Abstract
Stroke is the third most common cause of death globally and a leading cause of disability. The cellular and molecular changes following stroke and causes of neuronal death are not fully understood, and there are few effective treatments currently available. A rapid increase in the levels of reactive oxygen species (ROS) post stroke can overwhelm antioxidant defenses and trigger a series of pathophysiologic events including the inflammatory response, blood-brain barrier (BBB) disruption, apoptosis, and autophagy, ultimately leading to neuron degeneration and apoptosis. It is thought that beyond a certain age, the ROS accumulation resulting from stroke increases the risk of morbidity and mortality. In the present review, we summarize the role of oxidative stress (OS) as a link between aging and stroke pathogenesis. We also discuss how antioxidants can play a beneficial role in the prevention and treatment of stroke by eliminating harmful ROS, delaying aging, and alleviating damage to neurons.
Keywords: Stroke, Age, Oxidative stress, Review
Introduction
Stroke is an age-related disease that leads to neurologic dysfunction and is associated with high rates of disability and mortality.[[1], [2], [3]] Stroke can be classified into hemorrhagic stroke (HS) and ischemic stroke (IS), with the latter accounting for 87% of cases. HS is caused by bleeding in or around the brain,[4] whereas IS is caused by disruption of the brain's arterial blood flow by thrombosis, embolism, or cerebrovascular rupture, resulting in ischemic necrosis of brain tissue and loss of neuronal function.[[5], [6], [7]] IS is the third leading cause of death worldwide, with nearly 15 million people affected yearly.[8,9]
Post-stroke patient care involves correctly identifying the type of stroke based on clinical findings. IS patients have higher mean Glasgow Coma Scale scores than HS patients,[10] who frequently experience acute onset headaches. Computed tomography (CT) findings include a mass effect, hypodense lesions, hyperdense artery signs, and sulcus effacement in IS and hyperdense lesions in HS.[11] The prognosis of patients is determined by the type of stroke, degree and length of blockage or bleeding, and severity of neurodegeneration. The location of the lesion is also important in HS, which has a worse outcome than IS.[12] Age adversely affects IS pathophysiology and prognosis.[[13], [14], [15]] Currently, 11% of the world's population is over 60 years old, with the percentage expected to reach 22% by 2050.[16] Clarifying the pathophysiology of age-related IS is critical for developing new treatments.
Few pharmacotherapies are effective in mitigating the effects of stroke.[[17], [18], [19]] Revascularization therapies such as thrombolysis recombinant tissue plasminogen activator and endovascular thrombectomy have been shown to reduce the disability rate of patients with acute cerebral infarction within 24 h.[20,21] However, ischemia-reperfusion injury after revascularization therapy can worsen outcomes.
The pathophysiology of stroke is complex. The acute disruption or reduction of cerebral blood flow and resultant decrease in available oxygen causes focal or global damage to brain tissue, with characteristic biochemical and molecular changes that can lead to transient or permanent neurologic sequelae or death.[[22], [23], [24], [25]] The main products of the oxidative stress (OS) response - i.e., free radicals including reactive oxygen species (ROS) - can damage brain tissue and are an important pathologic mechanism of stroke. Antioxidants that remove free radicals can limit neuronal injury following stroke [[26], [27], [28]] and are thus a potential treatment for IS.
Overview of OS and the Antioxidant System
OS occurs when there is an imbalance between oxidation and antioxidation, which leads to neutrophil infiltration, increased protease secretion, and production of oxidative intermediates.[29] Under pathologic conditions such as brain hypoxia, oxygen free radicals accumulate and cause damage to cell membranes, especially that of mitochondria. This lead to neuronal dysfunction and death; thus, interventions that alleviate OS are a potential treatment strategy.[[30], [31], [32]] There are two types of antioxidant system in the body: enzymatic (which includes glutathione peroxidase [GSH-Px], glucose-6-phosphate dehydrogenase [G6PD], catalase [CAT], and superoxide dismutase [SOD]) and non-enzymatic (which includes vitamins and phenols). Their potential roles in stroke are discussed below.
Molecular Mechanisms of Stroke
Excitatory neurotoxicity, oxidative/nitrosative stress, mitochondrial dysfunction, and calcium overload are the main causes of stroke. Cellular damage from free radicals, mainly OS/nitrosative stress injury, plays a critical role in ischemia-reperfusion injury.[33] Under normal conditions, electrons produced by metabolism through the mitochondrial respiratory chain combine with oxygen and are reduced to water. Under hypoxia, an excess of electrons combine with iron and other molecules to produce free radicals.[34] Restoration of blood flow results in a sudden increase in oxygen content, leading to peroxide production via the reaction between electrons and oxygen molecules. Following cerebral ischemia/reperfusion, tissue acidification, cell membrane depolarization, calcium influx, neurotransmitter release, and inflammatory cell infiltration can generate free radicals that are inactivated by antioxidants.[35]
ROS are produced by various cellular structures and molecules including mitochondria, nicotinamide adenine dinucleotide phosphate (NADPH), nitric oxide synthase (NOS), and xanthine oxidase.[36] SOD, CAT, and GSH-Px are present at low levels in the brain and the concentration of free radicals in brain tissue during hypoxia and reperfusion can exceed the antioxidant capacity of these enzymes. The accumulation of ROS can lead to apoptosis, tissue inflammation, DNA damage, lipid peroxidation, and protein degeneration.[37]
Role of Mitochondria in OS
Mitochondrial dysfunction is linked to age-related disorders such as metabolic syndromes, neurodegenerative and cardiovascular diseases, and cancer.[38] Mitochondria regulate energy metabolism and maintain cellular homeostasis. Aging is associated with decreased mitochondrial activity and accumulation of damaged mitochondria in various tissues.[39]
Mitochondrial distribution and activity influence neuron morphogenesis and synaptogenesis, developmental and synaptic plasticity, and axogenesis. Axons and dendrites form synapses after neural stem cells divide and differentiate into neurons over the course of development.[40] Mitochondria participate in neuroplasticity through the formation of adenosine 5′-triphosphate, generation of ROS and reactive nitrogen species (RNS), induction of apoptosis, and maintenance of calcium homeostasis. ROS and RNS interact with mitochondrial proteins, lipids, and DNA, at least in part through their proximity.[41] OS induces the release of proapoptotic mitochondrial proteins into the cytosol through interactions between mitochondrial and extramitochondrial proteins that can be inhibited by other proteins and small molecules.[42]
Changes in mitochondrial dynamics and quality control can lead to mitochondrial damage, which in turn contributes to senescence. Thus, strategies that improve or restore these processes may prevent aging and age-related disorders.[43]
Role of OS in Stroke
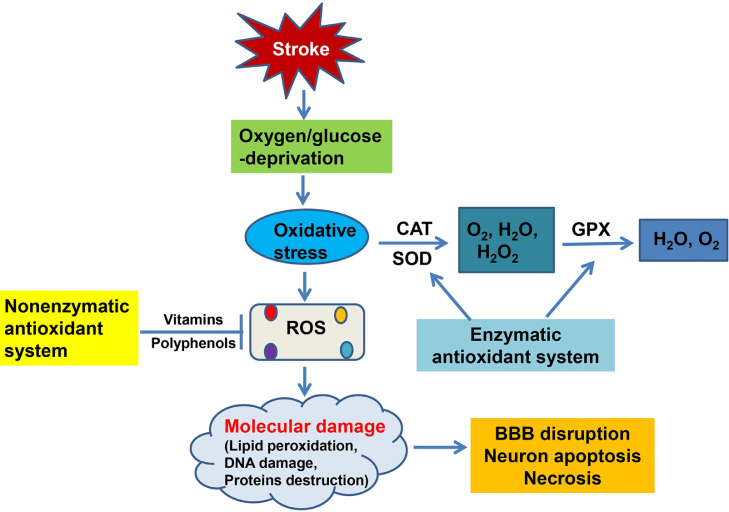
OS is a pathologic process resulting from an imbalance between ROS production and removal.[44] Excessive ROS in brain tissue can damage the mitochondrial membrane through lipid peroxidation, leading to destruction of the respiratory chain.[45] ROS in neurons damage mitochondrial DNA, inactivate enzymes and degrade proteins in mitochondria, and destroy cell membrane structure and function, causing neuronal death.[46] They can also affect cerebral blood flow, causing vasodilation and increasing endothelial cell permeability, causing damage to vascular endothelial cells, increasing blood-brain barrier (BBB) permeability, and leading to impairment of brain tissue microcirculation,[47] thereby aggravating the OS response and glutamate excitotoxicity.[48] The associated changes in mitochondrial dynamics can lead to upregulation of N-methyl-d-aspartate receptors and further enhance the OS response and induce neuronal death.[49] These processes can damage brain tissue and contribute to the pathogenesis of stroke (Figure 1).
Figure 1.
Schematic illustration of oxidative mechanisms, OS-mediated molecular damage, and antioxidant mechanisms in stroke.
BBB: Blood-brain barrier; CAT: Catalase; GSH-PX: Glutathione peroxidase; OS: Oxidative stress; ROS: Reactive oxygen species; SOD: Superoxide dismutase.
Relationship Between Aging, OS, and Stroke
OS is associated with aging and is a feature of aging-related vascular diseases including stroke.[50] Aging is itself a risk factor for worse prognosis following stroke.[[51], [52], [53], [54]] However, the molecular links between aging, OS, and stroke are not fully understood.[16] We speculate that age directly exacerbates stroke outcomes by inducing oxidative damage.
Byproducts of normal oxidative metabolism can cause damage to DNA, lipids, and proteins that contribute to aging.[55,56] Superoxide (O2), hydrogen peroxide (H2O2), and hydroxyl radicals are produced upon exposure to mutagens but are also byproducts of normal metabolism.[57,58] Lipid peroxidation produces mutagenic lipid epoxides, lipid hydroperoxides, lipoalkoxy and peroxide radicals, and enaldehydes. Singlet oxygen, a high-energy oxygen molecule, is produced by light-induced energy transfer, respiratory bursts of neutrophils, or lipid peroxidation.[59,60] Despite the activities of physiologic antioxidant defense systems, DNA may sustain oxidative damage [61] that cannot be repaired; meanwhile, other cellular repair systems such as the proteasomal degradation of damaged proteins may also decline with aging.[62] Adaptive responses to OS decrease with aging as a result of telomere dysfunction in cellular senescence and induction of senescence-associated secretory phenotypes (SASP) and dysregulation of metabolism.[[63], [64], [65]]
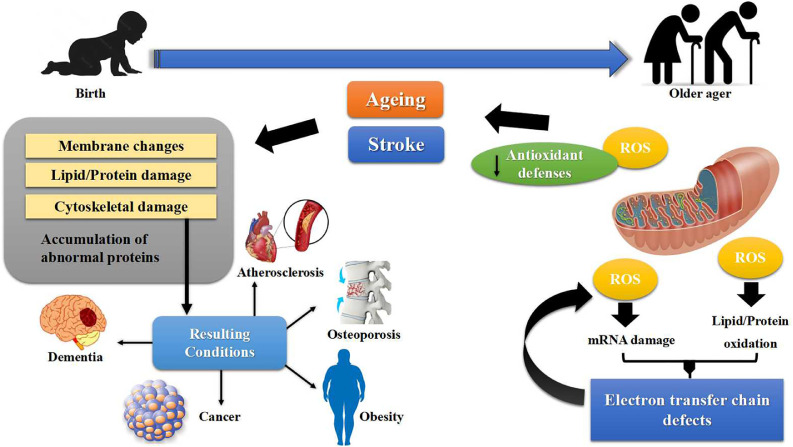
Increased OS and neuroinflammation in the aging hippocampus is a major cause of age-related cognitive decline and decreased neurogenesis and synaptic plasticity.[66] Post-ischemic microglia exhibit reduced interaction with neighboring neurons and polarization toward the infarct lesion, which may contribute to aging-associated vulnerability to and poorer recovery from IS.[67] Hallmarks of brain aging include OS, reduced adaptive neuroplasticity and resilience, aberrant neuronal network activity, impairment of DNA damage repair, and dysregulated energy metabolism.[68] Mitochondrial dysfunction under ischemic conditions increases with age and causes the loss of neurovasculature integrity and injury to brain tissue.[55] Aging can also damage collateral circulation and prevent brain revascularization, which is associated with increased endothelial NOS activity and decreased expression of inflammatory response markers, which result in the aggravation of stroke [69,70] (Figure 2).
Figure 2.
Proposed interactions between age-related diseases and stroke. ROS: Reactive oxygen species.
Antioxidants in Stroke
Relationship between aging, antioxidants, and stroke
The relationship between aging and antioxidants is complex and the rate of aging is thought to be more significant than chronological age in terms of disease risk.[71] Antioxidants, which slow the aging process by reducing or maintaining the levels of oxidizing molecules, can be obtained through the diet and may be included in cosmetic products to counter the skin cell-damaging effects of free radicals. Several biomolecules and compounds with redox activity have been identified that can slow the aging process.[36,[72], [73], [74], [75], [76]] Additionally, as OS is increased in the ischemic brain, antioxidants have been evaluated for their neuroprotective potential in stroke. In animal models, ischemic damage to brain tissue was mitigated by administering BBB-penetrating antioxidant molecules such as polyethylene glycol-conjugated SOD, CAT, and lazaroids; and infarct size was decreased in SOD transgenic mice but increased in SOD knockout mice compared with their wild-type counterparts.[73] A technical challenge when investigating the relationship between OS and stroke is the accurate quantification of free radicals generated in brain tissue.[75] Indirect evidence of OS during ischemia includes elevated levels of lipid peroxidation products and lower levels of antioxidants in tissues.[74] Additionally, it was reported that patients with stroke had lower plasma vitamin C and E levels than non-stroke patients, whereas the levels of thiobarbituric acid-reactive substances were elevated 2 days after the onset of cerebral ischemia.[36,76]
GSH-Px
GSH-Px, a peroxidative CAT, is a powerful radical scavenger that catalyzes the transformation of glutathione to oxidized glutathione, reducing toxic peroxide to nontoxic hydroxyl compounds that promote the breakdown of H2O2 into water and oxygen. Thus, GSH-Px can prevent oxidative damage to the cell membrane, thereby preserving cell structure and function.[77,78] Plasma GSH-Px activity was shown to be reduced in patients with acute IS [78] and a decrease in GSH-Px level is an independent risk factor for arterial IS.[79] GSH-Px deficiency increases extracellular OS, reduces bioavailable nitric oxide, and promotes platelet activation. In patients with acute IS, red blood cell GSH-Px activity was significantly reduced within 24 h after the onset of stroke symptoms compared with control patients,[80] and in an animal model, GSH-Px protected against ischemic brain injury whereas a reduction in GSH-Px level was associated with an increased risk of stroke.[81] These findings indicate that GSH-Px can serve as a biomarker for progression of reactive stroke and has clinical value for the differential diagnosis of stroke.
G6PD
G6PD is a rate-limiting enzyme in the pentose phosphate pathway (PPP), which plays an important role in neuronal survival during cerebral ischemia-reperfusion. G6PD deficiency has been linked to the development of stroke and is associated with poor prognosis following IS, increasing the risk of in-hospital mortality.[82] Additionally, stroke patients with G6PD deficiency may have worse safety outcomes with long-term low-dose aspirin therapy.[83] It was also reported that acute stroke patients with G6PD deficiency had a higher risk of poor clinical outcomes with thrombolysis therapy than in patients with normal G6PD levels.[84] Thus, G6PD level may reflect to some degree the extent of brain damage from stroke.
CAT
CAT is an oxygen radical-scavenging enzyme that mainly exists in tissues and erythrocytes. CAT metabolizes H2O2 to H2O and O2 and removes hydrogen from H2O2, causing the reaction of H2O2 with O2 and iron chelators to prevent damage to cell membranes by H2O2.[85,86] CAT levels were found to be lower in patients with acute IS than in healthy controls, and a reduction in CAT level exacerbated the cellular response to OS.[87]
SOD
SOD catalyzes the partitioning of superoxide anion free radicals to O2 and H2O2 [88] and is the main enzyme preventing cellular damage from oxygen radicals; its activity reflects the extent of damage involving lipid peroxidation.[89,90] Reduced SOD activity can lead to the accumulation of metabolites that induce oxidative damage.[91] Serum SOD levels in acute-phase IS may serve as a marker of stroke-associated infection; meanwhile, increased SOD levels can protect against brain injury.[92] In an animal model of IS, manganese SOD reduced infarct volume, improved neuronal function, and reduced OS and apoptosis.[93] This evidence suggests that SOD can reduce OS and has therapeutic benefits in preventing IS.
Non-enzymatic antioxidants
Non-enzymatic antioxidants including vitamins and phenols for the treatment of stroke are used in traditional Chinese medicine. Some of these are discussed in the following sections.
Vitamins
Vitamin C is a highly reductive polyhydroxy compound with antioxidant effects.[94] Ascorbate, the biologically active form, is critical for homeostasis and the regulation of neuron function[120]. Brain vitamin C content was shown to be closely related to cognitive decline and stroke severity.[95] An excess of free radicals can lead to lipid peroxidation and IS.[96] Vitamin C prevents atherosclerosis by reducing monocyte adhesion to the endothelium of blood vessels, reducing blood pressure, promoting vasodilation, increasing intravascular nitric oxide, and inhibiting low-density lipoprotein (LDL) peroxidation to reduce the risk of IS. Ascorbate was shown to protect neurons from glutamate excitotoxicity by modulating glutamate receptor activity and lowering the level of free radicals produced by glutamate release. High doses of vitamin C may decrease the severity of ischemia [97] and alleviate cognitive impairment in a rat model of sepsis via a protective mechanism involving suppression of inflammation and OS and modulation of heme oxygenase 1 (HO-1) signaling.[98] However, whether vitamin C can improve the outcome of patients with IS remains to be investigated in large-scale randomized controlled clinical trials.
Polyphenols
Phenolic compounds exert antioxidant effects through multiple mechanisms; these include downregulating key enzymes involved in ROS formation, combining with metal ions, and enhancing ROS clearance.[46,99] They may also protect plasma membrane structure and function by altering its properties to prevent the entry of oxidant molecules. Flavonoid polyphenolic compounds may inhibit peroxidase activity and neutrophil release.[100] Phenolic compounds regulate nitric oxide production by interacting with NOS, thereby reducing oxidative damage in cardiovascular disease.[101] In atherosclerosis, polyphenolic compounds inhibit LDL peroxidation to prevent IS,[102] and they also play an important role in preventing IS by protecting neurons from the OS response through inhibition of lipid peroxidation [103] (Figure 1).
Free radical products
Malondialdehyde (MDA) is the end product of fatty acid peroxidation under OS and indirectly reflects neuronal damage caused by free radicals.[104] Elevated plasma MDA levels have been reported in stroke patients.[105] Elevated plasma lipid H2O2 level in stroke patients at admission was shown to be correlated with the severity of neurologic defects.[106] Thus, MDA level is an indicator of stroke severity. Lipid peroxide (LPO) is produced by the reaction of oxygen radicals with polyunsaturated fatty acids and can serve as a marker of OS in cerebral venous sinus thrombosis and stroke,[48] and can also be used for the differential diagnosis of stroke.
Nanomaterial antioxidants in stroke treatment
Nanomaterials are used for drug delivery in the treatment of diseases; some have antioxidant properties and can potentially scavenge ROS following stroke. Nanoparticle drug delivery systems can increase the blood concentration and half-life of drugs and protect neurons from OS-induced death in the ischemic brain.[107] In one study, macrophage-disguised honeycomb manganese dioxide nanospheres loaded with fingolimod reduced OS and alleviated the inflammatory response and neuronal death.[108] Nanomaterial antioxidants thus offer the possibility of multitargeted treatment of IS.
Regulation of OS and antioxidant defense system by methylene blue (MB)
Clinical applications of the redox dye MB include the treatment of methemoglobinemia, ifosfamide encephalopathy, and septic shock.[109] MB is also a potent guanylate cyclase inhibitor; in isolated hepatocytes and HeLa cells, MB was shown to inhibit ethanol-induced redox changes and fat deposition,[110] and its impact has been investigated in cultured human erythrocytes. However, the effects of MB on OS are controversial.[111] MB reduced OS in rats treated with cyclosporine A but increased intracellular OS at concentrations up to 5 µM in cultured endothelium cells.[112] MB can also be cytotoxic over the long term.[113] MB exposure induced OS caused by increased ROS levels and decreased CAT, SOD, SOD, and total cellular antioxidant levels.[114]
Conclusions
This review discussed the central role of OS as a bridge between aging and stroke, and the potential role of antioxidant enzymes, compounds, or molecules in stroke treatment. Despite the contribution of OS to the pathology of stroke, pharmacologic agents that alleviate OS have had limited benefit for stroke patients thus far. However, there are opportunities for the development of effective treatments for stroke using neuroprotective agents and multitargeted approaches. The combination of anti-OS, anti-inflammatory, and other agents is a promising future research direction.
Author Contributions
Shengjie Feng, Miaoxian Yang, Shengpeng Liu, Yu He: Conceptualization and design. Shengjie Feng, Miaoxian Yang: Writing, Original draft preparation and Editing. Ye Gong,Shuixiang Deng: Writing-Reviewing, Supervision and Validation.
Acknowledgments
The authors thank Dr. Jixin Wu for English language editing.
Funding
This study was supported by a grant from the Shanghai Hospital Development Centre (grant number: SHDC2020CR3021A, to YG) and Science and Technology Commission of Shanghai Municipal (grant number: 21ZR1410700, to SD).
Ethics Statement
Not applicable.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Managing Editor: Jingling Bao
Contributor Information
Shuixiang Deng, Email: shuixiang2@126.com.
Ye Gong, Email: gong_ye@fudan.edu.cn.
References
- 1.Barthels D., Das H. Current advances in ischemic stroke research and therapies. Biochim Biophys Acta Mol Basis Dis. 2020;1866(4) doi: 10.1016/j.bbadis.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahman A.A., Amruta N., Pinteaux E., Bix G.J. Neurogenesis after stroke: a therapeutic perspective. Transl Stroke Res. 2021;12(1):1–14. doi: 10.1007/s12975-020-00841-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpkins A.N., Janowski M., Oz H.S., Roberts J., Bix G., Doré S., et al. Biomarker application for precision medicine in stroke. Transl Stroke Res. 2020;11(4):615–627. doi: 10.1007/s12975-019-00762-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGlinchey M.P., James J., McKevitt C., Douiri A., McLachlan S., Sackley C.M. The effect of rehabilitation interventions on physical function and immobility-related complications in severe stroke-protocol for a systematic review. Syst Rev. 2018;7(1):197. doi: 10.1186/s13643-018-0870-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 6.Campbell B.C.V., De Silva D.A., Macleod M.R., Coutts S.B., Schwamm L.H., Davis S.M., et al. Ischaemic stroke. Nat Rev Dis Primers. 2019;5(1):70. doi: 10.1038/s41572-019-0118-8. [DOI] [PubMed] [Google Scholar]
- 7.Spence J.D., Azarpazhooh M.R., Larsson S.C., Bogiatzi C., Hankey G.J. Stroke prevention in older adults: recent advances. Stroke. 2020;51(12):3770–3777. doi: 10.1161/STROKEAHA.120.031707. [DOI] [PubMed] [Google Scholar]
- 8.Sidorov E.V., Bejar C., Xu C., Ray B., Gordon D., Chainakul J., et al. Novel metabolites as potential indicators of ischemic infarction volume: a pilot study. Transl Stroke Res. 2021;12(5):778–784. doi: 10.1007/s12975-020-00876-z. [DOI] [PubMed] [Google Scholar]
- 9.Paul S., Candelario-Jalil E. Emerging neuroprotective strategies for treating ischemic stroke: an overview of clinical and preclinical studies. Exp Neurol. 2021;335 doi: 10.1016/j.expneurol.2020.113518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ojaghihaghighi S., Vahdati S.S., Mikaeilpour A., Ramouz A. Comparison of neurological clinical manifestation in patients with hemorrhagic and ischemic stroke. World J Emerg Med. 2017;8(1):34–38. doi: 10.5847/wjem.j.1920-8642.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stahl S., Schaller H.E. Springer; Berlin, Heidelberg: 2016. Verätzungen. in: verbrennungschirurgie; pp. 341–353. [DOI] [Google Scholar]
- 12.Abdu H., Tadese F., Seyoum G. Comparison of ischemic and hemorrhagic stroke in the medical ward of Dessie referral hospital, northeast Ethiopia: a retrospective study. Neurol Res Int. 2021;2021 doi: 10.1155/2021/9996958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang D., Bian Y., Zeng Z., Cui Y., Wang Y., Yu C. Associations between intensity, frequency, duration, and volume of physical activity and the risk of stroke in middle- and older-aged Chinese people: a cross-sectional study. Int J Environ Res Public Health. 2020;17(22):8628. doi: 10.3390/ijerph17228628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy-O'Reilly M., McCullough L.D. Age and sex are critical factors in ischemic stroke pathology. Endocrinology. 2018;159(8):3120–3131. doi: 10.1210/en.2018-00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balasubramanian P., Branen L., Sivasubramanian M.K., Monteiro R., Subramanian M. Aging is associated with glial senescence in the brainstem - implications for age-related sympathetic over activity. Ageing. 2021;13(10):13460–13473. doi: 10.18632/aging.203111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newgard C.B., Sharpless N.E. Coming of age: molecular drivers of ageing and therapeutic opportunities. J Clin Invest. 2013;123(3):946–950. doi: 10.1172/JCI68833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suri R., Rodriguez-Porcel F., Donohue K., Jesse E., Lovera L., Dwivedi A.K., et al. Post-stroke movement disorders: the clinical, neuroanatomic, and demographic portrait of 284 published cases. J Stroke Cerebrovasc Dis. 2018;27(9):2388–2397. doi: 10.1016/j.jstrokecerebrovasdis.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 18.Ajoolabady A., Wang S., Kroemer G., Penninger J.M., Uversky V.N., Pratico D., et al. Targeting autophagy in ischemic stroke: from molecular mechanisms to clinical therapeutics. Pharmacol Ther. 2021;225 doi: 10.1016/j.pharmthera.2021.107848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi Y.H., Laaker C., Hsu M., Cismaru P., Sandor M., Fabry Z. Molecular mechanisms of neuroimmune crosstalk in the pathogenesis of stroke. Int J Mol Sci. 2021;22(17):9486. doi: 10.3390/ijms22179486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herpich F., Rincon F. Management of acute ischemic stroke. Crit Care Med. 2020;48(11):1654–1663. doi: 10.1097/CCM.0000000000004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ospel J.M., Holodinsky J.K., Goyal M. Management of acute ischemic stroke due to large-vessel occlusion: JACC focus seminar. J Am Coll Cardiol. 2020;75(15):1832–1843. doi: 10.1016/j.jacc.2019.10.034. [DOI] [PubMed] [Google Scholar]
- 22.Du J., Yin G., Hu Y., Shi S., Jiang J., Song X., et al. Coicis semen protects against focal cerebral ischemia-reperfusion injury by inhibiting oxidative stress and promoting angiogenesis via the TGFβ/ALK1/Smad1/5 signaling pathway. Ageing. 2020;13(1):877–893. doi: 10.18632/aging.202194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vemuganti R. Stroke: molecular mechanisms and therapies. Neuromolecular Med. 2019;21(4):323–324. doi: 10.1007/s12017-019-08580-4. [DOI] [PubMed] [Google Scholar]
- 24.Sekerdag E., Solaroglu I., Gursoy-Ozdemir Y. Cell death mechanisms in stroke and novel molecular and cellular treatment options. Curr Neuropharmacol. 2018;16(9):1396–1415. doi: 10.2174/1570159X16666180302115544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He Z., Ning N., Zhou Q., Khoshnam S.E., Farzaneh M. Mitochondria as a therapeutic target for ischemic stroke. Free Radic Biol Med. 2020;146:45–58. doi: 10.1016/j.freeradbiomed.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Chen H., Yoshioka H., Kim G.S., Jung J.E., Okami N., Sakata H., et al. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal. 2011;14(8):1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan P.H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21(1):2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Manzanero S., Santro T., Arumugam T.V. Neuronal oxidative stress in acute ischemic stroke: sources and contribution to cell injury. Neurochem Int. 2013;62(5):712–718. doi: 10.1016/j.neuint.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 30.Achzet L.M., Davison C.J., Shea M., Sturgeon I., Jackson D.A. Oxidative stress underlies the ischemia/reperfusion-induced internalization and degradation of AMPA receptors. Int J Mol Sci. 2021;22(2):717. doi: 10.3390/ijms22020717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Y., Zhang S., Fan X. Role of polyphenols as antioxidant supplementation in ischemic stroke. Oxid Med Cell Longev. 2021;2021 doi: 10.1155/2021/5471347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao L., Wang K., Zhang P., Ren S., Sun J., Yang M., et al. Carbonyl reductase 1 attenuates ischemic brain injury by reducing oxidative stress and neuroinflammation. Transl Stroke Res. 2021;12(5):711–724. doi: 10.1007/s12975-021-00912-6. [DOI] [PubMed] [Google Scholar]
- 33.Ren J.X., Li C., Yan X.L., Qu Y., Yang Y., Guo Z.N. Crosstalk between oxidative stress and ferroptosis/oxytosis in ischemic stroke: possible targets and molecular mechanisms. Oxid Med Cell Longev. 2021;2021 doi: 10.1155/2021/6643382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCarty M.F., Lerner A. Nutraceutical induction and mimicry of heme oxygenase activity as a strategy for controlling excitotoxicity in brain trauma and ischemic stroke: focus on oxidative stress. Expert Rev Neurother. 2021;21(2):157–168. doi: 10.1080/14737175.2021.1861940. [DOI] [PubMed] [Google Scholar]
- 35.Chamorro Á., Dirnagl U., Urra X., Planas A.M. Neuroprotection in acute stroke: targeting Excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15(8):869–881. doi: 10.1016/s1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigo R., Fernández-Gajardo R., Gutiérrez R., Matamala J.M., Carrasco R., Miranda-Merchak A., et al. Oxidative stress and pathophysiology of ischemic stroke: novel therapeutic opportunities. CNS Neurol Disord Drug Targets. 2013;12(5):698–714. doi: 10.2174/1871527311312050015. [DOI] [PubMed] [Google Scholar]
- 37.Allen C.L., Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4(6):461–470. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- 38.Yan W., Diao S., Fan Z. The role and mechanism of mitochondrial functions and energy metabolism in the functional regulation of the mesenchymal stem cells. Stem Cell Res Ther. 2021;12(1):140. doi: 10.1186/s13287-021-02194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srivastava S. The mitochondrial basis of ageing and age-related disorders. Genes. 2017;8(12):398. doi: 10.3390/genes8120398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kauppila T.E.S., Kauppila J.H.K., Larsson N.G. Mammalian mitochondria and ageing: an update. Cell Metab. 2017;25(1):57–71. doi: 10.1016/j.cmet.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 41.Cenini G., Lloret A., Cascella R. Oxidative stress in neurodegenerative diseases: from a mitochondrial point of view. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/2105607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shpilka T., Haynes C.M. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat Rev Mol Cell Biol. 2018;19(2):109–120. doi: 10.1038/nrm.2017.110. [DOI] [PubMed] [Google Scholar]
- 43.Soane L., Solenski N., Fiskum G. Mitochondrial mechanisms of oxidative stress and apoptosis. Handbook of neurochemistry and molecular neurobiology-brain energetics. Integr Mol Cell Process. 2007;5:703–734. doi: 10.1007/978-0-387-30411-3_26. [DOI] [Google Scholar]
- 44.Narne P., Pandey V., Phanithi P.B. The interplay between mitochondrial metabolism and oxidative stress in ischemic stroke: an epigenetic connection. Mol Cell Neurosci. 2017;82:176–194. doi: 10.1016/j.mcn.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Lu M., Guo J., Wu B., Zhou Y., Wu M., Farzaneh M., et al. Mesenchymal stem cell-mediated mitochondrial transfer: a therapeutic approach for ischemic stroke. Transl Stroke Res. 2021;12(2):212–229. doi: 10.1007/s12975-020-00853-6. [DOI] [PubMed] [Google Scholar]
- 46.Li P., Stetler R.A., Leak R.K., Shi Y., Li Y., Yu W., et al. Oxidative stress and DNA damage after cerebral ischemia: potential therapeutic targets to repair the genome and improve stroke recovery. Neuropharmacology. 2018;134:208–217. doi: 10.1016/j.neuropharm.2017.11.011. Pt B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olloquequi J., Cornejo-Córdova E., Verdaguer E., Soriano F.X., Binvignat O., Auladell C., et al. Excitotoxicity in the pathogenesis of neurological and psychiatric disorders: therapeutic implications. J Psychopharmacol. 2018;32(3):265–275. doi: 10.1177/0269881118754680. [DOI] [PubMed] [Google Scholar]
- 48.Tiwari H.S., Misra U.K., Kalita J., Mishra A., Shukla S. Oxidative stress and glutamate excitotoxicity contribute to apoptosis in cerebral venous sinus thrombosis. Neurochem Int. 2016;100:91–96. doi: 10.1016/j.neuint.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Sun M.S., Jin H., Sun X., Huang S., Zhang F.L., Guo Z.N., et al. Free radical damage in ischemia-reperfusion injury: an obstacle in acute ischemic stroke after revascularization therapy. Oxid Med Cell Longev. 2018 doi: 10.1155/2018/3804979. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shao A., Lin D., Wang L., Tu S., Lenahan C., Zhang J. Oxidative stress at the crossroads of aging, stroke and depression. Aging Dis. 2020;11(6):1537–1566. doi: 10.14336/AD.2020.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang W., Jiang B., Sun H., Ru X., Sun D., Wang L., et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135(8):759–771. doi: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 52.Sposato L.A., Saposnik G. Gross domestic product and health expenditure associated with incidence, 30-day fatality, and age at stroke onset: a systematic review. Stroke. 2012;43(1):170–177. doi: 10.1161/STROKEAHA.111.632158. [DOI] [PubMed] [Google Scholar]
- 53.Albieri V., Olsen T.S., Andersen K.K. Risk of stroke in migraineurs using triptans. Associations with age, sex, stroke severity and subtype. EBioMedicine. 2016;6:199–205. doi: 10.1016/j.ebiom.2016.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grifoni E., Giglio D., Guazzini G., Cosentino E., Latini E., Dei A., et al. Age-related burden and characteristics of embolic stroke of undetermined source in the real world clinical practice. J Thromb Thrombolysis. 2020;49(1):75–85. doi: 10.1007/s11239-019-01951-5. [DOI] [PubMed] [Google Scholar]
- 55.Ames B.N., Shigenaga M.K., Hagen T.M. Oxidants, antioxidants, and the degenerative diseases of ageing. Proc Natl Acad Sci USA. 1993;90(17):7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ames B.N., Shigenaga M.K. Oxidants are a major contributor to ageing. Ann N Y Acad Sci. 1992;663:85–96. doi: 10.1111/j.1749-6632.1992.tb38652.x. [DOI] [PubMed] [Google Scholar]
- 57.Harman D. Free radical theory of ageing. Mutat Res. 1992;275(3–6):257–266. doi: 10.1016/0921-8734(92)90030-S. [DOI] [PubMed] [Google Scholar]
- 58.Fraga C.G., Shigenaga M.K., Park J.W., Degan P., Ames B.N. Oxidative damage to DNA during ageing: 8-hydroxy-2¢-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci USA. 1990;87(12):4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Angelova P.R., Esteras N., Abramov A.Y. Mitochondria and lipid peroxidation in the mechanism of neurodegeneration: finding ways for prevention. Med Res Rev. 2021;41(2):770–784. doi: 10.1002/med.21712. [DOI] [PubMed] [Google Scholar]
- 60.Kopitz J., Holz F.G., Kaemmerer E., Schutt F. Lipids and lipid peroxidation products in the pathogenesis of age-related macular degeneration. Biochimie. 2004;86(11):825–831. doi: 10.1016/j.biochi.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 61.Stadtman E.R. Protein oxidation and ageing. Science. 1992;257(5074):1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 62.Harman D. The ageing process. Proc Natl Acad Sci USA. 1981;78(11):7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bratic A., Larsson N.G. The role of mitochondria in ageing. J Clin Invest. 2013;123(3):951–957. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stefanatos R., Sanz A. The role of mitochondrial ROS in the ageing brain. FEBS Lett. 2018;592(5):743–758. doi: 10.1002/1873-3468.12902. [DOI] [PubMed] [Google Scholar]
- 65.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 66.Bettio L.E.B., Rajendran L., Gil-Mohapel J. The effects of ageing in the hippocampus and cognitive decline. Neurosci Biobehav Rev. 2017;79:66–86. doi: 10.1016/j.neubiorev.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 67.Shi L., Rocha M., Zhang W., Jiang M., Li S., Ye Q., et al. Genome-wide transcriptomic analysis of microglia reveals impaired responses in aged mice after cerebral ischemia. J Cereb Blood Flow Metab. 2020;40:S49–S66. doi: 10.1177/0271678x20925655. 1 Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mattson M.P., Arumugam T.V. Hallmarks of brain aging: adaptive and pathological modification by metabolic states. Cell Metab. 2018;27(6):1176–1199. doi: 10.1016/j.cmet.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rzechorzek W., Zhang H., Buckley B.K., Hua K., Pomp D., Faber J.E. Aerobic exercise prevented the rarefaction of pial collaterals and increased stroke severity that occurs with ageing. J Cereb Blood Flow Metab. 2017;37(11):3544–3555. doi: 10.1177/0271678X17718966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soliman A.M., Das S., Mahakkanukrauh P. Inflammatory molecular mediators and pathways involved in vascular aging and stroke: a comprehensive review. Curr Med Chem. 2022;29(34):552–5542. doi: 10.2174/0929867328666210901122359. [DOI] [PubMed] [Google Scholar]
- 71.Heydari S., Ghanbarzadeh S., Anoush B., Ranjkesh M., Javadzadeh Y., Kouhsoltani M., et al. Nanoethosomal formulation of gamma oryzanol for skin-ageing protection and wrinkle improvement: a histopathological study. Drug Dev Ind Pharm. 2017;43(7):1154–1162. doi: 10.1080/03639045.2017.1300169. [DOI] [PubMed] [Google Scholar]
- 72.Furue M., Uchi H., Mitoma C., Hashimoto-Hachiya A., Chiba T., Ito T., et al. Antioxidants for healthy skin: the emerging role of aryl hydrocarbon receptors and nuclear factor-erythroid 2-related factor-2. Nutrients. 2017;9(3):223. doi: 10.3390/nu9030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cherubini A., Polidori M.C., Bregnocchi M., Pezzuto S., Cecchetti R., Ingegni T., et al. Antioxidant profile and early outcome in stroke patients. Stroke. 2000;31(10):2295–2300. doi: 10.1161/01.str.31.10.2295. [DOI] [PubMed] [Google Scholar]
- 74.Zini I., Tomasi A., Grimaldi R., Vannini V., Agnati L.F. Detection of free radicals during brain ischemia and reperfusion by spin trapping and microdialysis. Neurosci Lett. 1992;138(2):279–282. doi: 10.1016/0304-3940(92)90933-x. [DOI] [PubMed] [Google Scholar]
- 75.Mecocci P., Boccardi V., Cecchetti R., Bastiani P., Scamosci M., Ruggiero C., et al. A long journey into ageing, brain ageing, and Alzheimer's disease following the oxidative stress tracks. J Alzheimer Dis. 2018;62(3):1319–1335. doi: 10.3233/JAD-170732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilton A. Risk factors for postoperative complications and in-hospital mortality following surgery for cervical spinal cord injury. Cureus. 2022;14(11):e31960. doi: 10.7759/cureus.31960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu N.N., Dong Z.L., Han L.L. MicroRNA-410 inhibition of the TIMP2-dependent MAPK pathway confers neuroprotection against oxidative stress-induced apoptosis after ischemic stroke in mice. Brain Res Bull. 2018;143:45–57. doi: 10.1016/j.brainresbull.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 78.Milanlioglu A., Aslan M., Ozkol H., Çilingir V., Nuri Aydın M., Karadas S. Serum antioxidant enzymes activities and oxidative stress levels in patients with acute ischemic stroke: influence on neurological status and outcome. Wien Klin Wochenschr. 2016;128(5–6):169–174. doi: 10.1007/s00508-015-0742-6. [DOI] [PubMed] [Google Scholar]
- 79.Voetsch B., Jin R.C., Bierl C., Benke K.S., Kenet G., Simioni P., et al. Promoter polymorphisms in the plasma glutathione peroxidase (GPx-3) gene: a novel risk factor for arterial ischemic stroke among young adults and children. Stroke. 2007;38(1):41–49. doi: 10.1161/01.STR.0000252027.53766.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Demirkaya S., Topcuoglu M.A., Aydin A., Ulas U.H., Isimer A.I., Vural O. Malondialdehyde, glutathione peroxidase and superoxide dismutase in peripheral blood erythrocytes of patients with acute cerebral ischemia. Eur J Neurol. 2001;8(1):43–51. doi: 10.1046/j.1468-1331.2001.00166.x. [DOI] [PubMed] [Google Scholar]
- 81.Ishibashi N., Prokopenko O., Reuhl K.R., Mirochnitchenko O. Inflammatory response and glutathione peroxidase in a stroke model. J Immunol. 2002;168(4):1926–1933. doi: 10.4049/jimmunol.168.4.1926. [DOI] [PubMed] [Google Scholar]
- 82.Ou Z., Chen Y., Li J., Ouyang F., Liu G., Tan S., et al. Glucose-6-phosphate dehydrogenase deficiency and stroke outcomes. Neurology. 2020;95(11):e1471–e1478. doi: 10.1212/WNL.0000000000010245. [DOI] [PubMed] [Google Scholar]
- 83.Chen Y., Li J., Ou Z., Zhang Y., Liang Z., Deng W., et al. Safety and efficacy of low-dose aspirin in ischemic stroke patients with different G6PD conditions. Int J Stroke. 2021;16(4):411–419. doi: 10.1177/1747493020950903. [DOI] [PubMed] [Google Scholar]
- 84.Wu X., Fu R., Tang Y., Shi L., Rong X., Guo J., et al. Intravenous thrombolysis for stroke patients with G6PD deficiency. J Stroke Cerebrovasc Dis. 2018;27(7):2026–2031. doi: 10.1016/j.jstrokecerebrovasdis.2018.02.060. [DOI] [PubMed] [Google Scholar]
- 85.Goyal M.M., Basak A. Human catalase: looking for a complete identity. Protein Cell. 2010;1(10):888–897. doi: 10.1007/s13238-010-0113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alfonso-Prieto M., Biarnés X., Vidossich P., Rovira C. The molecular mechanism of the catalase reaction. J Am Chem Soc. 2009;131(33):11751–11761. doi: 10.1021/ja9018572. [DOI] [PubMed] [Google Scholar]
- 87.Žitňanová I., Šiarnik P., Kollár B., Chomová M., Pazderová P., Andrezálová L., et al. Oxidative stress markers and their dynamic changes in patients after acute ischemic stroke. Oxid Med Cell Longev. 2016 doi: 10.1155/2016/9761697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pumpho A., Chaikeeree N., Saengsirisuwan V., Boonsinsukh R. Selection of the better dual-timed up and go cognitive task to be used in patients with stroke characterized by subtraction operation difficulties. Front Neurol. 2020;11:262. doi: 10.3389/fneur.2020.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaur N., Sharma A.K., Shakeel A., Kumar V., Singh A., Gupta A., et al. Therapeutic implications of superoxide dismutase and its importance in kinase drug discovery. Curr Top Med Chem. 2017;17(22):2495–2508. doi: 10.2174/1568026617666170307112837. [DOI] [PubMed] [Google Scholar]
- 90.Maier C.M., Sun G.H., Cheng D., Yenari M.A., Chan P.H., Steinberg G.K. Effects of mild hypothermia on superoxide anion production, superoxide dismutase expression, and activity following transient focal cerebral ischemia. Neurobiol Dis. 2002;11(1):28–42. doi: 10.1006/nbdi.2002.0513. [DOI] [PubMed] [Google Scholar]
- 91.Lin S.P., Tu C., Huang W., Wu Y., Lin P.Y., Ye S., et al. Acute-phase serum superoxide dismutase level as a predictive biomarker for stroke-associated infection. Int J Neurosci. 2020;130(2):186–192. doi: 10.1080/00207454.2019.1667790. [DOI] [PubMed] [Google Scholar]
- 92.Li L., Tovmasyan A., Sheng H., Xu B., Sampaio R.S., Reboucas J.S., et al. Fe porphyrin-based SOD mimic and redox-active compound, (OH)FeTnHex-2-PyP4+, in a rodent ischemic stroke (MCAO) model: efficacy and pharmacokinetics as compared to its Mn analogue, (H2O)MnTnHex-2-PyP5+ Antioxidants. 2020;9(6):467. doi: 10.3390/antiox9060467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang H.F., Guo F., Cao Y.Z., Shi W., Xia Q. Neuroprotection by manganese superoxide dismutase (MnSOD) mimics antioxidant effect and oxidative stress regulation in acute experimental stroke. CNS Neurosci Ther. 2012;18(10):811–818. doi: 10.1111/j.1755-5949.2012.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morelli M.B., Gambardella J., Castellanos V., Trimarco V., Santulli G. Vitamin C and cardiovascular disease: an update. Antioxidants. 2020;9(12):1227. doi: 10.3390/antiox9121227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang C.Y., Chen J.Y., Wu M.H., Hu M.L. Therapeutic treatment with vitamin C reduces focal cerebral ischemia-induced brain infarction in rats by attenuating disruptions of blood brain barrier and cerebral neuronal apoptosis. Free Radic Biol Med. 2020;155:29–36. doi: 10.1016/j.freeradbiomed.2020.05.015. [DOI] [PubMed] [Google Scholar]
- 96.Zhong S., Li L., Shen X., Li Q., Xu W., Wang X., et al. An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic Biol Med. 2019;144:266–278. doi: 10.1016/j.freeradbiomed.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 97.Zhang N., Zhao W., Hu Z.J., Ge S.M., Huo Y., Liu L.X., et al. Protective effects and mechanisms of high-dose vitamin C on sepsis-associated cognitive impairment in rats. Sci Rep. 2021;11(1):14511. doi: 10.1038/s41598-021-93861-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ge Y., Lin D., Cui B., Zhang L., Li S., Wang Z., et al. Effects of long noncoding RNA H19 on isoflurane-induced cognitive dysregulation by promoting neuroinflammation. Neuroimmunomodulation. 2022;29(2):117–127. doi: 10.1159/000519124. [DOI] [PubMed] [Google Scholar]
- 99.Brown J.E., Khodr H., Hider R.C., Rice-Evans C.A. Structural dependence of flavonoid interactions with Cu2+ ions: implications for their antioxidant properties. Biochem J. 1998;330:1173–1178. doi: 10.1042/bj3301173. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J Nutr Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yamagata K. Polyphenols regulate endothelial functions and reduce the risk of cardiovascular disease. Curr Pharm Des. 2019;25(22):2443–2458. doi: 10.2174/1381612825666190722100504. [DOI] [PubMed] [Google Scholar]
- 102.Rees A., Dodd G.F., Spencer J.P.E. The effects of flavonoids on cardiovascular health: a review of human intervention trials and implications for cerebrovascular function. Nutrients. 2018;10(12):1852. doi: 10.3390/nu10121852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bian Y., Yamashita T., Taira Y., Shang J., Tsunoda K., Feng T., et al. A polyphenolic complex attenuates inflammatory response and blood- brain barrier disruption. Curr Neurovasc Res. 2020;17(3):286–293. doi: 10.2174/1567202617666200517105727. [DOI] [PubMed] [Google Scholar]
- 104.Zeng J., Zhu L., Liu J., Zhu T., Xie Z., Sun X., et al. Metformin protects against oxidative stress injury induced by ischemia/reperfusion via regulation of the lncRNA-H19/miR-148a-3p/Rock2 axis. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/8768327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cojocaru I.M., Cojocaru M., Sapira V., Ionescu A. Evaluation of oxidative stress in patients with acute ischemic stroke. Rom J Intern Med. 2013;51(2):97–106. [PubMed] [Google Scholar]
- 106.Ferretti G., Bacchetti T., Masciangelo S., Nanetti L., Mazzanti L., Silvestrini M., et al. Lipid peroxidation in stroke patients. Clin Chem Lab Med. 2008;46(1):113–117. doi: 10.1515/CCLM.2008.011. [DOI] [PubMed] [Google Scholar]
- 107.Song G., Zhao M., Chen H., Lenahan C., Zhou X., Ou Y., et al. The role of nanomaterials in stroke treatment: targeting oxidative stress. Oxid Med Cell Longev. 2021;2021 doi: 10.1155/2021/8857486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li C., Zhao Z., Luo Y., Ning T., Liu P., Chen Q., et al. Macrophage-disguised manganese dioxide nanoparticles for neuroprotection by reducing oxidative stress and modulating inflammatory microenvironment in acute ischemic stroke. Adv Sci (Weinh) 2021;8(20) doi: 10.1002/advs.202101526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hotchkiss R.S., Moldawer L.L., Opal S.M., Reinhart K., Turnbull I.R., Vincent J.L. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.May J.M., Qu Z.C., Cobb C.E. Reduction and uptake of methylene blue by human erythrocytes. Am J Physiol Cell Physiol. 2004;286(6):C1390–C1398. doi: 10.1152/ajpcell.00512.2003. [DOI] [PubMed] [Google Scholar]
- 111.Rezzani R., Rodella L., Corsetti G., Bianchi R. Does methylene blue protect the kidney tissues from damage induced by ciclosporin a treatment? Nephron. 2001;89(3):329–336. doi: 10.1159/000046094. [DOI] [PubMed] [Google Scholar]
- 112.May J.M., Qu Z.C., Whitesell R.R. Generation of oxidant stress in cultured endothelial cells by methylene blue: protective effects of glucose and ascorbic acid. Biochem Pharmacol. 2003;66(5):777–784. doi: 10.1016/S0006-2952(03)00408-8. [DOI] [PubMed] [Google Scholar]
- 113.Yildiz H., Durmus A.S., Simsek H. Surgery-induced changes in red blood cell and plasma lipid peroxidation, enzymatic and non-enzymatic antioxidants, and blood haematology of female rats: protective role of methylene blue and vitamin E. Eur J Obstet Gynecol Reprod Biol. 2011;155(1):89–93. doi: 10.1016/j.ejogrb.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 114.Aksu B., Umit H., Kanter M., Guzel A., Aktas C., Civelek S., et al. Effects of methylene blue in reducing cholestatic oxidative stress and hepatic damage after bile-duct ligation in rats. Acta Histochem. 2010;112(3):259–269. doi: 10.1016/j.acthis.2008.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.