Abstract
For many years, the methods of cancer treatment are usually surgery, chemotherapy and radiation therapy. Although these methods help to improve the condition, most tumors still have a poor prognosis. In recent years, immunotherapy has great potential in tumor treatment. Chimeric antigen receptor T-cell immunotherapy (CAR-T) uses the patient's own T cells to express chimeric antigen receptors. Chimeric antigen receptor (CAR) recognizes tumor-associated antigens and kills tumor cells. CAR-T has achieved good results in the treatment of hematological tumors. In 2017, the FDA approved the first CAR-T for the treatment of B-cell acute lymphoblastic leukemia (ALL). In October of the same year, the FDA approved CAR-T to treat B-cell lymphoma. In order to improve and enhance the therapeutic effect, CAR-T has become a research focus in recent years. The structure of CAR, the targets of CAR-T treatment, adverse reactions and improvement measures during the treatment process are summarized. This review is an attempt to highlight recent and possibly forgotten findings of advances in chimeric antigen receptor T cell for treatment of hematological tumors.
Keywords: Chimeric antigen receptor, T cell immunotherapy, Hematological malignancies, Target therapy
Graphical abstract
The primary challenges in CAR-T immunotherapy include the presence of adverse side effects, an unfavorable tumor microenvironment (TME), and T-cell exhaustion. Furthermore, there is a pressing need to overcome the difficulty of expanding the production of clinical-grade cell therapies to ensure their accessibility and affordability for a larger global patient population.

1. Introduction
Chimeric Antigen Receptor T-cell therapy, commonly referred to as CAR-T therapy, represents a groundbreaking advancement in the field of immunotherapy for cancer treatment. The foundation for CAR-T therapy was laid in the 1980s when scientists began exploring ways to modify immune cells to target cancer. Early experiments involved modifying T cells to express tumor-specific receptors. These efforts, however, faced challenges in achieving clinical success [1]. In 1989, Zelig Eshhar, an Israeli immunologist, along with his team at the Weizmann Institute of Science, outlined a comparable strategy for reprogramming T cells to identify antigens independently of the major histocompatibility complex (MHC) [2]. In the early 2000s, researchers made a breakthrough by developing CARs. These synthetic receptors combined an extracellular antigen-binding domain, typically derived from an antibody, with intracellular T-cell activation domains. This design allowed T cells to recognize specific cancer antigens. Encouraged by the success in B-cell malignancies, researchers began exploring CAR-T therapy for other cancers. Clinical trials expanded to include non-Hodgkin lymphoma (NHL) and multiple myeloma. Additionally, efforts to target solid tumors intensified, although this area remains challenging [3]. CAR-T therapy involves the genetic modification of a patient's own T cells to equip them with a chimeric antigen receptor (CAR), which enables these cells to recognize and attack cancer cells with precision. Unlike traditional cancer treatments, such as chemotherapy and radiation, CAR-T therapy harnesses the power of the immune system to target and destroy cancer cells (Fig. 1).
Fig. 1.
History of cancer immunotherapy development. CAR-T therapy has a relatively short but impactful history, emerging in the early 21st century as a breakthrough in cancer treatment. The therapy gained prominence with the first FDA approval in 2017, marking a significant milestone in the field of immunotherapy.
Six CAR-T cell therapies have been granted approval for hematological cancers by the Food and Drug Administration since 2017. Four out of six CAR-T cell products are anti-CD19 CARs, and the two newest CAR-T cell products target BCMA (B-cell maturation antigen) [4]. Kymriah (tisagenlecleucel), Yescarta (axicabtagene ciloleucel), and Breyanzi (lisocabtagene maraleucel) are approved in the third line or later for patients with large B-cell lymphoma, but only Yescarta and Breyanzi are approved as second line therapies due to results from the phase 3 ZUMA-7 (NCT03391466) and TRANSFORM (NCT03575351) studies, respectively [[5], [6], [7], [8], [9], [10], [11], [12]].
Novartis' Kymriah, a CD19-directed genetically modified autologous T-cell immunotherapy, was the first CAR-T cancer immunotherapy to reach the U.S. market following FDA approval in 2017. The therapeutic is indicated for the treatment of relapsed/refractory (r/r) pediatric and young adult acute lymphoblastic leukemia, r/r diffuse large B-cell lymphoma, and r/r follicular lymphoma [13]. Approximately 15 % of pediatric and young adult patients with ALL (acute lymphoblastic leukemia) patients are refractory or relapse and Kymriah has a strong impact on this group of patients. In the pivotal ELIANA trial, which was conducted in pediatric and young adult patients with relapsed or refractory acute lymphoblastic leukemia (r/r ALL), the overall response rate (ORR), comprising complete remission (CR) and complete remission with incomplete count recovery (CRi), reached 82 % [14]. The most recent analysis of this study revealed a 5-year relapse-free survival rate of 49 % among patients who initially achieved CR or CRi following Kymriah administration [15]. These findings not only underscore the remarkable effectiveness observed in this particular patient group during the clinical trial but also validate the therapy's impressive real-world performance. In addition to cytokine release syndrome and neurological adverse reactions, other important identified safety risks with Kymriah therapy are infections, tumor lysis syndrome, and prolonged cytopenia, in particular, prolonged depletion of the target cells [16].
According to the data from the clinicaltrials.gov, China became the country with the most registered CAR T trials after 2017. Take IM19 an example, it consisted of an FMC63 scFv, 4-1BB and CD3ζ intracellular domain and was manufactured into a memory T-enriched formulation [17]. The clinical trial was conducted based by Beijing ImmunoChina Medical Science & Technology Co., Ltd. with the register number NCT03344705. It was demonstrated that one month and three months after IM19 treatment, tumor burden was decreased in 16 patients with r/r B-NHL. The 3-month CR was 50.0 %, and the 6- month CR was 40.9 %. Seven patients (31.8 %) showed ongoing response even after 1 year. IM19 with 4-1BB-based costimulatory domain showed effective and durable antitumor activity, however, very low toxicities for B cell lymphoma [18].
While the therapy has demonstrated remarkable success in clinical trials, it also presents unique challenges and considerations, including complex manufacturing processes, potential side effects, and ongoing research to expand its application beyond blood cancers. Despite these challenges, CAR-T therapy offers renewed hope for patients facing previously untreatable or relapsed cancers, representing a new frontier in the fight against this devastating disease [19].
2. CAR-T technology
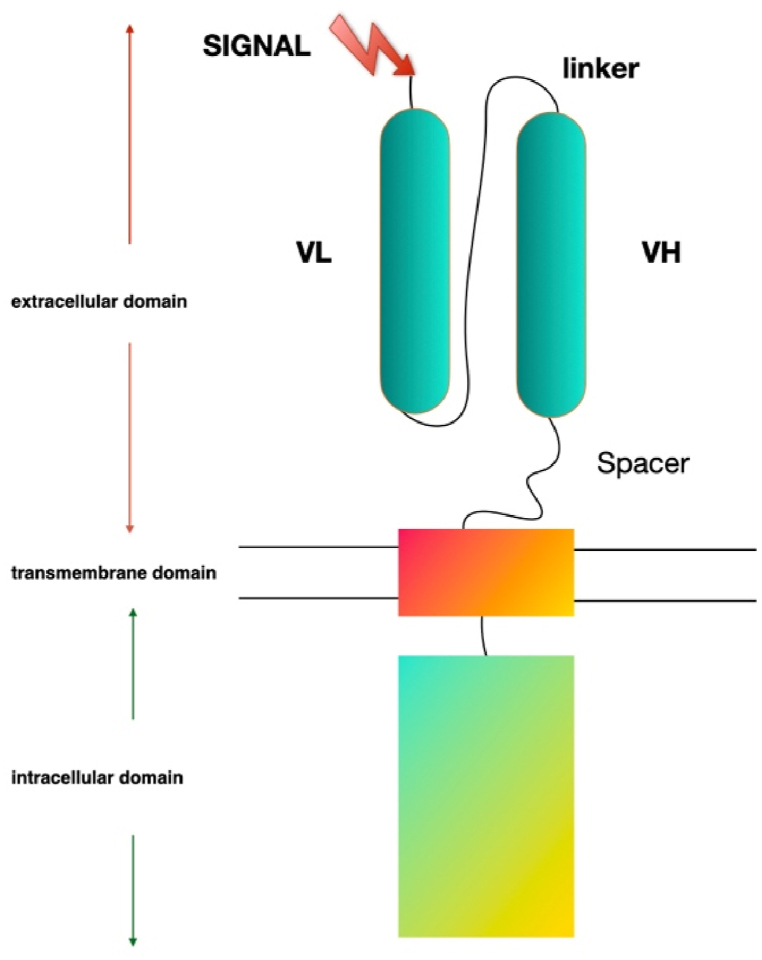
CART (chimeric antigen receptor T-cell) is composed of extracellular single-chain antibody fragments, transmembrane domains, and intracellular T cell signaling domains [[20], [21], [22]]. T cell receptors depend on antigens presented by antigen-presenting cells and present in the form of major histocompatibility complexes and antigen-peptide complexes [23]. The combination of TCR and MHC antigen peptide complex induces an intracellular cascade reaction: phosphorylated TCR recruits intracellular second messengers to provide the first signal, and the costimulatory molecules on the surface of T cells (CD28, CD27, CD134, CD137 or ICOS) and the respective receptors on APC (CD80, CD86, CD137L or ICOSL) combine to provide a second signal [24]. Unlike TCR-T, the extracellular antibody in CAR binds to the corresponding tumor antigen and recognizes, activates T cells in a non-MHC manner to exert anti-tumor effects [25] (Fig. 2).
Fig. 2.
The chimeric antigen receptor (CAR) T cell design. The single-chain variable fragment (scFv) of the CAR derived from the heavy and light chains of the antibody variable region, while the CAR CD3ζ domain is derived from the T cell receptor intracellular signaling domains. CARs of basic design can be divided into three regions as follows: i) Antibody-derived antigen-binding domain for antigen recognition binding; it usually contains a single-stranded variable fragment derived from an antibody; ii) transmembrane domain for anchor support to the plasma membrane; and iii) signaling domain for T-cell activation.
2.1. First-generation CAR-T cell therapy
The first-generation CAR-T cells were developed in the late 1980s and early 1990s and represent the earliest form of CAR T cell therapy [26,27]. These CAR T cells contain a single-chain variable fragment (scFv) that is specific for a particular antigen expressed on cancer cells. The scFv is typically derived from an antibody that recognizes the target antigen [[28], [29], [30]]. When the scFv binds to its target antigen on the cancer cell, it activates the T cell receptor (TCR) signaling pathway, which leads to T cell activation and destruction of the cancer cell [[31], [32], [33]]. However, first-generation CAR T cells are limited in their effectiveness because they do not have the ability to persist in the body and have a limited ability to penetrate solid tumors [34,35].
First-generation CAR T cells were also associated with significant toxicity and adverse effects, such as cytokine release syndrome (CRS) and neurotoxicity, which were caused by the activation of the immune system in response to the CAR T cells [36,37] Additionally, first-generation CAR T cells had limited efficacy against certain types of cancer and were not effective in patients with low levels of target antigen expression [34,[38], [39], [40], [41]].
Despite their limitations, the development of first-generation CAR T cells paved the way for subsequent generations of CAR T cell therapy and helped to establish the potential of CAR T cells as a powerful tool for cancer immunotherapy.
2.2. Second-generation CAR-T cell therapy
The second-generation CAR T cells were developed in the late 1990s and early 2000s as an improvement over the first-generation CAR T cells [[42], [43], [44], [45], [46], [47], [48], [49], [50]]. Second-generation CAR T cells have two domains: an extracellular antigen recognition domain and an intracellular signaling domain. They are designed with two key domains: an extracellular antigen recognition domain and an intracellular signaling domain. The extracellular domain is made up of a single-chain variable fragment (scFv), which is specific for a particular antigen expressed on the surface of cancer cells. The intracellular domain is made up of one or more co-stimulatory domains and a CD3ζ domain that transduces the activation signal.
The addition of co-stimulatory domains to the second-generation CAR T cells has been shown to significantly enhance their anti-tumor activity and persistence in vivo. Second-generation CAR T cells have demonstrated superior clinical outcomes compared to first-generation CAR T cells in the treatment of hematological malignancies, and have opened up new avenues for the development of CAR T cell therapy for solid tumors.
The scFv on the second-generation CAR T cells recognizes and binds to the target antigen on cancer cells. This binding triggers the activation of the CAR T cell and leads to the release of cytotoxic molecules that kill the cancer cell. However, the scFv alone is not sufficient to fully activate the T cell and provide sustained anti-tumor activity [51].
The second-generation CAR T cells are engineered with co-stimulatory domains that are able to activate and enhance the function of the T cell. The most commonly used co-stimulatory domains are CD28 and 4-1BB (also known as CD137), which are capable of promoting T cell proliferation, survival, and cytokine production [[52], [53]]. These co-stimulatory domains are located in the intracellular domain of the CAR, downstream of the CD3ζ domain [[51], [52], [53]].
Despite the improvements in efficacy, second-generation CAR T cells are still associated with toxicities such as cytokine release syndrome and neurotoxicity. Ongoing research is focused on optimizing the design of the CAR T cells to improve their safety and effectiveness [51].
2.3. Third-generation CAR-T cell therapy
The third-generation CAR T cells are designed to further enhance the efficacy and persistence of CAR T cell therapy. Third-generation CAR T cells have an extracellular antigen recognition domain, an intracellular signaling domain, and two or more co-stimulatory domains [[54], [55], [56], [57]].The extracellular domain of the third-generation CAR T cells is composed of a scFv that recognizes and binds to a specific antigen expressed on the surface of cancer cells. The intracellular domain of the CAR T cells is similar to that of the second-generation CAR T cells, containing a CD3ζ domain that transduces the activation signal, and one or more co-stimulatory domains [[58], [59], [60], [61]].
In addition to the co-stimulatory domains used in the second-generation CAR T cells (CD28 and 4-1BB), third-generation CAR T cells also incorporate additional co-stimulatory domains, such as OX40 or CD27. These additional co-stimulatory domains are able to further enhance the activation and proliferation of the CAR T cells, leading to improved anti-tumor activity and persistence [62,63]. The addition of multiple co-stimulatory domains to the third-generation CAR T cells has been shown to enhance the production of cytokines and increase the survival and proliferation of the CAR T cells. This has led to improved efficacy in the treatment of hematological malignancies, particularly in patients who have relapsed or become refractory to previous CAR T cell therapy [64,65].
However, third-generation CAR T cells are also associated with increased toxicity compared to second-generation CAR T cells, particularly with regard to the development of cytokine release syndrome and neurotoxicity. Therefore, efforts are ongoing to optimize the design of third-generation CAR T cells to minimize toxicity while maintaining their anti-tumor activity [66,67].
2.4. Fourth-generation CAR-T cell therapy
The fourth-generation CAR-T cells, also known as T cells redirected for antigen-unrestricted cytokine-initiated killing (TRUCKs), are designed to address some of the limitations of the earlier generations by combining the antigen recognition and T cell activation functions with the ability to secrete therapeutic molecules such as cytokines or antibodies [[68], [69], [70], [71], [72]].
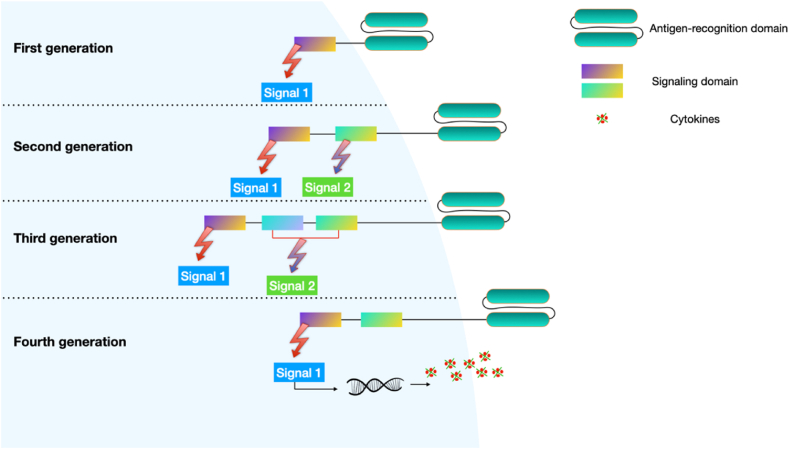
The fourth-generation CAR-T cells are engineered to express a CAR with two signaling domains, similar to the second-generation CAR-T cells (Fig. 3). One signaling domain is the activation domain that triggers T cell activation upon recognition of the tumor antigen, while the other is the costimulatory domain that enhances T cell proliferation and cytokine production. In addition, the fourth-generation CAR-T cells also have an inducible gene expression system that can be activated upon antigen recognition [68,73].
Fig. 3.
Earlier generations of CAR-T cells. Co-stimulatory signaling domains have been added to different generations of CAR T cells to improve their ability to produce more T cells after infusion and survive longer in the circulation.
This inducible gene expression system can be programmed to secrete therapeutic molecules, such as cytokines or antibodies, upon recognition of the tumor antigen. The therapeutic molecules secreted by the fourth-generation CAR-T cells can have a variety of functions, such as increasing T cell proliferation, enhancing T cell persistence, overcoming immunosuppression, or targeting tumor-specific antigens. For example, the CAR-T cells can be engineered to secrete cytokines like IL-12, which can enhance T cell proliferation and promote a more pro-inflammatory environment at the tumor site [35,73,74].
The fourth-generation CAR-T cells have been shown to have potent antitumor activity in preclinical studies. They can overcome some of the limitations of the earlier generations by enhancing the cytotoxicity of the CAR-T cells and recruiting other immune cells to the tumor site. In addition, the ability to secrete therapeutic molecules allows for the targeting of immunosuppressive factors in the tumor microenvironment, which may enhance the antitumor activity of the CAR-T cells [[75], [76], [77], [78], [79], [80], [81]].
Several clinical trials are underway to evaluate the safety and efficacy of the fourth-generation CAR-T cells in patients with hematological malignancies and solid tumors. These cells hold great promise for the treatment of cancer by combining the benefits of CAR-T cell therapy with the ability to secrete therapeutic molecules, which may further enhance their antitumor activity and overcome resistance mechanisms. However, further research is needed to optimize the design and manufacturing of these cells and to evaluate their long-term safety and efficacy [[82], [83], [84], [85]].
2.5. Fifth-generation CAR-T cell therapy
Fifth-generation CAR-T therapy is a complex and highly specialized form of immunotherapy that involves several steps, each of which is critical to the success of the treatment [69,[86], [87], [88], [89]].
The dual-targeting CAR used in fifth-generation CAR-T cell therapy is designed to recognize and bind to two different antigens on the surface of cancer cells [[90], [91], [92]]. This approach offers several advantages over earlier generations of CAR-T cell therapy that target only one antigen: i). By targeting two antigens, the CAR-T cells can more accurately distinguish between cancer cells and healthy cells, reducing the risk of off-target effects. ii). Enhanced efficacy: Dual-targeting CAR-T cells can achieve greater tumor cell killing than single-targeting CAR-T cells, as they are able to recognize a larger number of cancer cells [93,94]. iii). Reduced risk of resistance: Cancer cells can sometimes develop resistance to CAR-T cell therapy by downregulating or mutating the targeted antigen. By targeting two antigens, the CAR-T cells can still recognize and attack cancer cells even if one of the antigens is lost or mutated [95]. iv). TCR signaling: In fifth-generation CAR-T cell therapy, the modified TCR signaling pathway provides additional activation signals to the T cells. This helps to improve the T cells' ability to recognize and attack cancer cells, as well as to prevent the T cells from becoming exhausted and losing their anti-cancer activity over time. Specifically, the modified TCR provides signals that enhance T cell proliferation, survival, and cytokine production, which are all critical for effective anti-tumor responses. v). Cytokine release control: Cytokine release syndrome (CRS) is a potentially serious side effect of CAR-T cell therapy, in which the immune system releases large amounts of cytokines in response to the therapy. This can cause symptoms ranging from mild flu-like symptoms to life-threatening complications such as multiorgan failure. Fifth-generation CAR-T cells are designed to have better control over cytokine release, which can help to reduce the severity of CRS. There are several approaches to controlling cytokine release, including modifying the CAR-T cells to produce cytokine inhibitors, using low-dose conditioning regimens prior to CAR-T cell infusion, and administering corticosteroids or other immunomodulatory agents to manage CRS symptoms [96,97]. vi). Safety switches: Safety switches are included in fifth-generation CAR-T cell therapy to allow the therapy to be turned off in case of adverse effects or if the therapy is no longer needed. These switches include suicide genes, which can be activated to kill the CAR-T cells, as well as other mechanisms that can be used to selectively eliminate the CAR-T cells from the body. The inclusion of safety switches is an important safety feature of fifth-generation CAR-T cell therapy, as it allows the therapy to be rapidly stopped if necessary, minimizing the risk of adverse events [98].
Fifth-generation CAR-T cell therapy is an exciting advance in the field of immunotherapy, offering the potential for more effective and safer treatment of cancer. While this approach is still in the experimental stage, early results have been promising, and ongoing clinical trials are investigating its safety and efficacy in a range of cancer types (Fig. 4).
Fig. 4.
Five generations of CAR T-cell structure. i). The signaling domain of first-generation CARs only has a CD3ζ-derived signaling module. ii). Second-generation CARs are equipped with an extracellular antigen-recognizing domain combined with two intracellular domains: CD3z and an additional costimulatory domain (e.g., CD28 or 4-1BB). iii). Third-generation CARs are equipped with an extracellular antigen-recognizing domain combined with three intracellular domains: CD3z and two additional costimulatory domains. Co-stimulatory molecules include CD28, 4-1BB (CD137), CD27 and OX40 (CD134). iv). Fourth-generation CAR T cells are also referred to as TRUCKs, which inducibly express chemokines such as IL-12. v). Fifth-generation CARs consist of a novel co-stimulatory domain to activate some specific signaling pathways.
2.6. Off-the-shelf allogeneic T cell technology
Off-the-shelf allogeneic T cell technology involves the use of T cells from healthy donors that are genetically engineered to express a Chimeric Antigen Receptor (CAR) targeting specific tumor antigens. The use of allogeneic T cells allows for the creation of a standardized cell therapy product that can be manufactured and stored in advance, making it readily available for patients when needed [[99], [100], [101], [102]].
The process of generating off-the-shelf allogeneic T cell therapies typically involves collecting T cells from healthy donors, which are then genetically modified using viral vectors to express a CAR that recognizes a specific tumor antigen. The CAR T cells are then expanded in culture to generate a large number of cells, which are frozen and stored until needed for treatment [103].
One of the key advantages of off-the-shelf allogeneic T cell therapies is their potential to be readily available for patients, as they can be manufactured in advance and stored until needed. This is in contrast to patient-specific CAR T cell therapies, which require a lengthy manufacturing process that involves collecting T cells from the patient, genetically modifying them, and expanding them in culture [104,105].
Another potential advantage of off-the-shelf allogeneic T cell therapies is their potential to reduce the cost of CAR T cell therapy, as they can be manufactured in large quantities and distributed more widely. Additionally, the use of allogeneic T cells may allow for the development of CAR T cell therapies for patients who are unable to donate their own T cells due to factors such as prior chemotherapy, immunodeficiency, or advanced age [106,107].
However, there are also some challenges associated with the use of off-the-shelf allogeneic T cell therapies. One potential challenge is the risk of graft-versus-host disease, which occurs when the donor T cells attack the recipient's tissues. To mitigate this risk, various strategies are being developed, such as using gene editing techniques to remove certain T cell receptors that are associated with graft-versus-host disease [108].
In summary, off-the-shelf allogeneic T cell technology offers a promising approach to CAR T cell therapy that may provide greater access to this innovative treatment modality, while also potentially reducing costs and manufacturing timelines. However, continued research is needed to fully realize the potential of this technology and to address the challenges associated with its use.
3. CART manufacturing
The manufacturing of CAR-T therapies involves complex and highly specialized processes [109]. While the fundamental principles of CAR-T production are consistent across different clinical centers, there can be variations in specific protocols, equipment, and expertise (Fig. 5). Here are some key differences that can exist in CAR-T manufacture at various clinical centers:
Fig. 5.
A brief diagram of the CAR-T cells manufacturing process. It involves the initial collection of patient-derived leukocytes through leukapheresis. Subsequently, these T cells are transported to a biopharmaceutical facility for genetic modification, enabling them to express CARs on their surface. Following this, the engineered CAR-T cells undergo in vitro expansion before being sent back to the hospital for infusion into the patient.
Firstly, different clinical centers may have different manufacturing facilities, ranging from small-scale academic laboratories to large-scale commercial cell therapy manufacturing facilities. The size and sophistication of these facilities can impact the scale and efficiency of CAR-T production [110]. In addition, there are various manufacturing platforms for CAR-T production, such as retroviral or lentiviral transduction, and electroporation. Different centers may prefer or specialize in specific platforms based on their expertise and available equipment.
Secondly, the source of T cells used for CAR-T production can vary. Some centers use autologous T cells derived from the patient's own blood, while others explore allogeneic (donor-derived) approaches. Allogeneic CAR-Ts are more complex due to the need for immune matching and genetic engineering to avoid graft-versus-host disease. Moreover, the choice of target antigen may differ between clinical centers, depending on the type of cancer being treated and the available preclinical data.
Thirdly, the experience and expertise of the manufacturing team can greatly influence the success of CAR-T production. Some centers have extensive experience with CAR-T therapies and may have developed proprietary protocols. Furthermore, stringent quality control measures are essential to ensure the safety and efficacy of CAR-T products. These measures may vary in terms of the tests performed and the acceptance criteria used.
Fourthly, patient-specific factors, such as the patient's health status and disease stage, can influence the manufacturing process. Variations may occur in the pre-processing and post-processing steps. The availability of reagents, vectors, and other materials can differ between clinical centers. Supply chain logistics can impact the manufacturing timeline. Besides, clinical trials may have specific protocols and inclusion/exclusion criteria that affect CAR-T manufacturing. These criteria can vary between trials and centers.
Fifthly, clinical centers may have different methods for collecting and reporting data related to CAR-T manufacturing and patient outcomes. Additionally, the cost of CAR-T manufacturing can vary widely based on factors such as the scale of production and the location of the clinical center.
Despite these differences, there is ongoing collaboration and information sharing within the scientific and medical communities to standardize and optimize CAR-T manufacturing processes. This collaborative effort aims to ensure the consistent quality and safety of CAR-T therapies across different clinical centers and to expand access to these groundbreaking treatments for patients with cancer.
4. Targets of CAR-T treatment for hematological tumors
In the context of effective CAR-T therapy, the identification of appropriate tumor antigens plays a pivotal role, necessitating a careful equilibrium between effectiveness and safety aspects. Preferable antigens should meet the following criteria: (i) demonstrate high and uniform surface expression on tumor cells, (ii) maintain expression throughout various disease stages, (iii) hold significance in disease pathophysiology, (iv) exhibit minimal or no shedding into the bloodstream, (v) remain unaffected by specific treatment-induced pressures that might lead to down-regulation or elimination, and (vi) refrain from expression on normal tissues [111]. Currently there are several ongoing clinical trials assessing the safety and efficacy of CART immunotherapy in various malignancies (Fig. 6. & Table 1).
Fig. 6.
The target distribution of world's CAR-T clinical trials. Drug targets for hematological malignancies, such as BCMA, CD19, and CD22, continue to be the most popular target proteins, according to a 2022 report. Due to the competition among these popular targets, alternative novel targets, such as CLEC12A, GPRC5D, CD3, and CD7, have arisen in recent years. These alternatives show promise for revealing the potential for novel CAR-T discoveries.
Table 1.
Chimeric Antigen Receptor T-Cell Therapy for hematological tumors.
| Disease | Full name | Target | Clinical Trials |
|---|---|---|---|
| ALL | acute lymphoblastic leukemia | CD19/CD20/CD22/CD123 | NCT04012879; NCT04049383; NCT04094766; NCT04016129 |
| CLL | Chronic lymphoblastic leukemia | CD19 | NCT04007029; NCT03960840 |
| NHL | non-Hodgkin lymphoma | CD19/CD20 | NCT03790891; NCT03497533; NCT04169932 |
| ALCL | anaplastic large cell lymphoma | CD30 | NCT03383965; NCT04008394 |
| HL | Hodgkin lymphoma | CD19/CD30 | NCT01087294; NCT04134325 |
| MM | Multiple myeloma | CD269/CD138 | NCT03672318; NCT04182581; NCT03271632 |
CD19. CD19 is considered as an ideal target of CAR-T immunotherapy for the treatment of B-cell tumors. It can be specifically expressed on a variety of B-cell malignant tumor cells and B-cell precursor cells, but not on normal tissues and cells. The second-generation CD19 CAR-T (4-1BB/CD3ζ) was exploited to treat 2 children with B-cell ALL patients by Grupp et al. [46]. Both patients achieved complete remission, and one of them had a relapse, with blast cells that no longer expressed CD19, approximately 2 months after treatment. Another clinical trial targeting CAR to treat CD19+ B cell malignancy has shown great success, with 27 complete remission in totally 30 patients suffered relapsed or refractory pediatric acute lymphoblastic leukemia (ALL) [49]. In Yescarta (formerly known as KTE-C19) clinical trial, which involves 21 children and young adults with large B-cell NHL, CAR T cells were not detected beyond 68 days [112]. In addition to B cell ALL, CD19 is expressed on B cell CLL. Some CAR-Ts used in the treatment of B-cell ALL are also tested in the treatment of B-cell CLL, but the therapeutic effect on B-cell CLL is not so effective [113]. Kochenderfer et al. have reported anti-tumor effects of autologous CD19-directed CAR T cells in 10 patients with chronic lymphoma/leukemia [114]. One patient obtained a complete remission and none of them developed graft-versus-host disease (GVHD). The team then exploited CD19-specific CAR-T (CD28/CD3ζ) to treat patients with diffuse large B-cell lymphoma (DLBCL). Of the 15 patients, 8 cases had complete remission and 4 cases had partial remission [115]. Investigators from UPenn also reported their results from treating patients with DLBCL. 5 out of 13 evaluable patients achieved complete responses [116]. Similar studies were conducted at Memorial Sloan Kettering Cancer Center, with CD19-targeted CAR-T-cell therapy used to treat 8 patients with aggressive B-cell NHL and 5 patients experiencing complete responses [117]. Multiple myeloma is a cancer that forms in plasma cells, where cancerous plasma cells accumulate in the bone marrow and crowd out healthy blood cells. The study of CAR T therapy for multiple myeloma is still in its infancy. Some preclinical evidence led to a widespread effort to develop novel chimeric antigen receptors [[118], [119], [120]]. Patients with relapsed/refractory multiple myeloma experienced a high response to the B cell maturation antigen - targeted CAR T cells based on previous results from Greipp PR [121]. These findings suggest an exciting avenue for novel immunotherapies to treat multiple myeloma.
CD20. Expression patterns of CD20 are similar to those of CD19 and hence CARTs targeting CD20 can be an option for adoptive immunotherapy of hematological tumors. Currently, anti-CD20 CAR-T cell therapy have also been used as therapeutic treatments in serval studies [122]. In one phase I clinical trial, researchers aim to explore the efficacy of CD20-specific CAR autologous T cells for the treatment of patients suffering from relapsed indolent NHL and MCL (mast cell leukemia) [123]. The investigator demonstrated potential antitumor activity and safety, of adoptive T-cell therapy using this approach. It was unknown whether the second-generation of CAR-T treatment is effective in DLBCL patients. To address this, one research team used anti-CD20 CAR-T cell with 4-1BB for these patients and showed a promising effect of this novel treatment [124]. Of the recruited 7 patients with refractory advanced CD20+ DLBCL, one received complete remission and three received partial remission rates in initial report.
CD22. In addition to CD19 and CD20, CD22 is also currently being investigated in clinical trials and was shown to be one potential therapeutic target. For example, CD22-CAR T cells have been noted to have beneficial effects in patients with B-ALL diseases who were unsuitable to receive CD19-CAR T cell therapy [[125], [126], [127]]. However, there still lots of patients relapsed due to poor CAR T-cell persistence or resistance [128]. Hence, multiple means were utilized to enhance the anti-cancer activity of CD22-CAR T cells. Compared with other binding domains, a second-generation CAR in which the scFv binds a proximal CD22 epitope demonstrated superior antileukemic activity [129]. Interestingly, a CAR based on the same scFv targeting CD22 but armed with different linker lengths may induce different clinical responses. Despite similar binding affinities, the shorter linker CAR generated a stronger anti-tumor response which contributes to a series of new clinical trials (NCT03620058, NCT02650414) [130].
CD30. As a characteristic marker of malignant Reed–Sternberg cells in HL, CD30 is being studied extensively in clinical trials [[131], [132], [133], [134], [135], [136], [137]]. Brentuximab vedotin, which links an anti-CD30 antibody conjugated to monomethyl auristatin E (MMAE) developed for clinical use demonstrated it would be the first choice of patients with relapsed ALCL (anaplastic large cell lymphoma) who have not previously received it [138]. It is also the first successfully developed drug to be approved by FDA for patients with Hodgkin's lymphoma [139]. Far from conclusive, many varieties of research have demonstrated high expression of CD30 is present in about half of PTCL (peripheral T-cell lymphomas) patients [[140], [141], [142], [143]]. Therefore, CAR-T cell therapy targeting CD30 opens new avenues for the treatment of refractory or recurrent CD30-positive PTCL in the future.
RORI. RORI,a member of receptor tyrosine kinase family, is widely expressed in embryonic development and multiple human malignance [[144], [145], [146], [147], [148]]. In normal tissues, RORI expression was either negative or positive but is restricted only to adipocytes, basal epithelial lining of the esophagus, the surface and foveolar epithelial cells of the gastric antral mucosa, and in the duodenal mucosa [149,150]. This tumor-selective expression made RORI potentially serve as an alternative candidate for CAR T immunotherapy. Accumulating evidence suggests that ROR1 has intrinsic kinase activity thereby mediating bone metastasis of breast cancer via cross-talking with the Hippo-YAP pathway [151]. Present research by Lars W et al. suggest that ROR1-CAR T cells are capable of eliminating tumor cells which are harbored in crypt structures [152]. In addition, the safety profile of ROR1 CAR-T cells was similar to other reagent in previous studies, with no serious toxicity in a primate model [153]. A series of research suggest that ROR1 could be ideal candidates for CART immunotherapy though further studies are still needed to investigate the comprehensive utility of this therapy.
CD123. CD123 has emerged as a novel target for hematolymphoid malignancies. Firstly, CD123 is overexpressed broadly across hematolymphoid neoplasms, including acute myeloid leukemia, blastic plasmacytoid dendritic cell neoplasm, acute lymphoblastic leukemia, hairy cell leukemia, and systemic mastocytosis [154]. Secondly, varieties of promising data from trials showed that manipulation of CD123 has considerable promise as a strategy for the immunotherapy of cancer, especially CD123-targetting CAR T cells [155,156]. In view of this, attempts to develop CD123-targetting CAR T therapies that could directly target hematological malignancies expressing CD123 are ongoing. For example, Brett M's team developed a CD123 CAR consisting of a single-chain variable fragment derived from CD123-monoclonal antibody. And these CD123 CAR T cells have shown notable clinical efficacy in patients with a variety of hematological malignancies, including relapsed/refractory AML, plasmacytoid dendritic cell neoplasm [157,158]. However, their lack of selectivity is concerning as off-target effects have been noted since CD123 is dimly expressed on some normal hematopoietic cells and endothelial cells [159].
CD33. There has been a long-standing interest in targeting CD33 therapy. Some pre-clinical studies have demonstrated CD33-CAR T cell lead to sustained substantial anti-tumor activity and significant tumor eradication against AML cells [[160], [161], [162]]. In one early clinical trial, Wang et al. demonstrated that administration of autologous T cells with 38 % anti-CD33 CAR expression would display notable cytolytic functions against CD33+ blasts [163]. However, targeting CD33 might be problematic since normal progenitor cells, myeloid cells also express this protein and, therefore, effective treatment probably play a major causative role in prolonged cytopenia and delayed hematopoietic recovery [164].
5. Future direction
In recent years, CART therapy has received great attention and a number of recent breakthroughs have demonstrated tremendous potential to control cancer by engineered T cells [165,166]. On the whole,CART cells are characterized by recognizing specific antigens on the surface of target cells and then triggering cytotoxic T cell activation independent of MHC (major histocompatibility complex) molecules [167]. Overwhelming evidence published thus far strongly support the conclusion that chimeric antigen receptor T cells functions to fight against cancer. Of note, clinical trials using CAR T-cell therapy against solid tumors have not achieved the same success seen in hematological malignancies [168]. Therefore, over the past decade much attention has been paid to how CAR T-cell therapy fight against hematological malignancies. Perhaps the main barriers to effective CAR-T cell therapy is antigen heterogeneity, which impairs the detection of cancer cells by T cells [34]. This phenomenon has emerged as the critical obstacle to the durability of CAR-T cell therapy. For example, 70 %–90 % of B-ALL (B-cell acute lymphoblastic leukemia) patients have shown impressive response rate after receiving CD19 CAR T cells therapy but CD19-negative relapse occurs in 14 % of all adult B-ALL patients demonstrated by Jae H [[47], [48], [49],[169], [170], [171], [172]]. However, CD19-negative leukemia is gradually growing which leads to CAR-T failure for maintaining long term remission, especially in patients whose CAR T cells persist for a long time [49,[173], [174], [175]]. It is probable that various combinations of several mechanisms are contributing to antigen loss, including antigen escape and lineage switch [[176], [177], [178]]. Alternative targeting concept and combinatorial antigen recognition offer strategies to overcome antigen loss defect [179,180].
Here are more detailed explanations of the clear directions for chimeric antigen receptor T cell (CAR-T) therapy for the treatment of hematological malignancies: Firstly, the development of CAR-T cell therapies that target novel antigens beyond CD19, CD20, and BCMA is an important direction for CAR-T cell therapy. This may involve identifying new surface proteins or intracellular targets that are expressed on hematological malignancies but not on healthy tissues. Identifying novel targets may expand the range of cancers that can be treated with CAR-T cell therapy and reduce the risk of relapse due to antigen loss. Secondly, combination therapies with CAR-T cell therapy are another important direction. For example, combining CAR-T cells with immune checkpoint inhibitors or other targeted therapies may enhance the efficacy of CAR-T cell therapy and overcome resistance mechanisms. Combination therapies may also help to reduce the incidence of treatment-related adverse events. Thirdly, development of off-the-shelf allogeneic CAR-T cell products: The development of off-the-shelf allogeneic CAR-T cell products is an important direction for CAR-T cell therapy. This approach involves using T cells from healthy donors that are genetically modified to express a CAR, and can be manufactured in large quantities and stored for later use. This may help to reduce the cost of CAR-T cell therapy and increase its availability. Fourthly, improving the safety of CAR-T cell therapy is a critical direction, including the development of strategies to reduce the risk of cytokine release syndrome (CRS) and neurotoxicity, as well as the risk of graft-versus-host disease in the case of allogeneic CAR-T cell therapy. This may involve the development of CAR-T cell therapies that are more selective for tumor cells and less toxic to healthy tissues. Fifthly, broadening the applicability of CAR-T cell therapy: The broadening of the applicability of CAR-T cell therapy is another important direction. This may involve the use of CAR-T cell therapy in earlier stages of disease, combination with other treatments, or as part of the initial treatment regimen. For example, CAR-T cell therapy may be used in combination with chemotherapy or radiation therapy to improve the overall response rate.
In summary, the development of novel targets, combination therapies, the use of off-the-shelf allogeneic CAR-T cell products, enhancing safety, and broadening the applicability of CAR-T cell therapy are important directions for the field of CAR-T cell therapy for hematological malignancies.
Other challenges in targeting hematological malignancies with CAR-T therapy include on-target off-tumor effects, immune suppressive microenvironment and CAR-T cell-associated toxicities, such as strong cytokine-driven effects and neurotoxicity [181]. In conclusion, CAR-T therapies have unique specificity and has made great progress to deal with hematological malignancies as a highly effective form of adoptive cell therapy. Despite the potential challenges, there is no doubt that this type of immunotherapy is now standing on the threshold of great advances in the war against cancer.
6. Challenge and possible solutions
One of the major challenges of CAR-T cell therapy is antigen escape or loss, which occurs when the target antigen on tumor cells is downregulated or eliminated, resulting in relapse. Possible solutions include identifying new targets, targeting multiple antigens, and designing CAR-T cells with dual signaling domains to enhance T cell activation and proliferation [[182], [183], [184]].
CAR-T cell therapy can also cause severe toxicities such as cytokine release syndrome (CRS) and neurotoxicity. Possible solutions include optimizing the dose and timing of CAR-T cell infusion, using prophylactic medications to mitigate toxicities, and developing new CAR-T cell designs with better control over T cell activation and proliferation [185,186].
Manufacturing CAR-T cells is complex and expensive, and can lead to product variability and delays. Possible solutions include developing more efficient and automated manufacturing processes, improving quality control and assurance, and optimizing the selection and expansion of T cells. In addition, CAR-T cell therapy is not widely available due to its high cost and limited availability. Possible solutions include developing off-the-shelf allogeneic CAR-T cell products that can be used for multiple patients, improving reimbursement policies, and expanding the availability of CAR-T cell therapy to more treatment centers. Despite the high response rates of CAR-T cell therapy, some patients may still develop resistance or relapse. Possible solutions include combining CAR-T cell therapy with other treatments such as immune checkpoint inhibitors or targeted therapies, or designing CAR-T cells with improved persistence and durability [187,188] (see Fig. 7).
Fig. 7.
Major challenges in applying CAR-T cell therapy.
Several hurdles impede the effectiveness and accessibility of CAR T-cell therapy in clinical applications. The immunosuppressive microenvironment in solid tumors, characterized by components like myeloid-derived suppressor cells, regulatory T cells, tumor-associated macrophages, and cancer-associated fibroblasts, hinders CAR T-cell infiltration and activity. Recent research underscores that hematologic and solid malignancies have distinct requirements for CAR T-mediated cytotoxicity. Novel targets must be explored, as antigen loss is a significant contributor to relapses in hematological malignancies, and the identification of tumor-specific antigens is essential for extending CAR T therapy to solid tumors. Identifying and targeting new antigens will be pivotal in advancing cell-based therapies. Toxicity issues are addressed by employing antagonists targeting inflammatory cytokine signaling via IL-6 and IL-1 in clinical settings. Recent advancements propose that genetically modifying CAR T functionality through GM-CSF or IFN-γ could offer a broader approach to mitigate systemic toxicities. Additionally, current manufacturing processes are time-consuming and costly, demanding the development of rapid and cost-effective protocols to enhance the accessibility of CAR T-cell therapy.
In summary, the challenges of CAR-T cell therapy for hematological malignancies include antigen escape and loss, toxicities, manufacturing challenges, limited access, and resistance and relapse. Possible solutions include identifying new targets, optimizing dosing and timing, developing off-the-shelf allogeneic CAR-T cell products, improving manufacturing processes, and combining CAR-T cell therapy with other treatments.
Ethical approval
Not applicable.
Funding
This work was funded by the Shandong Province Natural Science Foundation, China grants ZR2022QH372.
Data availability statement
No data was used for the research described in the article.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Chao Wang: Software, Investigation. Jianpeng Wang: Methodology, Investigation, Data curation. Shusheng Che: Writing – original draft, Software. Hai Zhao: Writing – review & editing, Writing – original draft, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kuwana Y., Asakura Y., Utsunomiya N., Nakanishi M., Arata Y., Itoh S., Nagase F., Kurosawa Y. Expression of chimeric receptor composed of immunoglobulin-derived V resions and T-cell receptor-derived C regions. Biochem. Biophys. Res. Commun. 1987 doi: 10.1016/0006-291X(87)90502-X. [DOI] [PubMed] [Google Scholar]
- 2.Gross G., Waks T., Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. U. S. A. 1989 doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Styczyński J. A brief history of car-t cells: from laboratory to the bedside. Acta Haematol. Pol. 2020 doi: 10.2478/ahp-2020-0002. [DOI] [Google Scholar]
- 4.Chen Y.J., Abila B., Mostafa Kamel Y. CAR-T: what is next? Cancers. 2023;15 doi: 10.3390/cancers15030663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locke F.L., Miklos D.B., Jacobson C.A., Perales M.-A., Kersten M.-J., Oluwole O.O., Ghobadi A., Rapoport A.P., McGuirk J., Pagel J.M., Muñoz J., Farooq U., van Meerten T., Reagan P.M., Sureda A., Flinn I.W., Vandenberghe P., Song K.W., Dickinson M., Minnema M.C., Riedell P.A., Leslie L.A., Chaganti S., Yang Y., Filosto S., Shah J., Schupp M., To C., Cheng P., Gordon L.I., Westin J.R. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N. Engl. J. Med. 2022 doi: 10.1056/nejmoa2116133. [DOI] [PubMed] [Google Scholar]
- 6.Westin J., Oluwole O.O., Kersten M.J., Miklos D.B., Perales M.-A., Ghobadi A., Rapoport A., Sureda A., Jacobson C.A., Farooq U., van Meerten T., Ulrickson M.L., Elsawy M., Leslie L.A., Chaganti S., Dickinson M., Yang Y., Schupp M.A., To C.A., Locke F.L. Primary overall survival analysis of the phase 3 randomized ZUMA-7 study of axicabtagene ciloleucel versus standard-of-care therapy in relapsed/refractory large B-cell lymphoma. J. Clin. Oncol. 2023;41 doi: 10.1200/JCO.2023.41.17_suppl.LBA107. LBA107–LBA107. [DOI] [Google Scholar]
- 7.Westin J.R., Oluwole O.O., Kersten M.J., Miklos D.B., Perales M.-A., Ghobadi A., Rapoport A.P., Sureda A., Jacobson C.A., Farooq U., van Meerten T., Ulrickson M., Elsawy M., Leslie L.A., Chaganti S., Dickinson M., Dorritie K., Reagan P.M., McGuirk J., Song K.W., Riedell P.A., Minnema M.C., Yang Y., Vardhanabhuti S., Filosto S., Cheng P., Shahani S.A., Schupp M., To C., Locke F.L. Survival with axicabtagene ciloleucel in large B-cell lymphoma. N. Engl. J. Med. 2023;389:148–157. doi: 10.1056/NEJMoa2301665. [DOI] [PubMed] [Google Scholar]
- 8.Perales M.-A., Kuruvilla J., Snider J.T., Vadgama S., Blissett R., El-Moustaid F., Smith N.J., Patel A.R., Johnston P.B. The cost-effectiveness of axicabtagene ciloleucel as second-line therapy in patients with large B-cell lymphoma in the United States: an e aaaaconomic evaluation of the ZUMA-7 trial. Transplant Cell Ther. 2022;28 doi: 10.1016/j.jtct.2022.08.010. 750.e1-750.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nastoupil L.J., Kamdar M.K., Chavez J.C., Ghesquieres H., Ghosh M., Holmes H., López Guillermo A., Martinez-Lopez J., Novak U., Ribrag V., Roddie C., Waller E.K., Feldman T.A., Rosenthal A.C., Sauter C., Von Bonin M., Karmali R., Montheard S., Previtali A., Abramson J.S. Subgroup analyses of primary refractory (refr) vs early relapsed (rel) large B-cell lymphoma (LBCL) from the TRANSFORM study of lisocabtagene maraleucel (liso-cel) vs standard of care (SOC) as second-line (2L) therapy. J. Clin. Oncol. 2023 doi: 10.1200/jco.2023.41.16_suppl.7526. [DOI] [Google Scholar]
- 10.Abramson J.S., Solomon S.R., Arnason J., Johnston P.B., Glass B., Bachanova V., Ibrahimi S., Mielke S., Mutsaers P., Hernandez-Ilizaliturri F., Izutsu K., Morschhauser F., Lunning M., Crotta A., Montheard S., Previtali A., Ogasawara K., Kamdar M. Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of the phase 3 TRANSFORM study. Blood. 2023;141:1675–1684. doi: 10.1182/blood.2022018730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nastoupil L.J., Kamdar M.K., Chavez J.C., Ghesquieres H., Ghosh M., Holmes H., López Guillermo A., Martinez-Lopez J., Novak U., Ribrag V., Roddie C., Waller E.K., Feldman T.A., Rosenthal A.C., Sauter C., Von Bonin M., Karmali R., Montheard S., Previtali A., Abramson J.S. Subgroup analyses of primary refractory (refr) vs early relapsed (rel) large B-cell lymphoma (LBCL) from the TRANSFORM study of lisocabtagene maraleucel (liso-cel) vs standard of care (SOC) as second-line (2L) therapy. J. Clin. Oncol. 2023;41 doi: 10.1200/JCO.2023.41.16_suppl.7526. 7526–7526. [DOI] [Google Scholar]
- 12.Kamdar M., Solomon S.R., Arnason J., Johnston P.B., Glass B., Bachanova V., Ibrahimi S., Mielke S., Mutsaers P., Hernandez-Ilizaliturri F., Izutsu K., Morschhauser F., Lunning M., Maloney D.G., Crotta A., Montheard S., Previtali A., Stepan L., Ogasawara K., Mack T., Abramson J.S. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysi. Lancet. 2022;399:2294–2308. doi: 10.1016/S0140-6736(22)00662-6. [DOI] [PubMed] [Google Scholar]
- 13.Philippidis A. Kymriah, first CAR-T cancer immunotherapy approved by FDA. Clinical OMICs. 2017;4 doi: 10.1089/clinomi.04.05.09. 8–8. [DOI] [Google Scholar]
- 14.Rives S., Maude S.L., Hiramatsu H., Baruchel A., Bader P., Bittencourt H., Buechner J., Laetsch T., De Moerloose B., Qayed M., Stefanski H.E., Davis K.L., Martin P.L., Nemecek E., Peters C., Yanik G., Balduzzi A., Boissel N., Khaw S.L., Krueger J., Levine J., Davies S., Myers G.D., Yeo A., O'Donovan D., Ramos R., Pulsipher M., Grupp S. S112: tisagenlecleucel in pediatric and young adult patients (pts) with relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-all): final analyses from the ELIANA study. Hemasphere. 2022;6:13–14. doi: 10.1097/01.HS9.0000843344.19780.98. [DOI] [Google Scholar]
- 15.John S., Pulsipher M.A., Moskop A., Hu Z.-H., Phillips C.L., Hall E.M., Margossian S.P., Nikiforow S., Martin P.L., Oshrine B., Keating A.K., Rouce R.H., Tiwari R., Redondo S., Willert J., Agarwal A., Pasquini M.C., Grupp S.A. Real-world outcomes for pediatric and young adult patients with relapsed or refractory (R/R) B-cell acute lymphoblastic leukemia (ALL) treated with tisagenlecleucel: update from the center for international blood and marrow transplant research (CIBMTR) registry. Blood. 2021;138:428. doi: 10.1182/blood-2021-146393. [DOI] [Google Scholar]
- 16.Awasthi R., Maier H.J., Zhang J., Lim S. Kymriah® (tisagenlecleucel)–An overview of the clinical development journey of the first approved CAR-T therapy. Hum. Vaccines Immunother. 2023;19 doi: 10.1080/21645515.2023.2210046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ying Z., He T., Jin S., Wang X., Zheng W., Lin N., Tu M., Xie Y., Ping L., Liu W., Deng L., Ding Y., Hu X., Bu B., Lu X., Song Y., Zhu J. A durable 4-1BB-based CD19 CAR-T cell for treatment of relapsed or refractory non-Hodgkin lymphoma. Chin. J. Cancer Res. 2022 doi: 10.21147/j.issn.1000-9604.2022.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ying Z., He T., Jin S., Wang X., Zheng W., Lin N., Tu M., Xie Y., Ping L., Liu W., Deng L., Ding Y., Hu X., Bu B., Lu X., Song Y., Zhu J. A durable 4-1BB-based CD19 CAR-T cell for treatment of relapsed or refractory non-Hodgkin lymphoma. Chin. J. Cancer Res. 2022 doi: 10.21147/j.issn.1000-9604.2022.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitra A., Barua A., Huang L., Ganguly S., Feng Q., He B. From bench to bedside: the history and progress of CAR T cell therapy. Front. Immunol. 2023 doi: 10.3389/fimmu.2023.1188049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad U., Khan Z., Ualiyeva D., Amissah O.B., Noor Z., Khan A., Zaman N., Khan M., Khan A., Ali B. Chimeric antigen receptor T cell structure, its manufacturing, and related toxicities; A comprehensive review. Advances in Cancer Biology - Metastasis. 2022 doi: 10.1016/j.adcanc.2022.100035. [DOI] [Google Scholar]
- 21.Zhao Z., Chen Y., Francisco N.M., Zhang Y., Wu M. The application of CAR-T cell therapy in hematological malignancies: advantages and challenges. Acta Pharm. Sin. B. 2018;8:539–551. doi: 10.1016/j.apsb.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skorka K., Ostapinska K., Malesa A., Giannopoulos K. The application of CAR-T cells in haematological malignancies. Arch. Immunol. Ther. Exp. 2020;68:34. doi: 10.1007/s00005-020-00599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malissen B., Bongrand P. Early T cell activation: integrating biochemical, structural, and biophysical cues. Annu. Rev. Immunol. 2015 doi: 10.1146/annurev-immunol-032414-112158. [DOI] [PubMed] [Google Scholar]
- 24.Malissen B., Grégoire C., Malissen M., Roncagalli R. Integrative biology of T cell activation. Nat. Immunol. 2014 doi: 10.1038/ni.2959. [DOI] [PubMed] [Google Scholar]
- 25.Chmielewski M., Hombach A.A., Abken H. Antigen-specific T-cell activation independently of the MHC: chimeric antigen receptor-redirected T cells. Front. Immunol. 2013;4 doi: 10.3389/fimmu.2013.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majzner R.G., Mackall C.L. Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med. 2019;25:1341–1355. doi: 10.1038/s41591-019-0564-6. [DOI] [PubMed] [Google Scholar]
- 27.Blache U., Popp G., Dünkel A., Koehl U., Fricke S. Potential solutions for manufacture of CAR T cells in cancer immunotherapy. Nat. Commun. 2022;13:5225. doi: 10.1038/s41467-022-32866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad Z.A., Yeap S.K., Ali A.M., Ho W.Y., Alitheen N.B.M., Hamid M. ScFv antibody: principles and clinical application. Clin. Dev. Immunol. 2012 doi: 10.1155/2012/980250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujiwara K., Masutani M., Tachibana M., Okada N. Impact of scFv structure in chimeric antigen receptor on receptor expression efficiency and antigen recognition properties. Biochem. Biophys. Res. Commun. 2020 doi: 10.1016/j.bbrc.2020.03.071. [DOI] [PubMed] [Google Scholar]
- 30.Zhang C., Wang L., Zhang Q., Shen J., Huang X., Wang M., Huang Y., Chen J., Xu Y., Zhao W., Qi Y., Li Y., Ou Y., Yang Z., Qian C. Screening and characterization of the scFv for chimeric antigen receptor T cells targeting CEA-positive carcinoma. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1182409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laletin V., Bernard P.L., Costa Da Silva C., Guittard G., Nunes J.A. Negative intracellular regulators of T-cell receptor (TCR) signaling as potential antitumor immunotherapy targets. J Immunother Cancer. 2023 doi: 10.1136/jitc-2022-005845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jayaraman J., Mellody M.P., Hou A.J., Desai R.P., Fung A.W., Pham A.H.T., Chen Y.Y., Zhao W. CAR-T design: elements and their synergistic function. EBioMedicine. 2020 doi: 10.1016/j.ebiom.2020.102931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujiwara K., Masutani M., Tachibana M., Okada N. Impact of scFv structure in chimeric antigen receptor on receptor expression efficiency and antigen recognition properties. Biochem. Biophys. Res. Commun. 2020 doi: 10.1016/j.bbrc.2020.03.071. [DOI] [PubMed] [Google Scholar]
- 34.Sterner R.C., Sterner R.M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou A.J., Chen L.C., Chen Y.Y. Navigating CAR-T cells through the solid-tumour microenvironment. Nat. Rev. Drug Discov. 2021;20:531–550. doi: 10.1038/s41573-021-00189-2. [DOI] [PubMed] [Google Scholar]
- 36.Andrea A.E., Chiron A., Bessoles S., Hacein-Bey-Abina S. Engineering next-generation CAR-T cells for better toxicity management. Int. J. Mol. Sci. 2020;21:8620. doi: 10.3390/ijms21228620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han D., Xu Z., Zhuang Y., Ye Z., Qian Q. Current progress in CAR-T cell therapy for hematological malignancies. J. Cancer. 2021;12:326–334. doi: 10.7150/jca.48976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmermannova O., Caiado I., Ferreira A.G., Pereira C.-F. Cell fate reprogramming in the era of cancer immunotherapy. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.714822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martino M., Naso V., Loteta B., Canale F.A., Pugliese M., Alati C., Musuraca G., Nappi D., Gaimari A., Nicolini F., Mazza M., Bravaccini S., Derudas D., Martinelli G., Cerchione C. Chimeric antigen receptor T-cell therapy: what we expect soon. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms232113332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmermannova O., Caiado I., Ferreira A.G., Pereira C.F. Cell fate reprogramming in the era of cancer immunotherapy. Front. Immunol. 2021 doi: 10.3389/fimmu.2021.714822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudzki J.D., Wolf D. AML—is it time to drive a CAR(-T)? Memo - Magazine of European Medical Oncology. 2020;13:50–54. doi: 10.1007/s12254-020-00577-1. [DOI] [Google Scholar]
- 42.Holzinger A., Barden M., Abken H. The growing world of CAR T cell trials: a systematic review, Cancer Immunology. Immunotherapy. 2016;65:1433–1450. doi: 10.1007/s00262-016-1895-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tokarew N., Ogonek J., Endres S., von Bergwelt-Baildon M., Kobold S. Teaching an old dog new tricks: next-generation CAR T cells. Br. J. Cancer. 2019;120:26–37. doi: 10.1038/s41416-018-0325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadelain M., Brentjens R., Rivière I. The promise and potential pitfalls of chimeric antigen receptors. Curr. Opin. Immunol. 2009;21:215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brentjens R.J., Davila M.L., Riviere I., Park J., Wang X., Cowell L.G., Bartido S., Stefanski J., Taylor C., Olszewska M., Borquez-Ojeda O., Qu J., Wasielewska T., He Q., Bernal Y., Rijo I.V., Hedvat C., Kobos R., Curran K., Steinherz P., Jurcic J., Rosenblat T., Maslak P., Frattini M., Sadelain M. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013 doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., Teachey D.T., Chew A., Hauck B., Wright J.F., Milone M.C., Levine B.L., June C.H. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013 doi: 10.1056/nejmoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davila M.L., Riviere I., Wang X., Bartido S., Park J., Curran K., Chung S.S., Stefanski J., Borquez-Ojeda O., Olszewska M., Qu J., Wasielewska T., He Q., Fink M., Shinglot H., Youssif M., Satter M., Wang Y., Hosey J., Quintanilla H., Halton E., Bernal Y., Bouhassira D.C.G., Arcila M.E., Gonen M., Roboz G.J., Maslak P., Douer D., Frattini M.G., Giralt S., Sadelain M., Brentjens R. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014 doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N., Steinberg S.M., Stroncek D., Tschernia N., Yuan C., Zhang H., Zhang L., Rosenberg S.A., Wayne A.S., Mackall C.L. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015 doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F., Mahnke Y.D., Melenhorst J.J., Rheingold S.R., Shen A., Teachey D.T., Levine B.L., June C.H., Porter D.L., Grupp S.A. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014 doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porter D.L., Kalos M., Zheng Z., Levine B., June C. Chimeric antigen receptor therapy for B-cell malignancies. J. Cancer. 2011;2:331–332. doi: 10.7150/jca.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Stegen S.J.C., Hamieh M., Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat. Rev. Drug Discov. 2015;14:499–509. doi: 10.1038/nrd4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maher J., Brentjens R.J., Gunset G., Rivière I., Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nat. Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 53.Imai C., Mihara K., Andreansky M., Nicholson I.C., Pui C.-H., Geiger T.L., Campana D. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 54.Waibel R., Weber E., Stahel R.A. CD24, a signal-transducing molecule expressed on human B cells, is a major surface antigen on small cell lung carcinomas. Lung Cancer. 1993;10:131–132. doi: 10.1016/0169-5002(93)90369-9. [DOI] [PubMed] [Google Scholar]
- 55.Kochenderfer J.N., Feldman S.A., Zhao Y., Xu H., Black M.A., Morgan R.A., Wilson W.H., Rosenberg S.A. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J. Immunother. 2009;32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suerth J.D., Morgan M.A., Kloess S., Heckl D., Neudörfl C., Falk C.S., Koehl U., Schambach A. Efficient generation of gene-modified human natural killer cells via alpharetroviral vectors. J. Mol. Med. 2016;94:83–93. doi: 10.1007/s00109-015-1327-6. [DOI] [PubMed] [Google Scholar]
- 57.Schambach A., Bohne J., Chandra S., Will E., Margison G.P., Williams D.A., Baum C. Equal potency of gammaretroviral and lentiviral SIN vectors for expression of O6-methylguanine–DNA methyltransferase in hematopoietic cells. Mol. Ther. 2006;13:391–400. doi: 10.1016/j.ymthe.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 58.Hossian A.K.M.N., Hackett C.S., Brentjens R.J., Rafiq S. Multipurposing CARs: same engine, different vehicles. Mol. Ther. 2022;30:1381–1395. doi: 10.1016/j.ymthe.2022.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson P.C., Abramson J.S. Engineered T cells: CAR T cell therapy and beyond. Curr. Oncol. Rep. 2022;24:23–31. doi: 10.1007/s11912-021-01161-4. [DOI] [PubMed] [Google Scholar]
- 60.Rafiq S., Hackett C.S., Brentjens R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2020;17:147–167. doi: 10.1038/s41571-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hupperetz C., Lah S., Kim H., Kim C.H. CAR T cell immunotherapy beyond haematological malignancy. Immune Netw. 2022;22 doi: 10.4110/in.2022.22.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guedan S., Posey A.D., Shaw C., Wing A., Da T., Patel P.R., McGettigan S.E., Casado-Medrano V., Kawalekar O.U., Uribe-Herranz M., Song D., Melenhorst J.J., Lacey S.F., Scholler J., Keith B., Young R.M., June C.H. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight. 2018;3 doi: 10.1172/jci.insight.96976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qu J., Mei Q., Chen L., Zhou J. Chimeric antigen receptor (CAR)-T-cell therapy in non-small-cell lung cancer (NSCLC): current status and future perspectives, Cancer Immunology. Immunotherapy. 2021;70:619–631. doi: 10.1007/s00262-020-02735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han D., Xu Z., Zhuang Y., Ye Z., Qian Q. Current progress in CAR-T cell therapy for hematological malignancies. J. Cancer. 2021;12:326–334. doi: 10.7150/jca.48976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holstein S.A., Lunning M.A. CAR T‐cell therapy in hematologic malignancies: a voyage in progress. Clin. Pharmacol. Ther. 2020;107:112–122. doi: 10.1002/cpt.1674. [DOI] [PubMed] [Google Scholar]
- 66.Schubert M.-L., Schmitt A., Neuber B., Hückelhoven-Krauss A., Kunz A., Wang L., Gern U., Michels B., Sellner L., Hofmann S., Müller-Tidow C., Dreger P., Schmitt M. Third-generation CAR T cells targeting CD19 are associated with an excellent safety profile and might improve persistence of CAR T cells in treated patients. Blood. 2019;134 doi: 10.1182/blood-2019-125423. 51–51. [DOI] [Google Scholar]
- 67.Sievers S., Watson G., Johncy S., Adkins S. Recognizing and grading CAR T-cell toxicities: an advanced practitioner perspective. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.00885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chmielewski M., Abken H. TRUCKS, the fourth‐generation CAR T cells: current developments and clinical translation. Adv Cell Gene Ther. 2020;3:1–9. doi: 10.1002/acg2.84. [DOI] [Google Scholar]
- 69.Tokarew N., Ogonek J., Endres S., von Bergwelt-Baildon M., Kobold S. Teaching an old dog new tricks: next-generation CAR T cells. Br. J. Cancer. 2019;120:26–37. doi: 10.1038/s41416-018-0325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y., Adu-Berchie K., Brockman J.M., Pezone M., Zhang D.K.Y., Zhou J., Pyrdol J.W., Wang H., Wucherpfennig K.W., Mooney D.J. Cytokine conjugation to enhance T cell therapy. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2213222120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Haideri M., Tondok S.B., Safa S.H., Maleki A.H., Rostami S., Jalil A.T., Al-Gazally M.E., Alsaikhan F., Rizaev J.A., Mohammad T.A.M., Tahmasebi S. CAR-T cell combination therapy: the next revolution in cancer treatment. Cancer Cell Int. 2022;22:365. doi: 10.1186/s12935-022-02778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rahimmanesh I., Khanahmad H. Chimeric antigen receptor-T cells immunotherapy for targeting breast cancer. Res Pharm Sci. 2021;16:447. doi: 10.4103/1735-5362.323911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huynh D., Winter P., Märkl F., Endres S., Kobold S. Beyond direct killing—novel cellular immunotherapeutic strategies to reshape the tumor microenvironment. Semin. Immunopathol. 2023;45:215–227. doi: 10.1007/s00281-022-00962-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duan D., Wang K., Wei C., Feng D., Liu Y., He Q., Xu X., Wang C., Zhao S., Lv L., Long J., Lin D., Zhao A., Fang B., Jiang J., Tang S., Gao J. The BCMA-targeted fourth-generation CAR-T cells secreting IL-7 and CCL19 for therapy of refractory/recurrent multiple myeloma. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.609421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Z., Zhou J., Yang X., Liu Y., Zou C., Lv W., Chen C., Cheng K.K., Chen T., Chang L.-J., Wu D., Mao J. Safety and antitumor activity of GD2-Specific 4SCAR-T cells in patients with glioblastoma. Mol. Cancer. 2023;22:3. doi: 10.1186/s12943-022-01711-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luangwattananun P., Junking M., Sujjitjoon J., Wutti-in Y., Poungvarin N., Thuwajit C., Yenchitsomanus P. Fourth-generation chimeric antigen receptor T cells targeting folate receptor alpha antigen expressed on breast cancer cells for adoptive T cell therapy. Breast Cancer Res. Treat. 2021;186:25–36. doi: 10.1007/s10549-020-06032-3. [DOI] [PubMed] [Google Scholar]
- 77.Fang W., Fu Q., Zhao Q., Zheng Y., Liu L., Li Z., Dai X., Wang H., Zhu X., Zhao P., Lin M., Zhang H., Xiao J., Liu J., Tong Z., Wang Z., Liang T. Phase I trial of fourth-generation chimeric antigen receptor T-cells targeting glypican-3 for advanced hepatocellular carcinoma. J. Clin. Oncol. 2021;39 doi: 10.1200/JCO.2021.39.15_suppl.4088. 4088–4088. [DOI] [Google Scholar]
- 78.Batra S.A., Rathi P., Guo L., Courtney A.N., Fleurence J., Balzeau J., Shaik R.S., Nguyen T.P., Wu M.-F., Bulsara S., Mamonkin M., Metelitsa L.S., Heczey A. Glypican-3–Specific CAR T cells coexpressing IL15 and IL21 have superior expansion and antitumor activity against hepatocellular carcinoma. Cancer Immunol. Res. 2020;8:309–320. doi: 10.1158/2326-6066.CIR-19-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang W., Liu Y., Hu Y., Gao J. [Construction and function of Glypican-3-targeted fourth-generation chimeric antigen receptor T cells (secreting IL-7 and CCL19)] Sheng Wu Gong Cheng Xue Bao. 2020;36:979–991. doi: 10.13345/j.cjb.200106. [DOI] [PubMed] [Google Scholar]
- 80.Zhou X., Tu S., Wang C., Huang R., Deng L., Song C., Yue C., He Y., Yang J., Liang Z., Wu A., Li M., Zhou W., Du J., Guo Z., Li Y., Jiao C., Liu Y., Chang L.-J., Li Y. Phase I trial of fourth-generation anti-CD19 chimeric antigen receptor T cells against relapsed or refractory B cell non-hodgkin lymphomas. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.564099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma X., Shou P., Smith C., Chen Y., Du H., Sun C., Porterfield Kren N., Michaud D., Ahn S., Vincent B., Savoldo B., Pylayeva-Gupta Y., Zhang S., Dotti G., Xu Y. Interleukin-23 engineering improves CAR T cell function in solid tumors. Nat. Biotechnol. 2020;38:448–459. doi: 10.1038/s41587-019-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou X., Tu S., Wang C., Huang R., Deng L., Song C., Yue C., He Y., Yang J., Liang Z., Wu A., Li M., Zhou W., Du J., Guo Z., Li Y., Jiao C., Liu Y., Chang L.-J., Li Y. Phase I trial of fourth-generation anti-CD19 chimeric antigen receptor T cells against relapsed or refractory B cell non-hodgkin lymphomas. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.564099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Duan D., Wang K., Wei C., Feng D., Liu Y., He Q., Xu X., Wang C., Zhao S., Lv L., Long J., Lin D., Zhao A., Fang B., Jiang J., Tang S., Gao J. The BCMA-targeted fourth-generation CAR-T cells secreting IL-7 and CCL19 for therapy of refractory/recurrent multiple myeloma. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.609421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koneru M., O'Cearbhaill R., Pendharkar S., Spriggs D.R., Brentjens R.J. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16ecto directed chimeric antigen receptors for recurrent ovarian cancer. J. Transl. Med. 2015;13:102. doi: 10.1186/s12967-015-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma X., Shou P., Smith C., Chen Y., Du H., Sun C., Porterfield Kren N., Michaud D., Ahn S., Vincent B., Savoldo B., Pylayeva-Gupta Y., Zhang S., Dotti G., Xu Y. Interleukin-23 engineering improves CAR T cell function in solid tumors. Nat. Biotechnol. 2020;38:448–459. doi: 10.1038/s41587-019-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mehrabadi A.Z., Ranjbar R., Farzanehpour M., Shahriary A., Dorostkar R., Hamidinejad M.A., Ghaleh H.E.G. Therapeutic potential of CAR T cell in malignancies: a scoping review. Biomed. Pharmacother. 2022;146 doi: 10.1016/j.biopha.2021.112512. [DOI] [PubMed] [Google Scholar]
- 87.Lu J., Jiang G. The journey of CAR-T therapy in hematological malignancies. Mol. Cancer. 2022;21:194. doi: 10.1186/s12943-022-01663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim D., Cho J.-Y. Recent advances in allogeneic CAR-T cells. Biomolecules. 2020;10:263. doi: 10.3390/biom10020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu J., Jiang G. The journey of CAR-T therapy in hematological malignancies. Mol. Cancer. 2022 doi: 10.1186/s12943-022-01663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie B., Li Z., Zhou J., Wang W. Current status and perspectives of dual-targeting chimeric antigen receptor T-cell therapy for the treatment of hematological malignancies. Cancers. 2022;14:3230. doi: 10.3390/cancers14133230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cordoba S., Onuoha S., Thomas S., Pignataro D.S., Hough R., Ghorashian S., Vora A., Bonney D., Veys P., Rao K., Lucchini G., Chiesa R., Chu J., Clark L., Fung M.M., Smith K., Peticone C., Al-Hajj M., Baldan V., Ferrari M., Srivastava S., Jha R., Arce Vargas F., Duffy K., Day W., Virgo P., Wheeler L., Hancock J., Farzaneh F., Domning S., Zhang Y., Khokhar N.Z., Peddareddigari V.G.R., Wynn R., Pule M., Amrolia P.J. CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: a phase 1 trial. Nat. Med. 2021;27:1797–1805. doi: 10.1038/s41591-021-01497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hirabayashi K., Du H., Xu Y., Shou P., Zhou X., Fucá G., Landoni E., Sun C., Chen Y., Savoldo B., Dotti G. Dual-targeting CAR-T cells with optimal co-stimulation and metabolic fitness enhance antitumor activity and prevent escape in solid tumors. Nat. Can. (Ott.) 2021;2:904–918. doi: 10.1038/s43018-021-00244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spiegel J.Y., Patel S., Muffly L., Hossain N.M., Oak J., Baird J.H., Frank M.J., Shiraz P., Sahaf B., Craig J., Iglesias M., Younes S., Natkunam Y., Ozawa M.G., Yang E., Tamaresis J., Chinnasamy H., Ehlinger Z., Reynolds W., Lynn R., Rotiroti M.C., Gkitsas N., Arai S., Johnston L., Lowsky R., Majzner R.G., Meyer E., Negrin R.S., Rezvani A.R., Sidana S., Shizuru J., Weng W.-K., Mullins C., Jacob A., Kirsch I., Bazzano M., Zhou J., Mackay S., Bornheimer S.J., Schultz L., Ramakrishna S., Davis K.L., Kong K.A., Shah N.N., Qin H., Fry T., Feldman S., Mackall C.L., Miklos D.B. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat. Med. 2021;27:1419–1431. doi: 10.1038/s41591-021-01436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van der Schans J.J., van de Donk N.W.C.J., Mutis T. Dual targeting to overcome current challenges in multiple myeloma CAR T-cell treatment. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.01362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van der Schans J.J., van de Donk N.W.C.J., Mutis T. Dual targeting to overcome current challenges in multiple myeloma CAR T-cell treatment. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.01362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Balagopal S., Sasaki K., Kaur P., Nikolaidi M., Ishihara J. Emerging approaches for preventing cytokine release syndrome in CAR-T cell therapy. J. Mater. Chem. B. 2022;10:7491–7511. doi: 10.1039/d2tb00592a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hamieh M., Mansilla-Soto J., Rivière I., Sadelain M. Programming CAR T cell tumor recognition: tuned antigen sensing and logic gating. Cancer Discov. 2023;13:829–843. doi: 10.1158/2159-8290.CD-23-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Asmamaw Dejenie T., Tiruneh M., Medhin G., Dessie Terefe G., Tadele Admasu F., Wale Tesega W., Chekol Abebe E. Current updates on generations, approvals, and clinical trials of CAR T-cell therapy. Hum. Vaccines Immunother. 2022;18 doi: 10.1080/21645515.2022.2114254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Perez C., Gruber I., Arber C. Off-the-Shelf allogeneic T cell therapies for cancer: opportunities and challenges using naturally occurring “universal” donor T cells. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.583716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sinha D., Srihari S., Beckett K., Le Texier L., Solomon M., Panikkar A., Ambalathingal G.R., Lekieffre L., Crooks P., Rehan S., Neller M.A., Smith C., Khanna R. “Off-the-shelf” allogeneic antigen-specific adoptive T-cell therapy for the treatment of multiple EBV-associated malignancies. J Immunother Cancer. 2021 doi: 10.1136/jitc-2020-001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brudno J.N., Kochenderfer J.N. Off-the-shelf CAR T cells for multiple myeloma. Nat. Med. 2023;29:303–304. doi: 10.1038/s41591-022-02195-2. [DOI] [PubMed] [Google Scholar]
- 102.Berrien-Elliott M.M., Jacobs M.T., Fehniger T.A. Allogeneic natural killer cell therapy. Blood. 2023 doi: 10.1182/blood.2022016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martínez Bedoya D., Dutoit V., Migliorini D. Allogeneic CAR T cells: an alternative to overcome challenges of CAR T cell therapy in glioblastoma. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.640082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lulla P.D., Brenner M. Emerging challenges to cellular therapy of cancer. Cancer J. 2023;29:20–27. doi: 10.1097/PPO.0000000000000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tzannou I., Papadopoulou A., Naik S., Leung K., Martinez C.A., Ramos C.A., Carrum G., Sasa G., Lulla P., Watanabe A., Kuvalekar M., Gee A.P., Wu M.F., Liu H., Grilley B.J., Krance R.A., Gottschalk S., Brenner M.K., Rooney C.M., Heslop H.E., Leen A.M., Omer B. Off-the-shelf virus-specific T cells to treat BK virus, human herpesvirus 6, cytomegalovirus, Epstein-Barr virus, and adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J. Clin. Oncol. 2017;35:3547–3557. doi: 10.1200/JCO.2017.73.0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lulla P.D., Brenner M. Emerging challenges to cellular therapy of cancer. Cancer J. 2023;29:20–27. doi: 10.1097/PPO.0000000000000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Labanieh L., Mackall C.L. CAR immune cells: design principles, resistance and the next generation. Nature. 2023;614:635–648. doi: 10.1038/s41586-023-05707-3. [DOI] [PubMed] [Google Scholar]
- 108.An Y., Jin X., Zhang H., Zhang M., Mahara S., Lu W., Zhao M. Springer US; 2023. “Off-the-Shelf” Allogeneic CAR Cell Therapy—Neglected HvG Effect. [DOI] [PubMed] [Google Scholar]
- 109.Levine B.L., Miskin J., Wonnacott K., Keir C. Global manufacturing of CAR T cell therapy. Mol Ther Methods Clin Dev. 2017;4:92–101. doi: 10.1016/j.omtm.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaiser A.D., Assenmacher M., Schröder B., Meyer M., Orentas R., Bethke U., Dropulic B. Cancer Gene Ther.; 2015. Towards a Commercial Process for the Manufacture of Genetically Modified T Cells for Therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abbott R.C., Cross R.S., Jenkins M.R. Finding the keys to the CAR: identifying novel target antigens for T cell redirection immunotherapies. Int. J. Mol. Sci. 2020;21:515. doi: 10.3390/ijms21020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N., Steinberg S.M., Stroncek D., Tschernia N., Yuan C., Zhang H., Zhang L., Rosenberg S.A., Wayne A.S., Mackall C.L. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015 doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Braendstrup P., Levine B.L., Ruella M. The long road to the first FDA-approved gene therapy: chimeric antigen receptor T cells targeting CD19. Cytotherapy. 2020 doi: 10.1016/j.jcyt.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kochenderfer J.N., Dudley M.E., Carpenter R.O., Kassim S.H., Rose J.J., Telford W.G., Hakim F.T., Halverson D.C., Fowler D.H., Hardy N.M., Mato A.R., Hickstein D.D., Gea-Banacloche J.C., Pavletic S.Z., Sportes C., Maric I., Feldman S.A., Hansen B.G., Wilder J.S., Blacklock-Schuver B., Jena B., Bishop M.R., Gress R.E., Rosenberg S.A. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013 doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kochenderfer J.N., Dudley M.E., Kassim S.H., Somerville R.P.T., Carpenter R.O., Maryalice S.S., Yang J.C., Phan G.Q., Hughes M.S., Sherry R.M., Raffeld M., Feldman S., Lu L., Li Y.F., Ngo L.T., Goy A., Feldman T., Spaner D.E., Wang M.L., Chen C.C., Kranick S.M., Nath A., Nathan D.A.N., Morton K.E., Toomey M.A., Rosenberg S.A. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 2015 doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schuster S.J., Svoboda J., Dwivedy Nasta S., Porter D.L., Chong E.A., Mahnke Y., Lacey S.F., Melenhorst J.J., Chew A., Shah G., Hasskarl J., Litchman M., Wasik M.A., Landsburg D.J., Mato A.R., Garfall A.L., Frey N.V., Marcucci K.T., Shea J., McConville H., Zheng Z., Levine B.L., June C.H. Phase IIa trial of chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed or refractory CD19+ lymphomas. Blood. 2014 doi: 10.1182/blood.v124.21.3087.3087. [DOI] [Google Scholar]
- 117.Sauter C.S., Riviere I., Bernal Y., Wang X., Purdon T., Yoo S., Moskowitz C.H., Giralt S., Matasar M.J., Curran K.J., Park J.H., Sadelain M., Brentjens R.J. Phase I trial of 19-28z chimeric antigen receptor modified T cells (19-28z CAR-T) post-high dose therapy and autologous stem cell transplant (HDT-ASCT) for relapsed and refractory (rel/ref) aggressive B-cell non-Hodgkin lymphoma (B-NHL) J. Clin. Oncol. 2015 doi: 10.1200/jco.2015.33.15_suppl.8515. [DOI] [Google Scholar]
- 118.Gagelmann N., Riecken K., Wolschke C., Berger C., Ayuk F.A., Fehse B., Kröger N. Development of CAR-T cell therapies for multiple myeloma. Leukemia. 2020 doi: 10.1038/s41375-020-0930-x. [DOI] [PubMed] [Google Scholar]
- 119.D'Agostino M., Raje N. Anti-BCMA CAR T-cell therapy in multiple myeloma: can we do better? Leukemia. 2020;34:21–34. doi: 10.1038/s41375-019-0669-4. [DOI] [PubMed] [Google Scholar]
- 120.Carpenter R.O., Evbuomwan M.O., Pittaluga S., Rose J.J., Raffeld M., Yang S., Gress R.E., Hakim F.T., Kochenderfer J.N. Clinical Cancer Research; 2013. B-Cell Maturation Antigen Is a Promising Target for Adoptive T-Cell Therapy of Multiple Myeloma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Greipp P.R., Miguel J.S., Dune B.G.M., Crowley J.J., Barlogie B., Bladé J., Boccadoro M., Child J.A., Harousseau J.L., Kyle R.A., Lahuerta J.J., Ludwig H., Morgan G., Powles R., Shimizu K., Shustik C., Sonneveld P., Tosi P., Turesson I., Westin J. International staging system for multiple myeloma. J. Clin. Oncol. 2005 doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 122.Ramos C.A., Heslop H.E., Brenner M.K. CAR-T cell therapy for lymphoma. Annu. Rev. Med. 2016 doi: 10.1146/annurev-med-051914-021702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Till B.G., Jensen M.C., Wang J., Chen E.Y., Wood B.L., Greisman H.A., Qian X., James S.E., Raubitschek A., Forman S.J., Gopal A.K., Pagel J.M., Lindgren C.G., Greenberg P.D., Riddell S.R., Press O.W. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang Y., ying Zhang W., wang Han Q., Liu Y., ren Dai H., lei Guo Y., Bo J., Fan H., Zhang Y., jing Zhang Y., xia Chen M., chao Feng K., shun Wang Q., bing Fu X., dong Han W. Effective response and delayed toxicities of refractory advanced diffuse large B-cell lymphoma treated by CD20-directed chimeric antigen receptor-modified T cells. Clin. Immunol. 2014 doi: 10.1016/j.clim.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 125.Haso W., Lee D.W., Shah N.N., Stetler-Stevenson M., Yuan C.M., Pastan I.H., Dimitrov D.S., Morgan R.A., FitzGerald D.J., Barrett D.M., Wayne A.S., MacKall C.L., Orentas R.J. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013 doi: 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pan J., Niu Q., Deng B., Liu S., Wu T., Gao Z., Liu Z., Zhang Y., Qu X., Zhang Y., Liu S., Ling Z., Lin Y., Zhao Y., Song Y., Tan X., Zhang Y., Li Z., Yin Z., Chen B., Yu X., Yan J., Zheng Q., Zhou X., Gao J., Chang A.H., Feng X., Tong C. CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia. 2019 doi: 10.1038/s41375-019-0488-7. [DOI] [PubMed] [Google Scholar]
- 127.Fry T.J., Shah N.N., Orentas R.J., Stetler-Stevenson M., Yuan C.M., Ramakrishna S., Wolters P., Martin S., Delbrook C., Yates B., Shalabi H., Fountaine T.J., Shern J.F., Majzner R.G., Stroncek D.F., Sabatino M., Feng Y., Dimitrov D.S., Zhang L., Nguyen S., Qin H., Dropulic B., Lee D.W., Mackall C.L. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018 doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zanetti S.R., Velasco-Hernandez T., Gutierrez-Agüera F., Díaz V.M., Romecín P.A., Roca-Ho H., Sánchez-Martínez D., Tirado N., Baroni M.L., Petazzi P., Torres-Ruiz R., Molina O., Bataller A., Fuster J.L., Ballerini P., Juan M., Jeremias I., Bueno C., Menéndez P. A novel and efficient tandem CD19- and CD22-directed CAR for B-cell ALL. Mol. Ther. 2021 doi: 10.1016/j.ymthe.2021.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Haso W., Lee D.W., Shah N.N., Stetler-Stevenson M., Yuan C.M., Pastan I.H., Dimitrov D.S., Morgan R.A., FitzGerald D.J., Barrett D.M., Wayne A.S., Mackall C.L., Orentas R.J. Anti-CD22–chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–1174. doi: 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Singh N., Frey N.V., Engels B., Barrett D.M., Shestova O., Ravikumar P., Shyu A., Highfill S., Zhao L., Peng L., Granda B., Ramones M., Lu X.M., Christian D.A., Perazzelli J., Lacey S.F., Roy N., Burkhardt J.K., Colomb F., Abdel-Mohsen M., Young R.M., Brogdon J., Grupp S., June C.H., Maude S.L., Gill S.I., Ruella M. Single chain variable fragment linker length regulates CAR biology and T cell efficacy. Blood. 2019;134 doi: 10.1182/blood-2019-131024. 247–247. [DOI] [Google Scholar]
- 131.Younes A., Gopal A.K., Smith S.E., Ansell S.M., Rosenblatt J.D., Savage K.J., Ramchandren R., Bartlett N.L., Cheson B.D., De Vos S., Forero-Torres A., Moskowitz C.H., Connors J.M., Engert A., Larsen E.K., Kennedy D.A., Sievers E.L., Chen R. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J. Clin. Oncol. 2012 doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Younes A., Bartlett N.L., Leonard J.P., Kennedy D.A., Lynch C.M., Sievers E.L., Forero-Torres A. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N. Engl. J. Med. 2010 doi: 10.1056/nejmoa1002965. [DOI] [PubMed] [Google Scholar]
- 133.Younes A., Connors J.M., Park S.I., Fanale M., O'Meara M.M., Hunder N.N., Huebner D., Ansell S.M. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin's lymphoma: a phase 1, open-label, dose-escalation study. Lancet Oncol. 2013 doi: 10.1016/S1470-2045(13)70501-1. [DOI] [PubMed] [Google Scholar]
- 134.Moskowitz C.H., Nademanee A., Masszi T., Agura E., Holowiecki J., Abidi M.H., Chen A.I., Stiff P., Gianni A.M., Carella A., Osmanov D., Bachanova V., Sweetenham J., Sureda A., Huebner D., Sievers E.L., Chi A., Larsen E.K., Hunder N.N., Walewski J. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)60165-9. [DOI] [PubMed] [Google Scholar]
- 135.Pro B., Advani R., Brice P., Bartlett N.L., Rosenblatt J.D., Illidge T., Matous J., Ramchandren R., Fanale M., Connors J.M., Yang Y., Sievers E.L., Kennedy D.A., Shustov A. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J. Clin. Oncol. 2012 doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 136.Horwitz S.M., Advani R.H., Bartlett N.L., Jacobsen E.D., Sharman J.P., O'Connor O.A., Siddiqi T., Kennedy D.A., Oki Y. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014 doi: 10.1182/blood-2013-12-542142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fanale M.A., Horwitz S.M., Forero-Torres A., Bartlett N.L., Advani R.H., Pro B., Chen R.W., Davies A., Illidge T., Huebner D., Kennedy D.A., Shustov A.R. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral t-cell lymphomas: results of a phase i study. J. Clin. Oncol. 2014 doi: 10.1200/JCO.2013.54.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Younes A., Yasothan U., Kirkpatrick P. Brentuximab vedotin. Nat. Rev. Drug Discov. 2012;11:19–20. doi: 10.1038/nrd3629. [DOI] [PubMed] [Google Scholar]
- 139.Diefenbach C.S.M., Leonard J.P. American Society of Clinical Oncology Educational Book; 2012. Targeting CD30 in Hodgkin Lymphoma: Antibody-Drug Conjugates Make a Difference; pp. 162–166. [DOI] [PubMed] [Google Scholar]
- 140.Went P., Agostinelli C., Gallamini A., Piccaluga P.P., Ascani S., Sabattini E., Bacci F., Falini B., Motta T., Paulli M., Artusi T., Piccioli M., Zinzani P.L., Pileri S.A. Marker expression in peripheral T-cell lymphoma: a proposed clinical-pathologic prognostic score. J. Clin. Oncol. 2006;24:2472–2479. doi: 10.1200/JCO.2005.03.6327. [DOI] [PubMed] [Google Scholar]
- 141.Forero-Torres A., Leonard J.P., Younes A., Rosenblatt J.D., Brice P., Bartlett N.L., Bosly A., Pinter-Brown L., Kennedy D., Sievers E.L., Gopal A.K. A Phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br. J. Haematol. 2009;146:171–179. doi: 10.1111/j.1365-2141.2009.07740.x. [DOI] [PubMed] [Google Scholar]
- 142.Bisig B., de Reynies A., Bonnet C., Sujobert P., Rickman D.S., Marafioti T., Delsol G., Lamant L., Gaulard P., de Leval L. CD30-positive peripheral T-cell lymphomas share molecular and phenotypic features. Haematologica. 2013;98:1250–1258. doi: 10.3324/haematol.2012.081935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bossard C., Dobay M.P., Parrens M., Lamant L., Missiaglia E., Haioun C., Martin A., Fabiani B., Delarue R., Tournilhac O., Delorenzi M., Gaulard P., De Leval L. Immunohistochemistry as a valuable tool to assess CD30 expression in peripheral T-cell lymphomas: high correlation with mRNA levels. Blood. 2014 doi: 10.1182/blood-2014-07-584953. [DOI] [PubMed] [Google Scholar]
- 144.Baskar S., Ka Y.K., Hofer T., Levy J.M., Kennedy M.G., Lee E., Staudt L.M., Wilson W.H., Wiestner A., Rader C. Clinical Cancer Research; 2008. Unique Cell Surface Expression of Receptor Tyrosine Kinase ROR1 in Human B-Cell Chronic Lymphocytic Leukemia. [DOI] [PubMed] [Google Scholar]
- 145.Chang H., Jung W.Y., Kang Y., Lee H., Kim A., Kim B.H. Expression of ROR1, pAkt, and pCREB in gastric adenocarcinoma. Ann. Diagn. Pathol. 2015 doi: 10.1016/j.anndiagpath.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 146.Chien H.P., Ueng S.H., Chen S.C., Chang Y.S., Lin Y.C., Lo Y.F., Chang H.K., Chuang W.Y., Huang Y.T., Cheung Y.C., Shen S.C., Hsueh C. Expression of ROR1 has prognostic significance in triple negative breast cancer. Virchows Arch. 2016 doi: 10.1007/s00428-016-1911-3. [DOI] [PubMed] [Google Scholar]
- 147.Zhang H., Qiu J., Ye C., Yang D., Gao L., Su Y., Tang X., Xu N., Zhang D., Xiong L., Mao Y., Li F., Zhu J. ROR1 expression correlated with poor clinical outcome in human ovarian cancer. Sci. Rep. 2014 doi: 10.1038/srep05811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu Y., Yang H., Chen T., Luo Y., Xu Z., Li Y., Yang J. Silencing of receptor tyrosine kinase ROR1 inhibits tumor-cell proliferation via PI3K/AKT/mTOR signaling pathway in lung adenocarcinoma. PLoS One. 2015 doi: 10.1371/journal.pone.0127092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Balakrishnan A., Goodpaster T., Randolph-Habecker J., Hoffstrom B.G., Jalikis F.G., Koch L.K., Berger C., Kosasih P.L., Rajan A., Sommermeyer D., Porter P.L., Riddell S.R. Clinical Cancer Research; 2017. Analysis of ROR1 Protein Expression in Human Cancer and Normal Tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Deniger D.C., Yu J., Huls M.H., Figliola M.J., Mi T., Maiti S.N., Widhopf G.F., Hurton L.V., Thokala R., Singh H., Olivares S., Champlin R.E., Wierda W.G., Kipps T.J., Cooper L.J.N. Sleeping beauty transposition of chimeric antigen receptors targeting receptor tyrosine kinase-like orphan receptor-1 (ROR1) into diverse memory T-cell populations. PLoS One. 2015;10 doi: 10.1371/journal.pone.0128151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao Y., Zhang D., Guo Y., Lu B., Zhao Z.J., Xu X., Chen Y. Tyrosine kinase ROR1 as a target for anti-cancer therapies. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.680834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wallstabe L., Göttlich C., Nelke L.C., Kühnemundt J., Schwarz T., Nerreter T., Einsele H., Walles H., Dandekar G., Nietzer S.L., Hudecek M. ROR1-CAR T cells are effective against lung and breast cancer in advanced microphysiologic 3D tumor models. JCI Insight. 2019;4 doi: 10.1172/jci.insight.126345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Berger S.C., Sommermeyer D., Hudecek M., Berger M., Balakrishnan A., Paskiewicz P., Kosasih P., Rader C., Riddell S. Safety of targeting ROR1 for cancer immunotherapy with chimeric antigen receptor-modified T cells in a primate model. J Immunother Cancer. 2014;2:P3. doi: 10.1186/2051-1426-2-S3-P3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Perriello V.M., Gionfriddo I., Rossi R., Milano F., Mezzasoma F., Marra A., Spinelli O., Rambaldi A., Annibali O., Avvisati G., Di Raimondo F., Ascani S., Falini B., Martelli M.P., Brunetti L. CD123 is consistently expressed on NPM1-mutated AML cells. Cancers. 2021;13:496. doi: 10.3390/cancers13030496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mardiros A., Dos Santos C., McDonald T., Brown C.E., Wang X., Budde L.E., Hoffman L., Aguilar B., Chang W.C., Bretzlaff W., Chang B., Jonnalagadda M., Starr R., Ostberg J.R., Jensen M.C., Bhatia R., Forman S.J. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013 doi: 10.1182/blood-2012-12-474056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jacoby E., Shahani S.A., Shah N.N. Updates on CAR T-cell therapy in B-cell malignancies. Immunol. Rev. 2019;290:39–59. doi: 10.1111/imr.12774. [DOI] [PubMed] [Google Scholar]
- 157.Mardiros A., Dos Santos C., McDonald T., Brown C.E., Wang X., Budde L.E., Hoffman L., Aguilar B., Chang W.C., Bretzlaff W., Chang B., Jonnalagadda M., Starr R., Ostberg J.R., Jensen M.C., Bhatia R., Forman S.J. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013 doi: 10.1182/blood-2012-12-474056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Budde L., Song J.Y., Kim Y., Blanchard S., Wagner J., Stein A.S., Weng L., Del Real M., Hernandez R., Marcucci E., Shepphird J.K., Wang X., Wood B., Marcucci G., Brown C.E., Forman S.J. Remissions of acute myeloid leukemia and blastic plasmacytoid dendritic cell neoplasm following treatment with cd123-specific CAR T cells: a first-in-human clinical trial. Blood. 2017;130 doi: 10.1182/blood.V130.Suppl_1.811.811. 811–811. [DOI] [Google Scholar]
- 159.Gill S., Tasian S.K., Ruella M., Shestova O., Li Y., Porter D.L., Carroll M., Danet-Desnoyers G., Scholler J., Grupp S.A., June C.H., Kalos M. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor–modified T cells. Blood. 2014;123:2343–2354. doi: 10.1182/blood-2013-09-529537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pizzitola I., Anjos-Afonso F., Rouault-Pierre K., Lassailly F., Tettamanti S., Spinelli O., Biondi A., Biagi E., Bonnet D. Chimeric antigen receptors against CD33/CD123 antigens efficiently target primary acute myeloid leukemia cells in vivo. Leukemia. 2014 doi: 10.1038/leu.2014.62. [DOI] [PubMed] [Google Scholar]
- 161.Dutour A., Marin V., Pizzitola I., Valsesia-Wittmann S., Lee D., Yvon E., Finney H., Lawson A., Brenner M., Biondi A., Biagi E., Rousseau R. Antitumor effect of anti-CD33 chimeric receptor-expressing EBV-CTL against CD 33 + acute myeloid leukemia. Adv Hematol. 2012;(2012):1–10. doi: 10.1155/2012/683065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Marin V., Pizzitola I., Agostoni V., Attianese G.M.P.G., Finney H., Lawson A., Pule M., Rousseau R., Biondi A., Biagi E. Cytokine-induced killer cells for cell therapy of acute myeloid leukemia: improvement of their immune activity by expression of CD33-specific chimeric receptors. Haematologica. 2010 doi: 10.3324/haematol.2010.026310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang Q., Wang Y., Lv H., Han Q., Fan H., Guo B., Wang L., Han W. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol. Ther. 2015;23:184–191. doi: 10.1038/mt.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Maakaron J.E., Rogosheske J., Long M., Bachanova V., Mims A.S. CD33‐Targeted therapies: beating the disease or beaten to death? J. Clin. Pharmacol. 2021;61:7–17. doi: 10.1002/jcph.1730. [DOI] [PubMed] [Google Scholar]
- 165.Miliotou A.N., Papadopoulou L.C. CAR T-cell therapy: a new era in cancer immunotherapy. Curr. Pharmaceut. Biotechnol. 2018;19:5–18. doi: 10.2174/1389201019666180418095526. [DOI] [PubMed] [Google Scholar]
- 166.Sterner R.C., Sterner R.M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Whilding L.M., Maher J. CAR T-cell immunotherapy: the path from the by-road to the freeway? Mol. Oncol. 2015;9:1994–2018. doi: 10.1016/j.molonc.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.CAR T-cell therapy for solid tumors? Cancer Discov. 2018;8 doi: 10.1158/2159-8290.CD-ND2018-008. 1341–1341. [DOI] [PubMed] [Google Scholar]
- 169.Gardner R., Finney O., Smithers H., Leger K.J., Annesley C.E., Summers C., Brown C., Mgebroff S., Lindgren C., Spratt K., Oron A., Li D., Bleakley M., Park J.R., Jensen M.C. CD19CAR T cell products of defined CD4:CD8 composition and transgene expression show prolonged persistence and durable MRD-negative remission in pediatric and young adult B-cell ALL. Blood. 2016;128 doi: 10.1182/blood.V128.22.219.219. 219–219. [DOI] [Google Scholar]
- 170.Turtle C.J., Hanafi L.A., Berger C., Gooley T.A., Cherian S., Hudecek M., Sommermeyer D., Melville K., Pender B., Budiarto T.M., Robinson E., Steevens N.N., Chaney C., Soma L., Chen X., Yeung C., Wood B., Li D., Cao J., Heimfeld S., Jensen M.C., Riddell S.R., Maloney D.G. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 2016 doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brentjens R.J., Rivière I., Park J.H., Davila M.L., Wang X., Stefanski J., Taylor C., Yeh R., Bartido S., Borquez-Ojeda O., Olszewska M., Bernal Y., Pegram H., Przybylowski M., Hollyman D., Usachenko Y., Pirraglia D., Hosey J., Santos E., Halton E., Maslak P., Scheinberg D., Jurcic J., Heaney M., Heller G., Frattini M., Sadelain M. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011 doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Park J.H., Riviere I., Wang X., Purdon T., Sadelain M., Brentjens R.J. Impact of disease burden on long-term outcome of 19-28z CAR modified T cells in adult patients with relapsed B-ALL. J. Clin. Oncol. 2016 doi: 10.1200/jco.2016.34.15_suppl.7003. [DOI] [Google Scholar]
- 173.Gardner R., Finney O., Smithers H., Leger K.J., Annesley C.E., Summers C., Brown C., Mgebroff S., Lindgren C., Spratt K., Oron A., Li D., Bleakley M., Park J.R., Jensen M.C. CD19CAR T cell products of defined CD4:CD8 composition and transgene expression show prolonged persistence and durable MRD-negative remission in pediatric and young adult B-cell ALL. Blood. 2016;128 doi: 10.1182/blood.V128.22.219.219. 219–219. [DOI] [Google Scholar]
- 174.Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., Qayed M., De Moerloose B., Hiramatsu H., Schlis K., Davis K.L., Martin P.L., Nemecek E.R., Yanik G.A., Peters C., Baruchel A., Boissel N., Mechinaud F., Balduzzi A., Krueger J., June C.H., Levine B.L., Wood P., Taran T., Leung M., Mueller K.T., Zhang Y., Sen K., Lebwohl D., Pulsipher M.A., Grupp S.A. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Maude S.L., Teachey D.T., Rheingold S.R., Shaw P.A., Aplenc R., Barrett D.M., Barker C.S., Callahan C., Frey N.V., Nazimuddin F., Lacey S.F., Zheng Z., Levine B., Melenhorst J.J., Motley L., Porter D.L., June C.H., Grupp S.A. Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL. J. Clin. Oncol. 2016;34 doi: 10.1200/JCO.2016.34.15_suppl.3011. 3011–3011. [DOI] [Google Scholar]
- 176.Majzner R.G., Heitzeneder S., Mackall C.L. Harnessing the immunotherapy revolution for the treatment of childhood cancers. Cancer Cell. 2017;31:476–485. doi: 10.1016/j.ccell.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 177.Brown C.E., Mackall C.L. CAR T cell therapy: inroads to response and resistance. Nat. Rev. Immunol. 2019;19:73–74. doi: 10.1038/s41577-018-0119-y. [DOI] [PubMed] [Google Scholar]
- 178.Stirrups R. CAR T-cell therapy in refractory large B-cell lymphoma. Lancet Oncol. 2018;19:e19. doi: 10.1016/S1470-2045(17)30928-2. [DOI] [PubMed] [Google Scholar]
- 179.Hudecek M., Lupo-Stanghellini M.-T., Kosasih P.L., Sommermeyer D., Jensen M.C., Rader C., Riddell S.R. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin. Cancer Res. 2013;19:3153–3164. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Caruso H.G., Hurton L.V., Najjar A., Rushworth D., Ang S., Olivares S., Mi T., Switzer K., Singh H., Huls H., Lee D.A., Heimberger A.B., Champlin R.E., Cooper L.J.N. Cancer Res.; 2015. Tuning Sensitivity of CAR to EGFR Density Limits Recognition of Normal Tissue while Maintaining Potent Antitumor Activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Simon B., Uslu U. Fasten the seat belt: increasing safety of CAR T‐cell therapy. Exp. Dermatol. 2020;29:1039–1045. doi: 10.1111/exd.14131. [DOI] [PubMed] [Google Scholar]
- 182.Alsajjan R., Mason W.P. Bispecific T-cell engagers and chimeric antigen receptor T-cell therapies in glioblastoma: an update. Curr. Oncol. 2023;30:8501–8549. doi: 10.3390/curroncol30090619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hirschhorn D., Budhu S., Kraehenbuehl L., Gigoux M., Schröder D., Chow A., Ricca J.M., Gasmi B., De Henau O., Mangarin L.M.B. T cell immunotherapies engage neutrophils to eliminate tumor antigen escape variants. Cell. 2023;186:1432–1447. doi: 10.1016/j.cell.2023.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zheng J. CAR T-cell therapy in solid tumors: current review and future perspectives, highlights in science. Eng. Technol. 2023;54:517–527. [Google Scholar]
- 185.Chohan K.L., Siegler E.L., Kenderian S.S. CAR-T cell therapy: the efficacy and toxicity balance. Curr Hematol Malig Rep. 2023;18:9–18. doi: 10.1007/s11899-023-00687-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chinthapalli M.T., Lena L., Hoxha E., Jano D., Rucaj E., Hoxha A., Kazazi S., Hoxha K., Mamillo K. Management of emergency adverse effects of cart cell therapy in hematologic cancers. J. Pharm. Negat. Results. 2023:435–446. [Google Scholar]
- 187.Liu Y., Sperling A.S., Smith E.L., Mooney D.J. Optimizing the manufacturing and antitumour response of CAR T therapy. Nature Reviews Bioengineering. 2023;1:271–285. [Google Scholar]
- 188.Baron K.J., Turnquist H.R. Clinical manufacturing of regulatory T cell products for adoptive cell therapy and strategies to improve therapeutic efficacy. Organogenesis. 2023;19 doi: 10.1080/15476278.2022.2164159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.