rye (Secale cereale L., cv. Balbo) seedlings. Cross reactivity between monocotyledonous phytochrome and antidicotyledonous-phytochrome serum and between dicotyledonous phytochrome and antimonocotyledonous-phytochrome serum was always weak or not perceptible by this assay. Among the four dicotyledonous phytochromes examined, pea and bean were the most similar immunochemically as anticipated. Pea and lettuce phytochrome somewhat unexpectedly also exhibited similar immunochemical reactivity. Zucchini phytochrome by contrast was immunochemically distinct from pea, bean, and lettuce phytochrome, although it did react with all three antidicotyledonous-phytochrome sera. Initial attempts to identify immunoglobulins that would recognize phytochrome regardless of its source indicated that they may exist. Such immunoglobulins are of interest because they might react with one or more determinants that could be part of an active site of phytochrome. These immunoglobulins, once isolated, could thus serve as a potential probe for the active site of phytochrome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cordonnier M. M., Pratt L. H. Immunopurification and initial characterization of dicotyledonous phytochrome. Plant Physiol. 1982 Feb;69(2):360–365. doi: 10.1104/pp.69.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundiff S. C., Pratt L. H. Immunological and physical characterization of the products of phytochrome proteolysis. Plant Physiol. 1975 Feb;55(2):212–217. doi: 10.1104/pp.55.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundiff S. C., Pratt L. H. Phytochrome Characterization by Rabbit Antiserum against High Molecular Weight Phytochrome. Plant Physiol. 1975 Feb;55(2):207–211. doi: 10.1104/pp.55.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner G., Pike C. S., Rice H. V., Briggs W. R. "Disaggregation" of phytochrome in vitro-a consequence of proteolysis. Plant Physiol. 1971 Dec;48(6):686–693. doi: 10.1104/pp.48.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R. E., Pratt L. H. Phytochrome immunoaffinity purification. Plant Physiol. 1979 Aug;64(2):332–336. doi: 10.1104/pp.64.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H. Comparative immunochemistry of phytochrome. Plant Physiol. 1973 Jan;51(1):203–209. doi: 10.1104/pp.51.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice H. V., Briggs W. R. Immunochemistry of phytochrome. Plant Physiol. 1973 May;51(5):939–945. doi: 10.1104/pp.51.5.939. [DOI] [PMC free article] [PubMed] [Google Scholar]