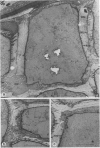
Abstract
The secretion of α-amylase from single isolated (Hordeum vulgare L. cv Himalaya) aleurone layers was studied in an automated flow-through apparatus. The apparatus, consisting of a modified sample analyzer linked to a chart recorder, automatically samples the flow-through medium at 1 minute intervals and assays for the presence of α-amylase. The release of α-amylase from aleurone layers begins after 5 to 6 hours of exposure to gibberellic acid and reaches a maximum rate after 10 to 12 hours. The release of α-amylase shows a marked dependence on Ca2+, and in the absence of Ca2+ it is only 20% of that in the presence of 10 millimolar Ca2+. Withdrawal of Ca2+ from the flow-through medium results in the immediate cessation of enzyme release and addition of Ca2+ causes immediate resumption of the release process. The effect of Ca2+ is concentration-dependent, being half-maximal at 1 millimolar Ca2+ and saturated at 10 millimolar Ca2+. Ruthenium red, which blocks Ca2+ but not Mg2+ efflux from barley aleurone layers, renders α-amylase release insensitive to Ca2+ withdrawal. Inhibitors of respiratory metabolism cause a burst of α-amylase release which lasts for 0.5 to 5 hours. Following this phase of enhanced α-amylase release, the rate of release declines to zero. Pretreatment of aleurone layers with HCl prior to incubation in HCN also causes a burst of α-amylase release, indicating that the inhibitor is affecting the secretion of α-amylase and not its movement through the cell wall. The rapid inhibition of α-amylase release upon incubation of aleurone layers at low temperature (5°C) or in 0.5 molar mannitol also indicates that enzyme release is dependent on a metabolically linked process and is not diffusion-limited. This conclusion is supported by cytochemical observations which show that, although the cell wall matrix of aleurone layers undergoes extensive digestion after gibberellin treatment, the innermost part of the cell wall is not degraded and could influence enzyme release.
Full text
PDF
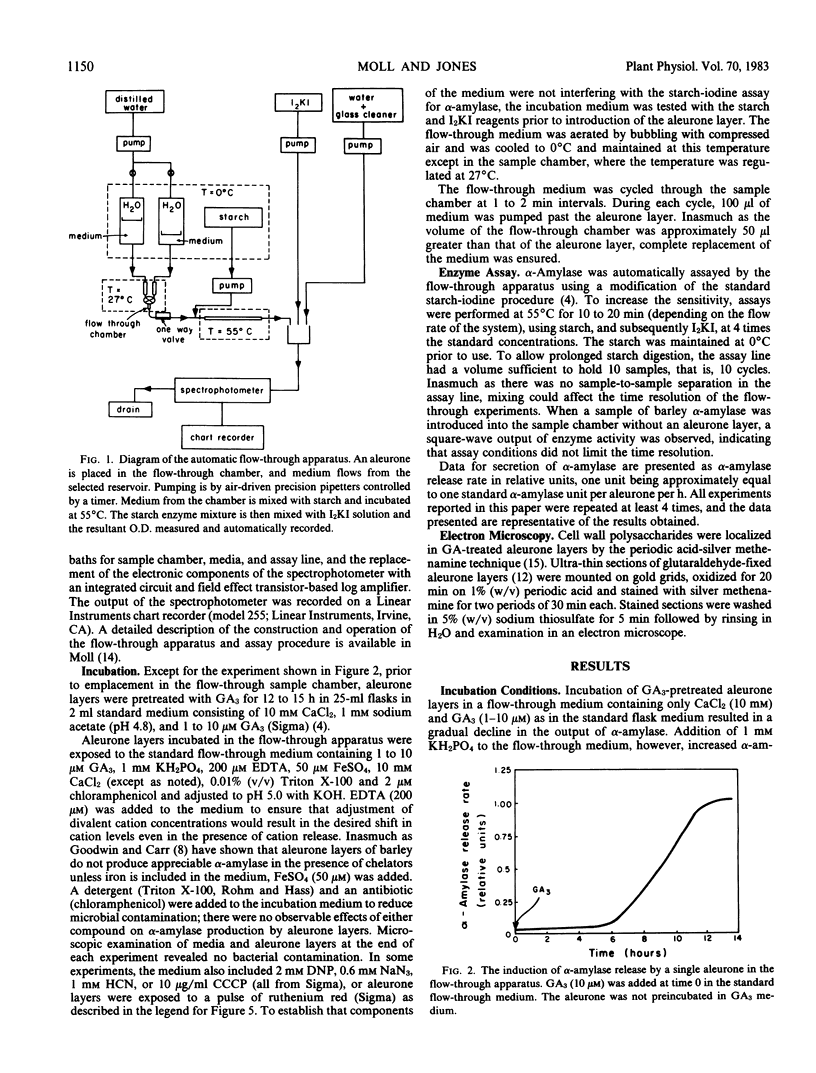
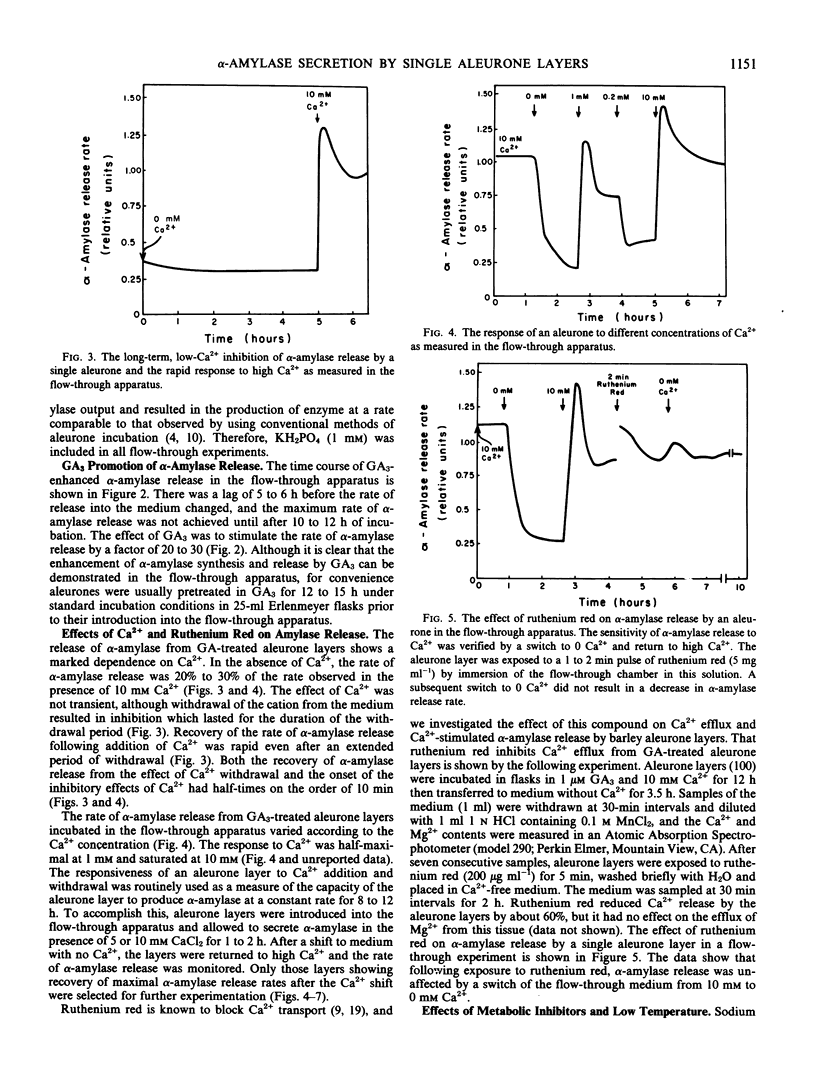
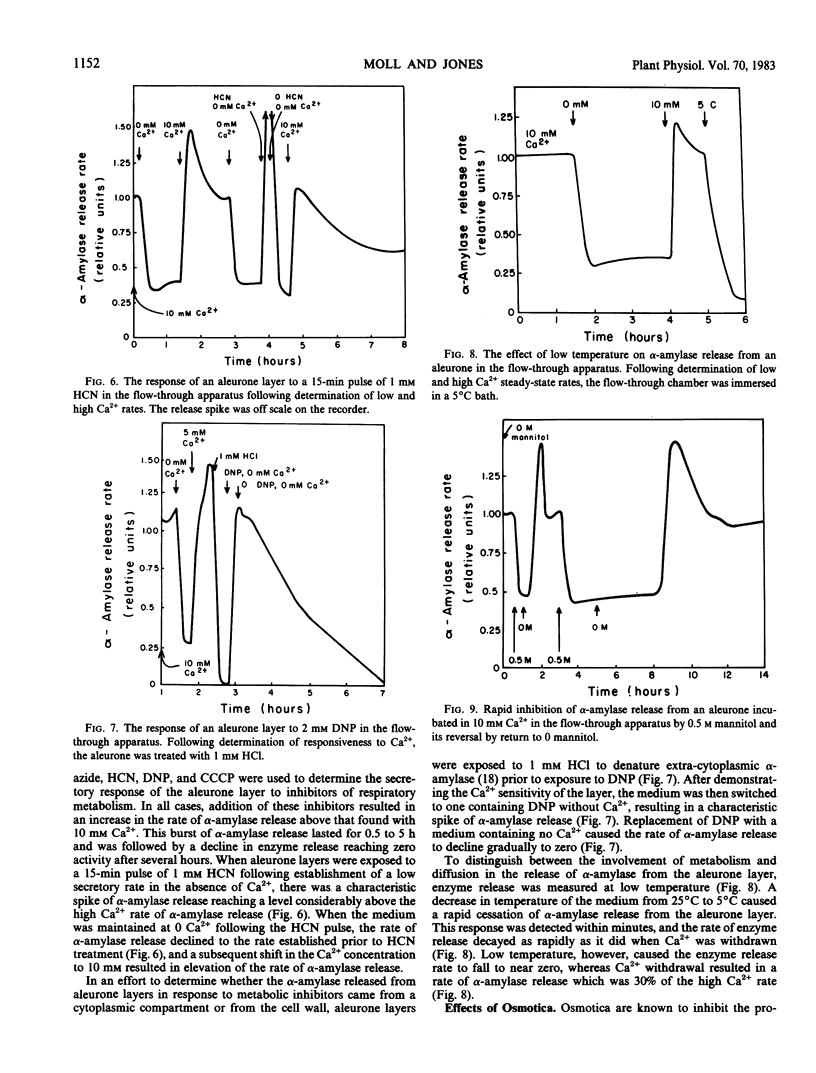



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. E., Jones R. L. Osmotic regulation of alpha-amylase synthesis and polyribosome formation in aleurone cells of barley. J Cell Biol. 1973 Nov;59(2 Pt 1):444–455. doi: 10.1083/jcb.59.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J. Mechanism of osmotic regulation of hydrolase synthesis in aleurone cells of barley: inhibition of protein synthesis. Biochem Biophys Res Commun. 1973 Jul 2;53(1):99–104. doi: 10.1016/0006-291x(73)91406-x. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J., Varner J. E. Gibberellic Acid-enhanced synthesis and release of alpha-amylase and ribonuclease by isolated barley and aleurone layers. Plant Physiol. 1967 Mar;42(3):398–406. doi: 10.1104/pp.42.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Varner J. E. Hormonal control of enzyme synthesis: on the mode of action of gibberellic Acid and abscisin in aleurone layers of barley. Plant Physiol. 1967 Jul;42(7):1008–1016. doi: 10.1104/pp.42.7.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Involvement of calcium in exocytosis and the exocytosis--vesiculation sequence. Biochem Soc Symp. 1974;(39):1–28. [PubMed] [Google Scholar]
- Hinds T. R., Raess B. U., Vincenzi F. F. Plasma membrane Ca2+ transport: antagonism by several potential inhibitors. J Membr Biol. 1981 Jan 30;58(1):57–65. doi: 10.1007/BF01871034. [DOI] [PubMed] [Google Scholar]
- Jones R. L., Armstrong J. E. Evidence for osmotic regulation of hydrolytic enzyme production in germinating barley seeds. Plant Physiol. 1971 Aug;48(2):137–142. doi: 10.1104/pp.48.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L. Gibberellic Acid and Ion Release from Barley Aleurone Tissue: Evidence for Hormone-dependent Ion Transport Capacity. Plant Physiol. 1973 Oct;52(4):303–308. doi: 10.1104/pp.52.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. K. Calcium translocation and control mechanisms for endocrine secretion. Symp Soc Exp Biol. 1979;33:225–249. [PubMed] [Google Scholar]
- Rambourg A. An improved silver methenamine technique for the detection of periodic acid-reactive complex carbohydrates with the electron microscope. J Histochem Cytochem. 1967 Jul;15(7):409–412. doi: 10.1177/15.7.409. [DOI] [PubMed] [Google Scholar]
- Varner J. E., Mense R. M. Characteristics of the process of enzyme release from secretory plant cells. Plant Physiol. 1972 Feb;49(2):187–189. doi: 10.1104/pp.49.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson E. L., Vincenzi F. F., Davis P. W. Ca 2+ -activated membrane ATPase: selective inhibition by ruthenium red. Biochim Biophys Acta. 1971 Dec 3;249(2):606–610. doi: 10.1016/0005-2736(71)90140-4. [DOI] [PubMed] [Google Scholar]