Abstract
Postsurgical adhesions are a common complication of surgical procedures that can lead to postoperative pain, bowel obstruction, infertility, as well as complications with future procedures. Several agents have been developed to prevent adhesion formation, such as barriers, anti‐inflammatory and fibrinolytic agents. The Food and Drug Administration (FDA) has approved the use of physical barrier agents, but they have been associated with conflicting clinical studies and controversy in the clinical utilization of anti‐adhesion barriers. In this review, we summarize the human anatomy of the peritoneum, the pathophysiology of adhesion formation, the current prevention agents, as well as the current research progress on adhesion prevention. The early cellular events starting with injured mesothelial cells and incorporating macrophage response have recently been found to be associated with adhesion formation. This may provide the key component for developing future adhesion prevention methods. The current use of physical barriers to separate tissues, such as Seprafilm®, composed of hyaluronic acid and carboxymethylcellulose, can only reduce the risk of adhesion formation at the end stage. Other anti‐inflammatory or fibrinolytic agents for preventing adhesions have only been studied within the context of current research models, which is limited by the lack of in‐vitro model systems as well as in‐depth study of in‐vivo models to evaluate the efficiency of anti‐adhesion agents. In addition, we explore emerging therapies, such as gene therapy and stem cell‐based approaches, that may offer new strategies for preventing adhesion formation. In conclusion, anti‐adhesion agents represent a promising approach for reducing the burden of adhesion‐related complications in surgical patients. Further research is needed to optimize their use and develop new therapies for this challenging clinical problem.
Keywords: abdominal adhesion, adhesion prevention, anti‐adhesion strategy, anti‐inflammatory, fibrinolytic agent, physical barrier, postsurgical adhesion
1. INTRODUCTION
Postsurgical adhesions (Figure 1) are a serious complication for patients as they form fibrin scar tissue between the surfaces of organs and the internal wall of the abdominal cavity. Common abdominal surgeries such as appendectomies, caesarean sections, and intestinal anastomoses are followed with the inevitable consequence of postsurgical adhesion formation for 89%–95% of patients following one or more open abdominal operations. 1 , 2 Other abdominal surgeries involving incorporation of implanted medical material such as hernia repair with mesh implantation is associated with higher postsurgical adhesion formation. 3 , 4 The hernia repair with polypropylene mesh implantation have been found to contribute to adhesion formation to abdominal viscera, especially with small bowel. 5 , 6 , 7 Common complications associated with postsurgical adhesions include small bowel obstruction, chronic pelvic pain, dyspareunia, infertility, and a higher risk of adhesion formation in subsequent surgical operations. 1 , 8 , 9 , 10 , 11 Despite improvements in surgical technique, such as the development of minimally invasive surgery, which reduces tissue trauma and external contamination; adhesions still occur in up to 45%–64% of abdominal operations. 1 , 2 , 9 , 12
FIGURE 1.
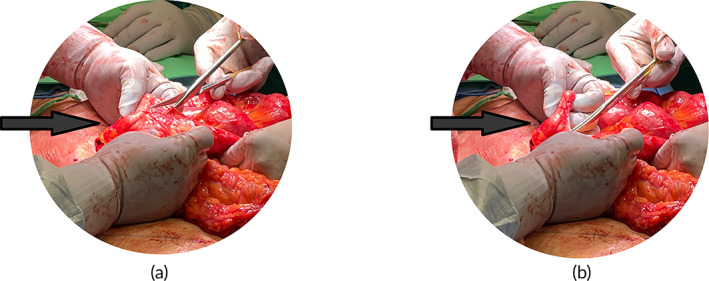
(a,b) Postsurgical adhesion lysis procedure during operation to treat ventral hernia recurrence (a. Before lysis procedure, b. After lysis procedure).
It has been postulated that postsurgical adhesions are triggered by tissue trauma or injury, and other contributory factors during the procedure, such as sharp, mechanical, or thermal irritation; ischemia; abrasion; and exposure to foreign bodies such as fiber, powder, irritating fluids, and airflow causing oxidative stress. 10 , 13 Additionally, it was observed in 1919 that healing of the peritoneum is different from external wound healing. 11 Postsurgical adhesion formation has been found as a dynamic process triggered by traumatized tissues and resulting in the cascade effect of fibrin exudate, cytokine production, cell migration, vascular edema, and the suppression of fibrinolytic activity. 11 This series of late cellular events includes the immune response and the fibrinolytic deposition forming a fibroblast‐rich scar tissue with collagen and extracellular matrix. 14 , 15 Whereas this late stage of adhesion formation is well understood, however, the dynamic and early events of postsurgical adhesion formation directly after surgery have not been well‐studied nor clearly understood. Thus, there are neither appropriate surgical procedures nor effective anti‐adhesion products currently available to prevent postsurgical adhesion formation. 15 , 16
By viewing adhesion tissue histologically, the composition of the internal scar tissue is known to contain a mixture of macrophages, eosinophils, red blood cells, tissue debris, mast cells, and fibroblasts. 11 , 17 , 18 Milligan and Raftery et al. (1973) reported that postsurgical adhesions also include nerve fibers and endothelial cells which can play a role in angiogenesis and the formation and stabilization of any postsurgical adhesions. 19 , 20 Yanez‐Mo et al. (2003) suggested an alternative explanation for etiology of postsurgical adhesions from myofibroblast metaplasia. 21 This concept has been supported by Sandoval et al. (2016), who determined that mesothelial cells undergo mesothelial‐to‐mesenchymal transition (MMT) 22 to become myofibroblast cells and deposit extracellular matrix (ECM) with subperitoneal fibroblasts. 2 , 11 , 19 , 23 On the other hand, Tsai et al. (2018) recently observed via lineage tracing in a rat model that the early events of adhesion formation relied on activation of mesothelial cells rather than fibroblasts depositing matrix. 14 , 15 , 16 Fischer et al. (2020) followed this previous study by creating a stressed mesothelial cell model to identify the cytoskeleton and calcium signaling channels that triggered the formation of postsurgical adhesion. 14 , 15
Based on our current understanding of the cellular mechanism, it has been identified that stressed mesothelial cells are a part of the earliest stages of the cascade towards triggering postsurgical adhesions. Current prevention methods that have been explored include physical barriers, gels, medications, as well as nanoparticles and gene therapy. 24 , 25 The most popular prevention devices approved by the Food and Drug Administration (FDA) have been Seprafilm® 26 and Interceed®, which function only as a physical barrier to prevent adhesion formation. 27 Since the introduction of Seprafilm® to the United States (US) market, multiple attempts have been made to petition the FDA for product recall due to concerns of complications associated with the inflammatory response of the implanted material such as peritonitis and severe foreign body reactions. 28 Nevertheless, FDA has found no consistent evidence to support such complications and thus, Seprafilm® remains the current golden standard to reduce adhesion clinically. Based on the findings of three clinical trials and other relevant studies, Seprafilm® was found to be only 2%–8% efficient in preventing adhesions. 28 , 29 , 30 , 31 Although there are many different materials proposed for the prevention of postsurgical adhesions, the lack of understanding of the cellular mechanism of adhesion formation has made it difficult to develop an effective anti‐adhesion treatment.
Most of the current literature review of postsurgical adhesions involves only the surgical pathogenesis perspective or the selection of polymers and materials in the prevention of postsurgical adhesions. The goal of this literature review is to establish the current clinical perspective of adhesion formation, the postsurgical human peritoneum response, and the pathogenesis involved in adhesion formation. This will allow alternative clinical strategies to address the current issues of postsurgical adhesion formation associated with implantable biomedical devices. The perspective of the literature reviews provides a linkage between the integration of current clinical practice with biomedical engineering research.
1.1. Clinical significance of abdominal adhesion prevention
Postsurgical adhesion‐related issues place a large economic burden on the US healthcare system and negatively impact the standard of living of patients who undergo abdominal surgeries as well as complicating future abdominal procedures for surgeons. 2 , 13 , 32 , 33 There are significant hospitalization expenditures in the US that are more than $1 billion dollars per year for the patients who undergo abdominal and gynecological surgeries for treatment of adhesion‐related issues. 33 Resulting from potentially prolonged hospitalization, patients are at an increased risk of internal organ injury, developing severe infections, and may require the need for physical rehabilitation before returning to normal daily functions.
Despite the economic impact of treating postsurgical adhesions, they are known to cause severe abdominal and pelvic pain, 34 small bowel‐obstruction, 17 complicate any secondary surgery, 20 and infertility 35 a for female patients. Although there are few studies that provide direct evidence to prove a correlation between chronic pelvic or abdominal pain and adhesions, nevertheless, some studies report that patients who experience adhesion lysis have less pain. 34 Cheong et al. (2014) report that adhesions may cause infertility and pelvic pain by blocking the fallopian tubes and preventing oocyte retrieval. 18 Ellis et al. (1999) reported that 64% of gynecological patients were readmitted within 10 years after surgery for adhesion‐related issues. 36 Additionally, the abdominal procedures involving biomedical device implantation such as hernia or pelvic organ prolapse repair can significantly increase the possibility of forming postsurgical adhesions because of the foreign body response (FBR). 7 , 35 , 37 The FBR is defined as a series of events initiated through the activity of foreign body giant cells that form scar tissue and/or a fibrous capsule. 37 According to the previous study, it is not clear whether there is overlap between postsurgical adhesions and FBR, in that both involve an inflammatory response to injury or an implanted foreign material. It has been concluded that the presence of implanted foreign materials, such as a mesh or a suture, may play a role in the development of adhesion formation. 38 , 39 A study by Artsen et al. (2020) found that the presence of a mesh implant can exacerbate postsurgical adhesion formation by promoting a sustained foreign body reaction in the surrounding tissues. 40 , 41 They have noticed that this reaction involves the recruitment of inflammatory cells, which can secrete cytokines and other factors that promote fibrosis and adhesion formation. 38 , 40 Thus, the use of a mesh in abdominal procedures has been associated with severe complications, including the formation of postsurgical adhesions. In a study by Klinge et al. (2014), patients who underwent abdominal surgery with mesh implantation had a higher incidence of complications includes postsurgical adhesions, infections, or chronic inflammation based on the mesh explants samples collagen Type I and III ratio. 42 Likewise, another study by Schreinemacher et al. (2013) found that patients who had mesh implanted with a layered coating develop less adhesion compared to mesh only. 4 These findings suggest that the use of a mesh in abdominal procedures may increase the risk of adhesion formation and adding a layered coating have a potential to decrease the risk of adhesion formation.
It is important to be aware of the potential issues for adhesions following abdominal procedures, especially those involving mesh implantation, as they can lead to serious complications. At the same time, adhesion formation can also increase the risk of internal bleeding requiring blood transfusions, or visceral damage requiring intervention from other subspecialities or critical care. There are no effective surgical techniques, surgical barriers, or pharmaceutical products that have been successfully demonstrated to reduce the risk of clinical postsurgical adhesions. This is due to the lack of understanding of the physiological mechanism. Given the pressing need to address the formation of postsurgical adhesions, it is imperative to gain a comprehensive understanding of the underlying developmental process. Doing so will facilitate the application of current biomedical anti‐adhesion technologies, and pave the way for the design of novel, more effective therapeutic strategies.
1.2. Peritoneum anatomy
The peritoneum is part of the abdominal cavity, which is the largest cavity among the three serosal cavities found in the human body. 43 The surface area of the peritoneum is equal to the skin, which is around 177 cm2/kg (body weight) for adults 17 with a surface area of approximately 1.8 m2. 44 The peritoneum serves to protect the abdominal cavity, minimize friction, facilitate free movement between abdominal viscera, resist or localize infection, and store fat, especially in the greater omentum. 17 , 44 The primary purpose of the peritoneal cavity is to provide a smooth surface layer over the abdominal viscera lined by a layer of mesothelial cells and a basal layer of connective tissue that is perfused with blood and lymphatic vessels to limit the mobility of viscera. 45 It is also recognized as a dynamic cellular membrane with more complex structure and physiological functions and is known to participate in the embryogenesis of the primitive gut. The peritoneum has been shown to have control over fluid and cell transport; serving as a physiological barrier; participating in the induction, modulation, and inhibition of the immune system; generating tissue repair and the formation of scars; as well as preventing both adhesion and tumoral dissemination. 43
As shown in Figure 2, the inferior abdominal cavity is composed of the visceral peritoneum and the peritoneum cavity. 43 The visceral peritoneum integrates with the outer serosal layers of organs, whereas the parietal peritoneum lines the inner surface of the abdominal wall as shown in Figure 2. 46 There are numerous elastic fibers, especially in the deeper layers of the parietal peritoneum, and comparatively few connective tissue cells. 46 The visceral layer of the mesothelial membranes covers the intra‐abdominal organs including liver, spleen, stomach, bowel, and the reproductive organs in females. 43 Figure 2 also illustrates that the serosa is composed of two layers, namely loose connective submesothelial tissue and a mesothelial layer. 17 , 43 The mesothelial layer is a monolayer of cells that is attached to the basal lamina of connective tissue. Because they are loosely bound together to form an intracellular bridge or matrix, the peritoneum is susceptible to trauma or injury. 17
FIGURE 2.
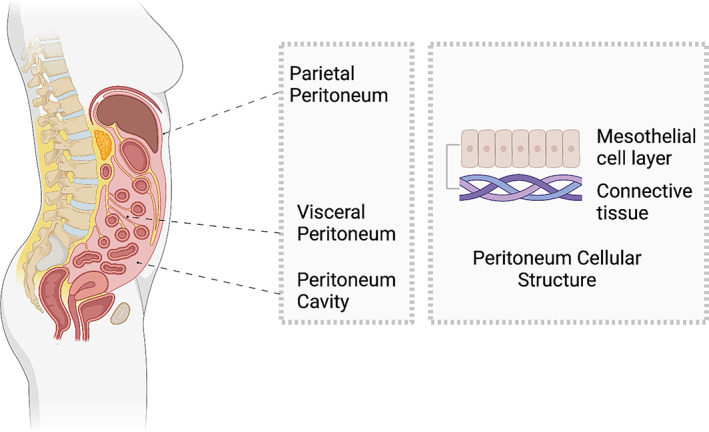
Anatomy and organization of the peritoneum including the visceral, parietal, and peritoneum cavities. The right‐hand image shows the cellular structure of two layers of mesothelial cells and connective tissue.
The extracellular matrix provides a suitable microenvironment for tissue regeneration including different types of collagen (Type I and III), fibronectin, glycoproteins, fibroblasts, macrophages, as well as vascular and lymphatic vessels. 47 The peritoneum forms a closed sac in the male and an open sac in the female because the ends of the fallopian tubes are not enclosed. 17 An interesting phenomenon is that the anatomy of the female peritoneum is different around the ovaries. Based on the histology of the tissue around the ovaries, a layer of cells called the germinal epithelium covers the ovarian surface with a dense, irregular connective tissue layer called the tunica albuginea which is continuous with the peritoneum. 45 Since the ovarian epithelium originates from the coelomic mesothelium, it therefore has similar structural, immunohistochemical and molecular characteristics as the peritoneal mesothelium. 47 , 48 The differences between female and male anatomy are due to the different types of tissue around the female ovaries. Referring to their histopathological observations, Beck et al. (1997) reported a higher frequency of postsurgical adhesions among female patients after abdominal/gynecological surgery compared to other types of operations in male patients. 49 In other respects, the peritoneum lining the abdominal wall and the visceral organs are similar to males. 43 Therefore, it is still not clear whether gender is a significant factor in developing adhesions. 50
2. PATHOPHYSIOLOGY
Postsurgical adhesion can occur throughout the human body following various types of operations, 51 such as cardiac, 52 peritoneum, 1 , 2 , 8 epidural, 53 as well as tendon procedures. 54 However, the pathophysiology of the different kinds of adhesion formation is still unclear as the tissue compositions are different between human cavities. 51 The complications with abdominal adhesions affect patients' quality of life and make future operations more difficult are major factors in addressing postsurgical adhesions in the abdominal cavity. Currently, the most special component of in the formation of postsurgical abdominal adhesions are the origins of mesothelial cells 16 and peritoneum large macrophages, 55 which have been introduced to initiate the early events in adhesion formation.
As we introduced the internal abdominal cellular composition, the mesothelial cell is the most special component in forming the peritoneum, which is a single layer of mesothelial cells 56 as it acts as a physiological barrier and participates in different biological activity. Therefore, the single layer of mesothelial cell function as a barrier or transportation medium by changing the apical‐basal polarity and intracellular junctions between the cells. 56 Discovered complexes between the cells includes tight junction, adherens, and gap junction as well as desmosomes. Tight junctions and adherens provide structural support and semipermeable properties in regulating ions, water, or other soluble nutrients. 56 However, the detailed pathway and interactions of these peritoneal components remain unknown. It has been found that the cell or fluids transport is known to regulate extracellular matrix (ECM) components through matrix metalloproteases (MMPs) and inhibitors (TIMPs) as well as generate procoagulant and fibrinolytic activity. 57 Injured mesothelial cell also receive an influx of cytokines resulting in neutrophil and macrophage activation. 58 In addition to the events involving postsurgical adhesions formation in the abdominal cavity, there are some similarities in other locations of the body that develop adhesions such as coagulation, inflammation, fibrinolysis, as well as ischemia.
Postsurgical adhesion formation is associated with the downregulation of both the fibrin deposition and fibrinolytic pathways during the wound‐healing process. When trauma or an incision occurs to the peritoneum, blood vessels increase their permeability and a release of histamines and cytokines initiate the inflammatory response. 11 , 59 The difference between normal and downregulated fibrinolytic activity depends on whether there is an ischemic condition. 59 Under ischemic conditions, fibrinolytic activity is suppressed, and fibrin cannot be resorbed during the fibroblast proliferation process, which leads to fibroblast infiltration and scar tissue formation. Based on our current understanding, postsurgical adhesions are caused by the combination of wound healing and a series of cascades that includes, but is not limited to, hypoxia, coagulation, inflammation, and fibrin degradation. 60 The cellular mechanism of these cascades is mainly dependent on the fibrinolytic system involving mesothelial cells. 8 , 18 , 60 Neither the degree and details of each event, nor the whole cascade related to adhesion formation have been studied systematically. Different studies agree that after fibrin clot formation, immune cells and fibroblasts migrate to the clot and attach themselves starting the formation of a tissue scaffold. Angiogenesis also forms around the tissue leading to the deposition of fibrin and subsequent postsurgical adhesion formation. 47 , 60 , 61 It has been known that the severity of acute inflammation is associated with adhesion formation. 16 Tsai et al. (2018) demonstrated that the early events of adhesion formation involve the mechanical injury of mesothelial cells, which triggers the direct recruitment of immune cells including neutrophils and monocytes through chemokine release such as CXCL‐1 and MCP‐1. 58 Following this mesothelial model, Fischer et al. (2020) identified a non‐specific calcium‐related signaling pathway, which is related to initiating the early events of mesothelial cells injury. 15 Thus, mesothelial cells have been recently recognized as a clue to understanding early events of adhesion formation as well as associated inflammatory response.
2.1. Overview
When postsurgical adhesions are formed internally within the abdominal cavity, the series of events at the cellular level are elusive and difficult to understand and observe directly. From observations using a mouse model, postsurgical adhesions result from the downregulation of fibrin deposition and fibrinolytic activity, which is triggered by hypoxia, coagulation, inflammation, and fibrin degradation. 60 The fibrinolytic system is the dependent factor in forming adhesions, which is supported by diZerega et al. (2000), who reported that the fibrin matrix generated during coagulation is associated with suppressed fibrinolysis. 11 However, the traumatized tissue sites reduce blood flow and create ischemia that promote local fibrin matrix generation. diZerega et al. (2000) also reported that the development of intraperitoneal adhesions is a complex and dynamic process and leads to traumatized tissue binding together and forming a fibrin bridge. 11 Over time, during the inflammatory response, the fibrin matrix is gradually replaced by vascular granulation tissue, which is characterized by the presence of macrophages, fibroblasts, and giant cells. 11 These bridges are then organized by the elements of wound repair, which leads to the formation of a rich vascular network and the incorporation of neuronal elements. The mechanism of adhesion formation has been summarized by Diamond (2016), who claims that the release of histamine, cytokines, and growth factors triggers the coagulation cascade and inflammatory response, which leads to the release of serosanguineous fluid and the recruitment of immune cells from traumatized tissue. 59 In addition, Sandoval et al. (2016) have reported that adhesion formation in humans consists of fibrin scar connective tissue, which is composed of extracellular matrix containing fibroblast‐like cells and capillaries. 22 The cellularity and collagen matrix formed by fibroblast cells can vary depending on the number of cells. They also concluded that in most human samples, they were able to identify preserved mesothelial cells. In addition to fibroblasts, endothelial cells, and mesothelial cells, along with mononuclear inflammatory cells were also observed and characterized, including lymphocytes, mast cells, and macrophages, which were confirmed by immunohistochemistry markers such as CD45, tryptase, and CD68. 22 Figure 3a provides a schematic illustration of the composition of adhesion scar tissue.
FIGURE 3.
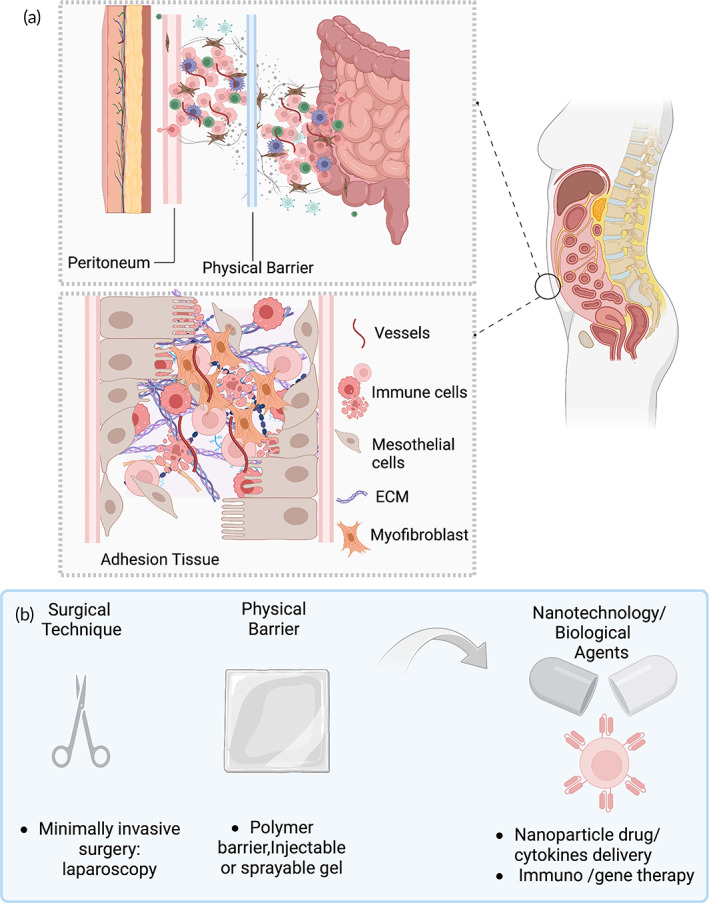
(a) A schematic overview of postsurgical adhesion composition and (b) prevention strategy of postsurgical adhesions.
Given the complexity and magnitude of adhesion formation, this major healthcare concern has been underappreciated. The current stage of preventing or reducing adhesions includes two major strategies involving surgical techniques and the use of adjuvants as shown in Figure 3b. 62 One improvement in surgical technique is the development of minimally invasive surgery or laparoscopic surgery, which reduces the extent of tissue trauma, improves hemostasis, and eliminates exposure to reactive oxygen species. 63 However, this particular study 63 concludes that the use of a laparoscopic technique does not reduce the frequency of adhesions compared to open surgery. This is because laparoscopic surgery is associated with longer operative times and high insufflation pressure, which can promote adhesion formation. 63 In terms of using adjuvants, such as barrier agents or drugs, including anti‐inflammatory or fibrinolytic agents, they have been developed to prevent or reduce the risk of adhesions. 62 While some of these adjuvants have shown promising results in animal models and clinical studies, there is no consensus on their efficacy and safety. Moreover, many adjuvants require expensive special expertise, and have side effects. The challenge of preventing or reducing adhesions is further compounded by the lack of good predictive animal models and the biochemical complexities of adhesiogenicity. Adhesions are a multifactorial process involving inflammation, angiogenesis, fibrosis, and matrix remodeling, which makes it difficult to develop targeted therapies. However, the potential method for preventing adhesions is still in development, and possible advanced bioengineering technology will direct future trends towards creating novel nanomaterials for drug delivery, developing, or modifying the biological components, such as utilizing nanoparticles or liposomes to deliver growth factors or cytokines and integrating nanotechnology with gene or immune therapy to regulate cellular events.
2.2. Physiological cellular mechanism
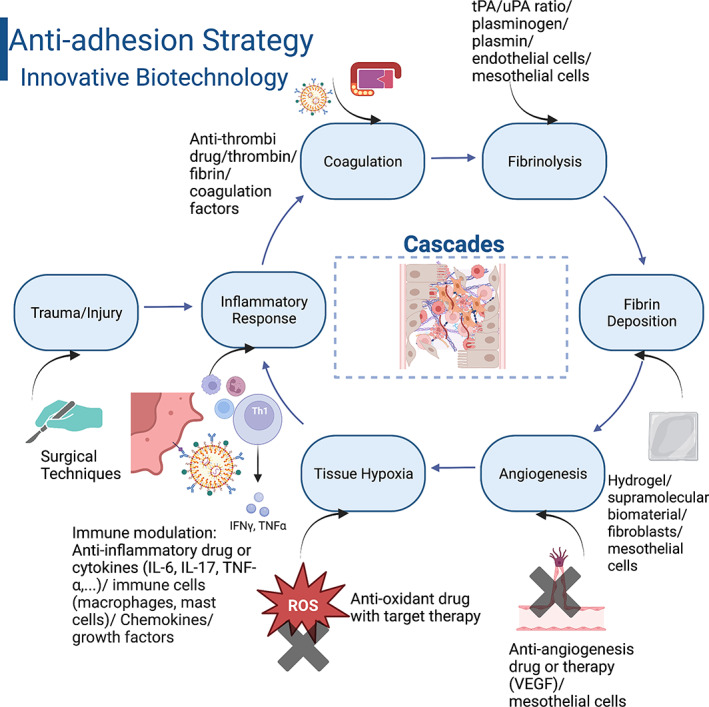
When trauma or a surgical incision causes an injury, it directly triggers a series of biological events or cascades which develops simultaneously in the peritoneum resulting in postsurgical adhesion formation. 11 , 16 , 18 , 23 , 60 The schematic Figure 4a provides multiple events and pathways, which can potentially lead to postsurgical adhesion formation as well as significant changes in cellular composition during the healing process. 20
FIGURE 4.
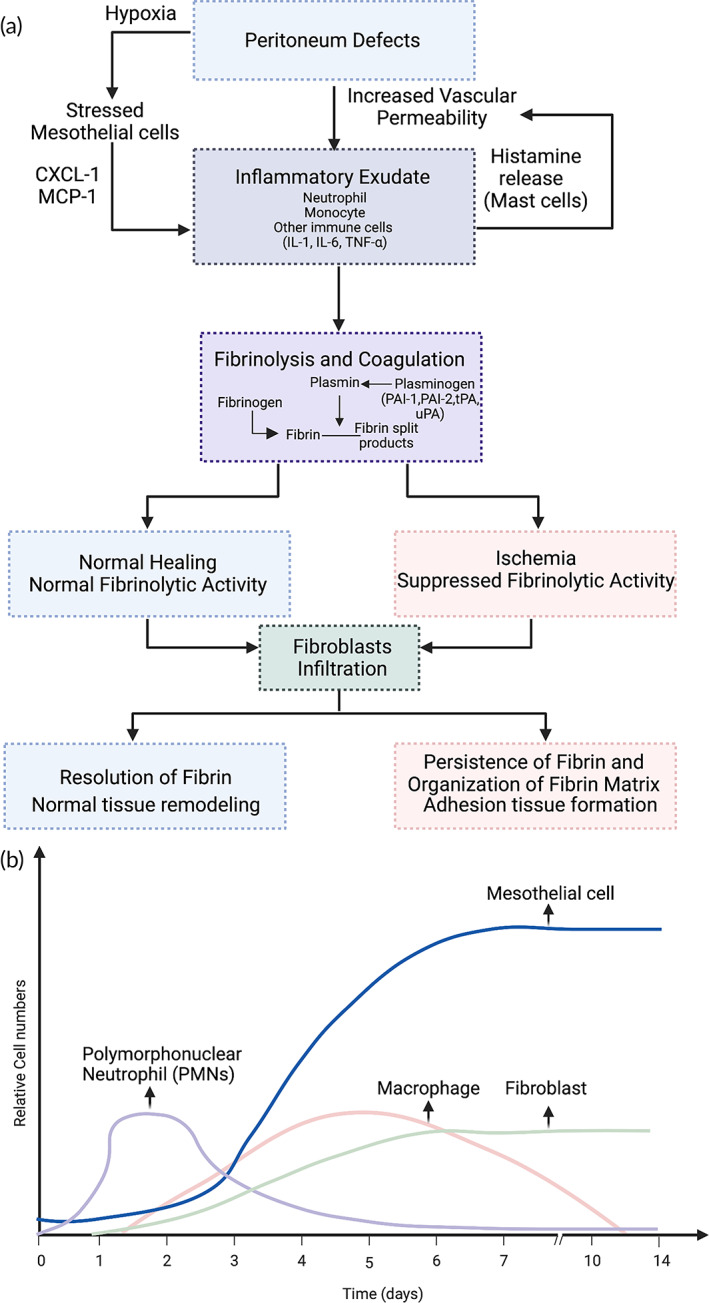
(a) Schematic illustration of the pathogenesis of adhesion formation showing the interactions between inflammation, ischemia or hypoxia, fibrinolysis, and coagulation. (b) Relative cellular numbers can change the rate of postoperative adhesion formation.
Peritoneal trauma or injury begins with the disruption of the stomal mast cells, which release histamine and kinins while increasing vascular permeability. 64 Platelet aggregation and plasma protein coagulation are essential components of the normal process of tissue repair. However, after surgical trauma or injury, abnormal regulation of fibrinolysis plays a significant role in adhesion development. The balance between fibrin deposition and fibrinolysis is determined by the level of plasmin. 65 Plasminogen activator inhibitor‐1 (PAI‐1) is one of the main inhibitors of fibrinolysis, whereas plasminogen activators (Pas) activate plasminogen and mediate fibrinolysis activator and tissue‐type plasminogen activator (tPA). 66 tPA provides a balance between pro‐coagulation and the fibrinolytic pathways, which are also regulated by PAI‐1. 66 When abdominal injury occurs with an imbalance between tPA and PAI‐1, this can potentially lead to increased fibrin generation and the deposition of a fibrinous matrix which results in adhesion formation. 66 Another important contributor related to the anticoagulation effect is antithrombin III which decreases thrombin activity. 65 Fibrinolysis reduces the fibrinous matrix at surgical sites. However, even with fibroblast migration to the surgical site, the persistence of fibrin deposits leads to additional ECM formation and the potential to induce tissue adhesions. 67 The increasing release of cytokines (IL‐1, IL‐6, and TNF‐α) from the inflammatory response could down‐regulate tPA activity, thereby decreasing the tPA/PAI ratio as well as the level of fibrinolytic activity. 68 As a result, the fibrin‐rich matrix accumulates in the damaged serosa areas and persists longer than necessary, hence creating an environment that promotes the migration of macrophages. As these structures develop more capillaries and deposit more ECM, they are responsible for the formation of permanent adhesions within a week of undergoing surgery. 69 The presence of a foreign body material, such as a mesh implant, or an infection will exacerbate this imbalance in fibrinolysis and result in excessive fibrin deposition and an increased risk of adhesion development. 67
Ischemia or tissue hypoxia can trigger the release of free radicals, leading to changes in cellular activity such as increased cell proliferation, suppressed apoptosis, altered glucose metabolism, and changes in gene expression of various factors. 16 , 70 , 71 , 72 , 73 During surgical procedures, the presences of free oxygen and nitrogen radicals can enhance DNA damage and increase the production of oxidized proteins. 64
During normal wound healing, fibroblasts undergo apoptosis, associated with low oxygen tension and oxidative stress. Subsequently, more fibroblasts are attached to the wound site and change their phenotype to myofibroblasts and remodel the tissue. Imudia et al. (2005) observed that hypoxia induces cell apoptosis in normal fibroblasts, but less apoptosis in adherent fibroblasts. 74 This is especially true when adherent fibroblast are exposed to hypoxia, because they can generate a higher degree of cell proliferation. 64 Other researchers, such as Ara et al. studied hypoxia in a rat animal models and found that due to an increased generation of reactive oxygen species (ROS), a significant number of free radicals was produced during the first 5 minutes after hypoxia, followed by the appearance of antioxidant enzymes. 64 , 75 Other studies have mentioned that free radicals promote the expression of growth factors, including transforming growth factor beta (TGF‐ β), IL‐6, type‐1 collagen, and vascular endothelial growth factor (VEGF). 59 , 76 In addition, tissue hypoxia induces the production of superoxide, and fibroblasts exposed to superoxide could produce profibrogenic factors, such as TGF‐β and collagen I, which will eventually contribute to adhesion formation. With respect to peritoneal adhesions, fibroblasts change their phenotype to an adherent phenotype under hypoxic conditions which results in the increased production of VEGF to enhance the reoxygenation of hypoxic tissue that these clots represent. 74
The adherent phenotype is characterized by an increase in the expression of fibronectin and type I and III collagen compared to normal fibroblasts, which are promoted by TGF‐β. The proteolysis of the deposited ECM is carried out by fibrinolytic and matrix metalloproteinase (MMP) systems, which are crucial in determine the fate of adhesions at this stage. 60 Hypoxia resulting in oxidative stress, is believed to play an important role in the pathogenesis of adhesion formation. 16 , 47 , 64 In addition, ensuring that the careful control of bleeding and the prevention of exposed tissue from drying is known to reduce the likelihood of adhesion formation. 77
The study of cellular events by diZerega, G. S and Raftery enabled the cellular sequence of activities on the healing peritoneum defects in rats to be observed using scanning electron microscopy. 11 , 19 As discussed earlier, the important components of cellular change during adhesion development are shown in Figure 4b. At defects in the internal peritoneum changes in the cellular level change includes the appearance of polymorphonuclear leukocytes (PMN) among the fibrin strands 12 h after injury. That indicates that PMN's start to migrate from blood vessels to the wound site and the recruit macrophages as part of inflammatory response 11 , 21 between 24 and 36 h of the initial injury. 11 , 19 A single layer of macrophages covers the fibrin supported platelet plug at 48 h (Day 2). A few mesenchymal cells and the mesothelial cell islets interconnected by desmosomes and tight junctions have been observed at Day 2. 11 At Day 3, while the macrophage is still the predominant cell type at the wound site, the surface also contains mesenchymal cells and a proliferation of fibroblasts in contact with each other. 11 The mesenchymal cells and fibroblasts were in contact with each other, and the macrophage cells amount has decreased at Day 4. 11 The earliest that the healing process appears to be complete is on Day 5 when a single layer of mesothelial cells has lined the surface. 11 On Day 5–6, the number of macrophages decreases directly, and by Day 8–10, a continuous layer of mesothelial cells has created a continuous basement membrane. 11
2.3. The role of the inflammatory response
An inflammatory response is activated by the peritoneum injury, in which the degree of acute inflammation appears to be associated with adhesion formation. Tsai et al. (2018) reported that an increased production of inflammatory mediators in the early inflammatory stage plays an important role in regulating ECM formation. 19 , 58 Additionally, activated platelets release various factors along with the fibrinogen and fibrin that are cleaved by thrombin and plasmin. These components act as chemoattractant for triggering the inflammatory response. 61 Several studies have demonstrated the role of inflammation in preventing adhesion formation. In one of them, Hu et al. (2013) reported that the use of an anti‐inflammatory drug reduced the formation of peritendinous adhesion following surgery in a rat model. 78 Adherent fibroblasts, associated with mRNA expression, were observed to increase their release of tumor necrosis factor‐alpha (TNF‐α) by 58% compared to normal fibroblasts, which increases the risk of surgical adhesions. 20 Like TNF‐α, interleukin‐6 (IL‐6) is a pro‐inflammatory cytokine, that generates a systemic inflammatory reaction. Both TNF‐α and IL‐6 are known to regulate the coagulation cascade and the formation of fibrin. 20 Recently, Uyama et al. (2019) have suggested that treatment with the IL‐6 receptor antibody reduces the risk of surgical adhesion formation. 79 Other inflammatory mediators, such as IL‐17 and interferon gamma (IFN‐γ), are also believed to serve as potential therapeutic target molecules for the prevention of surgical adhesions. 20 , 35 , 36 So, while anti‐inflammatory drugs as well as cytokines and small molecules have shown some efficiency in preventing adhesion formation, currently no specific target pathway has been identified, and no anti‐adhesion product developed that provides consistent results.
2.3.1. Mesothelial cell injury
Since the major cellular component of the peritoneum layer is a loose monolayer of mesothelial cells, the mesothelial cells have been studied and recognized as the main character in abdominal tissue repair and the inflammatory process with the secretion of inflammatory cytokines, growth factors, and ECM components. 20 Tsai et al. (2018) have reported that postsurgical adhesions originate from mesothelial cells. Podoplanin (PDPN) and mesothelin (MSLN) have been identified as the specific surface markers on the injured surfaces of mesothelial cells using a mouse model. 16 Foster et al. also (2020) found that JUN expression was strongly induced and correlated with significant MSLN expression, and the inhibition of JUN has been found to minimizes adhesion formation. 80 In another study, Fischer et al. (2020) reported that the calcium‐related signaling pathway of mesothelial cells initiates the formation of adhesions, suggesting that pathological changes of mesothelial cells act as the main component in the early adhesion cascade. 15 , 16 They also found that the calcium channel blocker drug is able to prevent in‐vitro changes to the mesothelial cell cytoskeleton as well as preventing adhesion in‐vivo model. 15
A number of years ago, Yanez‐Mo et al. (2003) observed that under the influence of the transcriptional repressor SNAIL1, peritoneal mesothelial cells gradually lose their epithelial morphology and exhibit a decline in the expression of cytokeratin and E‐cadherin, as they undergo a transition from epithelial to mesenchymal cells. 21 Most recently, Sandoval et al. (2016) confirmed that in the peritoneal cavity, mesothelial cells form a lining that undergoes a mesothelial‐to‐mesenchymal transition (MMT) in response to the pathological conditions. This process causes them to be transformed into myofibroblasts that are prevalent in peritoneal fibrotic tissue. 22 In support of these findings, Uyama et al. (2019) observed that in response to TNF‐ α, adhesion‐associated myofibroblasts expressed mainly IL‐6 and INF‐ γ that originated from mesothelial cells induced adhesion. 79
During adhesion formation, the release of vascular endothelial growth factor (VEGF) is an important event leading to neo‐angiogenesis. 81 Previous studies have indicated that the mesothelial cells can secret significant amounts of VEGF during the MMT process. 82 Animal experiments have shown that preventing the MMT can impede the development of peritoneal fibrosis and angiogenesis, which helps protect the peritoneal structure and function. 82 In another unique study, Strippoli et al. (2020) demonstrated that caveolin1 and yes‐associate protein (YAP) drive mechanically induced MMT transitions. This demonstrates that there is cooperation between biomechanical and biochemical signals in the triggering of MMT transitions and represents a novel potential opportunity to intervene in mechanically induced disorders causing peritoneal fibrosis, such as postsurgical adhesion development. 83
Injury to mesothelial cells is associated with the secretion of different cytokines, chemokines, and growth factors and the migration of immune cells to the site of injury, thereby initiating an acute inflammatory response. 84 Mesothelial cells produce a multitude of cytokines and growth factors which can regulate the inflammatory responses and tissue repair. The stimulation of mesothelial cells induces the pro‐inflammatory cytokines and chemokines to release IL‐1, IL‐8, monocyte chemoattractant protein‐1 (MCP‐1), and IFN‐ γ, all of which are associated with adhesion formation.
2.3.2. Macrophage recognition
The injured peritoneum has been observed through live intravital microscopy. By imaging living cells while they are in a complex multicellular environment, it has been possible to characterize the sequence of the cell recruitment. 85 A surprisingly finding by Zindel et al. (2021) is that monocytes and macrophage migrates to the injury sites and cover the lesion within 15 min of injury. 55 This finding is consistent with the previous study that showed macrophages are the most prevalent cell type within the injured peritoneum on Day 1 after adhesion induction. 86 Thus, the recruitment of monocytes followed by migration of macrophage was significantly faster than the recruitment of neutrophils. 55 So, this new finding changes the current understanding of initial adhesion formation cascades as it was previously believed that neutrophils played a significant role. There is a specific type of macrophage called large peritoneal macrophages (LPM) that is characterized by the expression of CD102 (Icam2) with transcription factor GATA6. 85 It undertakes the role in cleaning bacteria by phagocytosis and also participating in the adhesion formation process. 55 The observation of macrophages has recognized the importance of the passive transportation of the peritoneum fluids as it allows macrophages to aggregate rapidly after the injury and rapidly respond to Damage‐associated molecular patterns (DAMPs). 55 The cell–cell aggregates usually take place surrounded by blood vessel wall. 85 Peritoneal macrophages models have been proposed to be activated by DAMPs to Pattern recognition receptors (PRR). This activation can lead to the polarization of macrophages into an inflammatory M1 phenotype. 85 M1 macrophages play an important part in the degradation of the ECM during inflammatory response. 87 M1 macrophages produce pro‐inflammatory cytokines such as TNF and IL‐1, as well as reactive oxygen species and other mediators that promote inflammation and tissue damage. Rajab et al. (1997) suggested that enhanced peritoneal macrophages by protease peptone increased the activity of plasminogen and inhibited the development of adhesion formation. 88 Additionally, macrophage depletion and irritation/injury of the peritoneum resulted in peritoneal adhesion formation replicated in a mouse model. 89 A previous study suggested that prior depletion of macrophages could induce a significant neutrophil influx into the tissue in response to lipopolysaccharide. 87 Macrophages aggregation have participated in peritoneal wound healing process as well as cause inflammation. The role of macrophage aggregation is not clear whether it cause inflammatory or anti‐inflammatory. 85 The above findings suggest an important role of macrophages in adhesion phenotype, but their differentiation is still unknown. Macrophage function depends mainly on differentiation status, and its differentiation could affect wound healing in several organs. 90 A recent study demonstrated that although activated mesothelial cells induced the recruitment of monocytes through monocyte chemoattractant protein (MCP), the number of accumulated macrophages was markedly decreased throughout the adhesion formation process. 58 Macrophages could produce plasminogen activator inhibitors and tissue plasminogen activators that modulate fibrinolysis and inflammatory response. 91 Macrophage disappearance reaction (MDR) have been proposed by Nelson and Boyden, not as a specific reaction, but a response correlated to the increased inflammatory cytokines in the peritoneal fluids as well as the influx of pro‐inflammatory leukocytes. 85 However, there are still largely unknown changes in macrophage cellular events. Therefore, understanding of the functions and mechanisms of the various macrophage subpopulations can help to open new strategies for the prevention of surgical adhesion formation.
3. POSTSURGICAL ADHESION PREVENTION STRATEGIES
Despite significant efforts to understand the mechanism of postsurgical adhesion formation,
there is still insufficient evidence to fully explain all the cellular and molecular pathways involved in the formation of adhesions. Various strategies have been proposed to address this issue. Nevertheless, postsurgical adhesions continue to be reported. Even after adhesiolysis to remove the bands of scar tissue between adjoining organs and peritoneal structures, adhesion development still occurs in 80% of patients regardless of the type of procedure. 59 Thus, postsurgical adhesions continue to be an intractable issue for all surgeons, not only because there are currently no effective clinical procedures or products available to prevent postsurgical adhesion formation, but also because there are two main factors that need to be addressed. The first factor is the lack of understanding of the pathological mechanism of adhesion formation. The second factor is the absence of a reliable and reproducible in‐vitro model that can be used to study and evaluate the mechanism of postsurgical adhesion formation. Most potential anti‐adhesion candidates can only rely on in‐vivo models to evaluate the efficiency in preventing postsurgical adhesion, which brings in the question the accuracy and consistency of the experimental results.
In this chapter, we review and summarize the prevention strategies and for postsurgical adhesions. We discussed the current research on various forms of polymers, pharmaceutical product, and nanoparticles, as well as the potential use of gene therapy to prevent the formation of postsurgical adhesions.
3.1. Commercialized and FDA‐approved anti‐adhesion products
The application of a physical barrier is the most common and low‐cost method used in preventing postsurgical adhesions. In general, the traditional anti‐adhesion membranes work as a separating layer to avoid contact between surgical tissue sites and other organs. 59 , 92 They act as a boundary to inhibit the formation of tissue fibrin bridges during the tissue regeneration stage, hence reducing postsurgical adhesion formation. 28 , 93 Currently, there are three common forms of commercial products used to treat postsurgical adhesion; which include gel, films, and solutions. 92 Most of them rely on a biocompatible material that has the potential to prevent adhesion formation. For example, hyaluronic Acid (HA), oxidized regenerated cellulose (ORC), polylactic acid (PLA),and polyethylene glycol (PEG) are all possible candidates. However, none of them have been consistently proven to possess high efficacy for preventing adhesions. Additionally, there is a lack of studies about the long‐term effects and efficacy of the implanted anti‐barrier cytotoxicity or side effect profiles. The current data from clinical trials points to the efficiency rates of those products ranging from 26% to 70% in clinical studies. 16 , 78 , 85 , 86 , 87 , 88 Some other products such as Interceed® (Ethicon, Somerville, NJ) and Adept® (Ethicon, Somerville, NJ), have been approved by the FDA for gynecological surgery only.
To better understand how these materials play a role in preventing adhesions, numerous animal and human trials have been performed. Conflicting results from these studies have only raised further questions of the true efficacy of these materials. A major disadvantage of using a barrier method is the need for fixation to a particular anatomical site. The current clinical gold standard is Seprafilm®, which is composed of carboxymethyl cellulose (CMC) and HA. Even with Seprafilm®, the clinical significance of this barrier product is questionable in light of several petitions to the FDA for its recall. As a result, commercial barriers still have a long way to go before they can be used with confidence to resolve this problem or integrated new biotechnology into creating the next generation anti‐adhesion barriers.
Table 1 lists the commercialized products on the US and international markets, and describes their materials, product form, approval time, efficacy (according to clinical trials) and their claimed function and performance. Typically, the anti‐adhesion barrier should follow the principles listed as follows 27 :
Effectively separate tissues.
Has a long half‐life of at least 7 days after surgery within the peritoneal cavity.
Absorbed or metabolized without initiating a marked proinflammatory response.
Remain active and effective even when exposed to blood.
Does not compromise wound healing.
Does not promote infection.
TABLE 1.
Commercialized anti‐adhesion products
| Brand (company) | Material | Format | Approval | Intended use | Efficacy | Claimed property | Disadvantage |
|---|---|---|---|---|---|---|---|
| Coseal® (Baxter Healthcare Inc, Deerfield, IL) | Polyethylene glycol (PEG) | Hydrogel/sealant | CE‐2000 FDA‐2003 | Hemostasis product; cardiac surgeries | No toxicity 94 | Biodegradability; hemostasis 95 | Swelling may induce compressing; curing time needed |
| Repel‐CV® (SyntheMed Inc, Iselin, NJ) | Polylactic acid‐Polyethylene glycol (PLA‐PEG) | Film | FDA‐2009 | Pediatric cardiac surgery | 26% 96 | Low cell‐adhesion; flexible; biodegradability | Fixation difficulty; efficiency same as seprafilm |
| Hyalobarrier® (Nordic Pharma, Paris, France) | Hyaluronic acid (HA) | Hydrogel | CE‐1999 not approved in US | Abdomin‐pelvic area | 17.75% 97 | Biocompatibility; bioresorbability | Lower efficacy comparing with other products 98 |
| SprayShield® (Coviden; Dublin, Irland) | Polyethylene glycol (PEG) | Spray | CE‐2008 not seeking FDA | Surgical applications | 30% 89 | Bioresorbability hydrogel form onsite | Complications reported in few studies 98 |
| Adept® (Ethicon, Somerville, NJ) | Icodextrin (4%) | Solution | CE‐1999 FDA‐2006 | Gynecological surgery | 30% 99 | Delayed bioresorbability | Anastomotic wound healing problems 100 |
| Interceed® (Ethicon, Somerville, NJ) | Oxidized regenerated cellulose (ORC) | Film | FDA‐1989 | Gynecological surgery | 70% 86 , 101 | Low inflammatory response; mufcoadhesive; delayed bioresorbability | Fixation difficulty; hard to handle; efficiency rate is debating |
| Seprafilm (Baxter, Deerfield, IL) | Carboxymethyl cellulose (CMC)/hyaluronic acid (HA) | Film | FDA‐1996‐2022 | General surgery | 33% 102 | Low inflammatory response; muco‐adhesive; bioresorbability | Fixation; low efficiency for preventing adhesion |
Besides the FDA‐approved barrier products, there are still a lot of studies working on or attempting to improve the properties of biomaterials to prevent adhesions. Liao et al. (2023) summarized the studies for fabricating physical anti‐adhesion membrane by manipulating the surface of the biomaterials and exploring the combination of multifunction's biomaterials in order to reduce postsurgical adhesions. 51 Erdi et al. (2023) also take the advantages of current nanotechnology and introduced the most recent development of polymer types nanomaterials to utilize them as a more effective adjuvant surgical tool for surgical treatment, which has opened a new window in combining functional biomaterials to current clinical settings. 103
3.2. Cellular Modulation
Many studies have researched the use of biomaterials as a barrier‐type anti‐adhesion membrane for clinical practice. To date, the reported clinical efficacy of these biomaterials has been disappointing. Despite this, the inert property of barrier materials has a significant untapped potential to be a component in the integration of drugs and/or biological agents to improve the efficacy of preventing adhesions and further expand their clinical use. Thus, there are multiple studies currently being performed on assess the incorporation of different types of drugs targeting anti‐inflammatory, anticoagulative, and fibrinolytic properties, as well as growth factors in conjunction the biomaterials. However, the duration of these studies is limited to the short‐term effects of drug delivery. Further research needs to be performed in assessing long‐term mechanisms and clinical efficacy during the wound‐healing process.
Nanoparticles have been suggested as an alternative controlled drug delivery system because of their unique physical properties, which include uniform, modifiable particles, and various pore sizes allowing for a better control of the duration of drug delivery. Additionally, nanoparticles have other highly desirable properties including high surface area, large pore volume, and superior biocompatibility. 104 The use of biologically active substances loaded into nanoparticles offers an attractive opportunity to deliver anti‐adhesion therapeutics in a targeted and personalized manner. This approach may address and resolve the side effect of many agents. Additionally, nanoparticles can be incorporated into polymeric fibers and membranes, which would allow them to be used with current barrier methods. Table 2 lists the recent research that are utilizes nanoparticles and present their performance in both in‐vitro and in‐vivo models.
TABLE 2.
Anti‐Adhesion Research
| Function | Nano‐scale carrier | Drug/gene? | Membrane/solution | In‐vitro result | In‐vivo result | References |
|---|---|---|---|---|---|---|
| Fibroblasts proliferation; infection | Ag nanoparticles | N/A | Electrospun poly(l‐lactide) PLLA membrane | Less fibroblasts proliferation (Not related to adhesion pathology, can only prove no cell attachment for antibacterial property) | N/A (lack of evidence for proving anti‐adhesion property) | 105 |
| Fibroblasts attachment; infection; inflammatory response | Ag nanoparticles | Ibuprofen | Poly (ethylene glycol) (PEG)/Poly (caprolactone) (PCL) Shell Hyaluronic acid (HA) Core | Less cell attachment and good biocompatibility (not related to adhesion pathology, can only prove no cell attachment for antibacterial property). | Less inflammation and adhesion have been observed on core‐shell nanofibrous membranes directly as well as through histology (no inducing adhesion model and adhesion score to quantify the data). | 106 |
| Collagen Type I expression | Dextran glassy nanoparticles (DGNs) | bFGF | Electrospun PLLA membrane | Less multipotent C3H/10 T1/2 cell attachment with good biocompatibility | A blunt dissection for separating the tendons and peritendinous tissues for treated group compared to control with sharp dissection as well as quantifying the breaking force for all groups. | 107 |
| Collagen Type I and PCNA protein expression | PLGA Nanoparticles | Constructed an overexpressed bFGF+VEGFA genes with GFP in pEGFP‐N1 plasmid | PEI Polymer suspension | Low cytotoxicity on samples carried out with MTT cell viability test, and high transfection rate of the tenocytes cell culture determined the effectiveness of the gene delivery. | Adhesion score of white Leghorn chicken in‐vivo model significantly decreased with treatment and the tendon ultimate strength increased representing good healing results. | 108 |
| TGF‐β1 | Polylactic‐co‐glycolic acid (PLGA) Nanoparticles | miRNA based RNA interference (RNAi) plasmid | Polyethyleneimine (PEI) polymer suspension | Low cytotoxicity on samples carried out with MTT cell viability test. | Adhesion score, real‐time PCR, western blot of chicken tendon repair model with nanosphere/plasmid effectively inhibit the expression of TGF‐β1 for over 70% till week 6. | 109 |
| Phosphorylation of ERK1/2 and SMAD2/3 | N/A | Fresh amnions (include TGF‐β1, bFGF, VEGF, and PDGF) | PCL electrospun nanofibres | Higher cell viability and proliferation on tenocytes and fibroblasts showed a better proliferation activity compared to control, 7 days cell culture also showed ERK1/2 and SMAD2/3 protein expression higher than control. | Adhesion score macroscopic and microscopic decreased on PCL electrospun treated group compared to control and amnions group. Histological healing percent also showed the best on PCL electrospun group. | 110 |
| Down‐regulate proinflammatory gene and protein expression (Tgfb3 and Tgfbr2), up‐regulate inhibitory proteins Smad6 and Smad7, ghrelin induce blockage of TGF‐β signaling | N/A | Ghrelin | N/A | N/A | In‐vivo mice model showed that ghrelin inhibits the TGF‐β/Smads and p‐38 MAPK signaling pathways activation during the inflammatory response at the onset of injury before the granulation‐remodeling phase occurs, which significantly reduce the local inflammatory mediators and therefore prevent adhesion | 111 |
| Enhance fibrinolytic and enhance EGF‐HER1 signaling by regulating macrophage | N/A | Plasminogen activator inhibitor 1 | Carboxymethylcellulose (CMC) | N/A | The results have showed that the incidence of adhesions have been reduced but not eliminated in gauze induced adhesion rat model. The pharmacologic inhibition of PAI‐1 can prevent adhesion without the fear of bleeding, anastomotic disruption, and wound dehiscence. | 112 |
| PAI‐1 and tPA levels | N/A | tissue‐type plasminogen activator (tPA) | Poly(ε‐caprolactone)‐poly(ethylene glycol)‐poly(ε‐caprolactone) (PCEC) | N/A (Only drug release efficiency carried out in‐vitro experiment for a 14‐days slow release) | Adhesion score and frequency showed lower score for tPA‐hydrogel treated group, H&E staining as well as tPA and PAI‐1 level carried out with tPA‐hydrogel system | 113 |
| Fibrin deposition; angiogensis; macrophage infiltration | Peritoneal cell sheet (Include mesothelial cells and fibroblasts) | N/A | N/A | N/A | Peritoneal cell sheet transplantation prevents tissue adhesion, fibrin deposition, angiogenesis, lymphomagenesis, and macrophage infiltration in a rat cecal cauterization adhesion model. | 114 |
| Angiogenesis inhibitor, COX‐2 inhibitors drugs | N/A | Celecoxib, rofecoxib | Methylcellulose, silicone patch | N/A | Rats treated with selective and nonselective COX‐2 inhibitors had significantly fewer adhesions than control animals. Adhesions from mice treated with celecoxib had reduced microvessel density compared with rofecoxib, the nonselective COX inhibitors, and control animals. | 205 115 |
| Promote mesothelial cell proliferation; enhance fibrinolytic activity | N/A | Keratinocyte growth factor (KGF) | Sodium hyaluronate (HA) gel | N/A | The combination of KGF and HA can inhibit the severity of the fibrous changes in the adhesion induced rat model | 116 |
| Mesothelial cells Actin cytoskeleton (calcium channel); Anti‐adhesion | N/A | Small molecular calcium channel blocker (Bepridil, CK‐666, Rhosin, and Golgicide) | 2% Cellulose | Unstressed cytoskeleton effect of GFP labeled mesothelial cells in situ induced in‐vitro assay mesothelial cells with calcium channel blocker drug treatment. | Adhesion score of rats with different dosage of the calcium channel blocker treated and tested 27 targets of signaling pathway with single‐cell transcriptomics analyses to prove the adhesion related gene expression. | 15 |
| T cells in TLR4/MyD88/NF‐κB signaling pathway | Polylactic acid (PLA) nanoparticles | ligustrazine | Potassium dihydrogen phosphate/ sodium hydroxide solution suspension | N/A (Only drug release efficiency carried out in‐vitro experiment) | Adhesion score, Masson trichrome (MT) staining, ELISA, western blotting, Immunohistochemistry and PCR shows significantly lower adhesion formation due to inactivating the TLR4/MyD88/NF‐κB pathway as well as regulating the downstream cytokine expression. | 117 |
| Anti‐adhesion, immune cell infiltration | Mesoporous silica nanoparticles | Ibuprofen | Electrospun PLLA composite | N/A (Only drug release efficiency carried out in‐vitro experiment) | Adhesion score and H&E staining showed an improvement for anti‐adhesion and anti‐inflammatory results of treated group with drug‐loaded nanoparticles compared to control. | 78 |
| Anti‐adhesion | PLGA Nanoparticles | N/A | Aldehyde‐ and hydrazide‐modified hyaluronic acids (HA) | Low cytotoxicity on murine mesothelial cells | Adhesion score of rats and rabbits model showed no adhesion on over 60% of animals treated with this gel system compared to over 50% of animals on control group with severe adhesions. | 118 |
| Anti‐adhesion, reduce formation of intrauterine adhesion | N/A | N/A | Crosslinked hyaluronan gel | N/A | 60 patients were divided into two groups: Group 1 received curettage plus crosslinked hyaluronan gel (intervention group), and Group 2 received curettage alone (control group), Results showed hyaluronan gel reduce the formation of intrauterine adhesions in women after curettage for retained placental tissue after medically induced or spontaneous pregnancy loss in the second trimester | 119 |
| Anticoagulation | N/A | Heparin | Carboxymethylcellulose (CMC) | N/A | Adhesion score have significantly decreased with tiny doses of heparin, in combination with CMC 4% gel in two different animal models. (Two different types of animal models: Avascular knot model and Cecal abrasion model) | 120 |
| Antibacterial, hemostatic, anti‐inflammatory and biocompatibility | N/A | N/A | Nanochitosan membrane barrier | Higher adherence and biocompatibility (over 90%) on the material with a mouse fibroblasts L929 cell culture. | Rats' open abdominal cavity model proved nanochitosan membrane can reduce the invasion of inflammatory cells into the wound and inhibits the synthesis of collagen to prevent postoperative adhesions | 121 |
| Intracellular glutathione levels; Anti‐inflammatory; antioxidant | N‐acetylcysteine (NAC) nanoparticles | N/A | N/A | N/A | Adhesion score and C‐Reactive protein concentration showed a better result of lower doses of Nano‐NAC (50 and 75 mg/kg) compared to higher dose and control. | 122 |
| Anti‐inflammatory and anti‐oxidant; anti‐proliferation of fibroblast | N/A | Phlorotannin | Poly (ε‐caprolactone) film | Less fibroblasts proliferation and inhibit nitric oxide production (Not related to adhesion pathology, can only prove no cell attachment for antibacterial property) | N/A | 123 |
| Anti‐inflammatory, antioxidant, and antifibrotic effects | N/A | Pirfenidone | Carboxymethylcellulose (CMC) | N/A | Rat model revealed that pirfenidone has remarkable protective effects against postoperative intraabdominal adhesions and inflammation with a decreased mRNA expression levels and increased MMP‐9 concentration detected in peritoneal tissue. | 124 |
| Anti‐inflammatory | N/A | N/A | Human amniotic membrane | N/A | There were significantly fewer adhesions in the amniotic membrane group (2) versus hyaluronic acid (3) group (p = 0.01). | 125 |
| Anti‐inflammation and anti‐oxidation effects | N/A | 20(S)‐ginsenoside Rg3 | 20(S)‐ginsenoside Rg3‐loaded methoxy poly (ethylene glycol)‐block‐poly(L‐lactide‐co‐glycolide) (mPEG‐b‐PLGA) electrospun membrane (PEM/Rg3) | N/A | PEM/Rg3 membranes showed a significantly reduce of the peritoneal adhesion in rats model. | 126 |
| Anti‐inflammatory, promote healing, reduce fibrous adhesion | Nanomicelles | Lipophilic gold nanorods and curcumin | Lipophilic gold nanorods stabilized with the surfactant agent hexadecyltrimethylammonium bromide | N/A | The rat model showed controlled release of curcumin from these nanomicelles can effectively reduce adhesion formation while promoting tendon healing | 127 |
| Anti‐inflammatory; antioxidant; RhoA/ROCK signaling pathway | N/A | Rho GTPases | Sodium aescinate (AESS) | The cytotoxicity assay was used to decide the concentration of the AESS with cell proliferation inhibition condition. | AESS significantly reduces postoperative adhesion formation in a rat model; AESS increases the secretion and activity of tPA in peritoneal fluid and decreases FIB levels in plasma. AESS‐treated groups showed that the secretion, activity, and expression of tPA in rat peritoneum were notably increased. | 128 |
| Physical barrier for pericardial adhesion | PLA–PEG nanoparticles | N/A | HPMC‐C12 | N/A | Adhesion score of rats and sheep model treated with a PNP hydrogel adhesion barrier exhibited formulation‐dependent results, solid‐like PNP hydrogel formulations (G′ > G′′) 1:5, 1:10 and 2:10 all formed physical adhesion barriers that significantly reduced the incidence and severity of adhesions when compared with the untreated control group. | 129 |
According to the list, polymeric nanoparticles prepared from polylactic acid (PLA) and polyethylene glycol (PEG) are among the more popular options because of their ease of preparation and their FDA‐approved biocompatibility. Some studies claim that due to their unique nano‐scale morphology, the attachment of cells is significantly reduced. 129 Silver nanoparticles are another commonly used biomaterial for this purpose. The silver (Ag+) ion can reduce the incidence of infection due to its anti‐bacterial effect. 105 However, its cytotoxicity may raise concerns over long‐term use. Another newly emerging carrier involves the use of cell or cell‐derived products, which may further promote the biocompatibility and a more bio‐active content that can effectively regulate the cell responses. 110
There is wide range of different types of drugs that can be incorporated within these nanoparticles, and most of them are targeted to either modify the inflammatory response or to reduce the formation of fibrosis tissues. One of the common drugs, such as Ibuprofen is a candidate that works on the anti‐inflammatory process and result in less cell adhesion. This suggests that by blocking certain pathways one can prevent postsurgical adhesions from occurring. 78 , 106 Thus, instead of relying on a pro‐formulated medications strategy, the involvement of substances that cause cellular and/or cellular modulation effects may be able to better control these identified pathways more effectively. For example, Zhou et al. (2015) used TGF‐β1 mi‐RNA plasmids in a chicken tendon adhesion model to target and downregulate TGF‐β1. This study showed that using a plasmid‐nanoparticle as a non‐viral vector for gene therapy can potentially be beneficial in adhesion prevention. 109 Bianchi et al. (2016) applied ghrelin that can down‐regulate the proinflammatory gene and protein expression (TGF‐β3 and TGF‐β2), up‐regulate the inhibitory proteins Smad6 and Smad7, and induce blockage of TGF‐β signaling to reduce the local inflammatory response. 111 Growth factor is also used frequently in reducing adhesion formation, by promoting the growth of the normal cells in healing processes. 108 , 112 , 116
The in‐vivo animal model is currently the most sufficient and reliable method to test and evaluate anti‐adhesion candidates. Various animal models have been used in performing in‐vivo studies, however, the most common in‐vivo animal peritoneal model is the rat model. Additionally, there have been multiple methods introduced in previous studies to standardize adhesion inducing models, such as using visceral and parietal brushing, mechanical denuding and sutures, creating ischemia buttons as well as using electrocoagulation, etc. 130
The prevention of postsurgical adhesions through clinical approaches is currently limited to those biomedical devices that have been approved by the FDA. Only physical barrier types of commercial products have been applied clinically, leaving significant opportunities for further improvement. At the present time various studies that are being conducted that aim to enhance the efficacy of preventing adhesions through alternative pharmacological delivery methods. These approaches have the potential to achieve higher efficiency rates and potentially yield promising results for preventing adhesions in future clinical practice.
3.3. Future Therapeutic Strategy
From the current published literature, there are a significant number of studies that have introduced various biomaterials designed to prevent adhesion formation (Figure 5). These new biomaterials utilize a combination of physical, chemical, and/or biological properties to prevent the formation of adhesions. However, even with the creation of these innovative biomaterials, there has not been any significant clinical improvement in preventing or reducing the risk of adhesions. The only biomaterial which has made some level of advancement in impacting adhesion formation are physical barriers. For example, Stapleton et al. (2019) created a novel supramolecular polymeric hydrogel to reduce pericardial adhesion formation by allowing the hydrogel to completely encompass the heart while protecting it from the surrounding pericardium. 129
FIGURE 5.
Anti‐dhesion future strategy.
Even with these encouraging findings, many studies have been focused on the prevention of abdominal adhesions which has its own set of challenges and problems that need to be overcome. One of these issues is the significant difference in size and total surface area within the abdominal cavity when considering the peritoneum and all internal abdominal organs, such as stomach, liver, gallbladder, pancreas, small intestine, and large intestine. Given the fact that the previously explored approach of physical barriers and chemical barriers has had limited success, it is suggested that an alternative focus on a biological mechanism may provide a solution to the issue. The integration of biological compounds such as interferons into biocompatible materials could provide a targeted way to prevent the adhesion formation by disrupting the normal cascades of events.
Another issue that needs to be addressed involves validating the anti‐adhesive properties of any biomaterial from an in‐vitro viewpoint, given that the in‐vivo environment cannot be accurately replicated in the laboratory. Currently, the major limitation of previous studies is the use of an in‐vitro assay is to assess biocompatibility requirements or to establish the appropriate material concentration in preparation for future in‐vivo studies. Fischer et al. (2020) have reported that they have created an in‐vitro beads induced adhesion assay to mimic the postsurgical adhesions. This is a novel approach for developing an in‐vitro adhesion formation model. 15 Most of the studies listed in Table 2 used an in‐vivo approach as it provided a more standardized experimental model to study the combined impacts of inflammatory, coagulative, or fibroblastic activities on adhesion formation.
While anti‐adhesion membranes have been developed and used in clinical settings, there is still much research to be performed to fully understand the mechanisms of adhesion formation and develop effective solutions that prevent adhesion formation. There are also challenges associated with ensuring the safety and efficacy of anti‐adhesive biomaterials, particularly in long‐term applications. Ongoing research efforts are focused on addressing these challenges and developing novel biomedical devices that can prevent adhesion more safely and effectively.
4. CONCLUSION
In this paper, we have written an overview of the peritoneum anatomy, the pathophysiology of adhesion formation, as well as current strategies for preventing postsurgical adhesions. Based on our current understanding, postsurgical adhesions are triggered by trauma or tissue injury. Initial blood loss causes histamine release and increases the permeability of the vessels, followed by initiation of inflammatory response. At the same time, tissue hypoxia contributes to the mesothelial cells' stress and the release of the protein that generates inflammatory exudate. The wound‐healing process involves fibrinolysis and the coagulation cascade. Ischemia has been identified as an important factor that suppresses fibrinolytic activity and decreases fibroblasts infiltration resulting in the formation of persisting fibrin bands and adherent scar tissue.
Based on our current understanding of cellular and molecular adhesion mechanisms, different research studies have attempted to solve the issue by incorporating nanoparticles, anti‐inflammatory drugs, as well as fibrinolytic agents to intervene in the adhesion formation process. Several researchers have shown the positive results using animal studies either by applying nano‐micelles to deliver drugs or by using anti‐inflammatory drugs or fibrinolytic agents to modify or limit the adhesion cascades. However, due to the interdisciplinary nature of the research, no studies have designed the next‐generation anti‐adhesion barrier, nor have they been able to generate a clear understanding of the adhesion mechanism. Future studies will focus on utilizing a biomedical engineering platform to combine our current perspectives with the unique properties of advanced biomaterials using nanotechnology to develop a more effective and efficient anti‐adhesion barrier that can improve surgical outcomes.
AUTHOR CONTRIBUTIONS
Jiahui Chen: Conceptualization (lead); Writing – original draft (lead); review and editing (lead); Visualization (lead). Xiaoqi Tang: Writing – original draft (equal). Ziyu Wang: Writing – original draft (supporting). Arielle Perez: Resources (equal). Benjamin Yao: Writing – review and editing (equal). Ke Huang: Writing – review and editing (equal). Yang Zhang: Funding acquisition (equal); writing – review and editing (equal). Martin W King: Funding acquisition (equal); resources (equal); supervision (equal); writing – original draft (equal); writing – review and editing (equal).
FUNDING INFORMATION
We acknowledge the generous support from the National Institutes of Health (R21GM141675, R21GM141675) and the North Carolina Textile Foundation.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1002/btm2.10565.
Chen J, Tang X, Wang Z, et al. Techniques for navigating postsurgical adhesions: Insights into mechanisms and future directions. Bioeng Transl Med. 2023;8(6):e10565. doi: 10.1002/btm2.10565
Contributor Information
Jiahui Chen, Email: jchen75@ncsu.edu.
Martin W. King, Email: mwking2@ncsu.edu.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Lauder CIW, Garcea G, Strickland A, Maddern GJ. Abdominal adhesion prevention: still a sticky subject. Dig Surg. 2010;27(5):347‐358. doi: 10.1159/000314805 [DOI] [PubMed] [Google Scholar]
- 2. Menzies D, Ellis H. Intestinal obstruction from adhesions‐how big is the problem? Ann R Coll Surg Engl. 1990;72:60–63. [PMC free article] [PubMed] [Google Scholar]
- 3. Hooker GD, Taylor BM, Driman DK. Prevention of adhesion formation with use of sodium hyaluronate–based bioresorbable membrane in a rat model of ventral hernia repair with polypropylene mesh—a randomized, controlled study. Surgery. 1999;125(2):211‐216. doi: 10.1016/S0039-6060(99)70267-9 [DOI] [PubMed] [Google Scholar]
- 4. Schreinemacher MHF, van Barneveld KWY, Dikmans REG, Gijbels MJJ, Greve JWM, Bouvy ND. Coated meshes for hernia repair provide comparable intraperitoneal adhesion prevention. Surg Endosc. 2013;27(11):4202‐4209. doi: 10.1007/s00464-013-3021-5 [DOI] [PubMed] [Google Scholar]
- 5. Elliott MP, Juler GL. Comparison of Marlex mesh and microporous teflon sheets when used for hernia repair in the experimental animal. Am J Surg. 1979;137(3):342‐344. doi: 10.1016/0002-9610(79)90063-1 [DOI] [PubMed] [Google Scholar]
- 6. Bellón JM, Buján J, Contreras LA, Carrera‐San Martín A, Jurado F. Comparison of a new type of polytetrafluoroethylene patch (Mycro mesh) and polypropylene prosthesis (Marlex) for repair of abdominal wall defects. J Am Coll Surg. 1996;183(1):11‐18. [PubMed] [Google Scholar]
- 7. Mangir N, Roman S, Chapple CR, MacNeil S. Complications related to use of mesh implants in surgical treatment of stress urinary incontinence and pelvic organ prolapse: infection or inflammation? World J Urol. 2020;38(1):73‐80. doi: 10.1007/s00345-019-02679-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. MacFie J. Do intra‐abdominal adhesions cause pain?: do intra‐abdominal adhesions cause pain? Br J Surg. 2018;105(1):9‐10. doi: 10.1002/bjs.10731 [DOI] [PubMed] [Google Scholar]
- 9. Brüggmann D, Tchartchian G, Wallwiener M, Münstedt K, Tinneberg HR, Hackethal A. Intra‐abdominal Adhesions. Dtsch Aerztebl Int. 2010;5:769‐775. doi: 10.3238/arztebl.2010.0769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Penzias A, Bendikson K, Falcone T, et al. Postoperative adhesions in gynecologic surgery: a committee opinion. Fertil Steril. 2019;112(3):458‐463. doi: 10.1016/j.fertnstert.2019.06.027 [DOI] [PubMed] [Google Scholar]
- 11. diZerega GS. Peritoneal repair and postsurgical adhesion formation. Hum Reprod Update. 2001;7(6):547‐555. doi: 10.1093/humupd/7.6.547 [DOI] [PubMed] [Google Scholar]
- 12. Krielen P, Stommel MWJ, ten Broek RPG, van Goor H. Adhesive small bowel obstruction in the minimally invasive era. In: Garbuzenko DV, ed. Intestinal Obstructions. IntechOpen; 2020:Ch. 2. doi: 10.5772/intechopen.91896 [DOI] [Google Scholar]
- 13. Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Peritoneal Adhesions: etiology, pathophysiology, and clinical significance: recent advances in prevention and management. Dig Surg. 2001;18(4):260‐273. [DOI] [PubMed] [Google Scholar]
- 14. Rinkevich Y. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat Cell Biol. 2012;14:14‐1260. doi: 10.1038/ncb2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fischer A, Koopmans T, Ramesh P, et al. Postsurgical adhesions are triggered by calcium‐dependent membrane bridges between mesothelial surfaces. Nat Commun. 2020;11(1):3068. doi: 10.1038/s41467-020-16893-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsai JM, Sinha R, Seita J, et al. Surgical adhesions in mice are derived from mesothelial cells and can be targeted by antibodies against mesothelial markers. Sci Transl Med. 2018;10(469):eaan6735. doi: 10.1126/scitranslmed.aan6735 [DOI] [PubMed] [Google Scholar]
- 17. DiZerega GS. Peritoneum, peritoneal healing, and adhesion formation. In: Peritoneal Surgery. Springer; 2000;3‐37. doi: 10.1007/978-1-4612-1194-5 [DOI] [Google Scholar]
- 18. Chen YT, Chang YT, Pan SY, et al. Lineage tracing reveals distinctive fates for mesothelial cells and submesothelial fibroblasts during peritoneal injury. J Am Soc Nephrol. 2014;25(12):2847‐2858. doi: 10.1681/ASN.2013101079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raftery A. Regeneration of parietal and visceral peritoneum‐electron microscopical study. J Anat. 1973;115:375‐392. [PMC free article] [PubMed] [Google Scholar]
- 20. Hu Q, Xia X, Kang X, et al. A review of physiological and cellular mechanisms underlying fibrotic postoperative adhesion. Int J Biol Sci. 2021;17(1):298‐306. doi: 10.7150/ijbs.54403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yáñez‐Mó M, Ramírez‐Huesca M, Jiménez‐Heffernan JA, et al. Peritoneal dialysis and epithelial‐to‐mesenchymal transition of mesothelial cells. New Engl J Med. 2003;348:11‐413. [DOI] [PubMed] [Google Scholar]
- 22. Sandoval P, Jiménez‐Heffernan JA, Guerra‐Azcona G, et al. Mesothelial‐to‐mesenchymal transition in the pathogenesis of postsurgical peritoneal adhesions: mesothelial‐to‐mesenchymal transition and peritoneal adhesions. J Pathol. 2016;239(1):48‐59. doi: 10.1002/path.4695 [DOI] [PubMed] [Google Scholar]
- 23. Cheong YC. Peritoneal healing and adhesion formation/reformation. Hum Reprod Update. 2001;7(6):556‐566. doi: 10.1093/humupd/7.6.556 [DOI] [PubMed] [Google Scholar]
- 24. Dievernich A, Achenbach P, Davies L, Klinge U. Characterization of innate and adaptive immune cells involved in the foreign body reaction to polypropylene meshes in the human abdomen. Hernia. 2022;26(1):309‐323. doi: 10.1007/s10029-021-02396-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kamel RM. Prevention of postoperative peritoneal adhesions. Eur J Obstet Gynecol Reprod Biol. 2010;150(2):111‐118. doi: 10.1016/j.ejogrb.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 26. Lifecore Biomedical, Inc . Safety and Effectiveness data. Safety and effectiveness data (absorbable adhesion barrier). 2023. Published 2000. https://www.accessdata.fda.gov/cdrh_docs/pdf/P990015b.pdf
- 27. González‐Quintero VH, Cruz‐Pachano FE. Preventing adhesions in obstetric and gynecologic surgical procedures. Rev Obstet Gynecol. 2009;2(1):38‐45. [PMC free article] [PubMed] [Google Scholar]
- 28. Food and Drug Administration (FDA) . Docket Number FDA‐2015‐P‐2375. July 7, 2015. https://www.citizen.org/wp-content/uploads/2266_FDA-Final-Response-Seprafilm_January-29-2021.pdf
- 29. Leitao MM, Natenzon A, Abu‐Rustum NR, et al. Postoperative intra‐abdominal collections using a sodium hyaluronate‐carboxymethylcellulose (HA‐CMC) barrier at the time of laparotomy for ovarian, fallopian tube, or primary peritoneal cancers. Gynecol Oncol. 2009;115(2):204‐208. doi: 10.1016/j.ygyno.2009.08.005 [DOI] [PubMed] [Google Scholar]
- 30. Krill LS, Ueda SM, Gerardi M, Bristow RE. Analysis of postoperative complications associated with the use of anti‐adhesion sodium hyaluronate‐carboxymethylcellulose (HA‐CMC) barrier after cytoreductive surgery for ovarian, fallopian tube and peritoneal cancers. Gynecol Oncol. 2011;120(2):220‐223. doi: 10.1016/j.ygyno.2010.10.037 [DOI] [PubMed] [Google Scholar]
- 31. Bashir S, Ananth CV, Lewin SN, et al. Utilization and safety of sodium hyaluronate‐carboxymethylcellulose adhesion barrier. Dis Colon Rectum. 2013;56(10):1174‐1184. doi: 10.1097/DCR.0b013e31829ec889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sikirica V, Bapat B, Candrilli SD, Davis KL, Wilson M, Johns A. The inpatient burden of abdominal and gynecological adhesiolysis in the US. BMC Surg. 2011;11(1):13. doi: 10.1186/1471-2482-11-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ray NF, Denton WG, Thamer M, Henderson SC, Perry S. Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. J Am Coll Surg. 1998;186:186‐189. doi: 10.1016/S1072-7515(97)00127-0 [DOI] [PubMed] [Google Scholar]
- 34. Van den Beukel BAW, de Ree R, van Leuven S, et al. Surgical treatment of adhesion‐related chronic abdominal and pelvic pain after gynaecological and general surgery: a systematic review and meta‐analysis. Hum Reprod Update. 2017;23(3):276‐288. doi: 10.1093/humupd/dmx004 [DOI] [PubMed] [Google Scholar]
- 35. Arung W, Drion P, Cheramy JP, et al. Intraperitoneal adhesions after open or laparoscopic abdominal procedure: an experimental study in the rat. J Laparoendosc Adv Surg Tech A. 2012;22(7):651‐657. doi: 10.1089/lap.2012.0102 [DOI] [PubMed] [Google Scholar]
- 36. Ellis H. Adhesion‐related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet Lond Engl. 1999;353:353‐1480. doi: 10.1016/S0140-6736(98)09337-4 [DOI] [PubMed] [Google Scholar]
- 37. Anderson JM. Biological responses to materials. Annu Rev Mat Res. 2001;31(1):81‐110. doi: 10.1146/annurev.matsci.31.1.81 [DOI] [Google Scholar]
- 38. Tennyson L, Rytel M, Palcsey S, Meyn L, Liang R, Moalli P. Characterization of the T‐cell response to polypropylene mesh in women with complications. Am J Obstet Gynecol. 2019;220(2):187.e1‐187.e8. doi: 10.1016/j.ajog.2018.11.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klinge U, Dietz U, Fet N, Klosterhalfen B. Characterisation of the cellular infiltrate in the foreign body granuloma of textile meshes with its impact on collagen deposition. Hernia. 2014;18(4):571‐578. doi: 10.1007/s10029-014-1220-1 [DOI] [PubMed] [Google Scholar]
- 40. Artsen AM, Rytel M, Liang R, et al. Mesh induced fibrosis: the protective role of T regulatory cells. Acta Biomater. 2019;96:203‐210. doi: 10.1016/j.actbio.2019.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Artsen AM, Liang R, Meyn L, et al. T regulatory cells and TGF‐β1: predictors of the host response in mesh complications. Acta Biomater. 2020;115:127‐135. doi: 10.1016/j.actbio.2020.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Klosterhalfen B, Klinge U. Retrieval study at 623 human mesh explants made of polypropylene – impact of mesh class and indication for mesh removal on tissue reaction. J Biomed Mater Res. 2013;101(8):1393‐1399. doi: 10.1002/jbm.b.32958 [DOI] [PubMed] [Google Scholar]
- 43. Isaza‐Restrepo A, Martin‐Saavedra JS, Velez‐Leal JL, Vargas‐Barato F, Riveros‐Dueñas R. The peritoneum: beyond the tissue – a review. Front Physiol. 2018;9:738. doi: 10.3389/fphys.2018.00738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Baal JOAM, Van de Vijver KK, Nieuwland R, et al. The histophysiology and pathophysiology of the peritoneum. Tissue Cell. 2017;49(1):95‐105. doi: 10.1016/j.tice.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 45. Eroschenko VP, Fiore MSH d. Di Fiore's Atlas of Histology with Functional Correlations. 9th ed. Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 46. Capobianco A, Cottone L, Monno A, Manfredi AA, Rovere‐Querini P. The peritoneum: healing, immunity, and diseases. J Pathol. 2017;243(2):137‐147. doi: 10.1002/path.4942 [DOI] [PubMed] [Google Scholar]
- 47. Saed GM, Diamond MP. Molecular characterization of postoperative adhesions: the adhesion phenotype. J Am Assoc Gynecol Laparosc. 2004;11(3):307‐314. doi: 10.1016/S1074-3804(05)60041-2 [DOI] [PubMed] [Google Scholar]
- 48. Blaustein A. Peritoneal mesothelium and ovarian surface cells‐shared characteristics. Int J Gynecol Pathol. 1984;3(4):361‐375. [PubMed] [Google Scholar]
- 49. Beck DE. The role of Seprafilm bioresorbable membrane in adhesion prevention. Eur J Surg Suppl. 1997;577:49. [PubMed] [Google Scholar]
- 50. Weibel MA, Majno G. Peritoneal adhesions and their relation to abdominal surgery. A postmortem study. Am J Surg. 1973;126(3):345‐353. doi: 10.1016/S0002-9610(73)80123-0 [DOI] [PubMed] [Google Scholar]
- 51. Liao J, Li X, Fan Y. Prevention strategies of postoperative adhesion in soft tissues by applying biomaterials: based on the mechanisms of occurrence and development of adhesions. Bioact Mater. 2023;26:387‐412. doi: 10.1016/j.bioactmat.2023.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lin QY, Lang PP, Zhang YL, et al. Pharmacological blockage of ICAM‐1 improves angiotensin II‐induced cardiac remodeling by inhibiting adhesion of LFA‐1(+) monocytes. Am J Physiol Heart Circ Physiol. 2019;317(6):H1301‐H1311. doi: 10.1152/ajpheart.00566.2019 [DOI] [PubMed] [Google Scholar]
- 53. Hyun DM, Park CH, Kim Y, Choi SS. Successful electrode insertion for spinal cord stimulation after balloon adhesiolysis in a patient with epidural adhesion—a case report. Anesthesia Pain Med. 2021;16(2):191‐195. doi: 10.17085/apm.20084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wong JKF, Lui YH, Kapacee Z, Kadler KE, Ferguson MWJ, McGrouther DA. The cellular biology of flexor tendon adhesion formation: an old problem in a new paradigm. Am J Pathol. 2009;175(5):1938‐1951. doi: 10.2353/ajpath.2009.090380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zindel J, Peiseler M, Hossain M, et al. Primordial GATA6 macrophages function as extravascular platelets in sterile injury. Science. 2021;371(6533):1013. doi: 10.1126/science.abe0595 [DOI] [PubMed] [Google Scholar]
- 56. Herrick SE, Wilm B. Post‐surgical peritoneal scarring and key molecular mechanisms. Biomolecules. 2021;11(5):692. doi: 10.3390/biom11050692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mutsaers SE, Prêle CMA, Pengelly S, Herrick SE. Mesothelial cells and peritoneal homeostasis. Fertil Steril. 2016;106(5):1018‐1024. doi: 10.1016/j.fertnstert.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 58. Tsai JM, Shoham M, Fernhoff NB, et al. Neutrophil and monocyte kinetics play critical roles in mouse peritoneal adhesion formation. Blood Adv. 2019;3(18):2713‐2721. doi: 10.1182/bloodadvances.2018024026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Diamond MP. Reduction of postoperative adhesion development. Fertil Steril. 2016;106(5):994‐997.e1. doi: 10.1016/j.fertnstert.2016.08.029 [DOI] [PubMed] [Google Scholar]
- 60. Capella‐Monsonís H, Kearns S, Kelly J, Zeugolis DI. Battling adhesions: from understanding to prevention. BMC Biomed Eng. 2019;1(1):5. doi: 10.1186/s42490-019-0005-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cannata A, Petrella D, Russo CF, et al. Postsurgical intrapericardial adhesions: mechanisms of formation and prevention. Ann Thorac Surg. 2013;95(5):1818‐1826. doi: 10.1016/j.athoracsur.2012.11.020 [DOI] [PubMed] [Google Scholar]
- 62. Adhesions RB. Preventive strategies. Eur J Surg. 1997;163:32‐39. [PubMed] [Google Scholar]
- 63. Han ES, Scheib SA, Patzkowsky KE, Simpson K, Wang KC. The sticky business of adhesion prevention in minimally invasive gynecologic surgery. Curr Opin Obstet Gynecol. 2017;29(4):266‐275. doi: 10.1097/GCO.0000000000000372 [DOI] [PubMed] [Google Scholar]
- 64. Braun KM, Diamond MP. The biology of adhesion formation in the peritoneal cavity. Semin Pediatr Surg. 2014;23(6):336‐343. doi: 10.1053/j.sempedsurg.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 65. Fortin CN, Saed GM, Diamond MP. Predisposing factors to post‐operative adhesion development. Hum Reprod Update. 2015;21(4):536‐551. doi: 10.1093/humupd/dmv021 [DOI] [PubMed] [Google Scholar]
- 66. Koninckx PRMD, PhD GVMD, PhD UAMD, Adamyan LMD. Role of the peritoneal cavity in the prevention of postoperative adhesions, pain, and fatigue. Fertil Steril. 2016;106(5):998‐1010. doi: 10.1016/j.fertnstert.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 67. Haslinger B, Kleemann R, Toet KH, Kooistra T. Simvastatin suppresses tissue factor expression and increases fibrinolytic activity in tumor necrosis factor—activated human peritoneal mesothelial cells. Kidney Int. 2003;63(6):2065‐2074. doi: 10.1046/j.1523-1755.2003.t01-2-00004.x [DOI] [PubMed] [Google Scholar]
- 68. Herrick SE, Mutsaers SE. Mesothelial progenitor cells and their potential in tissue engineering. Int J Biochem Cell Biol. 2004;36(4):621‐642. doi: 10.1016/j.biocel.2003.11.002 [DOI] [PubMed] [Google Scholar]
- 69. Hellebrekers BWJ, Kooistra T. Pathogenesis of postoperative adhesion formation. Br J Surg. 2011;98(11):1503‐1516. doi: 10.1002/bjs.7657 [DOI] [PubMed] [Google Scholar]
- 70. Saed GM, Diamond MP. Hypoxia‐induced irreversible up‐regulation of type I collagen and transforming growth factor‐beta1 in human peritoneal fibroblasts. Fertil Steril. 2002;78(1):144‐147. [DOI] [PubMed] [Google Scholar]
- 71. Saed GM, Collins KL, Diamond MP. Transforming growth factors beta1, beta2 and beta3 and their receptors are differentially expressed in human peritoneal fibroblasts in response to hypoxia. Am J Reprod Immunol. 1989;48(6):387‐393. [DOI] [PubMed] [Google Scholar]
- 72. Saed GM, Munkarah AR, Diamond MP. Cyclooxygenase‐2 is expressed in human fibroblasts isolated from intraperitoneal adhesions but not from normal peritoneal tissues. Fertil Steril. 2003;79(6):1404‐1408. doi: 10.1016/S0015-0282(03)00257-7 [DOI] [PubMed] [Google Scholar]
- 73. Saed GM, Kruger M, Diamond MP. Expression of transforming growth factor‐β and extracellular matrix by human peritoneal mesothelial cells and by fibroblasts from normal peritoneum and adhesions: effect of Tisseel. Wound Repair Regen. 2004;12(5):557‐564. doi: 10.1111/j.1067-1927.2004.012508.x [DOI] [PubMed] [Google Scholar]
- 74. Imudia A, Kumar S, Saed G, Diamond M. Pathogenesis of intra‐abdominal and pelvic adhesion development. Semin Reprod Med. 2008;26(4):289‐297. doi: 10.1055/s-0028-1082387 [DOI] [PubMed] [Google Scholar]
- 75. Ara C, Kirimlioglu H, Karabulut AB, et al. Protective effect of melatonin against oxidative stress on adhesion formation in the rat cecum and uterine horn model. Life Sci. 1973;77(12):1341‐1350. doi: 10.1016/j.lfs.2005.01.024 [DOI] [PubMed] [Google Scholar]
- 76. Ambler DR, Fletcher NM, Diamond MP, Saed GM. Effects of hypoxia on the expression of inflammatory markers IL‐6 and TNF‐a in human normal peritoneal and adhesion fibroblasts. Syst Biol Reprod Med. 2012;58(6):324‐329. doi: 10.3109/19396368.2012.713439 [DOI] [PubMed] [Google Scholar]
- 77. Awonuga AO, Belotte J, Abuanzeh S, Fletcher NM, Diamond MP, Saed GM. Advances in the pathogenesis of adhesion development: the role of oxidative stress. Reprod Sci. 2014;21(7):823‐836. doi: 10.1177/1933719114522550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hu C, Liu S, Zhang Y, et al. Long‐term drug release from electrospun fibers for in vivo inflammation prevention in the prevention of peritendinous adhesions. Acta Biomater. 2013;9(7):7381‐7388. doi: 10.1016/j.actbio.2013.03.040 [DOI] [PubMed] [Google Scholar]
- 79. Uyama N, Tsutsui H, Wu S, et al. Anti‐interleukin‐6 receptor antibody treatment ameliorates postoperative adhesion formation. Sci Rep. 2019;9(1):17558. doi: 10.1038/s41598-019-54175-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Foster DS, Marshall CD, Gulati GS, et al. Elucidating the fundamental fibrotic processes driving abdominal adhesion formation. Nat Commun. 2020;11(1):4061. doi: 10.1038/s41467-020-17883-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Loureiro J, Aguilera A, Borras‐Cuesta F, et al. Blocking TGF‐β1 protects the peritoneal membrane from dialysate‐induced damage. J Am Soc Nephrol. 2011;22(9):1682‐1695. doi: 10.1681/ASN.2010111197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Loureiro J, Sandoval P, del Peso G, et al. Tamoxifen ameliorates peritoneal membrane damage by blocking mesothelial to mesenchymal transition in peritoneal dialysis. PLoS One. 2013;8(4):e61165. doi: 10.1371/journal.pone.0061165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Strippoli R, Sandoval P, Moreno‐Vicente R, et al. Caveolin1 and YAP drive mechanically induced mesothelial to mesenchymal transition and fibrosis. Cell Death Dis. 2020;11(8):647. doi: 10.1038/s41419-020-02822-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wang G, Wu K, Li W, et al. Role of IL‐17 and TGF‐β in peritoneal adhesion formation after surgical trauma. Wound Repair Regen. 2014;22(5):631‐639. doi: 10.1111/wrr.12203 [DOI] [PubMed] [Google Scholar]
- 85. Zwicky SN, Stroka D, Zindel J. Sterile injury repair and adhesion formation at serosal surfaces. Front Immunol. 2021;12:684967. doi: 10.3389/fimmu.2021.684967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Watanabe J, Ishida F, Ishida H, et al. A prospective multi‐center registry concerning the clinical performance of laparoscopic colorectal surgery using an absorbable adhesion barrier (INTERCEED®) made of oxidized regenerated cellulose. Surg Today. 2019;49(10):877‐884. doi: 10.1007/s00595-019-01816-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Beck‐Schimmer B, Schwendener R, Pasch T, Reyes L, Booy C, Schimmer R. Alveolar macrophages regulate neutrophil recruitment in endotoxin‐induced lung injury. Respir Res. 2005;6(1):61. doi: 10.1186/1465-9921-6-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ar'Rajab A, Dawidson I, Sentementes J, Sikes P, Harris R, Mileski W. Enhancement of peritoneal macrophages reduces postoperative peritoneal adhesion formation. J Surg Res. 1995;58(3):307‐312. doi: 10.1006/jsre.1995.1048 [DOI] [PubMed] [Google Scholar]
- 89. Banasiewicz T, Horbacka K, Karoń J, et al. Preliminary study with SprayShield ™ adhesion barrier system in the prevention of abdominal adhesions. Wideochir Inne Tech Maloinwazyjne. 2013;8(4):301‐309. doi: 10.5114/wiitm.2011.34884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sindrilaru A, Scharffetter‐Kochanek K. Disclosure of the culprits: macrophages—versatile regulators of wound healing. Adv Wound Care. 2013;2(7):357‐368. doi: 10.1089/wound.2012.0407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Burnett SH, Beus BJ, Avdiushko R, Qualls J, Kaplan AM, Cohen DA. Development of peritoneal adhesions in macrophage depleted mice. J Surg Res. 2006;131(2):296‐301. doi: 10.1016/j.jss.2005.08.026 [DOI] [PubMed] [Google Scholar]
- 92. Park H, Baek S, Kang H, Lee D. Biomaterials to prevent post‐operative adhesion. Materials. 2020;13(14):3056. doi: 10.3390/ma13143056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. De Wilde RL, Devassy R, Ten BRPG, et al. The future of adhesion prophylaxis trials in abdominal surgery: an expert global consensus. J Clin Med. 2022;11(6):1476. doi: 10.3390/jcm11061476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Healthcare B. Summary of safety and effectiveness data premarket approval application, PMA. 2003.
- 95. Bian S, Hao L, Qiu X, et al. An injectable rapid‐adhesion and anti‐swelling adhesive hydrogel for hemostasis and wound sealing. Adv Funct Mater. 2022;32(46):2270260. doi: 10.1002/adfm.202270260 [DOI] [Google Scholar]
- 96. Summary of Safety and Effectiveness Data . REPEL‐CV® Bioresorbable Adhesion Barrier Premarket Approval Application. https://www.accessdata.fda.gov/cdrh_docs/pdf7/P070005B.pdf
- 97. Acunzo G. Effectiveness of auto‐cross‐linked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic adhesiolysis: a prospective, randomized, controlled study. Hum Reprod. 2003;18(9):1918‐1921. doi: 10.1093/humrep/deg368 [DOI] [PubMed] [Google Scholar]
- 98. Sikar EY, Sikar HE, Top H, Aygit AC. Effects of hyalobarrier gel and Seprafilm in preventing peritendinous adhesions following crush‐type injury in a rat model. Ulusal Travma Acil Cerrahi Derg. 2019;25(2):93‐98. doi: 10.5505/tjtes.2018.54007 [DOI] [PubMed] [Google Scholar]
- 99. Brown CB, Luciano AA, Martin D, Peers E, Scrimgeour A, diZerega GS. Adept (icodextrin 4% solution) reduces adhesions after laparoscopic surgery for adhesiolysis: a double‐blind, randomized, controlled study. Fertil Steril. 2007;88(5):1413‐1426. doi: 10.1016/j.fertnstert.2006.12.084 [DOI] [PubMed] [Google Scholar]
- 100. Menzies D, Pascual MH, Walz MK, et al. Use of icodextrin 4% solution in the prevention of adhesion formation following general surgery: from the multicentre ARIEL registry. Ann R Coll Surg Engl. 2006;88(4):375‐382. doi: 10.1308/003588406X114730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nordic Adhesion Prevention Study Group . The efficacy of Interceed(TC7) for prevention of reformation of postoperative adhesions on ovaries, fallopian tubes, and fimbriae in microsurgical operations for fertility: a multicenter study. Fertil Steril. 1995;63(4):709‐714. doi: 10.1016/S0015-0282(16)57470-6 [DOI] [PubMed] [Google Scholar]
- 102. Diamond MP, Burns EL, Accomando B, Mian S, Holmdahl L. Seprafilm® adhesion barrier: (1) a review of preclinical, animal, and human investigational studies. Gynecol Surg. 2012;9(3):237‐245. doi: 10.1007/s10397-012-0741-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Erdi M, Sandler A, Kofinas P. Polymer nanomaterials for use as adjuvant surgical tools. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023;e1889:1‐27. doi: 10.1002/wnan.1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wilczewska AZ, Niemirowicz K, Markiewicz KH, Car H. Nanoparticles as drug delivery systems. Pharmacol Rep. 2012;64(5):1020‐1037. doi: 10.1016/S1734-1140(12)70901-5 [DOI] [PubMed] [Google Scholar]
- 105. Liu S, Zhao J, Ruan H, et al. Antibacterial and anti‐adhesion effects of the silver nanoparticles‐loaded poly(l‐lactide) fibrous membrane. Mat Sci Eng C Mat Biol Appl. 2013;33(3):1176‐1182. doi: 10.1016/j.msec.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 106. Shalumon KT, Sheu C, Chen CH, et al. Multi‐functional electrospun antibacterial core‐shell nanofibrous membranes for prolonged prevention of postsurgical tendon adhesion and inflammation. Acta Biomater. 2018;72:121‐136. doi: 10.1016/j.actbio.2018.03.044 [DOI] [PubMed] [Google Scholar]
- 107. Liu S, Qin M, Hu C, et al. Tendon healing and anti‐adhesion properties of electrospun fibrous membranes containing bFGF loaded nanoparticles. Biomaterials. 2013;34(19):4690‐4701. doi: 10.1016/j.biomaterials.2013.03.026 [DOI] [PubMed] [Google Scholar]
- 108. Yang QQ, Shao YX, Zhang LZ, Zhou YL. Therapeutic strategies for flexor tendon healing by nanoparticle‐mediated co‐delivery of bFGF and VEGFA genes. Colloids Surf B Biointerfaces. 2018;164:165‐176. doi: 10.1016/j.colsurfb.2018.01.031 [DOI] [PubMed] [Google Scholar]
- 109. Zhou Y, Zhu C, Wu YF, Zhang L, Tang JB. Effective modulation of transforming growth factor‐β1 expression through engineered microRNA‐based plasmid‐loaded nanospheres. Cytotherapy. 2015;17(3):320‐329. doi: 10.1016/j.jcyt.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 110. Liu C, Tian S, Bai J, et al. Regulation of ERK1/2 and SMAD2/3 pathways by using multi‐layered electrospun PCL‐amnion nanofibrous membranes for the prevention of postsurgical tendon adhesion. Int J Nanomedicine. 2020;15:927‐942. doi: 10.2147/IJN.S231538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bianchi E, Boekelheide K, Sigman M, Lamb DJ, Hall SJ, Hwang K. Ghrelin inhibits post‐operative adhesions via blockage of the TGF‐beta signaling pathway. PLoS One. 2016;11(4):e0153968. doi: 10.1371/journal.pone.0153968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Honjo K, Munakata S, Tashiro Y, et al. Plasminogen activator inhibitor‐1 regulates macrophage‐dependent postoperative adhesion by enhancing EGF‐HER1 signaling in mice. FASEB J. 2017;31(6):2625‐2637. doi: 10.1096/fj.201600871RR [DOI] [PubMed] [Google Scholar]
- 113. He T, Zou C, Song L, et al. Improving antiadhesion effect of thermosensitive hydrogel with sustained release of tissue‐type plasminogen activator in a rat repeated‐injury model. ACS Appl Mater Interfaces. 2016;8(49):33514‐33520. doi: 10.1021/acsami.6b13184 [DOI] [PubMed] [Google Scholar]
- 114. Kawanishi K, Yamato M, Sakiyama R, Okano T, Nitta K. Peritoneal cell sheets composed of mesothelial cells and fibroblasts prevent intra‐abdominal adhesion formation in a rat model. J Tissue Eng Regen Med. 2016;10(10):855‐866. doi: 10.1002/term.1860 [DOI] [PubMed] [Google Scholar]
- 115. Cahill RA. Prevention of intra‐abdominal adhesions using the antiangiogenic COX‐2 inhibitor celecoxib. Ann Surg. 2006;244(2):327‐328. doi: 10.1097/01.sla.0000230021.24473.cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wei G, Zhou C, Wang G, Fan L, Wang K, Li X. Keratinocyte growth factor combined with a sodium hyaluronate gel inhibits postoperative intra‐abdominal adhesions. Int J Mol Sci. 2016;17(10):1611. doi: 10.3390/ijms17101611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yang L, Lian Z, Zhang B, et al. Effect of ligustrazine nanoparticles on Th1/Th2 balance by TLR4/MyD88/NF‐kappa B pathway in rats with postoperative peritoneal adhesion. BMC Surg. 2021;21(1):211. doi: 10.1186/s12893-021-01201-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Yeo Y, Ito T, Bellas E, Highley CB, Marini R, Kohane DS. In situ cross‐linkable hyaluronan hydrogels containing polymeric nanoparticles for preventing postsurgical adhesions. Ann Surg. 2007;245(5):819‐824. doi: 10.1097/01.sla.0000251519.49405.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Can S, Kirpinar G, Dural O, et al. Efficacy of a new crosslinked hyaluronan gel in the prevention of intrauterine adhesions. J Soc Laparoendosc Surg. 2018;22(4):1‐6. doi: 10.4293/JSLS.2018.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Docherty JR, McCormick PA. A carboxymethylcellulose/heparin combination for the prevention of surgical adhesions. J Surg Res. 2017;213:228‐233. doi: 10.1016/j.jss.2017.02.066 [DOI] [PubMed] [Google Scholar]
- 121. Zhang S, Xu Z, Wen X, Wei C. A nano chitosan membrane barrier prepared via Nanospider technology with non‐toxic solvent for peritoneal adhesions' prevention. J Biomater Appl. 2021;36(2):321‐331. doi: 10.1177/08853282211008109 [DOI] [PubMed] [Google Scholar]
- 122. Shahzamani S, Jahandideh A, Abedi GR, Akbarzadeh A, Hesaraki S. Effect of N‐acetyl‐cysteine nanoparticles on intra‐abdominal adhesion after laparotomy in rats. Pol J Vet Sci. 2019;22(3):581‐588. doi: 10.24425/pjvs.2019.129967 [DOI] [PubMed] [Google Scholar]
- 123. Oh G, Choi I, Park WS, Jung W. Phlorotannin‐coated poly (ε‐caprolactone) film as a potential material for postsurgical adhesion prevention. J Appl Polym Sci. 2022;139(32):1‐11. doi: 10.1002/app.52756 [DOI] [Google Scholar]
- 124. Bayhan ZMD, Zeren SMD, Kocak FEMD, et al. Antiadhesive and anti‐inflammatory effects of pirfenidone in postoperative intra‐abdominal adhesion in an experimental rat model. J Surg Res. 2016;201(2):348‐355. doi: 10.1016/j.jss.2015.11.033 [DOI] [PubMed] [Google Scholar]
- 125. Kuckelman JP, Kononchik J, Smith J, et al. Human‐derived amniotic membrane is associated with decreased postoperative intraperitoneal adhesions in a rat model. Dis Colon Rectum. 2018;61(4):484‐490. doi: 10.1097/DCR.0000000000001037 [DOI] [PubMed] [Google Scholar]
- 126. Qiu R, Li J, Sun D, Li H, Qian F, Wang L. 20(S)‐Ginsenoside Rg3‐loaded electrospun membranes to prevent postoperative peritoneal adhesion. Biomed Microdevices. 2019;21(4):78. doi: 10.1007/s10544-019-0425-6 [DOI] [PubMed] [Google Scholar]
- 127. Zhang W, Li X, Franchini MC, et al. Controlled release of curcumin from curcumin‐loaded nanomicelles to prevent peritendinous adhesion during Achilles tendon healing in rats. Int J Nanomedicine. 2016;11:2873‐2881. doi: 10.2147/IJN.S103867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Liu W, Qin F, Wu F, et al. Sodium aescinate significantly suppress postoperative peritoneal adhesion by inhibiting the RhoA/ROCK signaling pathway. Phytomedicine (Stuttgart). 2020;69:153193. doi: 10.1016/j.phymed.2020.153193 [DOI] [PubMed] [Google Scholar]
- 129. Stapleton LM, Steele AN, Wang H, et al. Use of a supramolecular polymeric hydrogel as an effective post‐operative pericardial adhesion barrier. Nat Biomed Eng. 2019;3(8):611‐620. doi: 10.1038/s41551-019-0442-z [DOI] [PubMed] [Google Scholar]
- 130. Kraemer B, Wallwiener C, Rajab TK, Brochhausen C, Wallwiener M, Rothmund R. Standardised models for inducing experimental peritoneal adhesions in female rats. BioMed Res Int. 2014;2014:435056‐435058. doi: 10.1155/2014/435056 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


