Abstract
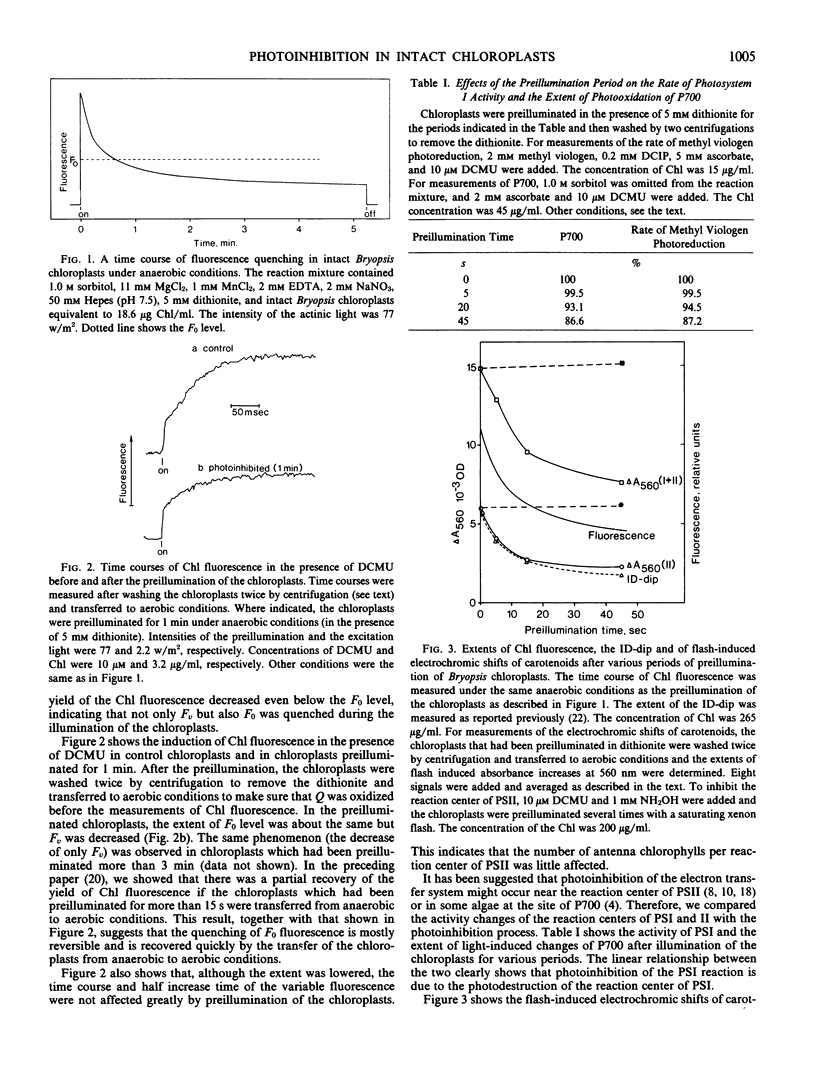
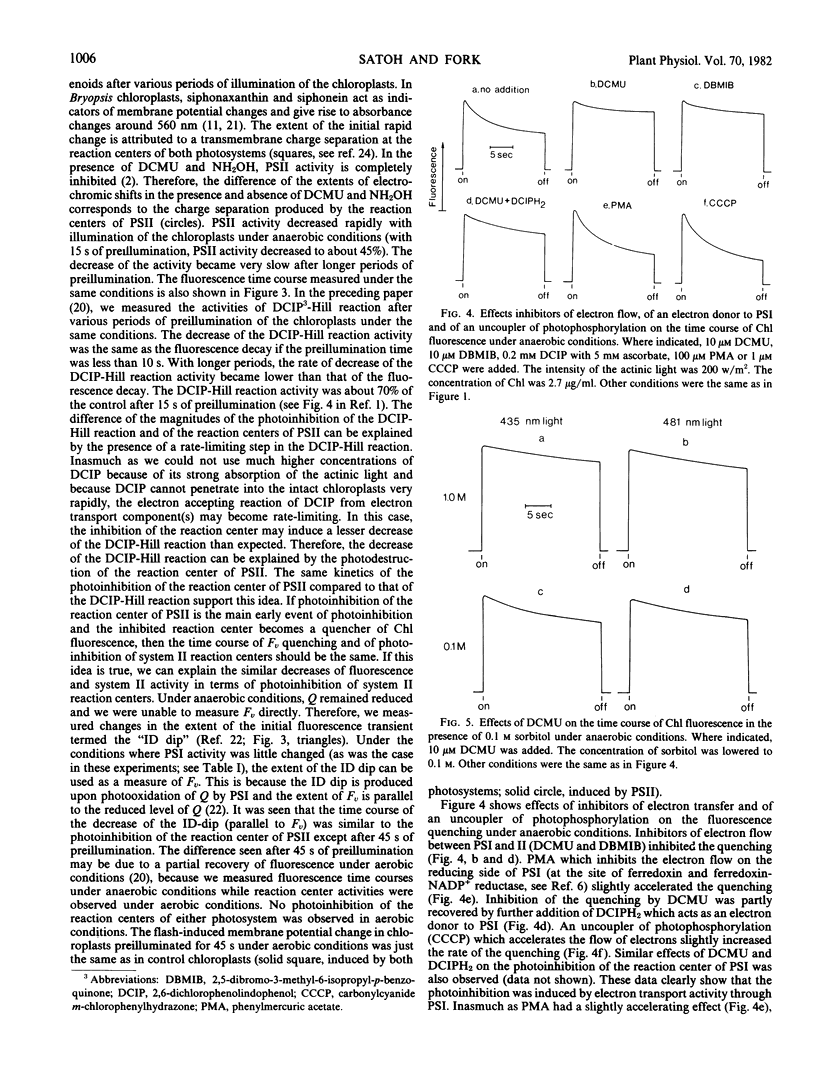
Illumination of intact Bryopsis corticulans chloroplasts under anaerobic conditions induced a decline of chlorophyll fluorescence and photoinhibition of Photosystems I and II. The time course of the light-induced decline of chlorophyll fluorescence and the decreases of activities of reactions sensitized by Photosystems I and II were compared. Photosystem I activity decreased in parallel with the disappearance of active P700. The time course of the destruction of the reaction center of Photosystem II was similar to that of photoinhibition of 2,6-dichlorophenolindophenol-Hill reaction.
It appears that the initial events in photoinhibition are the destruction of the reaction centers of Photosystems I and II and that the reaction centers that are inhibited become quenchers of chlorophyll fluorescence.
Effects of inhibitors of electron transfer and of an electron donor to Photosystem I showed that photoinhibition was related to Photosystem I activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennoun P. Réoxydation du quencher de fluorescence "Q" en présence de 3-(3,4-dichlorophényl)-1,1-diméthylurée. Biochim Biophys Acta. 1970 Sep 1;216(2):357–363. doi: 10.1016/0005-2728(70)90227-6. [DOI] [PubMed] [Google Scholar]
- Gerber D. W., Burris J. E. Photoinhibition and P700 in the Marine Diatom Amphora sp. Plant Physiol. 1981 Sep;68(3):699–702. doi: 10.1104/pp.68.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt R. C., Krogmann D. W. Inhibition of chloroplast reactions with phenylmercuric acetate. Plant Physiol. 1972 Mar;49(3):376–380. doi: 10.1104/pp.49.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. W., Kok B. Photoinhibition of Chloroplast Reactions. II. Multiple Effects. Plant Physiol. 1966 Jun;41(6):1044–1049. doi: 10.1104/pp.41.6.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. W., Kok B. Photoinhibition of chloroplast reactions. I. Kinetics and action spectra. Plant Physiol. 1966 Jun;41(6):1037–1043. doi: 10.1104/pp.41.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok B., Gassner E. B., Rurainski H. J. Photoinhibition of chloroplast reactions. Photochem Photobiol. 1966 Mar;4(2):215–227. doi: 10.1111/j.1751-1097.1965.tb05739.x. [DOI] [PubMed] [Google Scholar]
- Powles S. B., Osmond C. B. Photoinhibition of intact attached leaves of c(3) plants illuminated in the absence of both carbon dioxide and of photorespiration. Plant Physiol. 1979 Dec;64(6):982–988. doi: 10.1104/pp.64.6.982. [DOI] [PMC free article] [PubMed] [Google Scholar]


